Abstract
Metastasis is the leading cause of mortality in patients with breast cancer. In triple-negative breast cancer, high recurrence rates, increased invasive capacity of cells, and their aggressive ability to metastasize at secondary sites dictate patient survival. The Na+/H+ exchanger isoform 1 (NHE1) plays a critical role in controlling the metastatic potential of these cells. Its activity results in an elevation of intracellular pH and in extracellular acidification, a key step in the establishment of the tumor microenvironment. Here, we describe assays for characterization of Na+/H+ exchanger activity and its related downstream physiological effects on triple-negative breast cancer cells. Na+/H+ exchanger activity can be routinely and rapidly measured in live cells with a fluorometric assay that assesses changes in intracellular pH. Characterization of downstream cell effector function as a result of Na+/H+ exchanger activation can be evaluated by measuring directed cell migration and invasion. Cell migration is assessed with wound-healing assays, where a gap is introduced in a confluent monolayer of cells and the rate of gap closure is measured over time. Cell invasion is assessed in the short-term by transwell invasion assays that track cell movement through an extracellular matrix. Long-term invasiveness, growth and proliferation can be assessed with 3-D invasion assays using transwell inserts fitted with specialized scaffolds optimized for 3-D cell culture. Taken together these assays provide powerful tools for testing the effects of altering Na+/H+ exchanger activity with chemical inhibition on the metastatic capacity of breast cancer cells.
Keywords: Na+/H+ exchanger, Metastasis, Breast cancer, Intracellular pH
Abbreviations: BCECF-AM, 2′, 7-bis (2-carboxyethyl)-5(6) carboxyfluorescein acetoxymethyl ester; NHE1, Na+/H+ exchanger type 1 isoform; pHi, intracellular pH; EMD87580, 2-methyl-4,5-di-(methylsulfonyl)-benzoylguanidine; HMA, 5-(N, N-hexamethylene)-amiloride; TNBC, triple-negative breast cancer
Highlights
-
•
The Na+/H+ exchanger in breast cancer cells is assayed by rapid fluorometric assay.
-
•
Migration of triple negative breast cancer cells is assessed by wound healing.
-
•
Cell invasion is measured via transwell 3D invasion assays.
1. Introduction
Breast cancer is the most commonly diagnosed cancer among women [1], and it is the resulting metastasis that is the main cause of patient fatality [2], [3]. Triple-negative breast cancers in particular (negative for estrogen and progesterone receptors and human epidermal growth factor receptor-2 (HER2)) are the most problematic. They tend to be more aggressively metastatic and have high recurrence rates associated with resistance to standard chemotherapy treatments. This makes their characterization and development of treatments to stop metastasis most imperative, particularly since there are no therapies uniquely targeted toward this clinical breast cancer subtype.
A common property of tumor cells is intracellular alkalinization due to the activity of the membrane protein Na+/H+ exchanger (NHE) [4]. NHE is present in the plasma membrane and removes 1 intracellular H+ in exchange for 1 extracellular Na+ [4], [5], [6], [7], [8]. The increased pHi, which is mediated by NHE1 (NHE isoform 1), is associated with the proliferation and transformation tumor cells [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]. In particular, recently NHE1 has been shown to play a critical role of in the growth and metastasis of triple-negative breast cancer cells [19], [20], [21], [22], [23]. It is thought that, in addition to affecting intracellular pH, activity of NHE1 acidifies the extracellular microenvironment of tumor cells [8], [17], [18] which facilitates the digestion and remodeling of the extracellular matrix [18], [24], [25], critical to metastasis.
We have recently shown that disruption of the NHE1 gene inhibits the ability of MDA-MB-231 breast cancer cells to form in vivo xenograft tumors in athymic nude mice [19]. Also, NHE1 inhibition had a synergistic effect with the commonly used chemotherapy agent paclitaxel, resulting in a dramatic decrease in migratory and invasive potential of breast cancer cells. While these results marked a novel inroad in the fight against breast cancer, much remains to be done and NHE1 remains a viable research target in this regard. It is of note that NHE1 is also a clinical target in ischemic heart diseases and heart hypertrophy. However, the use of NHE1 inhibitors in the cardiovascular clinical setting has been disappointing with detrimental side effects [26], [27], and the development of new NHE1 inhibitors may be called for. In any case, NHE1 inhibitor development for use in the cardiovascular or the cancer setting will require careful analysis of their inhibitory behavior and downstream effects.
The purpose of this report is to outline and make readily accessible to other investigators assays for NHE1 activity and for downstream effects of NHE1 on triple-negative breast cancer (TNBC) cell behavior. The downstream behavior includes cell migration and invasion. It is hoped that this information will facilitate more active research into this area, leading to a more rapid development of clinical treatments to reduce metastasis in triple-negative breast cancer.
2. Materials and methods
2.1. Materials
2′, 7-bis (2-carboxyethyl)-5(6) carboxyfluorescein acetoxymethyl ester (BCECF-AM) was from Molecular Probes, Inc. (Eugene, OR). All the other chemicals used were of analytical grade and were acquired from Fisher Scientific (Ottawa, ON, Canada), Sigma (St. Louis, MO, USA) or BDH (Toronto, ON, Canada). Nigericin was obtained from multiple sources including Sigma and Caymen Chemicals (Ann Arbor, Michigan, USA). Coverslips were Red Label® Micro Cover Glasses from Thomas Scientific (Swedesboro, N.J., USA). Fluorometer cuvettes were made of polymethacrylate (Sigma).
2.2. Cell lines and culture conditions
Parental MDA-MB-231 cells were authenticated by DNA analysis (DDC Medical, Ohio). They showed >95% homology to the ATCC STR profile. Culture conditions were as described earlier [19]. Briefly, all cells were cultured in high-glucose (4500 mg/L) modified DMEM (HyClone) supplemented with 10% fetal calf serum (HyClone), 10 mM HEPES, and 1000 units/ml penicillin/streptomycin (Gibco) under 5% CO2, 37 °C and high humidity. Starvation media was supplemented with 0.2% serum but otherwise identical in composition.
2.2.1. Measurement of NHE1 activity
NHE1 activity was determined using a PTI Deltascan spectrofluorometer [19], [28], [29]. Experiments are described briefly and details of solutions and additions are contained in Table 1. Part A can be considered to be growth of MDA-MB-231 cells on coverslips and loading with the fluorescent dye BCEC-AM. Cells are grown in normal growth medium as described above. Two sterilized coverslips (Thomas® Red Label® Micro Cover Glasses) were previously added to 35 mm dishes. MDA-MB-231 cells are grown until approximately 80–90% confluent. BCECF-AM (2′,7′-bis(2-carboxyethyl)-5(6) carboxyfluorescein-acetoxymethyl ester) (Molecular Probes Inc., Eugene, OR, USA) is added to a final concentration of 2.5 μg/ml (1 μl of 1 mg/ml stock in DMSO), is added to the cells while the cells are in 400 μl normal tissue culture media (in the absence of serum). The cells are maintained at 37 °C for approximately 20 min. During this incubation period BECEF-AM is de-esterified and becomes trapped within the cell [30], [31]. It then fluoresces dependent upon intracellular pH (described below). BCECF-AM is stored as a 1 mg/ml stock in DMSO at −20 °C in a darkened container.
Table 1.
Protocol for measurement of pHi in MDA-MB-231 cells on coverslips. Cells are grown on coverslips as describe in the text. They are placed in a thermostatically controlled cuvette with constant stirring.
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Equilibration | 2.5 ml | “Normal1” sodium containing buffer for equilibration |
| 2 | Ammonium Cl | 30 μl | Final of 30 mM (2.5 M Stock), 3 min |
| 3 | Acidification | 2.5 ml | Sodium free2 buffer, 30 s |
| 4 | Recovery | 2.5 ml | “Normal1” sodium containing buffer |
| 5 | pH Calibration | 2.5 ml | 3pH 8.0 Calibration Buffer + 4nigericin |
| 6 | pH Calibration | 2.5 ml | 3pH 7.0 Calibration Buffer + 4nigericin |
| 7 | pH Calibration | 2.5 ml | 3pH 6.0 Calibration Buffer + 4nigericin |
1Normal Buffer.
135 mM NaCl.
5 mM KCl.
1.8 mM CaCl2.
1 mM MgSO4.
5.5 mM Glucose.
10 mM Hepes.
Warm solution to 37 °C and adjust pH of warm solution to 7.3 using KOH and HCl.
2Na+-free buffer.
135 mM N-Methyl glucamine.
5 mM KCl.
1.8 mM CaCl2.
1 mM MgSO4.
5.5 mM Glucose.
10 mM Hepes.
Warm solution to 37 °C and adjust pH of warm solution to 7.3 using KOH and HCl.
3pH Calibration Buffer.
5 mM N-Methyl glucamine.
135 mM KCl.
1.8 mM CaCl2.
1 mM MgSO4.
5.5 mM Glucose.
10 mM Hepes.
Warm solutions to 37 °C and adjust pH of warmed solution to 6, 7, and 8 using KOH and HCl.
4Nigericin. 10 mM stock in ethanol, stored in −20 °C. 2.5 μl is added to 2.5 of pH.
2.2.2. Acute acidification and recovery
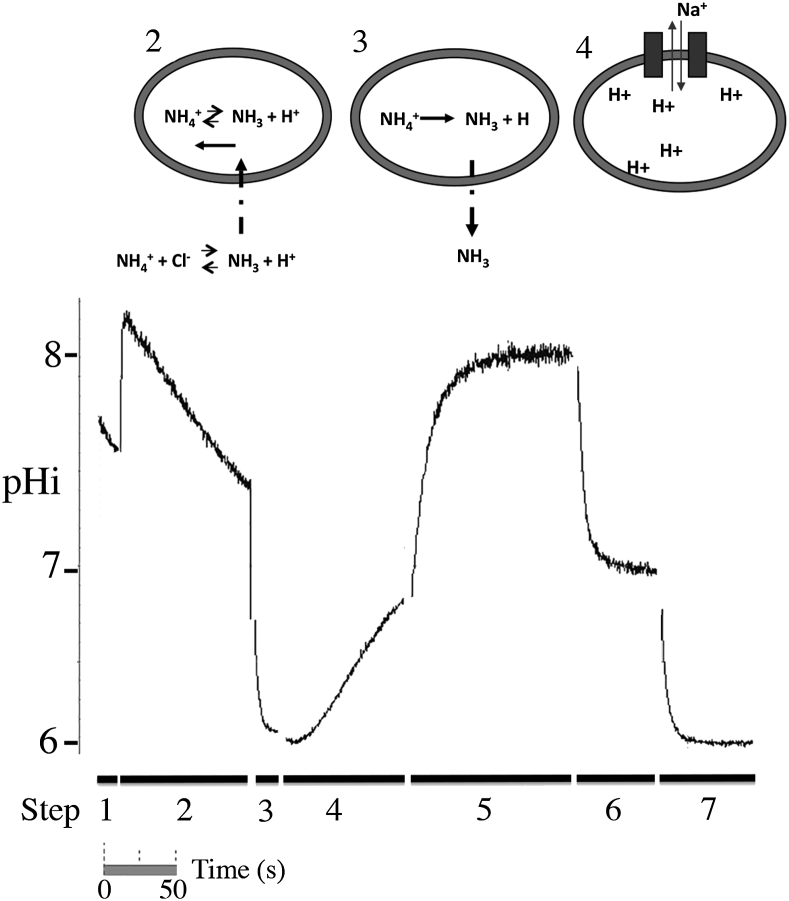
Part B is the measurement of intracellular pH changes in the cell, upon an induced, acute acid load. The outline is described below, with details included in Table 1. One of the pair of coverslips is removed from the petri dish. It is manually transferred to a Teflon coverslip holder that has a thin grove on the side in which the coverslip is wedged. The coverslip holder was made in-house and designed to fit in a standard spectrofluorometer cuvette. It is also designed to hold the coverslip such that it is at approximately 45° to the excitation light source, and does not reflect the exciting light source directly into the photometer measuring emission. It has a spacer on the bottom allowing for stirring of the surrounding solution. The coverslip is placed in a solution of “normal” buffer containing 135 mM NaCl (Table 1, Fig. 1) for 2–3 min. This is a simple equilibration stage. Next, a series of changes or additions are used to induce an acute acid load. All solutions are kept at 37 °C. Ammonium chloride is next (step 2) added from a concentrated stock solution. This initially alkalinizes the cell and after approximately 3 min, the pH returns to near resting pHi (Fig. 1). The amount of ammonium chloride can be increased or decreased to vary the level of acidification later produced. This is followed by a brief rinse and 20–30 s stay in sodium free buffer (step 3). The rapid removal of extracellular ammonium chloride shifts the intracellular equilibrium towards acid production, resulting in a large acid load in the cell (Fig. 1). pHi may decline to approximately 6.0. (The sequence of events leading to acidification is outlined in Fig. 1). Once the cells are acidified, they are returned to sodium-containing buffer to demonstrate Na+/H+ exchanger activity (step 4). The initial rate of recovery in the first 20 s is measured after recovery begins. The recovery can be allowed to continue for several minutes though the rate of alkalinisation declines with increasing pHi.
Fig. 1.
Na+/H+ exchanger activity in MDA-MB-231 cells. Changes in intracellular pH (pHi) in response to acid loading induced by ammonium chloride. An example of trace illustrating the changes in pHi from an acute acid load followed by recovery and pHi calibration is shown. Periods of NH4Cl, NaCl and Na-free solution are indicated and correspond with Table 1. Step 1 is an equilibration step allowing the cells to recovery from loading of BCECF. In Step 2, ammonium chloride is added. This initially alkalinizes the cell as uncharged NH3 is more permeable than the other cations. This makes an equilibrium shifted towards NH4+ in the cell. Over a period of a few minutes, the equilibrium balances with further diffusion. In step 3, external ammonium chloride is removed and replaced with a sodium-free buffer. NH3 diffuses out rapidly, shifting the intracellular equilibrium to produce protons (H+). This solution is then changed to sodium-containing solution (Step 4), which induces the activity of the Na+/H+ exchanger. pH calibration (Steps 5–7) is with nigericin-containing solutions at specific pH's. Nigericin is a H+, K+-ionophore that equilibrates external with internal pH.
2.2.3. pH calibration curve
For every coverslip of cells, a pH calibration curve is made which is then used to convert the emissions to intracellular pH28. After the final recovery (step 4 above) cells are transferred to 3 successive pH calibration buffers (Table 1, Fig. 1). In each pH calibration buffer, 2.5 μl of nigericin is added to a final concentration of 10 μM. Readings are taken at each pH. Cells are allowed to remain at the first pH calibration buffer for 2–3, and ½-1 min each successive pH calibration solution.
2.2.4. pHi measurement
Intracellular pH measurement is via dual excitation, single emission ratio. This helps ensure a measurement that is independent of the amount of cells or the amount of BCECF that has been taken up by the cells. Excitation is at wavelengths 440 and 502.5 nm. Emission is at 528.7 nm. Slit widths of excitation should be restricted as much as possible, ideally less than 5 nm for excitation. Excitation at 440 nm is at the isosbestic point, and results in an emission that is independent of pHi, while excitation at 502.5 nm will cause an emission that is dependent on pHi. Taking the ratio of these two therefore gives a reading that varies with pHi and is not dependent on dye concentration or cell number. Instrumentation for measurement of emission can vary.
2.2.5. General considerations
An experienced user can generally carry out two independent assays on two machines simultaneously, with the timing of the assays slightly staggered. Up to 24 assays can be done in one day. We have found that inclusion of a small stirring bar below the cuvette greatly improves readings. Anomalous readings can be caused by several factors. Loss of cells on the coverslip can give too weak a signal. However, this has never been a problem with this cell type. An alternative anomalous reading can be from cells loaded with too much BCECF. This can result in an emission that reaches the maximum of the instrument, and would give erroneous ratios. This is easily prevented by loading less BCECF into the cells, either by reducing the time, or by lowering the loading concentration. An occasional examination of the individual emissions wavelengths from each excitation wavelength can be used to ensure this is not occurring. Nigericin is a H+, K+ ionophore used to calibrate pHi. Extensive washing of the coverslip holder should be used to ensure that there is no trace contamination with residual nigericin in future experiments. When experiments are done with acutely treated cells, it is usually permissible to compare simple changes in the rate of pHi. However, when examining cells that have been chronically treated, or when comparing different cells lines this may not be adequate. Buffering capacity of cells should be determined and proton flux calculated to ensure that the apparent changes in pHi, are due to Na+/H+ exchanger activity and not due to changes in cell buffering. Measurement of cell buffering and proton flux is described earlier [30].
2.2.6. Assessment of cell migration
The effect of NHE1 activity on cell migration can be assessed with a wound-healing assay. Equal numbers of cells (0.5 × 106) are seeded into multi-well plates (generally 12–24 well plates; greater starting numbers of cells can be plated for 6 well plates) and allowed to grow to confluence. A scratch (wound) is induced in the monolayer with a pipette tip (which can be facilitated by attached the tip to an aspirator). Culture media is then aspirated and the wells are washed with 1X phosphate-buffered saline (PBS) to remove unattached cells. To assess NHE1 activity in triple-negative breast cancer cells, serum starvation over 24 h is utilized to induce a hyper-activation of exchanger activity. Control wells will contain culture media with 10% serum, while serum-deprived wells will contain media with only 0.2% serum. All other culture conditions remain unchanged. At this stage, any chemical agonists or inhibitors being tested can be introduced into culture media at appropriate concentrations. A light microscope with imaging software is required to take bright-field images of the induced gap (wound) in the cell monolayer at 0 h. Imaging software should allow for arbitrary measurements of the rate of gap closure at periodic intervals (0, 6, 12, 18, 24, 36, 48 h) over time (Fig. 2a). A minimum of three wells per treatment, three images per well, and three measurements per image is required for a good representation of experimental outcome over multiple independent experiments.
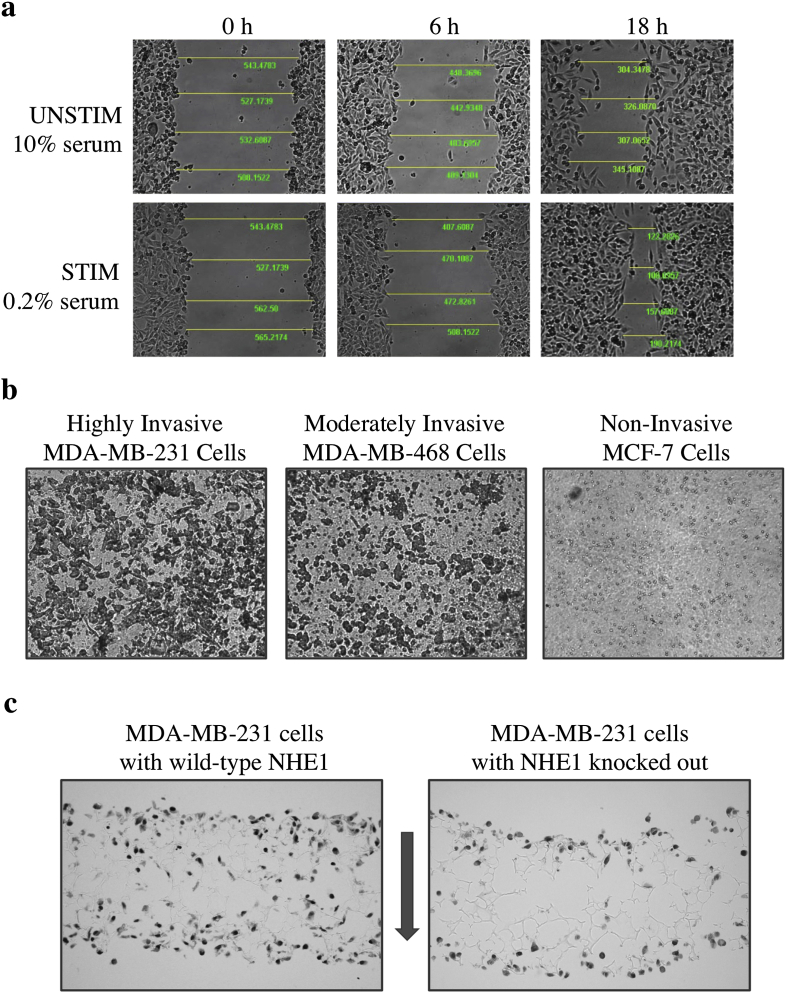
Fig. 2.
Assessing effector functionality mediated by Na+/H+ exchanger activity in MDA-MB-231 cells. a, Cell migration assays. Rate of cell migration over time is measured by rate of gap closure in a wound-healing assay. Imaging software can be used to take arbitrary measurements of gap distance to track gap closure and compare between treatments. Here, NHE1 activity is upregulated by serum starvation (STIM, 0.2% serum), and the resultant increase in the rate of cell migration at 18 h can thus be quantified. b, Cell invasion assays. Rate of cell invasion through Matrigel® matrix-coated transwell inserts can be determined by quantifying the numbers of stained invading cells attached to the underside of the transwell insert. For comparison, highly invasive MDA-MB-231 cells are shown with moderately invasive MDA-MB-468 cells and non-invasive MCF-7 cells. c, 3-D cell invasion assays. Long-term invasion, growth and proliferation of cells can be assessed with 3-D culture over 7 days. Cells are seeded in transwell inserts fitted with Alvetex® scaffold membranes that allow for cell growth in three dimensions, emulating cell growth and behavior in vivo. Here, we demonstrate the effect of NHE1 expression on long-term invasion where MDA-MB-231 cells expressing endogenous wild-type NHE1 are able to traverse the scaffold and grow and proliferate within it, while cells lacking NHE1 are only minimally invasive and viable.
2.2.7. General considerations
Optimization experiments for cytotoxicity of any chemical compounds, particular ion exchange inhibitors, should be carried out prior to their use in cell-based assays. Ensure that cell monolayers are not compromised and cells do not lift off in response to treatment with drugs. If so, wells may need to be coated with gelatin or poly-d-lysine prior to plating cells to ensure maximal adherence to the plastic. When testing multiple cell lines, rates of proliferation also need to be taken into consideration. If some cells proliferate at a higher rate than others, the rate of migration can be skewed by proliferation of cells inducing gap closure. In this instance, using mitomycin-C or another inhibitor of cell proliferation is recommended.
2.2.8. Assessment of cell invasion
NHE1 activity is key in cell invasion that occurs through the extracellular matrix [17]. In vitro, the ability of invasive cells to digest through the extracellular matrix is tested with transwell invasion assays, similar to a Boyden-chamber principle. Sterile transwell permeable inserts (Costar®, Corning) have polycarbonate membranes with 8 μm pores (pore size varies according to cell type used), and are 6.5 mm in diameter for a 24-well plate. Inserts are coated with Matrigel® Matrix (BD Biosciences), a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma that can be used to mimic the extracellular matrix in vitro. Matrigel® matrix concentrations from manufacturer can vary with batches; as such, optimization is necessary to determine the concentration of matrix to coat inserts. We used 50 μl of a 1:10 dilution of 8.0 mg/ml concentration of Matrigel® matrix to coat inserts. Equal cells numbers (generally 1 × 105 cells) are added to each matrix-coated insert in 0.2% serum-containing culture media; total volume of cell suspension and matrix coating should not exceed 100 μl. Any drugs being tested should be added to the cells at this point. Inserts (upper chamber) are placed in reservoir wells (lower chamber) of a 24-well plate. Each reservoir well should contain up to 800 μl of complete media containing 10% serum to induce cell movement through chemotaxis. Cells are incubated for 24–48 h to determine their invasive capacity. Invasive cells will digest through the matrix, and pass through the pores, adhering to the underside of the polycarbonate membrane of the insert, while non-invasive cells will remain in the upper chamber. Post-invasion, media is removed from inserts with a pipette and any non-invasive cells and matrix coating are removed with a cotton swab. Inserts are washed twice in PBS. Cells on the underside of inserts are then fixed by placing them in another 24-well plate in wells containing 3.7% paraformaldehyde for 2 min, prior to washing twice with PBS. Cells are permeabilized by placing inserts in wells containing 100% ice-cold methanol for 20 min, prior to washing twice with PBS. To stain cells, inserts are placed in wells containing Giemsa for 15 min. Inserts are washed 2–3 times in PBS to remove traces of dye and swabbed dry with a cotton bud. A light microscope with imaging software is required to take bright-field images of invasive cells (Fig. 2b). To visualize cells, inserts can be imaged directly by placing them on a glass slide, or the membrane can be excised using a scalpel and mounted on a slide with suitable mounting media and a coverslip. Imaging software can be used to count the number of invading cells, or cells can be counted manually. A minimum of three inserts per treatment, and three field images per insert is required for a good representation of experimental outcome over multiple independent experiments.
2.2.9. General considerations
Matrigel® matrix coating of inserts may need to be standardized prior to running invasion assays. Several dilutions of Matrigel® may need to be tested depending on the cell type used and the duration of the invasion assay. Inserts (of the size specifications described above) should not be coated with more than 50 μl of matrix suspension. It is recommended that any drugs being tested is added to media in both upper and lower chambers to assess their impact on invasion. Care should be taken in washing steps to minimize loss of cells.
2.2.10. Assessment of 3-D cell invasion
The effect of NHE1 activity on cell invasion can be assessed in the long term by using scaffold inserts that allow cells to undergo 3-dimensional growth instead of the polycarbonate porous inserts described above, where cells grow in a 2-dimensional monolayer. 3-D invasion assays utilize Alvetex® scaffold inserts (Reinnervate, UK) in a multi-well format. Here, sterile 6-well polystyrene scaffold inserts were used. Alvetex® scaffolds have a thickness of 200 μm and are highly porous. Prior to seeding cells, inserts were primed for cell culture by soaking in 70% ethanol, washed in PBS, and then coated with 0.8 mg/ml Matrigel® matrix per manufacturer's recommendations. 1 × 106 cells in a volume of 100 μl of complete culture media was added to the top of scaffolds and cells were allowed to adhere for 90 min prior to gently flooding wells with media supplemented with 10% serum (up to 10 ml per well in a 6-well format). Any drug treatments should be added at this stage. Media should be changed every 2–3 days; invasion assays for MDA-MB-231 TNBC cells can range from 7 to 21 days. For a study of long-term invasion dependent on Na+/H+ exchange activity, 7 days is sufficient. After this period, membrane scaffolds are excised from inserts with a scalpel and embedded in paraffin, taking care to maintain their top-to-bottom orientation so as to track cell invasion through the insert. Embedded scaffolds are then longitudinally sectioned into 10 μm thick slices, stained with hematoxylin and eosin, and mounted on glass slides for visualization with bright-field microscopy (Fig. 2c). Numbers of invading cells can be counted for a semi-quantitative representation of data. A minimum of three inserts per treatment, and three field images per insert is required for a good representation of experimental outcome over multiple independent experiments.
2.2.11. General considerations
Over time, invasive cells seeded on top of inserts will invade through the scaffold, growing and proliferating within it. Non-invasive cells will show limited movement, growth and proliferation within the scaffold (Fig. 2c). Hence, overall assay duration needs to be optimized depending on cell type, especially if any drugs are used. Serum starvation is not recommended for long-term experiments greater than 3 days.
3. Discussion
In this study, we describe several assays used for the determination of NHE1-related activity in the triple-negative breast cancer cell line MDA-MB-231. As noted above, NHE1-mediated increase in pHi is associated with the proliferation and transformation tumor cells [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18] and NHE1 has been shown to play a critical role in the growth and metastasis of TNBC cells [19], [20], [21], [22], [23]. Elucidation of the cause of elevated NHE1 activity and the development of new and improved potent and specific inhibitors of the protein are thus paramount in treatment of the disease. These require characterization of the activity of the protein and its induced downstream physiological effects.
Assay of NHE1 activity in MDA-MB-231 cells is relatively rapid. An experienced technician can complete over 20 assays in one day. We have used the same procedures on other types of breast cancer cells including hormone receptor-positive MCF-7 and triple-negative MDA-MB-468 breast cancer cells. There is no reason to believe that other similar cells types cannot be similarly assayed in this manner. Furthermore, the assessment of cell effector functions like directed migration and invasion is a key in determining the effects of novel inhibitors of NHE1 activity in these cells. Here, we describe assays to evaluate the rates of cell migration and invasion using well-established methodologies that have been modified specifically for triple-negative breast cancer cell lines. In addition, we present a robust new method of assessing long-term in vitro invasiveness, growth and proliferation of these cells in 3-D culture that closely mimics cell growth and behavior in vivo. We were the first to demonstrate that knocking out NHE1 in MDA-MB-231 TNBC cells drastically limits their capacity for long-term invasion in 3-D culture [19]. Testing the efficacies of novel NHE1 inhibitors in these assays can therefore provide important knowledge prior to moving into an in vivo animal model system.
In the search for therapies specifically aimed towards triple-negative breast cancer, NHE1 provides a promising target. NHE1 activity becomes dysregulated at the onset of oncogenic transformation, arguably prior to, or concurrent with, the altered metabolism of cancer cells associated with tumorigenesis [4]. This dysregulation leads to cellular alkalinization and extracellular acidification that promotes the establishment of the tumor microenvironment. Recent evidence suggests that the development of resistance seen with the use of chemotherapy drugs is facilitated by the acid pH of the tumor microenvironment [32]. Routinely used chemotherapy drugs are generally basic and become protonated and neutralized in an acidic milieu, compromising their efficacy and ability to reach intracellular targets. We have previously demonstrated that, in MDA-MB-231 cells, selective inhibition of NHE1 activity with EMD87580 (2-methyl-4,5-di-(methylsulfonyl)-benzoylguanidine) or HMA (5-(N, N-hexamethylene)-amiloride) increased the efficacy of paclitaxel-mediated cell death [19]. These NHE1 inhibitors had a synergistic effect on paclitaxel therapy, augmenting its anti-metastatic effects at low doses. We conclude that the development of more potent and specific NHE1 inhibitors that can modulate the pH of the tumor microenvironment to prevent or limit cancer progression independent of chemotherapy drugs is an important and necessary next step in anti-cancer therapeutics. The assays described in this manuscript, give insights into the effects of NHE1 inhibitors directly on NHE1 activity, and into its downstream behavior promoting metastasis. They should prove most useful in testing novel NHE1 inhibitors for future anti-metastatic use.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
Supported by the Women and Children's Health Research Institute and by the Canadian Institutes of Health Research (CIHR) 97816.
Contributor Information
Schammim Ray Amith, Email: amith@ualberta.ca.
Jodi Marie Wilkinson, Email: jmwilkin@ualberta.ca.
Larry Fliegel, Email: lfliegel@ualberta.ca.
References
- 1.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Weigelt B., Peterse J.L., van't Veer L.J. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 3.Redig A.J., McAllister S.S. Breast cancer as a systemic disease: a view of metastasis. J. Intern Med. 2013;274(2):113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reshkin S.J., Bellizzi A., Caldeira S., Albarani V., Malanchi I., Poignee M., Alunni-Fabbroni M., Casavola V., Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000;14(14):2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- 5.Fliegel L. The Na+/H+ exchanger isoform 1. Int. J. Biochem. Cell Biol. 2005;37(1):33–37. doi: 10.1016/j.biocel.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Malo M.E., Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can. J. Physiol. Pharmacol. 2006;84(11):1081–1095. doi: 10.1139/y06-065. [DOI] [PubMed] [Google Scholar]
- 7.Slepkov E.R., Rainey J.K., Sykes B.D., Fliegel L. Structural and functional analysis of the Na(+)/H(+) exchanger. Biochem. J. 2007;401(3):623–633. doi: 10.1042/BJ20061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harguindey S., Orive G., Luis Pedraz J., Paradiso A., Reshkin S.J. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin–one single nature. Biochim. Biophys. Acta. 2005;1756(1):1–24. doi: 10.1016/j.bbcan.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Noel J., Pouyssegur J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. Am. J. Phyiol. 1995;268:C283–C296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- 10.Wakabayashi S., Shigekawa M., Pouyssegur J. Molecular physiology of vertabrate Na+/H+ exchangers. Physiol. Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Hagag N., Lacal J.C., Graber M., Aaronson S., Viola M.V. Microinjection of ras p21 induces a rapid rise in intracellular pH. Mol. Cell Biol. 1987;7(5):1984–1988. doi: 10.1128/mcb.7.5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillies R.J., Martinez-Zaguilan R., Martinez G.M., Serrano R., Perona R. Tumorigenic 3T3 cells maintain an alkaline intracellular pH under physiological conditions. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7414–7418. doi: 10.1073/pnas.87.19.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perona R., Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature. 1988;334(6181):438–440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- 14.Rotin D., Steele-Norwood D., Grinstein S., Tannock I. Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res. 1989;49:205–211. [PubMed] [Google Scholar]
- 15.Cardone R.A., Casavola V., Reshkin S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer. 2005;5(10):786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 16.Stock C., Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009;458(5):981–992. doi: 10.1007/s00424-009-0677-8. [DOI] [PubMed] [Google Scholar]
- 17.Busco G., Cardone R.A., Greco M.R., Bellizzi A., Colella M., Antelmi E., Mancini M.T., Dell'Aquila M.E., Casavola V., Paradiso A., Reshkin S.J. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010;24(10):3903–3915. doi: 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- 18.Stock C., Cardone R.A., Busco G., Krahling H., Schwab A., Reshkin S.J. Protons extruded by NHE1: digestive or glue? Eur. J. Cell Biol. 2008;87(8–9):591–599. doi: 10.1016/j.ejcb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Amith S.R., Wilkinson J.M., Baksh S., Fliegel L. Na+/H+ exchanger (NHE1) as a novel co-adjuvant target in paclitaxel therapy of triple-negative breast cancer cells. Oncotarget. 2015;6(2):1262–1275. doi: 10.18632/oncotarget.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amith S.R., Fliegel L. Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res. 2013;73(4):1259–1264. doi: 10.1158/0008-5472.CAN-12-4031. [DOI] [PubMed] [Google Scholar]
- 21.Reshkin S.J., Bellizzi A., Albarani V., Guerra L., Tommasino M., Paradiso A., Casavola V. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na(+)/H(+) exchange, motility, and invasion induced by serum deprivation. J. Biol. Chem. 2000;275(8):5361–5369. doi: 10.1074/jbc.275.8.5361. [DOI] [PubMed] [Google Scholar]
- 22.Reshkin S.J., Bellizzi A., Cardone R.A., Tommasino M., Casavola V., Paradiso A. Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) NHE isoform 1 in human breast cancer cells. Clin. Cancer Res. 2003;9(6):2366–2373. [PubMed] [Google Scholar]
- 23.Brisson L., Driffort V., Benoist L., Poet M., Counillon L., Antelmi E., Rubino R., Besson P., Labbal F., Chevalier S., Reshkin S.J., Gore J., Roger S. NaV1.5 Na+ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013;126(Pt 21):4835–4842. doi: 10.1242/jcs.123901. [DOI] [PubMed] [Google Scholar]
- 24.Reshkin S.J., Greco M.R., Cardone R.A. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos. Trans. R. Soc. Lond B Biol. Sci. 2014;369(1638):20130100. doi: 10.1098/rstb.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greco M.R., Antelmi E., Busco G., Guerra L., Rubino R., Casavola V., Reshkin S.J., Cardone R.A. Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe. Oncol. Rep. 2014;31(2):940–946. doi: 10.3892/or.2013.2923. [DOI] [PubMed] [Google Scholar]
- 26.Odunewu-Aderibigbe A., Fliegel L. The Na(+)/H(+) exchanger and pH regulation in the heart. IUBMB Life. 2014;66(10):679–685. doi: 10.1002/iub.1323. [DOI] [PubMed] [Google Scholar]
- 27.Karmazyn M. NHE-1: still a viable therapeutic target. J. Mol. Cell Cardiol. 2013;61:77–82. doi: 10.1016/j.yjmcc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Slepkov E.R., Rainey J.K., Li X., Liu Y., Cheng F.J., Lindhout D.A., Sykes B.D., Fliegel L. Structural and functional characterization of transmembrane segment IV of the NHE1 isoform of the Na+/H+ exchanger. J. Biol. Chem. 2005;280(18):17863–17872. doi: 10.1074/jbc.M409608200. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Singh D., Fliegel L. The Na+/H+ antiporter potentiates growth and retinoic- acid induced differentiation of P19 embryonal carcinoma cells. J. Biol. Chem. 1997;272:26545–26549. doi: 10.1074/jbc.272.42.26545. [DOI] [PubMed] [Google Scholar]
- 30.Silva N.L., Wang H., Harris C.V., Singh D., Fliegel L. Characterization of the Na+/H+ exchanger in human choriocarcinoma (BeWo) cells. Pflugers Arch. Eur. J. Physiol. 1997;433:792–802. doi: 10.1007/s004240050347. [DOI] [PubMed] [Google Scholar]
- 31.Ding J., Rainey J.K., Xu C., Sykes B.D., Fliegel L. Structural and functional characterization of transmembrane segment VII of the Na+/H+ exchanger isoform 1. J. Biol. Chem. 2006;281(40):29817–29829. doi: 10.1074/jbc.M606152200. [DOI] [PubMed] [Google Scholar]
- 32.Taylor S., Spugnini E.P., Assaraf Y.G., Azzarito T., Rauch C., Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist. Updates. 2015;23:69–78. doi: 10.1016/j.drup.2015.08.004. [DOI] [PubMed] [Google Scholar]