Abstract
Toxic heavy metals such as cadmium (Cd) and copper (Cu) are global problems that are a growing threat to the environment. Despite some heavy metals are required for plant growth and development, others are considered toxic elements and do not play any known physiological role in plant cells. Elevated doses of Cd or Cu cause toxicity in plants and generate damages due to the stress condition and eventually cause a significant reduction in quantity and quality of crop plants. The nitric oxide (NO) donor sodium nitroprusside (SNP) is reported to alleviate the toxicity of some heavy metals like Cd and Cu. In the current study, the role of NO in alleviating stresses of Cd and Cu was investigated in in vitro-grown tobacco (Nicotiana tabacum) Based on plant growth, total chlorophyll contents, contents and activities of rubisco and rubisco activase. According to the results of this study, the growth and total chlorophyll contents of Cd/Cu stressed plants were hugely decreased in the absence of SNP, while the supplementation of SNP resulted in a significant increase of both fresh weight and total chlorophyll contents. Remarkable reductions of Rubisco and rubisco activase contents and activities were observed in Cd and Cu-induced plants. SNP supplementation showed the highest contents and activities of rubisco and rubisco activase compared to the control and Cu/Cd-stressed plants. Taken together, our findings suggest that SNP could play a protective role in regulation of plant responses to abiotic stresses such as Cd and Cu by enhancing Rubisco and Rubisco activase.
Keywords: Nitric oxide, Cadmium, Rubisco, Rubisco activase, Sodium nitroprusside, Chlorophyll
Abbreviations: ATP, Adenosine triphosphate; BTP, Bis tris phosphate; Cd, Cadmium; Cu, Copper; DMF, N, N-Dimethylformamide; DTT, Dithiothreitol; EDTA, Ethylenediaminetetraacetic acid; GSH, Glutathione; KCl, Potassium chloride; KHCO3, Potassium bicarbonate; MBT, Mercaptabemzathiazol; MgCl2, Magnesium chloride; NaCl, Sodium chloride; NADH, Nicotinamide adenine dinucleotide (reduced form); NaHCO3, Sodium bicarbonate (Sodium hydrogen carbonate); (NH4)2SO4, Ammonium sulphate; NO, Nitric oxide; PEG 10K, Polyethylene glycol 10,000; PEP, Phosphoenolpyruvate; PGK, Phosphoglycerate kinase; PK, Pyruvate kinase; PMSF, Phenylmethanesulfonyl fluoride; PVPP, Polyvinylpolypyrrolidone; Rubisco, Ribulose 1,5bisphosphate carboxylase/oxygenase; RuBP, Ribulose 1,5-bisphosphate; SNP, Sodium nitroprusside
Graphical abstract
Highlights
-
•
Heavy metal ions are believed to act as growth inhibitors and environmental disruptors.
-
•
Nitric oxide (NO) plays a functional role in regulation of plant responses to abiotic stresses.
-
•
Rubisco involved in the process of atmospheric carbon fixation in photosynthesis.
-
•
Rubisco Catalyzes 2 types of reactions (carboxylation and oxygenation).
-
•
Rubisco activase removes bound RuBP from inactive, decarboxylated Rubisco in an ATP-dependent reaction.
1. Introduction
It is well known that plants play an essential role in the environment and in fulfilling human needs. Nevertheless, crop plants production is seriously hampered by influential abiotic stresses. Therefore, it is necessary to figure out how these abiotic stresses can be in a cost-effective and eco-friendly way by utilizing growth regulators and signaling molecules. This research article mainly focuses on alleviation of heavy metal toxicity and enhancement of plant physiological and biochemical characteristics. Toxic heavy metals such as cadmium (Cd) and copper (Cu) are global problems that are a growing threat to the environment. Despite some heavy metals, specifically the micronutrients such as Fe, Mg, Mn, Cu and Mo are required for plant growth and development, others are consider as toxic elements and do not play any known physiological role in plant cells [1]. Heavy metal plays both the role of important components for the upkeep of normal physiological functions and toxic agents with damaging consequences when present in inappropriate amounts [2]. Nevertheless, at high concentrations all heavy metals can accumulate over time in plant tissues, cause toxicity and inhibit plant growth [3]. Among these toxic elements Cd and Cu are the most commonly reported toxic heavy metals [4]. Cd is a widespread trace pollutant that causes abiotic stress in plants. This element enters water or agricultural soils mainly from industrial processes and then accumulates overtime in plant's tissues and eventually transferred to the food chain [5]. Cu mainly accumulates in soil due to some human activities such as application of sewage sludge, application of fertilizers, mining, agricultural wastes and industrial activities [6]. The influence of Cu toxicity is largely on growth of plant root and morphology. In plants, the toxicity of both Cd and Cu is associated with plant growth inhibition and imbalances in many macro- and micronutrient levels. The presence of toxic elements like Cd and Cu in plants results in many physiological modifications affecting both nitrogen and carbohydrate metabolism [7], [8], [9]. Many authors reported growth reduction and some essential biochemical components under Cd and Cu toxicity. Benavides et al. [10] observed a reduction in plant growth under heavy metal toxicity. In a similar study, Rodríguez-Serrano et al. [11] observed a decrease in the glutathione (GSH) content under Cd stress. Thus, heavy metal toxicity is now being considered as a serious challenge facing researchers in biochemistry field. In order to cope with various heavy metal stresses and avoid heavy metals toxicity, plants possess natural defense systems which cellular and molecular responses as well adjust the free metal contents [12]. Abiotic stresses can also be managed by the exogenous application of some plant regulators or signaling molecules such as nitric oxide (NO). NO is considered as a biological messenger that plays an important role in regulation of various physiological processes in plants including plant growth, development and response to biotic and abiotic stress factors [13]. The most commonly used donor of NO is sodium nitroprusside (SNP). In recent decades, a growing number of authors have reported the effects of exogenously applied SNP on alleviating or reducing heavy metal toxicity in plants, but knowledge of physiological mechanisms of NO in overcoming heavy metal stress is insufficient, and some of the available results contradict one another. However, several studies reported that exogenous application of NO counteracts Cd toxicity through regulating cellular distribution of excess Cd and accumulation in plant cell wall. Xiong et al. [14] imputed the NO-induced Cd tolerance to the distribution of Cd in the cell walls of rice plants. Rubisco, formally known as ribulose-1,5-bisphosphate carboxylase/oxygenase, is the primary carboxylating enzyme that used by all photosynthetic organisms to fix atmospheric carbon dioxide [15]. Atmospheric carbon fixation or carbon assimilation is the conversion process of inorganic carbon (carbon dioxide form the atmosphere) to organic carbon (energy compounds) such as carbohydrates needed for growth and development. The process of carbon assimilation is completed by rubisco, almost of the total carbon atoms that are present in the bodies of living organisms have passed through the active site of Rubisco [16]; [17]. However, Rubisco allows oxygen to react with RuBP (ribulose-1, 5-bisphosphate) in addition to carbon dioxide, producing phosphoglycolate, and thereby also initiates photorespiration process. The most abundant protein in the biosphere, Rubisco comprises eight large (56 KDa) subunits and eight small (14 KDa) subunits respectively. Rubisco activase as the name implies, is an enzyme that activates rubisco in ATP dependent reaction. Rubisco activase is believed to power conformational changes in Rubisco that promote the release of inhibitory sugar phosphates from the catalytic site. The regulation of Rubisco activation state could differ depending on the temperatures [18]; [19]. Barta et al. [20] figured out that high temperature caused aggregation of Rubisco activase characterized by disruption of secondary structure content and insoluble protein formation. In this study, the effect of NO donor SNP application on growth, chlorophyll content, rubisco and rubisco activase contents and activities were determined in Cd and Cu stressed tobacco plants grown in vitro.
2. Materials and methods
2.1. Chemicals and apparatuses
MS medium was purchased from Duchefa Biochemie (Haarlem, Netherland), while other chemicals were obtained from Sigma Chemical Co. (St Louis, MO, USA). Vortex mixer, shaker, centrifuge 5415, deep freezer, ice maker, Refrigerator high speed centrifuge (Kontron T-324), Fraction collector (Bio-Rad 2110), ELISA microplate reader (Bio-Rad 680), autoclave, UV–visible spectrophotometer (GeneQuant 100) and hot plate and magnetic stirrer were used in this experiment.
2.2. Growth media
Plant growth medium of Murashige and Skoog [21] was supplemented with 0.05 mM of SNP as a preliminary experiment result and 0.2 mM of each Cd and Cu [22] in addition to the control treatment. The pH value was adjusted to 5.8 using pH meter. Then MS medium poured into culture jars, swirled around, then culture glass bottles containing growth media were sterilized at 121°C for 15 min using autoclave.
2.3. Planting
Tobacco seeds were aseptically cultivated in culture jars to achieve primary explants. Each 3 explants (3 cm in length) were cultivated on the culture medium. All culture bottles were placed in aseptic condition under room temperature (28°C) for 50 days. Then data were targeted measurements. The experiment was replicated three times.
2.4. Determination of growth (fresh weight)
Tobacco growth was estimated by measuring fresh weights of tobacco roots, leaves and stems, and then total fresh weight of tobacco was calculated. To measure the fresh weight of tobacco, 50 days old tobacco plants were smoothly uprooted from the artificial medium and separated into roots, leaves and stems using sterilized forceps and scissors. Each of the three parts of the plant was put in petri dishes then fresh masses were measured using a digital balance.
2.5. Determination of total chlorophyll content
Total chlorophyll was estimated by adopting the method described by Inskeep and Bloom [23]. Three fresh and fully expanded leaves were extracted overnight with N, N-Dimethylformamide (DMF). solution at 4°C and then the extract was centrifuged at 8000 × g for 5 min. The absorbance of the supernatant was measured at 647 and 664.5 nm. The following equation was used to estimate the content of total chlorophyll in tobacco leaf.
2.6. Isolation of rubisco and rubisco activase
Rubisco was isolated from the tobacco leaves according to the method of Wang et al. [24]. Tobacco leaves were grinded using liquid nitrogen. then rubisco extracted from powder of tobacco leaf tissues by the grinding buffer containing 50 mM BTP (pH 7.2), 10 mM NaHCO3, 10 mM MgCl2, 1 mM EDTA, 0.5 mM ATP, 10 mM DTT, 1 mM PMSF, 1 mM benzamidine, 0.01 mM leupeptin, 1.5% PVPP and 3 mM MBT. The solution was filtered from the leaf slurry through cheesecloth. The extract was centrifuged at 16,000 rpm for 40 min. After centrifugation, (NH4)2SO4 powder was added into the supernatant to 35% saturation. Using magnetic stirred, the supernatant stirred for 30 min. The supernatant and pellet were collected by centrifugation at 8000 × g for 8 min. The supernatant contains rubisco, and the resuspended pellet contains rubisco activase. (NH4)2SO4 powder was added into the supernatant up to 55% saturation. The pellet collected by centrifugation at 8000 rpm for 8 min was resuspended in 5 ml of 50 mM Tricine (pH 8.0), 10 mM NaHCO3, 10 mM MgCl2, 10 mM DTT, and 2 mM MBT (buffer A), and 50% PEG-10K was added to a final concentration of 17%. The resulting precipitate was collected by centrifugation at 8000 rpm for 8 min and resuspended in buffer A. Resuspended solution was loaded onto a Q-Sepharose column eluted with 20 mM BTP (pH 7.2) containing 0.1–0.5 M NaCl gradient. After passing through Q-Sepharose column, fractions were collected and assayed at 280 nm for rubisco content and rubisco activity. For rubisco activase isolation, Above obtained pellet was resuspended in 20 mM BTP (pH 7.2), 10 mM MgCl2, 0.2 mM ATP, and 2 mM MBT (buffer B) and 50% (w/v) PEG-10K was added to a final concentration of 18%, stirred for 5 min, and centrifuged at 8000 rpm for 8 min resulted pellet was dissolved in 2.5 ml of buffer B. Solution was cleared by spinning at 10,000 rpm for 10 min. Pellet was resuspended again in 2.5 ml buffer B solution. The collected supernatants were loaded on a Q-Sepharose column eluted with 20 mM BTP (pH 7.2) containing 0.0–0.5 M NaCl gradient. After passing through Q-Sepharose column fractions were collected and assayed for rubisco activase content and activity. Unless stated otherwise, all protein purification processes were carried out at 4C°.
2.7. Determination of rubisco and rubisco activase contents
Rubisco content was determined according to the method described by Wishnick and Lane [25]. Using spectrophotometer, the optical density was measured at 280 nm. The estimation of rubisco activase content was done by adopting the method described by Bradford [26] using bovine serum albumin as a standard and the optical density was measured at 595 nm.
2.8. Assay of rubisco and rubisco activase activities
Rubisco activity was spectrophotometrically determined at 340 nm by adopting the method of Racker [27]. The medium contained 1 M Tris–HCl buffer (pH 7.2), 6 mM NADH, 0.1 M GSH, 0.5 M KHCO3, 0.5% glyceraldehyde-3- phosphate dehydrogenase, 10 units/20 μl 3-phosphoglycerate kinase, 0.05% α-glycerophosphate dehydrogenase-triose phosphate isomerase, 0.025 M ribulose 1,5-bisphosphate (RuBP), 0.2 M ATP, 0.5 M MgCl2, and isolated rubisco extract in a total volume of 0.25 ml. Rubisco activase activity was estimated as the ability to produce ADP in an ATP-dependent reaction in absorbance at 340 nm according to the method explained by Robinson and Portis [28]. The isolated rubisco activase solution was added to a total volume of 0.2 ml of the activation reaction mixture containing 50 mM Tricine-KOH (pH 8.0), 20 mM KCl, 5 mM MgCl2, 2.5 mM ATP, 1 mM phosphoenolpyruvate, 0.3 mM NADH, 40 units/ml pyruvate kinase, and 40 units/ml L-lactic dehydrogenase.
2.9. Statistical analysis
The experiment was replicated three times. Results are presented as the mean ± standard error (SE). Significant differences were determined by one-way analysis of variance (ANOVA) using SPSS 12.0 software.
3. Results
3.1. Nitric oxide alleviates tobacco growth inhibition under Cd/Cu stresses
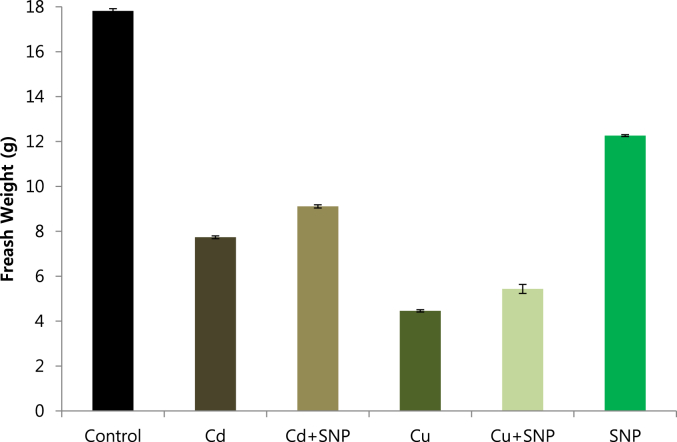
Influence of SNP (NO donor) on the growth of tobacco plants treated with heavy metals (Cd/Cu) was investigated and statistical analysis was performed. Results showed that the growth was inhibited in tobacco plants, which were under Cd and Cu stresses while SNP application alleviated the negative effects of these stresses. Growth of tobacco plants was inhibited in the presence of Cd and Cu. especially in Cd-stressed plants, the effect of heavy metal toxicity was clearly seen as yellowish-brown color on the old leaves of tobacco plants. Supplying SNP to Cd and Cu stressed plants promoted the growth of tobacco and all SNP treated tobacco plants were observed with fully green leaves (Fig. 1). In order to confirm these results, total fresh weight of tobacco plants was measured. Total fresh weight was significantly reduced under Cd and Cu toxicity. While fresh weight increased by SNP supplementation. The lowest fresh weight was observed in plants grown under Cu toxicity, application of 0.05 mM SNP, 0.2 mM Cu + 0.05 mM SNP and 0.2 mM Cu + 0.05 mM SNP increased the fresh weight of tobacco plants compared to Cd and Cu-stressed plants. The highest fresh weights were reported from the treatments of SNP, Cd + SNP and Cu + SNP respectively (Fig. 2).
Fig. 1.
Effect of 0.05 mM SNP on growth of 6 weeks old tobacco plants grown on MS medium under 0.2 mM Cd and 0.2 mM Cu stress conditions. Tobacco plants were maintained at 28 °C under light for 16 h (800 uM/m2/s PFD) and dark for 8 h.
Fig. 2.
Effect of 0.05 mM SNP on fresh weight of tobacco plants grown on MS medium under 0.2 mM Cd and 0.2 mM Cu stress conditions. The bars represent standard errors (SE).
3.2. NO increases chlorophyll contents in Cd/Cu-stressed tobacco plants
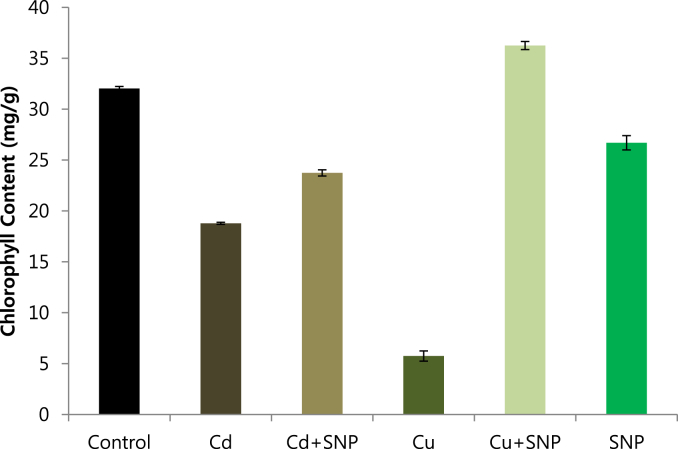
The results showed that total chlorophyll content was significantly reduced by heavy metal (Cd/Cu) stresses and increased by SNP application. Typical symptoms of Cu and Cd toxicity were yellowing and drying on the leaves of tobacco plants, while leaves of SNP supplemented plants were fully green. However, the observed morphological discerption of tobacco leaves indicates the influence of Cd, Cu and SNP treatments on total chlorophyll content of tobacco plants. According to the results, the lowest contents of total chlorophyll were reported from the tobacco plants which were exposed to Cd and Cu stresses. Especially Cu-induced tobacco plants showed a significant reduction in total chlorophyll content. While SNP application increased contents of total chlorophyll compared to tobacco plants grown under Cd and Cu stresses. Compared with the control plants, supplementing SNP with Cd had no significant differences in total chlorophyll content. When SNP was added to the Cu-induced plants, total chlorophyll content was higher than the control. In all cases, our results indicate that content of total chlorophyll was reduced by both metals (Cd/Cu) and increased by SNP supplementation (Fig. 3).
Fig. 3.
Effects of 0.05 mM SNP on Total chlorophyll content of tobacco plants grown on MS medium under 0.2 mM Cd and 0.2 mM Cu stress conditions. The bars represent standard errors (SE).
3.3. Influence of SNP on content and activity of rubisco in Cd/Cu-stressed tobacco plants
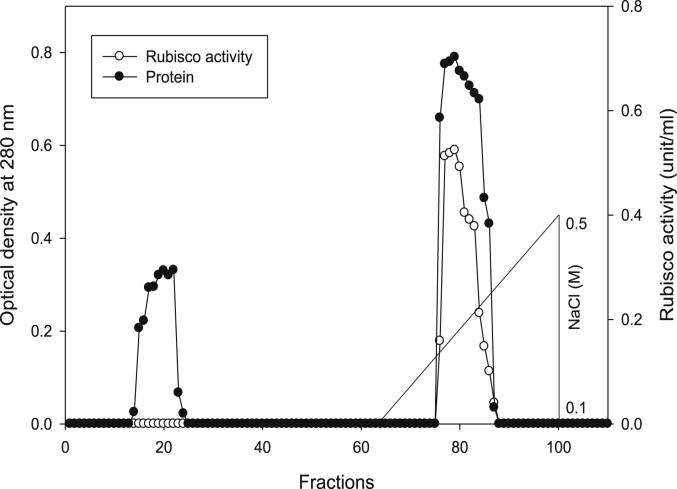
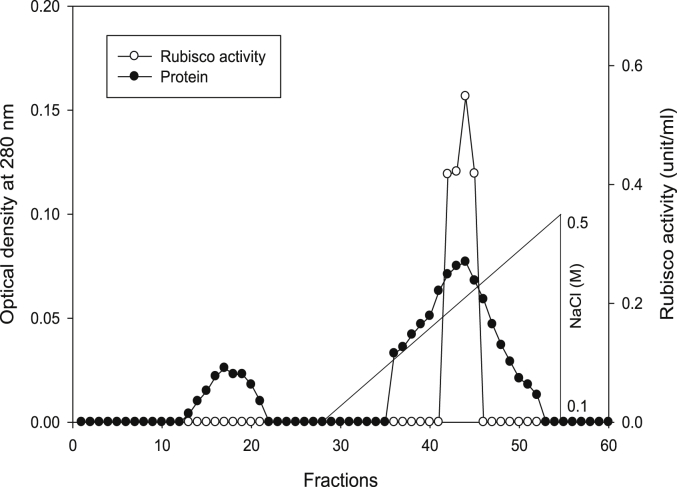
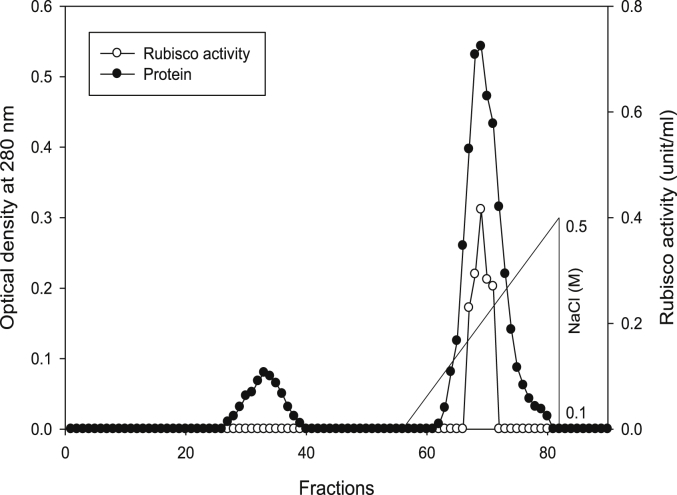
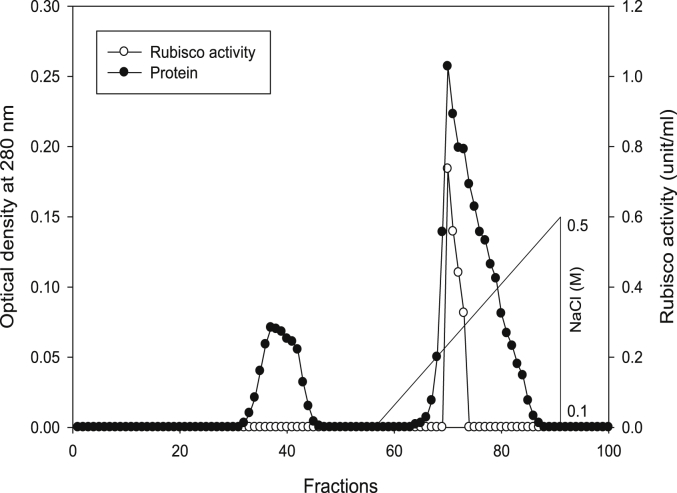
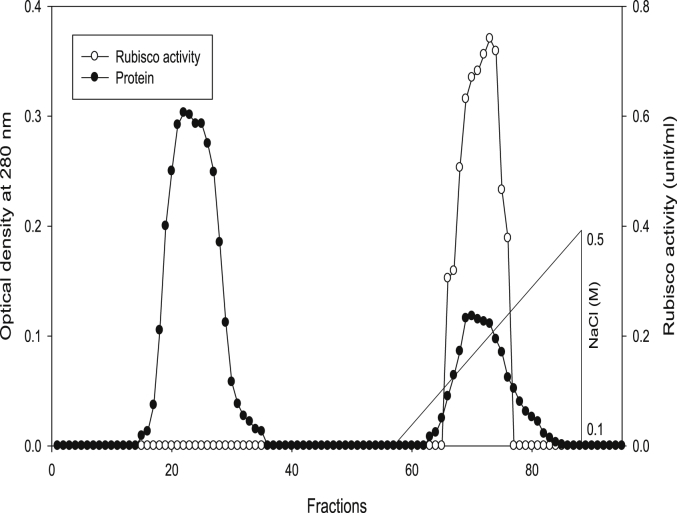
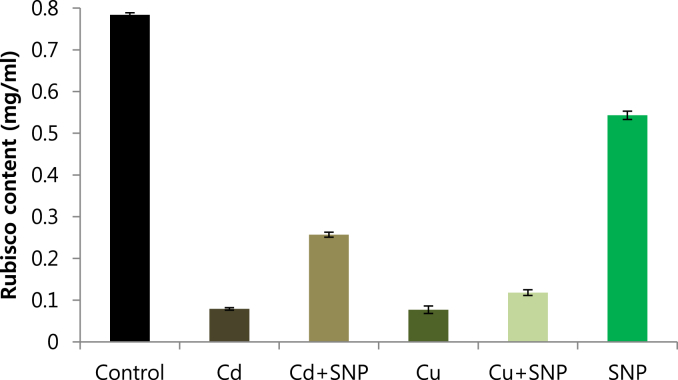
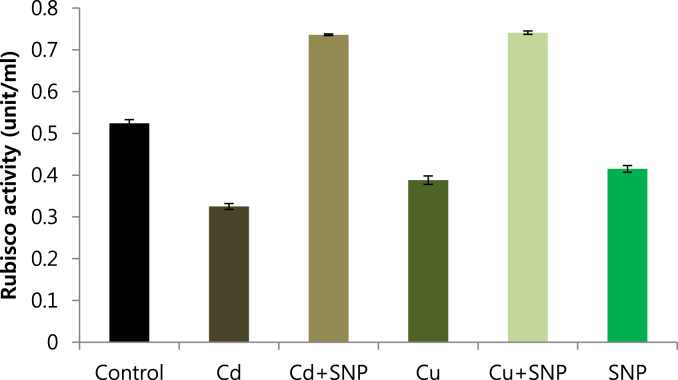
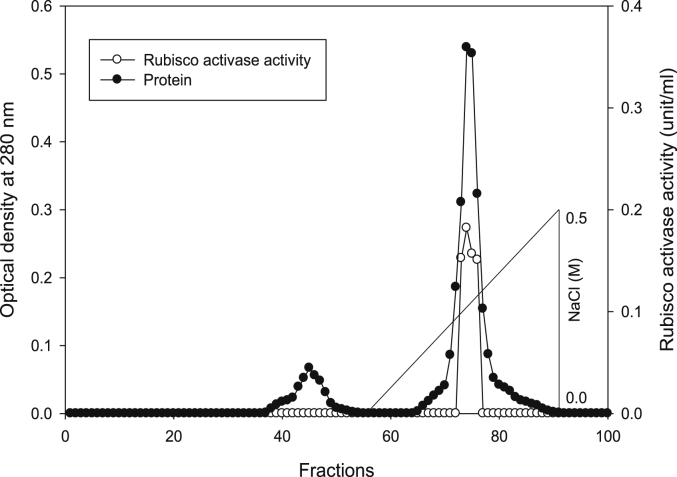
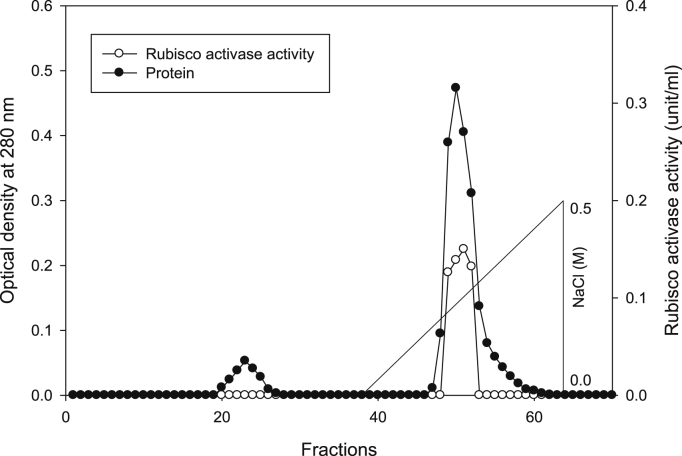
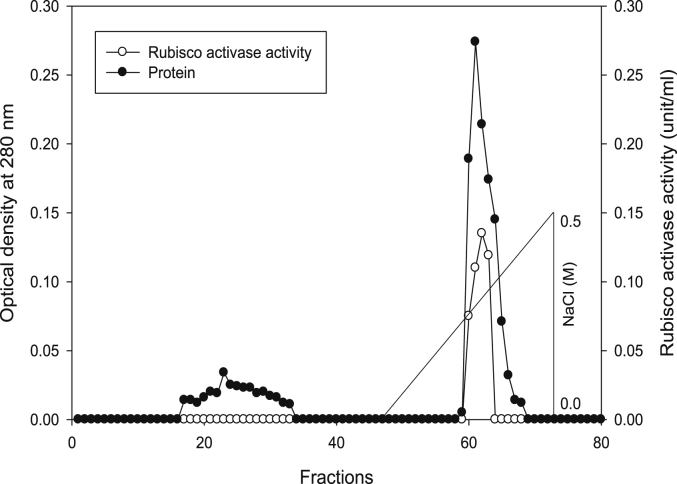
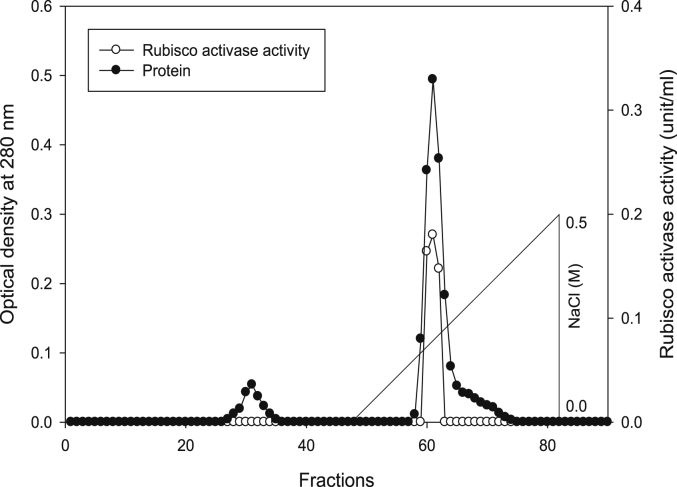
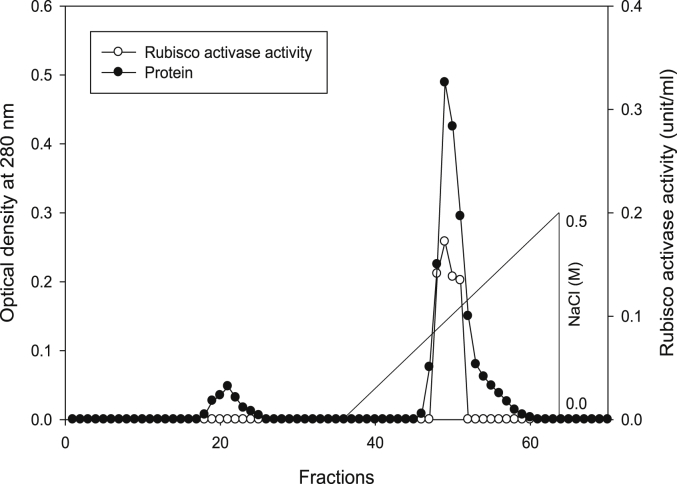
Using Q-sepharose column, Rubisco was isolated from leaves of tobacco plants grown under conditions of control (Fig. 4), 0.2 mM Cd (Fig. 5), 0.2 mM Cu (Fig. 6), 0.05 mM SNP (Fig. 7), 0.2 mM Cd + 0.05 mM SNP (Fig. 8), 0.2 mM Cu + 0.05 mM SNP (Fig. 9), respectively. According to the results, rubisco content was remarkably decreased by Cd and Cu treatments. Tobacco plants treated with 0.05 mM SNP showed the highest content of rubisco compared to those grown under Cd and Cu stresses. However, applying SNP to Cu-induced plants did not show significant differences. Rubisco content of Cd-stressed tobacco plants was remarkably increased by SNP application. In both Cd and Cu-stressed plants, content of rubisco was increased by SNP compared to heavy metal-stressed plants (Fig. 10). Rubisco activity was also reduced by both Cd and Cu. the highest reduction of rubisco activity was observed in Cd-stressed plants. Compared to the control, the biggest values of rubisco activity were reported from the treatments of 0.2 mM Cu + 0.05 mM SNP and 0.2 mM Cd + 0.05 mM SNP respectively (Fig. 11). However, tobacco plants treated with Cd, Cu and SNP did not show significant differences.
Fig. 4.
Elution profiles for tobacco leaf protein and rubisco activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with control. Fractionation was done on Q-Sepharose column. The column was eluted with BTP (pH 7.2) containing 0.1–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, while the straight line indicates NaCl gradient.
Fig. 5.
Elution profiles for tobacco leaf protein and rubisco activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cd in addition to the control. Fractionation was done on Q-Sepharose column. The column was eluted with BTP (pH 7.2) containing 0.1–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, while the straight line indicates NaCl gradient.
Fig. 6.
Elution profiles for tobacco leaf protein and rubisco activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cu in addition to the control. Fractionation was done on Q-Sepharose column. The column was eluted with BTP (pH 7.2) containing 0.1–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, while the straight line indicates NaCl gradient.
Fig. 7.
Elution profiles for tobacco leaf protein and rubisco activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with SNP in addition to the control. Fractionation was done on Q-Sepharose column. The column was eluted with BTP (pH 7.2) containing 0.1–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, while the straight line indicates NaCl gradient.
Fig. 8.
Elution profiles for tobacco leaf protein and rubisco activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cd + SNP in addition to the control. Fractionation was done on Q-Sepharose column. The column was eluted with BTP (pH 7.2) containing 0.1–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, while the straight line indicates NaCl gradient.
Fig. 9.
Elution profiles for tobacco leaf protein and rubisco activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cu + SNP in addition to the control. Fractionation was done on Q-Sepharose column. The column was eluted with BTP (pH 7.2) containing 0.1–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, while the straight line indicates NaCl gradient.
Fig. 10.
Effects of 0.05 mM SNP on Rubisco content of tobacco grown on MS medium under 0.2 mM Cd and 0.2 mM Cu stress conditions, the bars represent standard errors (SE).
Fig. 11.
Effect of 0.05 mM SNP on Rubisco activity of tobacco grown on MS medium under 0.2 mM Cd and 0.2 mM Cu stress conditions, the bars represent standard errors (SE).
3.4. Influence of SNP on content and activity of rubisco activase in Cd/Cu-stressed tobacco plants
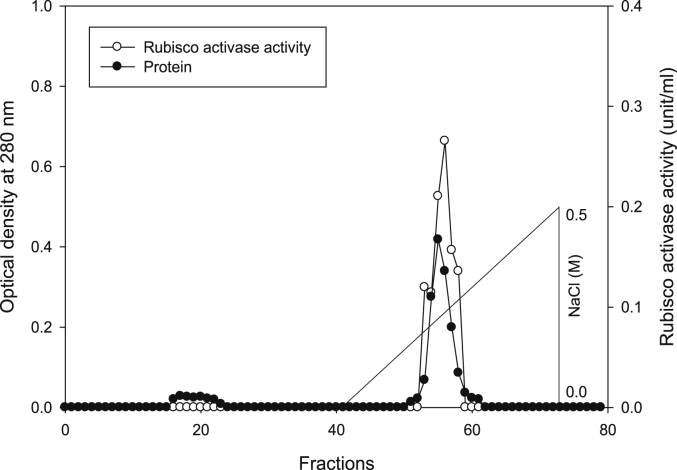
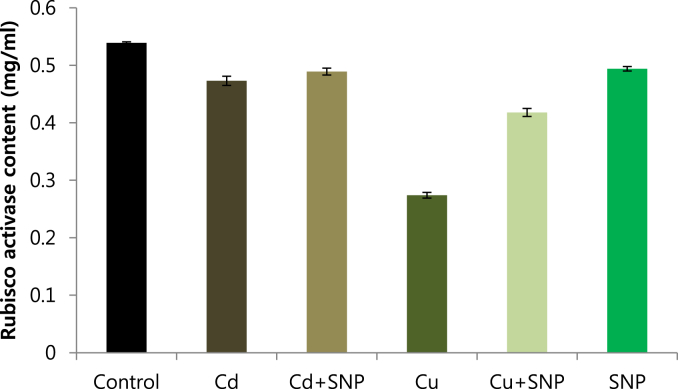
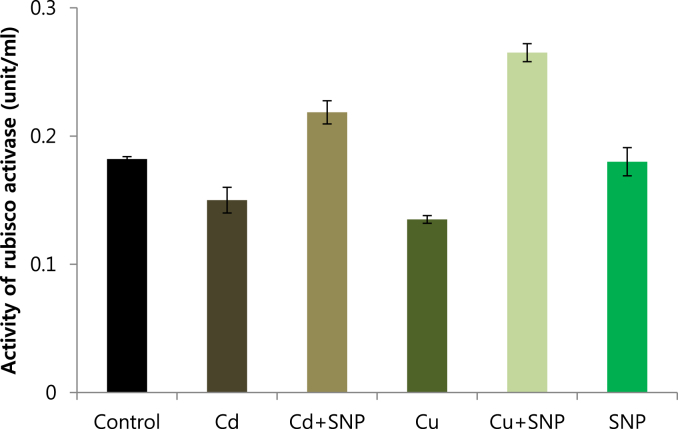
Rubisco activase was isolated from leaves of in vitro grown tobacco plants by using Q-sepharose column. Plants were subjected to control (Fig. 12), 0.2 mM Cd (Fig. 13), 0.2 mM Cu (Fig. 14), 0.05 mM SNP (Fig. 15), 0.2 mM Cd + 0.05 mM SNP (Fig. 16), 0.2 mM Cu + 0.05 mM SNP (Fig. 17), respectively. The results showed that both heavy metals (Cd/Cu) remarkably reduced the content of rubisco activase. A significant reduction of rubisco activase content was reported from the plants grown under Cu toxicity. While SNP application increased the content of rubisco activase compared to Cu-induced plants. Compared to Cd-stressed plants, SNP application had no significant differences (Fig. 18). Supplying SNP increased rubisco activase activity in Cu-stressed plants and Cd-stressed plants respectively. Stresses of Cd and Cu decreased rubisco activase contents as compared to the control. The lowest rubisco activase activity was observed in Cu-induced tobacco plants while the highest value of rubisco activase activity was reported from the treatment of 0.2 mM Cu + 0.05 mM SNP. In all cases, rubisco activase activity was reduced by both heavy metal stresses and increased by SNP supplementation (Fig. 19).
Fig. 12.
Elution profiles for tobacco leaf rubisco activase contents and activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with control. Fractionation was done on Q-Sepharose column. The column eluted with BTP (pH 7.2) containing 0.0–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, whilst the straight line indicates NaCl gradient.
Fig. 13.
Elution profiles for tobacco leaf rubisco activase contents and activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cd in addition to the control. Fractionation was done on Q-Sepharose column. The column eluted with BTP (pH 7.2) containing 0.0–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, whilst the straight line indicates NaCl gradient.
Fig. 14.
Elution profiles for tobacco leaf rubisco activase contents and activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cu in addition to the control. Fractionation was done on Q-Sepharose column. The column eluted with BTP (pH 7.2) containing 0.0–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, whilst the straight line indicates NaCl gradient.
Fig. 15.
Elution profiles for tobacco leaf rubisco activase contents and activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with SNP in addition to the control. Fractionation was done on Q-Sepharose column. The column eluted with BTP (pH 7.2) containing 0.0–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, whilst the straight line indicates NaCl gradient.
Fig. 16.
Elution profiles for tobacco leaf rubisco activase contents and activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cd + SNP in addition to the control. Fractionation was done on Q-Sepharose column. The column eluted with BTP (pH 7.2) containing 0.0–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, whilst the straight line indicates NaCl gradient.
Fig. 17.
Elution profiles for tobacco leaf rubisco activase contents and activities. Rubisco was isolated from leaves of in vitro grown tobacco plants treated with Cu + SNP in addition to the control. Fractionation was done on Q-Sepharose column. The column eluted with BTP (pH 7.2) containing 0.0–0.5 M NaCl gradient. After passing through anion exchanger column fractions were collected and assayed at 280 nm. Closed circles (●) represent protein, open circles (○) represent rubisco activity, whilst the straight line indicates NaCl gradient.
Fig. 18.
Effect of 0.05 mM SNP on Rubisco activase content of tobacco grown on MS medium 0.2 mM Cd and 0.2 mM Cu stress condition. The bars represent standard errors (SE).
Fig. 19.
Effect of 0.05 mM SNP on Rubisco activase activity of tobacco grown on MS medium under 0.2 mM Cd and 0.2 mM Cu stress condition. The bars represent standard errors (SE).
4. Discussion
It is well known that all abiotic stresses limit the growth and productivity of plants. In the recent decades, several studies have been carried out to understand the natural tolerance mechanisms in crop plants in order to alleviate negative impacts of these abiotic stress factors. Among the abiotic factors, heavy metals such as Cd and Cu are considered as serious stress factors which cause huge reduction in production of plants. Cd is a non-essential microelement for plant growth, but is taken up by roots in high doses and accumulates in the upper part of plants such as stems and leaves, thereby causes disturbances in metabolic processes, disrupting some physiological mechanisms, inhibiting growth, affecting crop yield and eventually threatening food safety all over the world. In the previous decades, many studies have been conducted on alleviation of Cd toxicity by applying plant regulators and possible mechanisms of the protective function of these hormones on Cd toxicity [29], [30], [31], but there have been no definitive results yet. Cu is considered as an important micronutrient for plant development and production. Although, concentrated levels of Cu cause toxicity in plant physiology and ultimately result in reduction of plant productivity and yield. In the past years, many authors reported that high doses of Cu inhibit plant growth and reduce the productivity. Lidon and Henriques [32] found that Cu stress inhibits plant growth and decreases the production. Therefore, heavy metal toxicity is now being recognized as a serious challenge that facing researchers in plant physiology and biochemistry field. In order to cope with these heavy metal stresses and avoid their toxicity, plants possess physiological defense systems which cellular and molecular responses as well adjust the free metal contents to tolerate toxicity of heavy metals. In addition to plant's natural tolerance mechanisms, there are some signaling molecules are known to play a crucial role in alleviation of heavy metal toxicity. Sodium nitroprusside (SNP) a donor of nitric oxide (NO) is recognized as one of the most important signaling molecules which can be used to overcome the toxicity of heavy metals. Recently, a huge number of researches about SNP functional role in alleviation of heavy metal stresses have been carried out. In many reports SNP was shown to promote the plant response to abiotic stresses, such as drought, heat, salinity, heavy metals and oxidative agents [33]. Other many reports are also suggesting the functional role of SNP in plant physiology [34], [35], [36], [37]. Supplementation of NO could significantly enhance wheat seeds germination and alleviate oxidative stress against Cu toxicity [38]. In soybean plants subjected to cadmium chloride (0.2 mM), the exogenous application of SNP promoted plant growth and recovered damage caused by this metal [39]. In addition, it was also observed that treating seedlings with 100 mM SNP protected sunflower leaves against Cd-induced oxidative stress condition [40]. In the current research we studied SNP role in alleviation of both Cd and Cu toxicity by investigating fresh weight, total chlorophyll content, rubisco content, rubisco activity, rubisco activase content and rubisco activase activity in tobacco plants grown in in vitro. According to the results, we found that all investigated characteristics and biochemical components were notably retarded by inducing Cd and Cu treatments and increased by SNP application. Supplying 0.2 mM of both Cd and Cu resulted in morphological disorders. And the growth of tobacco plants was clearly disrupted. While supplementation of SNP promoted the plant growth by recovering the toxic damages of both metals. In order to confirm these results, total fresh weight of tobacco was measured. The fresh weights of tobacco plants exposed to Cd and Cu were remarkably reduced compared to the control. SNP application showed an increase of total fresh weight in tobacco plants compared to those grown under both Cd and Cu stresses. The lowest fresh weight was observed in Cu-induced plants while the highest fresh weight was reported from SNP treated plants. In similar studies, SNP promoted the growth of several crops against Cd toxicity, such as wheat [41], sunflower [42] and rice [43]. However, the observed increase of total fresh weight in Cu, Cd and SNP treated plants indicates that SNP plays a protective role in alleviation of heavy metal toxicity. Total chlorophyll content was also affected by Cd and Cu stresses. Morphologically, leaves that were fully green turned to yellowish-brown and light green in Cd and Cu stressed tobacco plants. This morphological description indicates that the presence of both metals resulted in a huge reduction in total chlorophyll contents. After chlorophyll determination, the results showed that total chlorophyll content was significantly reduced by Cu toxicity. While in SNP supplemented plants the content of total chlorophyll was higher than the control. Plants grown under Cd + SNP treatment did not show significant differences in total chlorophyll content. Cd treatment showed a significant decrease in total chlorophyll as compared with the control. These results are in agreement with Yuanjie et al. [44] who reported that Cu significantly decrease total chlorophyll, chl. a, chl. b, and car as compared with the control. The applications of different concentrations of SNP changed the chlorophyll contents in ryegrass. In another research, a similar reduction in total chlorophyll content under Cu toxicity was observed in Atriplex halimus [45] and mangrove plant seedlings [46]. In this study, Cd-treated plants showed lower contents of total chlorophyll compared to those were supplemented with SNP. In similar results, Chen et al. [47] detected that NO stability of the subcellular structure under Cd stress contributed to its effective role in preventing Cd-induced leaf chlorosis and inhibition of photosynthesis in barely seedlings. Our results indicated that SNP played a crucial role in the increase of total chlorophyll content in Cd and Cu-stressed tobacco plants. Therefore, SNP might be responsible for the enhancement of heavy metal tolerance in plants. Rubisco and rubisco activase were also investigated in the research. Rubisco is an important protein that involved in atmospheric carbon fixation, whereby CO2 is converted to organic carbon (energy compounds). And rubisco activase is an enzyme which activates rubisco. In this study, contents of rubisco, activity of rubisco, contents of rubisco activase and activity of rubisco activase were investigated. Using Q-Sepharose column, rubisco and rubisco activase were isolated from leaves of tobacco plants treated with 0.2 mM Cd, 0.2 mM Cu, 0.05 mM SNP, 0.2 mM Cd + 0.05 mM SNP, 0.2 mM Cu + 0.05 mM SNP in addition to the control (untreated plants). Results of the present study showed that rubisco content was remarkably retarded by toxicity of Cd and Cu compared to the control. And the lowest contents of rubisco were observed when tobacco plants were exposed to Cu stress. Compared to the both Cd and Cu-stressed plants, SNP application significantly increased the contents of rubisco. Highest contents of rubisco were reported from treatments of 0.05 mM SNP, 0.2 mM Cd + 0.05 mM SNP, 0.2 mM Cu + 0.05 mM SNP, respectively. Rubisco activity was reduced by Cd stress while Cu treatment had no significant differences compared to the SNP treatment. Rubisco activity in Cd, Cu and SNP treatments was lower than the control. In tobacco plants grown under SNP supplemented with Cd and Cu conditions, rubisco activity was higher than the control. The highest rubisco activity was observed when SNP was applied to Cu-induced tobacco plants. Due to the important role of rubisco activase in process of rubisco activation, we investigated contents and activities of rubisco activase. Rubisco activase content was significantly decreased by Cu stress, while Cd treatment did not show significant differences compared to the control. Rubisco activase contents were increased in tobacco plants grown under SNP, Cd + SNP and Cu + SNP, respectively. Rubisco activase activity was also retarded by Cd and Cu stresses, while in SNP supplemented with Cu treatment rubisco activase activity was higher than the control. Compared to cd and Cu-stressed plants, rubisco activase activity was increased by SNP, Cd + SNP and Cu + SNP, respectively. Despite insufficiency of related studies, a few similar results have been previously reported. Roh and Chin [48] observed that the content and activity of rubisco activase decreased by Cd. However, the observed increase of rubisco and rubisco activase contents and activities in SNP treated plants indicates that SNP could overcome toxic damages generated by heavy metals.
5. Conclusion
In conclusion, induction of both metals Cd and Cu resulted in a significant reduction of tobacco growth, total chlorophyll content, rubisco content and activity, rubisco activae content and activity. While supplementation of SNP resulted in growth enhancement, as well increased the contents and activities of all investigated biochemical components. Thus, our results indicate that SNP application might alleviate Cd and Cu toxicities and regulate plant growth and development.
Conflict of interest
No conflict of interest.
Contributor Information
Alaaldin Idris H. Khairy, Email: alaidris86@gmail.com.
Kwang Soo Roh, Email: rks@kmu.ac.kr.
References
- 1.Benzarti S., Mohri S., Ono Y. Plant response to heavy metal toxicity: comparative study between the hyperaccumulator Thlaspi caerulescens (ecotype Ganges) and nonaccumulator plants: lettuce, radish, and alfalfa. Environ. Toxicol. 2008;23:607–616. doi: 10.1002/tox.20405. [DOI] [PubMed] [Google Scholar]
- 2.Nies D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 3.Rascio N., Navari-Izzo F. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 2011;180:169–181. doi: 10.1016/j.plantsci.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Goldbold D.L., Huttermann A. Effect of zinc, cadmium and mercury on root elongation of Picea abies (Karst) seedlings and the significance of these metals to forest die-back. Environ. Pollut. 1985;38:375–381. [Google Scholar]
- 5.Wagner G.J. Accumulation of cadmium in crop plants and its consequences to human health. Adv. Agron. 1993;51:173–212. [Google Scholar]
- 6.Sauve S., Cook N., Hendershot W.H., McBride M.B. Linking plant tissue concentrations and soil copper pools in urban contaminated soils. Environ. Pollut. 1996;94:153–157. doi: 10.1016/s0269-7491(96)00081-4. [DOI] [PubMed] [Google Scholar]
- 7.Greger M., Bertell G. Effects of Ca2+ and Cd2+ on the carbohydrate metabolism in sugar beet (Beta vulgaris) J. Exp. Bot. 1992;43:167–173. [Google Scholar]
- 8.Hernandez L.E., Garate A., Carpena-Ruiz R. Effects of cadmium on the uptake, distribution and assimilation of nitrate in Pisum sativum. Plant Soil. 1997;189:97–106. [Google Scholar]
- 9.Chaffei C., Gouia H., Gorbel M.H. Nitrogen metabolism of tomato under cadmium stress conditions. J. Plant Nutr. 2003;26:1617–1634. [Google Scholar]
- 10.Benavides M.P., Gallego S.M., Tomaro M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005;17:21–34. [Google Scholar]
- 11.Rodríguez-Serrano M., Romero-Puertas M.C., Pazmiño D.M. Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009;150:229–243. doi: 10.1104/pp.108.131524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002;53:1–11. [PubMed] [Google Scholar]
- 13.Jhanji S., Setia R.C., Kaur N. Role of nitric oxide in cadmium-induced stress on growth, photosynthetic components and yield of Brassica napus L. J. Environ. Biol. 2012;33:1027–1032. [PubMed] [Google Scholar]
- 14.Xiong J., An L., Lu H., Zhu C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta. 2009;230:755–765. doi: 10.1007/s00425-009-0984-5. [DOI] [PubMed] [Google Scholar]
- 15.Portis A.R., Jr. Regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:415–437. [Google Scholar]
- 16.Taylor T.C., Andersson I. The structure of the complex between rubisco and its natural substrate ribulose 1,5-bisphosphate. J. Mol. Biol. 1997;265:432–444. doi: 10.1006/jmbi.1996.0738. [DOI] [PubMed] [Google Scholar]
- 17.Cleland W.W., Andrews T.J., Gutteridge S., Hartman F.C., Lorimer G.H. Mechanism of Rubisco: the carbamate as general base. Chem. Rev. 1998;98:549–561. doi: 10.1021/cr970010r. [DOI] [PubMed] [Google Scholar]
- 18.Portis A.R., Jr. Rubisco activase—Rubisco’s catalytic chaperone. Photosynth. Res. 2003;75:11–27. doi: 10.1023/A:1022458108678. [DOI] [PubMed] [Google Scholar]
- 19.Bayramov S., Guliyev N. Changes in Rubisco activase gene expression and polypeptide content in Brachypodium distachyon. Plant Physiol. Biochem. 2014;81:61–66. doi: 10.1016/j.plaphy.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Barta C., Dunkle A.M., Wachter R.M., Salvucci M.E. Structural changes associated with the acute thermal instability of Rubisco activase. Arch. Biochem. Biophys. 2010;499:17–25. doi: 10.1016/j.abb.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Murashige T., Skoog F.A. Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- 22.Son J.A., Damodaran P.N., Roh K.S. Influence of exogenous application of glutathione on rubisco and rubisco activase in heavy metal-stressed tobacco plants grown in vitro. Saudi J. Biol. Sci. 2014;21:89–97. doi: 10.1016/j.sjbs.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inskeep W.P., Bloom P.R. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z.Y., Snyder G.W., Esau B.D., Portis A.R., Jr., Ogren W.L. Species-dependent variation in the interaction of substratebound ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) and rubisco activase. Plant Physiol. 1992;100:1858–1862. doi: 10.1104/pp.100.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wishnick M., Lane M.D. Ribulose diphosphate carboxylase from spinach leaves. Methods Enzymol. 1971;23:570–577. [Google Scholar]
- 26.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 27.Racker E. Ribulose diphosphate carboxylase from spinach leaves. Methods Enzymol. 1962;5:266–270. [Google Scholar]
- 28.Robinson S.P., Portis A.R., Jr. Adenosine triphosphate hydrolysis by purified rubisco activase. Arch. Biochem. Biophys. 1989;268:93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- 29.El-Enany A.E. Alleviation of cadmium toxicity on maize seedlings by calcium. Biol. Plant. 1995;37:93–99. [Google Scholar]
- 30.Suzuki N. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotech. 2005;22:19–25. [Google Scholar]
- 31.Zhang X.R., Yang H.Q., Sui J., Qiao H.T., Jiang Q.Q., Ran K. Calcium protects grape leaves against cadmium stress by root treatment. Acta Horti. Sin. 2008;35:1405–1410. [Google Scholar]
- 32.Lidon F.C., Henriques F.S. Effects of copper toxicity on growth and the uptake and translocation of metals in rice plants. J. Plant Nutri. 1993;16:1449–1464. [Google Scholar]
- 33.Gould K.S., Klinguer A., Pugin A., Wendehenne D. Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ. 2003;26:1851–1862. [Google Scholar]
- 34.Wilson I.D., Ribiero D.M., Bright J., Harrison J., Desikan R. Role of nitric oxide in regulating stomatal apertures. Plant Signal. Behav. 2009;4:467–469. doi: 10.4161/psb.4.5.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford N.M. Mechanism of nitric oxide synthesis in plants. J. Exp. Bot. 2006;57:471–478. doi: 10.1093/jxb/erj050. [DOI] [PubMed] [Google Scholar]
- 36.Leitner M., Vandelle E., Gaupels F., Bellin D., Delledonne M. NO signals in the haze: nitric oxide signalling in plant defence. Curr. Opin. Plant Biol. 2009;12:451–458. doi: 10.1016/j.pbi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Hu K.D., Hu L.Y., Li Y.H., Zhang F.Q., Zhang H. Protective role of nitric oxide on germination and antioxidant metabolism in wheat seeds under copper stress. Plant Growth Regul. 2007;53:173–183. [Google Scholar]
- 38.Rao M.V., Davis K.R. The physiology of ozone induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- 39.Orozco-Cardenas M.L., Ryan C.A. Nitric Oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 2002;130:487–493. doi: 10.1104/pp.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagendorf A., Uchida A.T., Hibino T., Takabe T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002;163:515–523. [Google Scholar]
- 41.Singh H.P., Batish D.R., Kaur G., Arora K., Kohli R.K. Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ. Exp. Bot. 2008;63:158–167. [Google Scholar]
- 42.Laspina N.V., Groppa M.D., Tomaro M.L., Benavides M.P. Nitric oxide protects sunflower leaves against Cd induced oxidative stress. Plant Sci. 2005;169:323–330. [Google Scholar]
- 43.Panda P., Nath S., Chanu T.T., Sharma G.D., Panda S.K. Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.) Acta Physiol. Plant. 2011;33:1737–1747. [Google Scholar]
- 44.Yuanjie D., Linlin X.U., Quanhui W., Zhenyi F., Jing K., Xiaoying B. Effects of exogenous nitric oxide on photosynthesis, antioxidative ability, and mineral element contents of perennial ryegrass under copper stress. J. Plant Interact. 2013;9:402–411. [Google Scholar]
- 45.Brahim L., Mohamed M. Effects of copper stress on antioxidative enzymes, chlorophyll and protein content in Atriplex halimus. Afr. J. Biotechnol. 2011;50:10143–10148. [Google Scholar]
- 46.Zhang F.Q., Wang Y.S., Lou Z.P., Dong G.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) Chemotherapy. 2007;67:44–50. doi: 10.1016/j.chemosphere.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Chen F., Wang F., Sun H.Y., Cai Y., Mao W.H., Zhang G.P., Vincze E., Wu F.B. Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeum vulgare) J. Plant Growth Regul. 2010;29:394–408. [Google Scholar]
- 48.Roh K.S., Chin H.S. Cadmium toxicity and calcium effect on growth and photosynthesis of tobacco. J. Life Sci. 2005;15:453–460. [Google Scholar]