Abstract
A galactose-specific lectin from Bauhinia monandra leaves (BmoLL) has been purified through ammonium sulfate fractionation followed by guar gel affinity chromatography column. This study aimed to evaluate the potential anti-inflammatory and antinociceptive activity of pure BmoLL in mice. Anti-inflammatory activity was evaluated by 1% carrageenan-induced inflammation in mice treated with BmoLL. Acetic acid-induced abdominal writhing and hot plate methods evaluated antinociceptive activity. BmoLL significantly inhibited the carrageenan-induced paw edema by 47% (30 mg/kg) and 60.5% (60 mg/kg); acetylsalicylic acid (ASA, 100 mg/kg) showed inhibition of 70.5%, in comparison to controls. Leukocyte migration, an immune response to the inflammation process, was significantly reduced in presence of BmoLL; in mice treated with ASA the decrease in leukocyte migration was similar to 15 mg/kg of the lectin. BmoLL at doses of 15, 30 and 60 mg/kg significantly reduced the number of animal contortions by 43.1, 50.1 and 71.3%, respectively. BmoLL leukocyte migration was significantly reduced; in mice treated with ASA the decrease in leukocyte migration was similar to 15 mg/kg of the lectin. BmoLL at doses of 15, 30 and 60 mg/kg significantly reduced the number of animal contortions by 43.1, 50.1 and 71.3%, respectively. The lectin (30 and 60 mg/kg) showed a significant effect in the hot plate assay. BmoLL anti-inflammatory and antinociceptive effects were dose-dependent. The search for new and natural compounds, with minimal side effects, to control pain and inflammation, is constantly increasing. BmoLL has great potential as a natural anti-inflammatory product that can be explored for pharmacological purposes.
Keywords: Analgesic potential, Bauhinia monandra, Galactose-specific lectin, Paw edema
Abbreviations: ASA, acetylsalicylic acid; BmoLL, Bauhinia monandra leaf lectin; BmoRoL, Bauhinia monandra roots lectin; CcL, Caulerpa cupressoides; CFAL, Clitoria fairchildiana; COX, cyclooxygenase; HA, hemagglutinating activity; PEG2, prostaglandins
Graphical abstract
Highlights
-
•
BmoLL inhibited the carrageenan-induced paw edema.
-
•
BmoLL significantly reduced the number of animal contortions.
-
•
BmoLL anti-inflammatory and antinociceptive effects in a dose dependent manner.
1. Introduction
Lectins are proteins or glycoproteins of non-immunogenic origin that possess carbohydrate-binding sites able to interact reversibly and specifically with sugars through hydrogen bonding, hydrophobic interactions and Van der Waals forces [1], [2]. These proteins have been isolated from plants, microorganisms, higher fungus, lichens and animals [3]. They are molecules of recognition ability within a cell, between cells or organisms, and the interactions between carbohydrates and lectins are an integral part of the host defense system [4]. In plants, lectins are present in different tissues [5] and act as defense proteins against phytophagous insects [6].
Studies focusing on the applications of plant lectins have identified biological activities that may confer diverse pharmacological properties to pure preparations obtained from several vegetal parts such as seeds [7], [8], bark [9] or leaves [10]. Lectins can be insecticide [11], [12] and, among others, have antifungal [13], [10], antibacterial [14], [10], [15], antiproliferative [16], antiplatelet aggregation [17], [18], anti-diabetic [19], antiparasite [20], [7], wound healing [21] and analgesic activities [22]. Also, these proteins from different sources showed anti-inflammatory [23], [4] and anti-nociceptive properties [4], [24]. A large amount of lectins are found in Leguminosae plants, mainly in the subfamilie Papilionoideae, but few lectins are from subfamilies Caesalpiniodeae and Mimosoideae [25], [26].
The genus Bauhinia (Caesalpiniodeae) is largely found in tropical regions, including Africa, Asia and South America; in Brazil, this plant is known as “cow's hoof” due to its leaf shapes [27]. Plants from this genus acted as anti-diabetic agents [28] and cause prolongation of blood coagulation clotting [29]. Bauhinia monandra is a plant popularly known in Brazil as “cow's hoof” and is widely used in folk medicine for diabetes treatment; leaf extracts of B. monandra are sources of natural antioxidants [30]. The Bauhinia lectins have been isolated from B. purpurea [31], B. monandra [32], B. variegata [33], [26], B. bauhinioides [34], B. forficata [18] and B. ungulata [8]. Two galactose-specific lectins were isolated from B. monandra; the first was reported by Coelho and Silva [27] and was purified from leaves (BmoLL); the other lectin was purified from secondary roots (BmoRoL) and showed termiticidal activity on Nasutitermes corniger and antifungal activities against phytopathogenic species of Fusarium [32]. Previous studies have reported some biological activities of BmoLL, such as an insecticidal action [11], but there is no much information about the pharmacological activity of this lectin.
The inflammatory process consists in a protective response to foreign and noxious stimulation and serves to eliminate the initial cause of cell injury as well as the necrotic cells resulting from the original aggression. This defense mechanism is constituted by an innate system of cellular and humoral responses to an injury [35], [36]. The classification of antinociceptive drugs is usually based on their mechanism of action either on the central nervous system or on the peripheral nervous system [36]. The therapies currently available to treat inflammation and pain are associated with side effects and low efficacy. Thus, there is a great demand for new and more effective anti-inflammatory compounds [4], [35]. Herbal medicines represent an alternative source of drugs to diseases treatments. The present study aimed to investigate the in vivo anti-inflammatory and antinociceptive potential of BmoLL in mice.
2. Materials and methods
2.1. Chemicals and reagents
Acetylsalicylic acid (ASA), propylene glycol, and λ-carrageenan were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Acetic acid, ether, chloroform, acetone, methanol and other solvents were from Merck (Darmstadt, Germany).
2.2. Sample collection and BmoLL purification
A voucher specimen of B. monandra, number 57462, is available at the Herbarium “Dárdano de Andrade Lima” of the “Instituto Agronômico de Pernambuco – IPA”, from Recife City, State of Pernambuco, Brazil. B. monandra leaves were collected from trees in Recife city, washed with distilled water, dried at 28 °C for 2–3 days, powdered, and extracted (10%, w/v) at 4 °C for 16 h in 10 mM citrate phosphate buffer containing 150 mM NaCl, pH 6.5. The extract was fractionated with ammonium sulfate (60% saturation) at 28 °C for 4 h; after centrifugation (12,000 × g, 4 °C, 15 min), the precipitate was ressuspended in distilled water and dialyzed in the buffer. The sample was applied into a guar gel affinity column made with guar gum (Guaran; Sigma Chemical Co., St. Louis, MO, USA); the column was previously equilibrated with the buffer. After removal of unbound proteins, BmoLL was eluted with 330 mM galactose in the buffer (flow rate of 0.3 mL/min), according to Coelho and Silva [27]. Hemagglutinating activity (HA) of BmoLL was evaluated according to Coelho et al. [2]; protein concentration was quantified [37] and purified lectin was stored at −20 °C.
2.3. Animals
Male Swiss white mice (Mus musculus), 60 days old, weighing 30 ± 5 g were obtained from the Laboratório de Imunopatologia Keizo Asami (LIKA) from the Universidade Federal de Pernambuco (UFPE). The animals were separated into groups (N = 6) and housed in cages at a constant temperature of 22 ± 2 °C, with 12 h dark–light cycle, with free access to standard chow (Labina, Agribands Brazil Ltd.) and water ad libitum. The animals were fasted for 4 h prior use for determination of anti-inflammatory and antinociceptive activities.
All experimental procedures were performed according to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and the study was approved by the Ethics Committee for Animal Care and Use (CEUA) of the Centro de Ciências Biológicas of UFPE (Of. number 259/10).
2.4. Assay of BmoLL anti-inflammatory activity in carrageenan-induced edema
Carrageenan was used to induce mice paw edema to evaluate the anti-inflammatory effect of BmoLL. The edema was induced by the injection of freshly prepared solution of 1% carrageenan (0.1 mL) in 0.9% NaCl, into the sub plantar tissue of the right-hind paw of each mouse, half hour prior to intraperitoneal administration of BmoLL [38]. Lectin doses were chosen according to a pilot experiment; 3 groups of animals (N = 6) were treated with 15, 30 and 60 mg/kg BmoLL (i.p.), a positive control group received 100 mg/kg acetylsalicylic acid (ASA), and the negative control group received just vehicle (0.9% NaCl).
The paw volume was measured by one caliper rule (Kanon – Staineless Mardened) at 0, 1, 2, 3 and 4 h immediately after the sub plantar injection of carrageenan, and the data obtained for each animal were recorded. Increase in paw thickness measured the difference in paw at 0 h and at the respective experimental hours. The results obtained for the various groups were reported as mean ± SD and expressed in millimeter. The percentage of inflammation inhibition was calculated according to equation (1) (Eq. (1)), where Vf and Vi represent the initial and final paw thickness, respectively.
| (1) |
2.5. Assay of anti-inflammatory activity by carrageenan-induced peritonitis
Peritonitis was evaluated as described by Foster et al. [39]. Groups of animals (N = 6) were deprived of food overnight and pre-treated intraperitoneally with 0.9% NaCl (negative control), ASA (positive control, 100 mg/kg) and BmoLL (15, 30 and 60 mg/kg). After 1 h the animals received an intraperitoneal (i.p.) injection of 1% carrageenan (1 mL). Four hours later, the animals were sacrificed by cervical dislocation, saline (2 mL) containing 1 mM EDTA was injected into the peritoneal cavity and immediately a brief massage was made; the peritoneal exudate was withdrawn and used for total leukocyte cell, mainly neutrophils, counting in a Cell Counter (ABX MICROS 60). The results were expressed as the number of leukocytes × 103/mm3.
2.6. Assay of antinociceptive activity by acetic acid-induced abdominal writhing
Abdominal writhing was based on a contraction from the abdominal muscle together with a stretching, induced by agent nociceptive (0.8% acetic acid) with intraperitoneal injection [40]. Groups of male mice received intraperitoneal injection of BmoLL (15, 30 and 60 mg/kg), ASA (100 mg/kg) and saline (negative control group) 1 h prior to administration of acetic acid (0.8%, i.p.). The number of writhing reflexes was counted for 20 min. A significant reduction of writhes in animals treated with BmoLL compared to those in the negative control group was considered as an antinociceptive response.
2.7. Assay of antinociceptive activity by hot plate test
The hot plate test was done applying the central analgesic activity against thermal stimuli [41]. The animals were individually placed in a hot plate heated at 55 ± 1 °C and the response was measured at 0, 30, 60, 90 and 120 min after the first thermal stimulus. The maximum exposure time of the animal on the hot plate was 60 s to avoid damage. A negative control group was treated with saline, a positive control group was treated with morphine (1 mg/kg, i.p.), and the assayed groups received BmoLL (15, 30 and 60 mg/kg; i.p.) 1 h before performing experiments.
2.8. Statistical analysis
The results obtained for the various groups were presented as mean ± standard deviation (SD). Statistical evaluation was carried out by one-way analysis of variance ANOVA, followed by Tukey's post hoc test with the significance level set at P < 0.05 using PRISMA (GraphPad Software, Inc., San Diego, CA, version 5.01).
3. Results
3.1. Anti-inflammatory activity by carrageenan-induced paw edema and carrageenan-induced peritonitis
Injection of 1% carrageenan into the mice hind paw induced edema and reached a maximum level at 1 h after administration, and then the edema decreased over the subsequent hours, except when BmoLL was administered at a dose of 15 mg/kg. Administration of BmoLL showed a significant (P < 0.05) anti-inflammatory activity (BmoLL 30 and 60 mg/kg) reducing the paw edema in a dose dependent manner (Table 1). Two hours after carrageenan application a reduction of the paw edema was observed with animals treated by BmoLL 30 and 60 mg/kg. After 4 h, a significant paw edema reduction was observed with BmoLL in these doses. The animals treated with ASA 100 mg/kg (positive control) showed the best result after 3 h of carrageenan application.
Table 1.
Effect of B. monandra leaf lectin (BmoLL) in the thickness of the right posterior paw edema of mice.
| Treatment | Edema thickness (mm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | Edema inhibition (%) | T3 | Edema inhibition (%) | T4 | Edema inhibition (%) | |
| Negative control | 4.15 ± 0.14 | 5.27 ± 0.33 | 5.04 ± 0.28 | – | 4.79 ± 0.11 | – | 4.71 ± 0.07 | – |
| ASA | 4.16 ± 0.18 | 4.91 ± 0.15 | 4.62 ± 0.18 | 48 | 4.35 ± 0.16¥ | 70 | 4.35 ± 0.14¥ | 65 |
| BmoLL (15 mg/kg) | 3.86 ± 0.51 | 3.61 ± 0.22 | 4.18 ± 0.16 | – | 4.29 ± 0.26¥ | 32.6 | 4.19 ± 0.25¥ | 40.9 |
| BmoLL (30 mg/kg) | 2.92 ± 0.2 | 3.92 ± 0.2 | 3.83 ± 0.26* | 18 | 3.25 ± 0.27* | 47.3 | 3.08 ± 0.20* | 69.6 |
| BmoLL (60 mg/kg) | 2.92 ± 0.2 | 4.5 ± 0.77 | 3.83 ± 0.41* | 17 | 3.17 ± 0.26* | 60.5 | 3.08 ± 0.20* | 69.6 |
Values are expressed as mean ± SD. ANOVA: P < 0.05; *P < 0.001, in relation to control group. Animals treated 1 h before induction of edema. T: time (h). T0: Animals with paw in normal conditions, without treatment; T1: Animal in treatment (after 1 h of carrageenan application) and with paw edema beginning; T2, T3 and T4: Animals in treatment (after 2, 3 and 4 h of carrageenan application) and with the paw edema reduction. ASA: Acetylsalicylic acid.
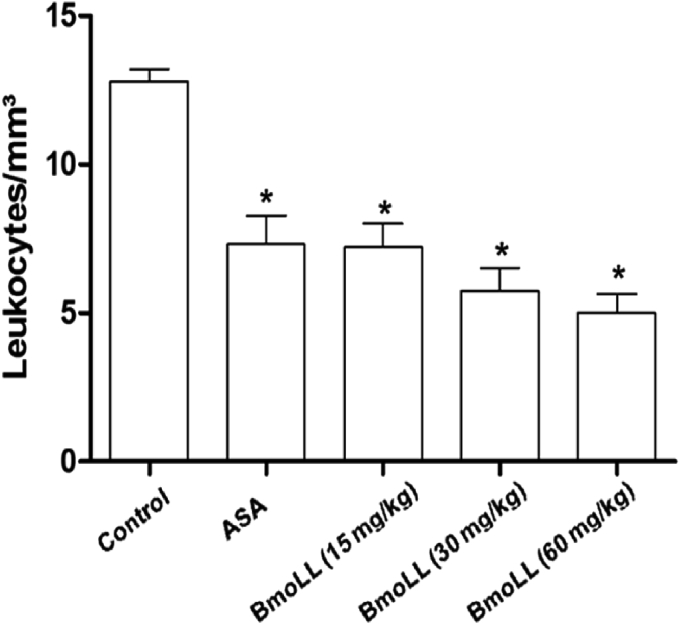
Leukocyte migration through activated venular walls is a fundamental immune response to an inflammation process that is prerequisite to the entry of effector cells such as neutrophils, monocytes, and effector T cells to sites of infection, injury, and stress [42]. The induction of inflammation with carrageenan into the peritoneal cavity caused a migration of leukocytes. In a dose-dependent manner, BmoLL (15, 30 and 60 mg/kg) significantly reduced the leukocyte migration (43.5, 54.9, and 60.9%, respectively) when compared with the negative control group (saline), as shown in Fig. 1. In the peritoneal fluid of the group treated with ASA (100 mg/kg) there was a decrease in leukocyte cellular migration, similar to the group treated with BmoLL 15 mg/kg. BmoLL showed a significant (P < 0.05) anti-inflammatory activity in the mice peritoneal cavity.
Fig. 1.
Effect of BmoLL in leukocyte migration in the model of carrageenan-induced peritonitis. Negative control: NaCl 0.9%; ASA: acetylsalicylic acid (positive control). Values are expressed as mean ± standard deviation. Significant difference in comparison to the control group: *P < 0.0001.
3.2. Antinociceptive activity by acetic acid-induced abdominal writhing and by hot plate test
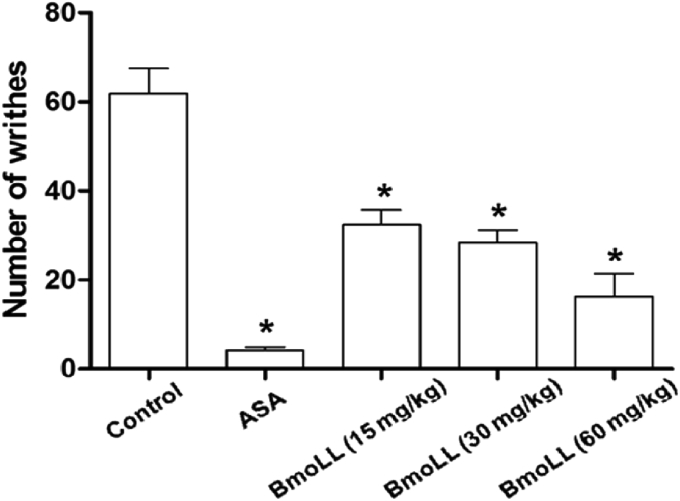
The administration of BmoLL in different concentrations (15, 30 and 60 mg/kg) showed a significant reduction (43.1, 54.2 and 71.3%, respectively) in the writhing induced by acetic acid administration, compared with the negative control group treated with saline (Fig. 2). The group treated with ASA (100 mg/kg), the positive control for anti-nociception, showed significant decrease (92.7%) of writhing.
Fig. 2.
Effect of BmoLL in the number of writhes in the model of nociception induced by acetic acid. ASA, acetylsalicylic acid. Values are expressed as mean ± standard deviation. Significant difference in comparison to the control group: *P < 0.0001.
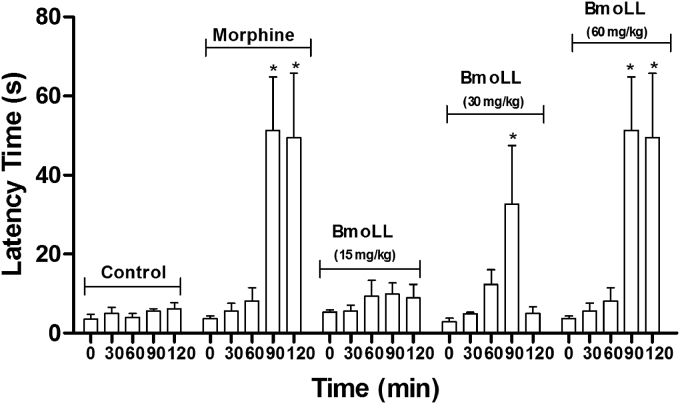
Fig. 3 shows the analgesic response of groups treated with three concentrations of BmoLL. The group of mice treated with a dose of 30 and 60 mg/kg (i.p.) revealed a significant analgesic response (P < 0.001) compared to the negative control (saline), in the form of increased reaction time of mice to jump or lick paws in hot plate method after 90 min MacDonald et al. [41]. The result with BmoLL 60 mg/kg was similar to that observed for the group treated with the standard drug (morphine 1 mg/kg) after 90 and 120 min. There was no analgesic response for the group treated with low dose of BmoLL (15 mg/kg).
Fig. 3.
Analgesic effect of BmoLL in latency time in the hot plate assay on mice. ASA, acetylsalicylic acid. Values are expressed as mean ± standard deviation. Significant difference in comparison to the control group: *P < 0.001.
4. Discussion
Carrageenan-induced paw edema is a most-valuable model to investigate anti-inflammatory effect of natural and chemical products. Inflammatory reactions, characterized by vascular and cellular events, have an important role in the activation of cyclooxygenase (COX) resulting in the release of inflammatory mediators such as prostaglandins and leukotrienes. Considering the significant effect presented by BmoLL to reduce substantially paw edema induced by carrageenan, analyzed at different times, we can assume that this lectin may have inhibitory action on the release of inflammatory mediators. Sisenando et al. [43] showed that BmoLL was unable to produce genotoxicity or cytotoxicity effects.
The anti-inflammatory activity of BmoLL corroborate with the lectin purified from the seeds of Clitoria fairchildiana (CFAL), which showed anti-inflammatory activity of 64% on paw edema induced by carrageenan [22]. Previous studies showed that some lectins have anti-inflammatory activity by reducing the rolling and adhesion of neutrophils on the endothelium, which may possibly be due to a competitive inhibition of glycosylated selectin binding sites on the membranes of leukocytes and endothelial cells. Thus, it is suggested that lectins can inhibit the vascular inflammation mediated by adhesion of immune cells [44], [45]. Therefore, we can suppose that the anti-inflammatory potential of BmoLL may be a result of vascular inflammation inhibition by adhesion of immune cells to endothelium.
In addition to the vascular inflammation processes, the migration of leukocytes is a key factor for promoting the inflammatory process [46]. Thus, by using the model of peritoneum inflammation to evaluate the migration of leukocytes in inflammatory response it was demonstrated in this study that BmoLL significantly reduced cell infiltration in all assayed doses by 43.1, 54.2 and 71.3%. Leite et al. [22] showed that lectin from the seeds of C. fairchildiana (CFAL) also decreased the leukocytes number in the peritoneal cavities by about 29%. Experimental studies have demonstrated the inhibition of leukocyte migration by different anti-inflammatory mechanisms. Napimoga et al. [46] demonstrated that Lonchocarpus sericeus lectin decreased leukocyte migration and inflammatory response via inhibition of cytokine and chemokine production. In this context, lectin from Luetzelburgia auriculata seeds promotes reduction of inflammation by inhibiting adhesion and rolling of leukocytes, and modulates histamine and prostaglandins (PEG2) in inflammation animal models [47]. Therefore, we can suppose that the peritoneum inflammation inhibition mediated by administration of BmoLL may be a result of reduction of cytokines and chemokines release involved with the leukocytes migration.
The experimental model of writhing by injecting of an irritant product such as acetic acid in mice is a chemical method used to assess nonspecific central and peripheral analgesic activity [48] of the test product, which infers from the reduction in writhing frequency. There will be an increase in the peritoneal fluid by inducing the release of chemical mediators and therefore a direct stimulus via activation of chemo sensitive nociceptors [49]. Therefore, the analgesic effect presented by BmoLL may be due to the anti-inflammatory effect in the inhibition of these mediators.
Recently, studies have shown that plant lectins can display antinociceptive effect via lectin domain interaction [22]. Considering the clear association between inflammatory processes and development of pain, a classical sign of inflammation, the BmoLL antinociceptive effects may be associated directly with decreasing inflammation. These results corroborated with the C. fairchildiana lectin that also showed significant antinociceptive effects, at all doses tested (0.1, 1 and 10 mg/kg) and this effect also could be associated to anti-inflammatory action [22]. Lectins from seeds of L. sericeus and Canavalia boliviana showed antinociceptive activity associated with inhibitory effects on leukocyte migration and hipernociception via inhibition of cytokine and chemokine production [46], [50]. Lectin from the green marine alga Caulerpa cupressoides (CcL, 3, 9 or 27 mg/kg) also reduced the number of writhes induced by acetic acid by 37.2%; 53.5% and 86.0%, respectively [4].
On the other hand, model nociception from a thermal stimulus is commonly used for evaluating the central analgesic effect. In the present work, we could observe that BmoLL was able to increase the latency time and the best results were found when the animals were treated with higher doses (30 and 60 mg/kg). Consistent with the findings reported here, other studies with lectins obtained from Brythamnion seaforthii [51], C. boliviana [50], and Canna limbata [52] observed a significant increase in the latency time of animals on the hot plate. In this method, the thermal sensation stimulates sensory fibers run through synapses to the cortex, resulting in the perception of warmth. The antinociceptive activity of BmoLL is already associated with peripheral analgesic activity. Our findings may also be attributed to a direct BmoLL effect on specific central nervous system receptors related to opioid molecules which may act as an antagonist binding to stereo specific and saturable receptor sites in the brain, spinal cord and other tissues, providing the relieve of pain, as proposed with Canavalia brasiliensis lectin by Pires et al. [53]. Therefore, our study demonstrates that BmoLL have antinociceptive effect not only on the peripheral system, but also on the central nervous system. Moreover, reinforcing the hypothesis that antinociceptive response of lectin may be explained by their specificity for different sugars, which could enhance the lectin-carbohydrate interaction and cell receptors.
5. Conclusions
BmoLL has potent anti-inflammatory and antinociceptive properties and this could explain the basis for the use of B. monandra in folk medicine. Furthermore, the results provided experimental evidence for the effectiveness of the traditional use of B. monandra in treating diseases associated with inflammation and pain, and that BmoLL may have potential to be used as a natural anti-inflammatory product for pharmacological purposes.
Conflict of interest statement
The authors declare that there are no conflicts of interest pertaining to the material in this manuscript.
Authors' contributions
This work was carried out in collaboration among all authors. Authors JKLC, CSFA and TFSA managed the experimental work; AFSS and JAT did the literature searches and wrote the first draft of the manuscript. Authors VLML and LCBBC designed the study and managed the study performed. All authors read and approved the final manuscript.
Acknowledgments
The authors express their gratitude to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for research grants and fellowships (VLML and LCBBC). Also, the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) are acknowledged for financial support. In addition, we are grateful to Grant SFRH/BPD/37349/2007 from the Foundation for Science and Technology and POPH/FSE, awarded to AFSS.
Contributor Information
Vera L.M. Lima, Email: lima.vera.ufpe@gmail.com.
Luana C.B.B. Coelho, Email: lcbbcoelho@gmail.com.
References
- 1.Santos A.F.S., Silva M.D.C., Napoleão T.H., Paiva P.M.G., Correia M.T.S., Coelho L.C.B.B. Current Topics in Peptide & Protein Research. 2014. Lectins: function, structure, biological properties and potential applications; pp. 41–62. [Google Scholar]
- 2.Coelho L.C.B.B., Santos A.F.S., Napoleão T.H., Correia M.T.S., Paiva P.M.G. Protein purification by affinity chromatography. In: Ahmad R., editor. Protein Purification. InTech, Open Access Publisher; Rijeka: 2012. pp. 53–72. [Google Scholar]
- 3.Santos A.F.S., Napoleão T.H., Bezerra R.F., Carvalho E.V.M.M., Correia M.T.S., Paiva P.M.G., Coelho L.C.B.B. Strategies to obtain lectins from distinct sources. In: Berhardt L.V., editor. vol. 63. Nova Science Publishers Inc.; 2013. pp. 33–60. (Advances in Medicine and Biology). [Google Scholar]
- 4.Vanderlei E.S.O., Patoilo K.K.N.R., Lima N.A., Lima A.P.S., Rodrigues J.A.G., Silva L.M.C.M., Lima M.E.P., Lima V., Benevides N.M.B. Antinociceptive and anti-inflammatory activities of lectin from the marine green alga Caulerpa cupressoides. Int. Immunopharmacol. 2010;10:1113–1118. doi: 10.1016/j.intimp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Santos A.F.S., Luz L.A., Argolo A.C.C., Teixeira J.A., Paiva P.M.G., Coelho L.C.B.B. Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem. 2009;44:504–508. [Google Scholar]
- 6.Vandenborre G., Smagghe G., Damme E.J.M.V. Plant lectins as defense proteins against phytophagous insects. Phytochemistry. 2011;72:1538–1550. doi: 10.1016/j.phytochem.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Melo C.M.L., Lima A.L.R., Beltrão E.I.C., Cavalcanti C.C.B., Melo-Júnior M.R., Montenegro S.M.L., Coelho L.C.B.B., Correia M.T.S.C., Carneiro-Leão A.M.A. Potential effects of Cramoll 1,4 lectin on murine Schistosomiasis mansoni. Acta Trop. 2011;118:152–158. doi: 10.1016/j.actatropica.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Silva H.C., Pinto L.S., Teixeira E.H., Nascimento K.S., Cavada B.S., Silva A.L.C. BUL: a novel lectin from Bauhinia ungulata L. seeds with fungistatic and antiproliferative activities. Process Biochem. 2014;49:203–209. [Google Scholar]
- 9.Araújo R.M.S., Ferreira R.S., Napoleão T.H., Carneiro-da-Cunha M.G., Coelho L.C.B.B., Correia M.T.S., Oliva M.L.V., Paiva P.M.G. Crataeva tapia bark lectin is an affinity adsorbent and insecticidal agent. Plant Sci. 2012;183:20–26. doi: 10.1016/j.plantsci.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Costa R.M.P.B., Vaz A.F.M., Oliva M.L.V., Coelho L.C.B.B., Correia M.T.S., Carneiro-Da-Cunha M.G. A new mistletoe Phthirusa pyrifolia leaf lectin with antimicrobial properties. Process Biochem. 2010;45:526–533. [Google Scholar]
- 11.Macedo M.L.R., Freire M.G.M., Silva M.B.R., Coelho L.C.B.B. Insecticidal action of Bauhinia monandra leaf lectin (BmoLL) against Anagasta kuehniella (Lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (Coleoptera: Bruchidae) Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2007;46(4):486–498. doi: 10.1016/j.cbpa.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Paiva P.M.G., Pontual E.V., Napoleão T.H., Coelho L.C.B.B. vol. 2. Nova Science Publishers Inc.; 2013. Larvae of pests and vectors as targets of plant lectins and trypsin inhibitors; pp. 7–10. (Lectins and Trypsin Inhibitors from Plants: Biochemical Characteristics and Adverse Effects on Insect Larvae). [Google Scholar]
- 13.Vaz A.F.M., Costa R.M.P.B., Melo A.M.M.A., Oliva M.L.V., Santana L.A., Silva-Lucca R.A., Coelho L.C.B.B., Coreia M.T.S. Biocontrol of Fusarium species by a novel lectin with low ecotoxicity isolated from Sebastiania jacobinensis. Food Chem. 2010;119:1507–1513. [Google Scholar]
- 14.Oliveira M.D.L., Andrade C.A.S., Santos-Magalhães N.S., Coelho L.C.B.B., Teixeira J.A.C., Carneiro-da-Cunha M.G., Correia M.T.S. Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett. Appl. Microbiol. 2008;46:371–376. doi: 10.1111/j.1472-765X.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 15.Nunes E.S., Souza M.A.A., Vaz A.F.M., Santana G.M.S., Gomes F.S., Coelho L.C.B.B., Paiva P.M.G., Silva R.M.L., Silva-Lucca R.A., Oliva M.L.V., Guarnieri M.C., Correia M.T.S. Purification of a lectin with antibacterial activity from Bothrops leucurus snake venom. Comp. Biochem. Physiol. Part B. 2011;159:57–63. doi: 10.1016/j.cbpb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Aranda-Souza M.A., Rossato F.A., Costa R.A.P., Figueira T.T., Castilho R.F., Guarniere M.C., Nunes E.S., Coelho L.C.B.B., Correia M.T.S., Vercesi A.E. A lectin from Bothrops leucurus snake venom raises cytosolic calcium levels and promotes B16-F10 melanoma necrotic cell death via mitochondrial permeability transition. Toxicon. 2014;82:97–103. doi: 10.1016/j.toxicon.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Ganguly P., Fossett N.G. Inhibition of thrombin-induced platelet aggregation by a derivate of wheat germ agglutinin. Evidence for a physiologic receptor of thrombin in human platelets. Blood. 1981;57:343–352. [PubMed] [Google Scholar]
- 18.Silva M.C.C., Santana L.A., Mentele R., Ferreira R.S., Miranda A., Silva-Lucca R.A., Sampaio M.U., Correia M.T.S., Oliva M.L.V. Purification, primary structure and potential functions of a novel lectin from Bauhinia forficate seeds. Proc. Biochem. 2012;47:1049–1059. [Google Scholar]
- 19.Rocha A.A., Araújo T.F.S., Fonseca C.S.M., Mota D.L., Medeiros P.L., Paiva P.M.G., Coelho L.C.B.B., Correia M.T.S., Lima V.L.M. Lectin from Crataeva tapia bark improves tissue damages and plasma hyperglycemia in alloxan-induced diabetic mice. Evid. Based Complement. Altern. Med. 2013;3 doi: 10.1155/2013/869305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes M.P., Inada N.M., Chiaratti M.R., Araújo F.F., Meirelles F.V., Correia M.T.S., Coelho L.C.B.B., Alves M.J.V., Gadelha F.R., Vercesi A.E. Mechanism of Trypanosoma cruzi death induced by Cratylia mollis seed lectin. J. Bioenerg. Biomembr. 2010;42:69–78. doi: 10.1007/s10863-010-9268-9. [DOI] [PubMed] [Google Scholar]
- 21.Coriolano M.C., Melo C.M.L., Silva F.O., Schirato G.V., Porto C.S., Santos P.J.P., Correia M.T.S., Porto A.L.F., Carneiro-Leão A.M.A., Coelho L.C.B.B. Parkia pendula seed lectin: potential use to treat cutaneous wounds in healthy and immune compromised mice. Appl. Biochem. Biotechnol. 2014;172:2682–2693. doi: 10.1007/s12010-013-0692-2. [DOI] [PubMed] [Google Scholar]
- 22.Leite J.F.M., Assreuy A.M.S., Mota M.R.L., Bringel P.H.S.F., Lacerda R.R., Gomes V.M., Cajazeiras J.B., Nascimento K.S., Pessoa H.L.F., Gadelha C.A.A., Delatorre P., Cavada B.S., Santi-Gadelha T. Antinociceptive and anti-inflammatory effects of a lectin like substance from Clitoria fairchildiana R. howard seeds. Molecules. 2012;17:3277–3290. doi: 10.3390/molecules17033277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araújo L.C.C., Aguiar J.S., Napoleão T.H., Mota F.V.B., Barros A.L.S., Moura M.C., Coriolano M.C., Coelho L.C.B.B., Silva T.G., Paiva P.M.G. Evaluation of cytotoxic and anti-inflammatory activities of extracts and lectins from Moringa oleifera seeds. PLoS ONE. 2013;8(12):e81973. doi: 10.1371/journal.pone.0081973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivanor R.L.C., Chaves H.V., Val D.R., Freitas A.R., Lemos J.C., Rodrigues J.A.G., Pereira K.M.A., Araújo I.W.F., Bezerra M.M., Benevides N.M.B. A lectin from the green seaweed Caulerpa cupressoides reduces mechanical hyper-nociception and inflammation in the rat temporo mandibular joint during zymosan-induced arthritis. Int. Immunopharmacol. 2014;21:34–43. doi: 10.1016/j.intimp.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Silva J.A., Damico D.C.S., Baldasso P.A., Mattioli M.A., Winck F.V., Fraceto L.F., Novello J.C., Marangoni S. Isolation and biochemical characterization of a galactoside binding lectin from Bauhinia variegata candida (BvcL) seeds. Protein J. 2007;26:193–201. doi: 10.1007/s10930-006-9061-0. [DOI] [PubMed] [Google Scholar]
- 26.Pinto L.S., Nagano C.S., Oliveira T.M., Moura T.R., Sampaio A.H., Debray H., Pinto V.P., Dellagostin A.O., Cavada B.S. Purification and molecular cloning of a new galactose-specific lectin from Bauhinia variegata seeds. J. Biosci. 2008;33:355–363. doi: 10.1007/s12038-008-0055-2. [DOI] [PubMed] [Google Scholar]
- 27.Coelho L.C.B.B., Silva M.B.R. Simple method to purify milligram quantities of the galactose-specific lectin from the leaves of Bauhinia monandra. Phytochem. Anal. 2000;11:295–300. [Google Scholar]
- 28.Fuentes O., Arancibia-Avila P., Alan J. Hypoglycemic activity of Bauhinia candicans in diabetic induced rabbits. Fitoterapia. 2004;75:527–532. doi: 10.1016/j.fitote.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Oliva M.L.V., Silva M.C.C., Sallai R.C., Brito M.V., Sampaio U.M. A novel sub classification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie. 2010;92:1667–1673. doi: 10.1016/j.biochi.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Argolo A.C.C., Sant'Ana A.E.G., Pletsch M., Coelho L.C.B.B. Antioxidant activity of leaf extracts from Bauhinia monandra. Bioresour. Technol. 2004;95:229–233. doi: 10.1016/j.biortech.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Young N.M., Watson D.C., Williams R.E. Lectins and legume chemotaxonomy: characterization of the N-acetyl-d-galactosamine specific lectin of Bauhinia purpurea. Fed. Eur. Biochem. Soc. 1985;182:403–406. [Google Scholar]
- 32.Souza J.D., Silva M.B.R., Argolo A.C.C., Napoleão T.H., Sá R.A., Correia M.T.S., Paiva P.M.G., Silva M.D.C., Coelho L.C.B.B. A new Bauhinia monandra galactose-specific lectin purified in milligram quantities from secondary roots with antifungal and termiticidal activities. Int. Biodeterior. Biodegrad. 2011;65:696–702. [Google Scholar]
- 33.Lin P., Ng T.B. Preparation and biological properties of a melibiose binding lectin from Bauhinia variegata seeds. J. Agric. Food Chem. 2008;56:10481–10486. doi: 10.1021/jf8016332. [DOI] [PubMed] [Google Scholar]
- 34.Silva H.C., Bari A.U., Pereira-Junior F.N., Simoes R.C., Barroso-Neto I.L., Nobre C.B., Preira M.G., Nascimento K.S., Rocha B.A., Delatorre P., Nagano C.S., Assereuy A.M., Cavada B.S. Purification and partial characterization of a new pro-inflammatory lectin from Bauhinia bauhinioides Mart (Caesalpinoideae) seeds. Protein Pept. Lett. 2011;18:396–402. doi: 10.2174/092986611794653987. [DOI] [PubMed] [Google Scholar]
- 35.Gil A.L.A.P., Navarro L.B., Vera M.P., Petricevich V.L. Anti-inflammatory and antinociceptive activities of the ethanolic extract of Bougainvillea xbuttiana. J. Ethnopharmacol. 2012;144:712–719. doi: 10.1016/j.jep.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Yu H.L., Zhang F., Li Y.J., Gong G.H., Quan Z.S. Anti-inflammatory and antinociceptive effects of 6-(4-chlorophenoxy)-tetrazolo[5,1-a]phthalazine in mice. Pharmacol. Rep. 2012;64:1155–1165. doi: 10.1016/s1734-1140(12)70912-x. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Winter C.A., Risley E.A., Nuss G.W. Carrageenan-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 39.Foster M.E., Johnson C.D., Bilings P.J., Davies P.W., Leaper D.J. Intraoperative antegrade lavage and anastomotic healing in acute colonic obstruction. Dis. Colon Rectum. 1986;29:255–259. doi: 10.1007/BF02553031. [DOI] [PubMed] [Google Scholar]
- 40.Koster R., Anderson M., Beer E.J. Acetic acid for analgesic screening. Fed. Proc. 1959;18:412–417. [Google Scholar]
- 41.MacDonald A.D., Woolfe G., Bergel F., Morrison A.L., Paroli E., Rinderknecht H. Analgesis action of pethidine and related compounds. Brit. J. Pharmacol. Chemother. 1946;1:4–14. [PMC free article] [PubMed] [Google Scholar]
- 42.Nourshargh S., Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Sisenando H.A., Macedo M.F., Saturnino A.C., Coelho L.C.B.B., Medeiros S.R. Evaluation of the genotoxic potential of Bauhinia monandra leaf lectin (BmoLL) Food Chem. Toxicol. 2009;47:303–308. doi: 10.1016/j.fct.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Assreuy A.M.S., Shibuya M.D., Martins G.J., de Souza M.L.P., Cavada B.S., Moreira R.A., Oliveira J.T.A., Ribeiro R.A., Flores C.A. Anti-inflammatory effect of glucose-mannose binding lectins isolated from Brazilian beans. Mediat. Inflamm. 1997;6:201–210. doi: 10.1080/09629359791695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocha B.A.M., Delatorre P., Oliveira T.M., Benevides R.G., Pires A.F., Sousa A.S., Souza L.A.G., Assereuy A.M.S., Debray M.H., Azevedo W.F., Sampaio A.H., Cavada B.S. Structural basis for both pro- and anti-inflammatory response induced by mannose-specific legume lectin from Cymbosema roseum. Biochimie. 2011;93:806–816. doi: 10.1016/j.biochi.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Napimoga M.H., Cavada B.S., Alencar N.M., Mota M.L., Bittencourt F.S., Alves-Filho J.C., Grespan R., Gonçalves R.B., Clemente-Napimoga J.T., Freitas A., Parada C.A., Ferreira S.H., Cunha F.Q. Lonchocarpus sericeus lectin decreases leukocyte migration and mechanical hypernociception by inhibiting cytokine and chemokines production. Int. Immunopharmacol. 2007;7:824–835. doi: 10.1016/j.intimp.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Alencar N.M.N., Oliveira R.S.B., Figueiredo J.G., Cavalcante I.J.M., Matos M.P.V., Cunha F.Q., Nunes J.V.S., Bomfim I.R., Ramos M.V. An anti-inflammatory lectin from Luetzelburgia auriculata seeds inhibits adhesion and rolling of leukocytes and modulates histamine and PGE2 action in acute inflammation models. Inflamm. Res. 2010;56:245–254. doi: 10.1007/s00011-009-0092-9. [DOI] [PubMed] [Google Scholar]
- 48.Dannerman P.J. In: Kohn D.K., Sally K., Wixson B., White W.J., John G., editors. vol. 6. Academic Press; USA: 1977. pp. 83–99. (Monitoring of Analgesia in Anesthesia and Analgesia in Laboratory Animals). [Google Scholar]
- 49.Zhang S., Wong Y.R., Ong C.N., Shen H.M. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 50.Figueiredo J.G., Bitencourt F.S., Beserra I.G., Teixeira C.S., Luz P.B., Bezerra E.H., Mota M.R., Assreuy A.M., Cunha F.Q., Cavada B.S., Alencar N.M. Antinociceptive activity and toxicology of the lectin from Canavalia boliviana seeds in mice. Naunyn Schmied. Arch. Pharmacol. 2009;380:407–414. doi: 10.1007/s00210-009-0448-2. [DOI] [PubMed] [Google Scholar]
- 51.Vieira L.A.P., Freitas A.L.P., Feitosa J.P.A., Silva D.C., Viana G.S.B. The alga Bryothamnion seaforthii contains carbohydrates with antinociceptive activity. Braz. J. Med. Biol. Res. 2004;37:1071–1079. doi: 10.1590/s0100-879x2004000700017. [DOI] [PubMed] [Google Scholar]
- 52.Araújo T.S., Teixeira C.S., Falcão M.A.P., Pinto Junior V.R., Santiago M.Q., Benevides R.G., Delatorre P., Martins J.L., Alexandre-Moreira M.S., Cavada B.S., Campesato E.A., Rocha B.A.M. Anti-inflammatory and antinociceptive activity of chitin-binding lectin from Canna Limbata seeds. Appl. Biochem. Biotechnol. 2013;171:1944–1955. doi: 10.1007/s12010-013-0470-1. [DOI] [PubMed] [Google Scholar]
- 53.Pires A.F., Assreuy A.M., Lopes E.A., Celedônio N.R., Soares C.E., Rodrigues N.V., Sousa P.L., Benevides R.G., Nagano C.S., Cavada B.S., Leal-Cardoso J.H., Coelho-de-Souza N.A., Santos C.F. Opioid-like antinociceptive effects of oral administration of a lectin purified from the seeds of Canavalia brasiliensis. Fundam. Clin. Pharmacol. 2013;27:201–209. doi: 10.1111/j.1472-8206.2011.00987.x. [DOI] [PubMed] [Google Scholar]