Abstract
Endophytic fungi have been described as producers of important bioactive compounds; however, they remain under-exploited as exopolysaccharides (EPS) sources. Therefore, this work reports on EPS production by submerged cultures of eight endophytes isolated from Piper hispidum Sw., belonging to genera Diaporthe, Marasmius, Phlebia, Phoma, Phyllosticta and Schizophyllum. After fermentation for 96 h, four endophytes secreted EPS: Diaporthe sp. JF767000, Diaporthe sp. JF766998, Diaporthe sp. JF767007 and Phoma herbarumJF766995. The EPS from Diaporthe sp. JF766998 differed statistically from the others, with a higher percentage of carbohydrate (91%) and lower amount of protein (8%). Subsequently, this fungus was grown under submerged culture for 72, 96 and 168 h (these EPS were designated EPSD1-72, EPSD1-96 and EPSD1-168) and the differences in production, monosaccharide composition and apparent molecular were compared. The EPS yields in mg/100 mL of culture medium were: 3.0 ± 0.4 (EPSD1-72), 15.4 ± 2.2 (EPSD1-96) and 14.8 ± 1.8 (EPSD1-168). The EPSD1-72 had high protein content (28.5%) and only 71% of carbohydrate; while EPSD1-96 and EPSD1-168 were composed mainly of carbohydrate (≈95 and 100%, respectively), with low protein content (≈5%) detected at 96 h. Galactose was the main monosaccharide component (30%) of EPSD1-168. Differently, EPSD1-96 was rich in glucose (51%), with molecular weight of 46.6 kDa. It is an important feature for future investigations, because glucan-rich EPS are reported as effective antitumor agents.
Keywords: Endophytes, EPS, HPSEC/RID, Monosaccharide composition
Graphical abstract
Highlights
1. Introduction
Endophytic fungi colonize, for all or part of their life cycle, the internal plant tissues without causing apparent harm to their host [1] and their ecological functions attract increasing attention [2]. The endophyte and host plant establish a harmonious symbiotic system interaction in which the microorganisms obtain energy, nutrients and shelter, while they protect the hosts against pathogens, herbivores and insects and induce plant growth or defense mechanisms [3], [4], [5]. Throughout the world researchers have been shown that endophytes are potential producers of novel and biologically active substances [6], as aliphatic compounds, alkaloids, flavonoids, peptides and steroids [7].
However, endophytes remain under-exploited as producers of exopolysaccharides (EPS) with biotechnological properties [8]. These macromolecules are sugar polymers containing more than 20 monosaccharide units joined by glycosidic linkages [9]. Microbial EPS have been studied for decades due to their interesting physicochemical and rheological properties with novel functionality not found in polymers produced by algae or plants [10]. They are synthesized intracellularly throughout growth or during the late logarithmic or stationary phase and then secreted into the culture medium in the form of slime [10], [11], [12]. The great diversity of their structures and functional roles is closely associated with differences in the sequences of monomeric units, glycosidic linkages and different types of branching [13].
In laboratory, the fungal EPS production has many advantages compared to polysaccharides extracted from fruiting bodies: easy isolation and purification avoiding the use of harsh extraction steps, minor production cost and huge production in short time [13], [14]. One homogeneous system that provides a source of EPS is the submerged culture [15], in which filamentous fungi exhibit different morphological growth forms ranging from dispersed mycelial filaments to pellets (densely interwoven mycelial masses) [16].
In previous studies, we reported the isolation, molecular identification, antimicrobial and proteolytic activities of endophytic fungi isolated from leaves of the medicinal plant Piper hispidum Sw., popularly called as “platanillo-de-cuba” (Cuba), “cordoncillo” (Mexico) and “falso-jaborandi” (Brazil) [17], [18], [19], [20]. In this study, eight P. hispidum endophytes were investigated to find the most potent EPS source and to evaluate its EPS production in submerged culture at different cultivation times. Fungal endophytes belonging to the genera Diaporthe, Marasmius, Phlebia, Phoma, Phyllosticta and Schizophyllum were selected. There are no reports in the literature about EPS produced by endophytes from the above-mentioned genera.
2. Materials and methods
2.1. Reagents and culture media
Potato dextrose agar (PDA) medium was purchased from HiMedia Laboratories (Mumbai, MH, India). Analytical standards and trifluoroacetic acid (TFA), were purchased from Sigma–Aldrich Company (St. Louis, MO, USA). Other chemicals were of analytical grade. Vogel's minimal salts medium (VMSM) was prepared according to Vogel [21].
2.2. Endophytic fungi
The endophytic ascomycetes and basidiomycetes used (Table 1) belong to the fungal culture collection of the Laboratory of Microbial Biotechnology, State University of Maringá, Brazil. They were isolated from healthy leaves of the medicinal plant P. hispidum located in the Dr. Luis Teixeira Mendes Forest Garden, a remnant of semideciduous forest in the municipality of Maringá, Paraná State, southern Brazil (23º26′5.10″S, 51º57′59.46″W). Molecular identification was based on sequencing of the ITS1-5.8S-ITS2 region of rDNA [17] and the sequences were submitted to the GenBank database. Fungi were maintained on PDA at 4 °C and subcultured at three-month intervals. The Castellani method [22] was used for permanent maintenance.
Table 1.
Endophytic fungi used for the screening of EPS production.
| Phylum | Order | Species | GenBank accession no. |
|---|---|---|---|
| Ascomycota | Botryosphaeriales | Phyllosticta capitalensis | JF766988 |
| Ascomycota | Diaporthales | Diaporthe sp. | JF767000 |
| Ascomycota | Diaporthales | Diaporthe sp. | JF766998 |
| Ascomycota | Diaporthales | Diaporthe sp. | JF767007 |
| Ascomycota | Pleosporales | Phoma herbarum | JF766995 |
| Basidiomycota | Agaricales | Marasmius cladophyllus | JF767003 |
| Basidiomycota | Agaricales | Schizophyllum commune | JF766994 |
| Basidiomycota | Polyporales | Phlebia sp. | JF766997 |
Endophytes were isolated and molecularly identified by Orlandelli et al. [17].
2.3. Culture conditions and preparation of EPS
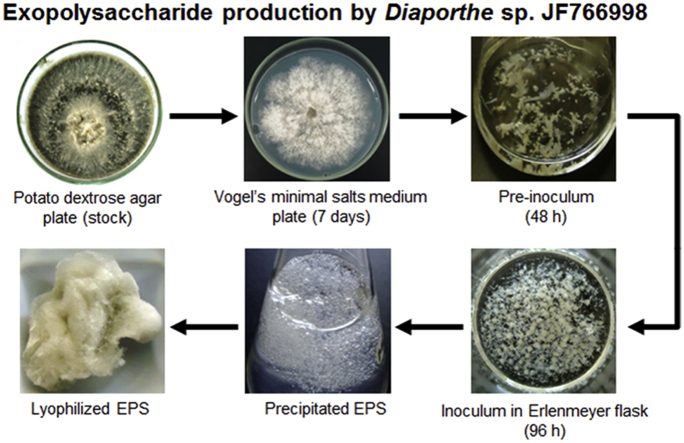
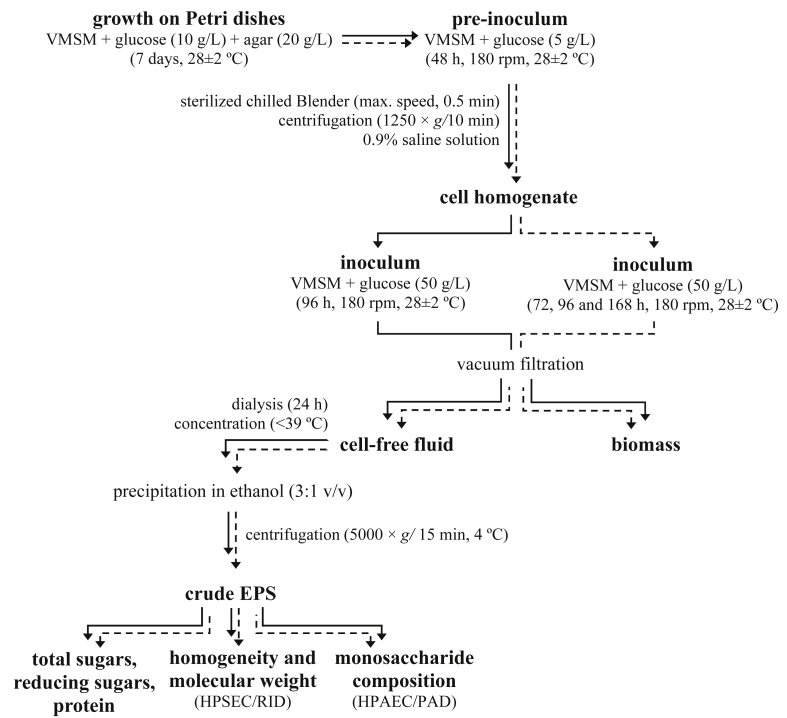
The submerged culture conditions for EPS production was performed as previously described by Steluti et al. [23] (Fig. 1), with some modifications: pre-inoculum was prepared from seven-day-old cultures of endophytes grown on agar plates containing VMSM, agar (20 g/L) and glucose (10 g/L) at 28 ± 2 °C. Then the pre-inoculum was homogenized (sterilized chilled Blender) for 0.5 min at maximum speed and centrifuged (1250 × g for 10 min) to cell separation. After, the cell homogenate was recovered, diluted with sterilized 0.9% saline solution to an absorbance of 0.4–0.5 at 400 nm. For the inoculum, 4-mL aliquots of the cell homogenate were transferred to 500-mL Erlenmeyer flasks containing 100 mL of VMSM and only glucose (50 g/L) as carbon source. Three replicate flasks per experiment were incubated at 28 ± 2 °C on an orbital shaker at 180 rpm for 96 h.
Fig. 1.
Diagrammatic scheme outlining the protocol for studies on EPS from P. hispidum endophytes. VMSM = Vogel's minimum salts medium. Solid arrows: steps followed for the screening of EPS sources. Dashed arrows: steps followed for the optimization of EPS production by Diaporthe sp. JF766998.
Cell-free extracellular fluid was obtained after removal of the fungal mycelia by vacuum filtration. The material was then extensively dialyzed (MW cut-off 12,000 Da) against distilled water for 24 h. The dialysate was concentrated under reduced pressure (<39 °C) in a rotary evaporator and treated with 3 volumes of absolute ethanol. The precipitates were recovered by centrifugation (5000 × g for 15 min at 4 °C) and dissolved in deionized water. Aliquots of each material were used for the determination of sugars and protein content. The rest of EPS was lyophilized and stored at −20 °C.
2.4. EPS production by Diaporthe sp. JF766998 under different cultivation time
Diaporthe sp. JF766998 was grown under submerged culture for 72, 96 and 168 h (Fig. 1) and the influence of cultivation time on the production (total sugars, reducing sugars, protein and EPS yield), monosaccharide composition and apparent molecular weight of EPS was evaluated.
2.5. Analytical techniques
Total sugars were determined by the phenol-sulfuric acid method [24] and reducing sugars were measured by the dinitrosalicylic acid (DNS) method [25]. d-glucose was used as the standard in both assay procedures. Protein was determined using the Bradford method [26] with bovine serum albumin as standard.
2.6. Determination of EPS homogeneity and apparent molecular weight
Aliquots of each EPS were dissolved in deionized water (1 mg/mL) and filtered through a Millipore nitrocellulose membrane with 0.22-μm pore size. Homogeneity was determined by high performance steric exclusion chromatography (HPSEC) coupled to a refractive index (RI) detector, model RID 10A, and UV–vis detector (Shimadzu Company, Kyoto, KYT, Japan). The chromatography system consisted of an HPLC pump, model 10AD, a manual injection valve (Shimadzu) fitted with a 200-μL loop and an Ultrahydrogel column (7.8 × 300 mm) system (Waters) with exclusion limit of 7 × 106, 4 × 105, 8 × 104 and 5 × 103 Da arranged in series. The mobile phase was 0.1 M NaNO3 with sodium azide (0.03%), and the flow rate was 0.6 mL/min. Data analysis was performed using LC solution software (Shimadzu Company, Kyoto, KYT, Japan). A standard curve of dextran with MW of 1400, 1100, 670, 500, 410, 266, 150, 77.8, 72.2, 50, 40.2, and 9.4 kDa was made to determine the apparent molecular weight (MWapp) of EPS.
2.7. Determination of monosaccharide composition
Lyophilized samples (0.05 mg of total sugar) were hydrolyzed with 0.3 mL of 2 M TFA in a sealed tube at 121 °C for 2 h. After hydrolysis, the solution was dried under vacuum, and the residue dissolved in 0.5 mL of water and dried again. The dissolution–evaporation cycle was repeated until complete evaporation of TFA. Finally, the residue was dissolved in 0.5 mL of deionized water and a 0.025-mL diluted aliquot was analyzed by high performance anion-exchange chromatography with pulsed amperometric detection (HPAEC/PAD) on a Dionex DX 500 Chromatograph (Dionex Company, Sunnyvale, CA, USA). Neutral monosaccharides were separated isocratically (0.014 M NaOH) using a CarboPac PA1 column (4 × 250 mm) equipped with a PA1 guard column using a flow rate of 1.0 mL/min. Elution was performed using water (eluent 1) and 14% 0.2 M NaOH (eluent 2). After 20 min, the column was regenerated with 100% eluent 2 for 15 min, followed by a return to 0.014 M NaOH. Monosaccharide quantification was carried out from peak area measurements using response factors obtained with monosaccharide standards.
2.8. Statistical analysis
The production of EPS by all endophytic fungi and the production of EPS by Diaporthe sp. JF766998 under different cultivation time were analyzed by ANOVA (analysis of variance) and means of triplicates were compared with a t-test (p < 0.05) using the statistical program SISVAR 5.3.
3. Results and discussion
3.1. Screening of EPS production by endophytic fungi
Microbial biosynthesis is affected by culture medium composition and cultivation conditions [13], [15]. The endophytes Marasmius cladophyllus JF767003, Phlebia sp. JF766997, Phyllosticta capitalensis JF766988 and Schizophyllum commune JF766994 did not grow after 96 h (planned 48 h and additional 48 h) of pre-inoculum cultivation, suggesting that the protocol employed herein was not favorable for carbohydrate production. Non-endophytic strains of the same genera/species are able to secrete EPS when grown under different conditions of submerged culture [27], [28], [29], [30], [31].
Four endophytic ascomycetes were able to secrete EPS under the culture conditions tested: Diaporthe sp. JF767000 (EPSD), Diaporthe sp. JF766998 (EPSD1), Diaporthe sp. JF767007 (EPSD2) and Phoma herbarum JF766995 (EPSP). The yield of EPSD1 was significantly (p < 0.05) higher than that of EPSD, EPSD2 and EPSP (Table 2). Also, a higher amount of carbohydrate and lower amount of protein (92% and 8%, respectively) were found in EPSD1 when compared with the other three EPS (≤83% total sugars and ≥14% protein).
Table 2.
Production and apparent molecular weight of EPS secreted by endophytic ascomycetes after submerged fermentation for 96 h.
| Endophytes | EPS code | pHf | EPS yield (mg)∗ | Quantification (%) |
HPSEC/RID |
|||
|---|---|---|---|---|---|---|---|---|
| TS | RS | P | RT (min) | MWapp (kDa) | ||||
| Diaporthe sp. JF767000 | EPSD | 5.5 | 7.9 ± 0.0b | 82.6 | 0.6 | 16.8 | 40.9 | 4.8 × 103 |
| 53.2 | 46.6 | |||||||
| Diaporthe sp. JF766998 | EPSD1 | 4.5 | 17.6 ± 2.1a | 91.0 | 1.0 | 8.0 | 52.8 | 40.0 |
| Diaporthe sp. JF767007 | EPSD2 | 4.5 | 10.9 ± 2.2b | 83.0 | 3.0 | 14.0 | 53.4 | 38.0 |
| Phoma herbarumJF766995 | EPSP | 5.0 | 2.7 ± 0.2c | 80.0 | 0.0 | 20.0 | 52.5 | 47.0 |
Means of triplicates (means ± standard deviation) of EPS secreted in flasks containing 100 mL of culture medium. Different (online) letters indicate that the means are significantly different according to a t-test (p < 0.05). pHf = final pH (initial pH 5.8). TS = total sugars, RS = reducing sugar, P = protein, RT = retention time, MWapp = apparent molecular weight.
Among the EPS secreted by three endophytes from the order Diaporthales (EPSD, EPSD1 and EPSD2), the fungus Diaporthe sp. JF766998 appeared to be the most promising due to the higher yield and carbohydrate content of EPSD1. Also, it contained about half the protein quantified in EPSD and EPSD2 (Table 2). Maziero et al. [28] found marked differences in the yields of EPS produced by two or more strains from the same genus (Ganoderma, Lentinus, Pleurotus or Psilocybe). Diamantopoulou et al. [32] suggested that the fungal synthesis of polysaccharides could be a strain-dependent process, a fact that explain the differences in EPS secreted by closely related species cultivated under the same conditions.
Selbmann et al. [33] showed that sorbitol, maltose, sucrose and starch were more efficient (12.3–12.5 g/L of EPS) than glucose (11.6 g/L EPS) as carbon source for the Antarctic fungus P. herbarum CCFEE. It suggests that yields of EPSP, from the endophyte P. herbarum JF766995 (Table 2), could be increased using other substrates. Glucose is biologically the most effective source of energy [22] and, not coincidentally, growth on glucose-based submerged cultures is largely employed for the screening of microbial sources of polysaccharides [22], [32], [34], [35]. Therefore, this substrate, at a concentration of 50 g/L, was chosen as sole carbon source for EPS production by P. hispidum endophytes.
According to some authors [12], [36], [37], EPS produced under submerged conditions can be conjugated to other components such as proteins, lipids and nucleic acids, that commonly co-precipitate in ethanol. Earlier studies reported that other fungal genera secreted EPS containing a high amount (≈14–26%) of protein [27], [38], [39], corroborating the results obtained herein for EPSD, EPSD2 and EPSP (14–20% protein).
The homogeneity and MWapp of EPSD, EPSD1, EPSD2 and EPSP were determined by HPSEC/RID (Table 2). EPSD1 exhibited a single and symmetric peak (not shown) similar to that reported for the EPS (designated FO1) secreted by the endophytic fungus Fusarium oxysporum Y24-2 [40]. According to Chen et al. [41], the symmetry observed is probably due to the high solubility of this EPS. On the other hand, the single and polydisperse peaks observed for EPSD2 and EPSP are consistent with the peak of Fr–I (EPS secreted by Phellinus linteus) detected by the SEC/MALLS system [42]. The EPSD elution profile by HPSEC/RID analysis showed two peaks with MWapp of 4.8 × 103 and 46.6 kDa (Table 2), suggesting the presence of at least two EPS. Data corroborating the EPSD elution profile are scarce for fungi but was reported for bacteria [43], [44], [45].
3.2. EPSD1 production by Diaporthe sp. JF766998 for different cultivation times
The screening of endophytic sources of EPS (Table 2) highlighted the production obtained for Diaporthe sp. JF766998. Therefore, this fungus was grown under submerged culture for 72, 96 and 168 h. The influence of cultivation time on the production, monosaccharide composition and MWapp of EPSD1 was examined. The EPS obtained were designated EPSD1-72, EPSD1-96 and EPSD1-168. As seen in Fig. 2, increase in cultivation time resulted in higher amount of fungal biomass, but the same was not observed for EPS production. As confirmed in Table 3, the yield of EPSD1-96 (15.4 ± 2.2 mg/100 mL of liquid medium) was slightly higher than the obtained for EPSD1-168 (14.8 ± 1.8 mg/100 mL), although this difference was not statistically significant. For all cultivation time tested, the reducing sugars value (measured as reducing sugars) was near to zero, indicating that the carbon source was almost totally consumed during the fungal fermentation. The presence of protein components could be probably related to constitutive enzymes secreted into the culture medium.
Fig. 2.
Morphological aspects of EPSD1 production by Diaporthe sp. JF766998 grown under submerged culture for 72, 96 and 168 h. PDA = potato dextrose agar medium. VMSM = Vogel's minimal salts medium.
Table 3.
Production and monosaccharide composition of EPSD1 from Diaporthe sp. JF766998 after submerged fermentation for 72, 96 and 168 h.
| EPS code | pHf | EPS yield (mg)∗ | Quantification (%) |
Monosaccharide composition (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TS | RS | P | Glc | Gal | Man | Fuc | GlcN | |||
| EPSD1-72 | 5.5 | 3.0 ± 0.4b | 71.0 | 0.5 | 28.5 | 84 | 11 | <1 | 5 | <1 |
| EPSD1-96 | 4.5 | 15.4 ± 2.2a | 94.7 | 0.5 | 4.8 | 51 | 31 | 16 | 2 | <1 |
| EPSD1-168 | 4.5 | 14.8 ± 1.8a | 99.8 | 0.2 | 0.0 | 30 | 42 | 22 | 4 | 2 |
Means of triplicates (means ± standard deviation) of EPS secreted in 100 mL of culture medium. Different (online) lettersindicate that the means are significantly different according to a t-test (p < 0.05). pHf = final pH (initial pH 5.8). TS = total sugars, P = protein, RS = reducing sugars, Glc = glucose, Gal = galactose, Man = mannose, Fuc = fucose, GlcN = glucosamine.
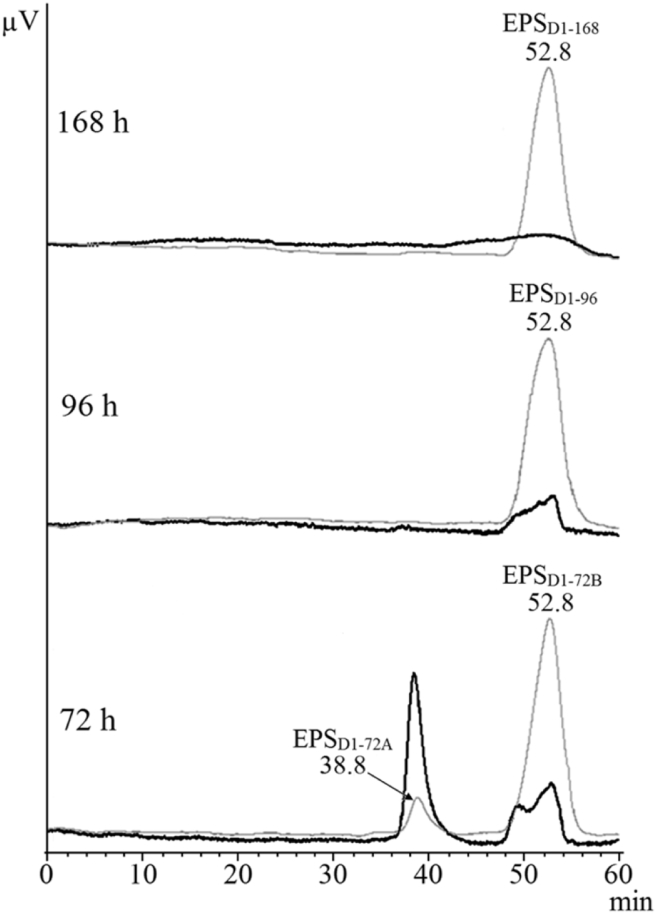
The monosaccharide composition of EPSD1-96 and EPSD1-168 was similar (Table 3); however, glucose was the main component in EPSD1-96 (51%) while galactose was the predominant sugar in EPSD1-168 (42%). In addition, both EPS exhibited similar profile on the HPSEC/RID analysis: single and symmetric peaks (at 52.8 min) with MWapp of 40.0 kDa (Fig. 3). Considering that a short production time is more economically viable, submerged fermentation for 96 h would be advantageous for Diaporthe sp. JF766998. The monosaccharide composition of EPSD1-96 was glucose, galactose, mannose and fucose in a molar ratio of 25:15:8:1. In contrast, the phytopathogen Phomopsis (= Diaporthe) foeniculi secreted two EPS: a galactan and a mannan [46]. The predominance of glucose in the EPS produced by the endophyte Diaporthe sp. JF766998 can be considered interesting for the investigation of biological activities, because several homo- and heteropolysaccharides with high glucose content were found to be more effective antitumor agents, as reviewed by Ferreira et al. [47].
Fig. 3.
Elution profile of EPSD1 analyzed by HPSEC/RID coupled to a UV–vis detector. Diaporthe sp. JF766998 was grown under submerged culture for 72, 96 and 168 h, and EPS were designated EPSD1-72, EPSD1-96 and EPSD1-168, respectively. Aliquot of EPS injected: 200 μL (1 mg/mL). Gel permeation columns with exclusion limit of 7 × 106, 4 × 105, 8 × 104 and 5 × 103 Da arranged in series. Flow rate = 0.6 mL/min. Eluent: 0.1 M NaNO3 with sodium azide (0.03%). UV–vis 280 nm ( ), RID (
), RID ( ).
).
EPSD1-72 yield was significantly (p < 0.05) lower than that obtained for EPSD1-96 and EPSD1-168 (Table 3). Although the monosaccharide composition of EPSD1-72 was mainly glucose, this preparation contained less carbohydrate (72%) and more protein (28%) than did the EPS obtained after 96 and 168 h of cultivation. On HPSEC/RID analysis (Fig. 3), EPSD1-72 showed an elution profile with two peaks called EPSD1-72A (38.8 min) and EPSD1-72B (52.8 min), with MWapp of 5 × 103 and 46.6 kDa, respectively. Probably, the UV–vis detection obtained for EPSD1-72A is an indication that a protein or glycoprotein was secreted when the fungus (Diaporthe sp. JF766998) remained in shaker flasks for 72 h. Krcmar et al. [27] reported that glucose was the main component of the EPS secreted by Phlebia radiata Fr.79 ATCC 64658, which was composed of ≈20% protein, indicating that a mixture of glucan and glycoprotein was secreted. For Botryosphaeria (= Lasiodiplodia) sp. MAMB-05, the chromatogram profile on Sepharose CL 4B indicated an EPS-glycoprotein association when the fungal inoculum was incubated for 72 h [38], like that suggested herein for EPSD1-72.
4. Conclusions
Endophytes are important sources of bioactive compounds, but should be further explored as EPS producers. This present study suggests that four P. hispidum endophytes, particularly Diaporthe sp. JF766998, are capable of producing these polymers. Among the three cultivation times tested (72, 96 and 168 h) for this fungus, 96 and 168 h resulted in EPS yield of 15.4 ± 2.2 and 14.8 ± 1.8 mg/100 mL culture medium, respectively, with apparent molecular weight of 46.6 kDa. It suggested that a short incubation time (96 h) could be more economically viable. The EPS secreted by Diaporthe sp. JF766998 cultivated for 96 h contained mainly carbohydrate (≈95%) and a low percentage of protein (≈5%), with glucose as the main monosaccharide component. This high glucose content is interesting for future investigations of the biological properties of this EPS.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 311534/2014-7 and 447265/2014-8), Fundação Araucária (276/2014) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 05/53879-3) for financial support. R.C. Orlandelli thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the doctoral scholarship. Dr. A. Leyva helped with English editing of the manuscript.
References
- 1.Petrini O. Fungal endophytes in tree leaves. In: Andrews J.H., Hirano S.S., editors. Microbial Ecology of Leaves. Springer; New York: 1991. pp. 179–197. [Google Scholar]
- 2.Xie X.G., Dai C.C. Degradation of a model pollutant ferulic acid by the endophytic fungus Phomopsis liquidambari. Bioresour. Technol. 2015;179:35–42. doi: 10.1016/j.biortech.2014.11.112. [DOI] [PubMed] [Google Scholar]
- 3.Firáková S., Šturdíková M., Múčková M. Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia. 2007;62:251–257. [Google Scholar]
- 4.Wang Y., Dai C.C. Endophytes: a potential resource for biosynthesis, biotransformation, and biodegradation. Ann. Microbiol. 2011;61:207–215. [Google Scholar]
- 5.Alvin A., Miller K.I., Neilan B.A. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol. Res. 2014;169:483–495. doi: 10.1016/j.micres.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gokul Raj K., Manikandan R., Arulvasu C., Pandi M. Anti-proliferative effect of fungal taxol extracted from Cladosporium oxysporum against human pathogenic bacteria and human colon cancer cell line HCT 15, Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015;138:667–674. doi: 10.1016/j.saa.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Yu H., Zhang L., Li L., Zheng C., Guo L., Li W. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol. Res. 2010;165:437–449. doi: 10.1016/j.micres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Mahapatra S., Banerjee D. Structural elucidation and bioactivity of a novel exopolysaccharide from endophytic Fusarium solani SD5. Carbohydr. Polym. 2012;90:683–689. doi: 10.1016/j.carbpol.2012.05.097. [DOI] [PubMed] [Google Scholar]
- 9.Nelson D.L., Cox M.M. sixth ed. WH Freeman and Company; New York: 2013. Lehninger Principles of Biochemistry. [Google Scholar]
- 10.Joshi A.A., Kanekar P.P. Production of exopolysaccharide by Vagococcus carniphilus MCM B-1018 isolated from alkaline Lonar Lake, India. Ann. Microbiol. 2011;61:733–740. [Google Scholar]
- 11.Jenkins R.O., Hall J.H. Production and applications of microbial exopolysaccharides. In: van Balken J.A.M., van Dam R.C., editors. Biotechnological Innovations in Chemical Synthesis. Butterworth-Heinemann Limited; Oxford: 1997. pp. 193–231. [Google Scholar]
- 12.A. Mishra, B. Jha, Microbial exopolysaccharides, in: E. Rosenberg, E.F. DeLong, F. Thompson, S. Lory, E. Stackebrandt (Eds.), The Prokaryotes: Applied Bacteriology and Biotechnology, Springer, Berlin - Heidelberg, pp. 179–192.
- 13.Mahapatra S., Banerjee D. Fungal exopolysaccharide: production, composition and applications. Microbiol. Insights. 2013;6:1–16. doi: 10.4137/MBI.S10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan B., Chi X., Zhang R. Optimization of exopolysaccharides production from a novel strain of Ganoderma lucidum CAU5501 in submerged culture. Braz. J. Microbiol. 2012;43:490–497. doi: 10.1590/S1517-83822012000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elisashvili V. Submerged cultivation of medicinal mushrooms: bioprocesses and products (review) Int. J. Med. Mushrooms. 2012;14:211–239. doi: 10.1615/intjmedmushr.v14.i3.10. [DOI] [PubMed] [Google Scholar]
- 16.Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004;22:189–259. doi: 10.1016/j.biotechadv.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Orlandelli R.C., Alberto R.N., Rubin Filho C.J., Pamphile J.A. Diversity of endophytic fungal community associated with Piper hispidum (Piperaceae) leaves. Genet. Mol. Res. 2012;11:1575–1585. doi: 10.4238/2012.May.22.7. [DOI] [PubMed] [Google Scholar]
- 18.Orlandelli R.C., Alberto R.N., Almeida T.T., Azevedo J.L., Pamphile J.A. In vitro antibacterial activity of crude extracts produced by endophytic fungi isolated from Piper hispidum Sw. J. App. Pharm. Sci. 2012;2:137–141. [Google Scholar]
- 19.Orlandelli R.C., Almeida T.T., Alberto R.N., Specian V., Azevedo J.L., Pamphile J.A. In vitro inhibition of Xanthomonas axonopodis pv. phaseoli by crude ethyl acetate extracts of endophytic fungi isolated from Piper hispidum Sw. Acta Adv. Agr. Sci. 2014;2:1–8. [Google Scholar]
- 20.Orlandelli R.C., Almeida T.T., Alberto R.N., Polonio J.C., Azevedo J.L., Pamphile J.A. Antifungal and proteolytic activities of endophytic fungi isolated from Piper hispidum Sw. Braz. J. Microbiol. 2015;46:359–366. doi: 10.1590/S1517-838246220131042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel H.J. A convenient growth medium for Neurospora crassa. Genet. Bull. 1956;13:42–43. [Google Scholar]
- 22.Castellani A. Maintenance and cultivation of common pathogenic fungi in sterile distilled water; further research. J. Trop. Med. Hyg. 1967;70:181–184. [Google Scholar]
- 23.Steluti R.M., Giese E.C., Piggato M.M., Sumiya A.F.G., Covizzi L.G., Job A.E., Cardoso M.S., Corradi da Silva M.L., Dekker R.F.H., Barbosa A.M. Comparison of botryosphaeran production by the ascomyceteous fungus Botryosphaeria sp., grown on different carbohydrate carbon sources, and their partial structural features. J. Basic Microbiol. 2004;44:480–486. doi: 10.1002/jobm.200410415. [DOI] [PubMed] [Google Scholar]
- 24.Dubois N., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 25.Miller G. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- 26.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Krcmar P., Novotny C., Marais M.F., Joseleau J.P. Structure of extracellular polysaccharide produced by lignin-degrading fungus Phlebia radiata in liquid culture. Int. J. Biol. Macromol. 1999;24:61–64. doi: 10.1016/s0141-8130(98)00072-5. [DOI] [PubMed] [Google Scholar]
- 28.Maziero R., Cavazzoni V., Bononi V.L.R. Screening of basidiomycetes for the production of exopolysaccharide and biomass in submerged culture. Rev. Microbiol. 1999;30:77–84. [Google Scholar]
- 29.Sassaki G.L., Ferreira J.C., Glienke-Blanco C., Torri G., de Toni F., Gorin P.A.J., Iacomini M. Pustulan and branched β-galactofuranan from the phytopathogenic fungus Guignardia citricarpa, excreted from media containing glucose and sucrose. Carbohydr. Polym. 2002;48:385–389. [Google Scholar]
- 30.Kumari M., Survase S.A., Singhal R.S. Production of schizophyllan using Schizophyllum commune NRCM. Bioresour. Technol. 2008;99:1036–1043. doi: 10.1016/j.biortech.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Hao L., Xing X., Li Z., Zhang J., Sun J., Jia S., Qiao C., Wu T. Optimization of effect factors for mycelial growth and exopolysaccharide production by Schizophyllum commune. Appl. Biochem. Biotechnol. 2010;160:621–631. doi: 10.1007/s12010-008-8507-6. [DOI] [PubMed] [Google Scholar]
- 32.Diamantopoulou P., Papanikolaou S., Komaitis M., Aggelis G., Philippoussis A. Patterns of major metabolites biosynthesis by different mushroom. Bioprocess Biosyst. Eng. 2014;37:1385–1400. doi: 10.1007/s00449-013-1112-2. [DOI] [PubMed] [Google Scholar]
- 33.Selbmann L., Onofri S., Fenice M., Federici F., Petruccioli M. Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Res. Microbiol. 2002;153:585–592. doi: 10.1016/s0923-2508(02)01372-4. [DOI] [PubMed] [Google Scholar]
- 34.Li P., Luo C., Sun H., Lu S., Mou Y., Peng Y., Zhou L. In vitro antioxidant activities of polysaccharides from endophytic fungus Fusarium oxysporum Dzf17. Afr. J. Microbiol. Res. 2011;5:5990–5993. [Google Scholar]
- 35.Ma X., Zhang H., Peterson E.C., Chen L. Enhancing exopolysaccharide antioxidant formation and yield from Phellinus species through medium optimization studies. Carbohydr. Polym. 2014;107:214–220. doi: 10.1016/j.carbpol.2014.02.077. [DOI] [PubMed] [Google Scholar]
- 36.Corradi da Silva M.L., Izeli N.L., Martinez P.F., Silva I.R., Constantino C.J.L., Cardoso M.S., Barbosa A.M., Dekker R.F.H., Silva G.V.J. Purification and structural characterisation of (1→3;1→6)-β-d-glucans (botryosphaerans) from Botryosphaeria rhodina grown on sucrose and fructose as carbon sources: a comparative study. Carbohydr. Polym. 2005;61:10–17. [Google Scholar]
- 37.Xiang Y., Xu X., Li J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food. Chem. 2012;134:1899–1905. doi: 10.1016/j.foodchem.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 38.Barbosa A.M., Steluti R.M., Dekker R.F.H., Cardoso M.S., Corradi da Silva M.L. Structural characterization of botryosphaeran: a (1→3;1→6)-β-d-glucan produced by the ascomyceteous fungus, Botryosphaeria sp. Carbohydr. Res. 2004;338:1691–1698. doi: 10.1016/s0008-6215(03)00240-4. [DOI] [PubMed] [Google Scholar]
- 39.Smiderle F.R., Olsen L.M., Ruthes A.C., Czelusniak P.A., Santana-Filho A.P., Sassaki G.L., Gorin P.A.J., Iacomini M. Exopolysaccharides, proteins and lipids in Pleurotus pulmonarius submerged culture using different carbon sources. Carbohydr. Polym. 2012;87:368–376. doi: 10.1016/j.carbpol.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 40.Guo S., Mao W., Li Y., Tian J., Xu J. Structural elucidation of the exopolysaccharide produced by fungus Fusarium oxysporum Y24-2. Carbohydr. Res. 2013;365:9–13. doi: 10.1016/j.carres.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Siu K.C., Cheung Y.C., Wu J.Y. Structure and properties of a (1→3)-β-d-glucan from ultrasound-degraded exopolysaccharides of a medicinal fungus. Carbohydr. Polym. 2014;106:270–275. doi: 10.1016/j.carbpol.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 42.Hwang H.J., Kim S.W., Choi J.W., Yun J.W. Production and characterization of exopolysaccharides from submerged culture of Phellinus linteus KCTC 6190. Enzyme Microb. Tech. 2003;33:309–319. [Google Scholar]
- 43.Singh R.P., Shukla M.K., Mishra A., Kumari P., Reddy C.R.K., Jha B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr. Polym. 2011;84:1019–1026. [Google Scholar]
- 44.Llamas I., Amjres H., Mata J.A., Quesada E., Béjar V. The potential biotechnological applications of the exopolysaccharide produced by the halophilic bacterium Halomonas almeriensis. Molecules. 2012;17:7103–7120. doi: 10.3390/molecules17067103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salazar N., Ruas-Madiedo P., Prieto A., Calle L.P., Los Reyes-Gavilán C.G. Characterization of exopolysaccharides produced by Bifidobacterium longum NB667 and its cholate-resistant derivative strain IPLA B667dCo. J. Agric. Food Chem. 2012;60:1028–1035. doi: 10.1021/jf204034n. [DOI] [PubMed] [Google Scholar]
- 46.Corsaro M.M., de Castro C., Evidente A., Lanzetta R., Molinaro A., Mugnai L. Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculi. Carbohydr. Res. 1998;308:349–357. doi: 10.1016/s0008-6215(98)00085-8. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira I.C., Vaz J.A., Vasconcelos M.H., Martins A. Compounds from wild mushrooms with antitumor potential. Anticancer Agents Med. Chem. 2010;10:424–436. doi: 10.2174/1871520611009050424. [DOI] [PubMed] [Google Scholar]