Abstract
Medullary thyroid carcinoma (MTC) is an infrequent calcitonin-producing neuroendocrine tumor that initiates from the parafollicular C cells of the thyroid gland. Several genetic and epigenetic alterations are collaterally responsible for medullary thyroid carcinogenesis. In this review article, we shed light on all the genetic and epigenetic hallmarks of MTC. From the genetic perspective, RET, HRAS, and KRAS are the most important genes that are characterized in MTC. From the epigenetic perspective, Ras-association domain family member 1A, telomerase reverse transcriptase promoter methylations, overexpression of histone methyltransferases, EZH2 and SMYD3, and wide ranging increase and decrease in non-coding RNAs can be responsible for medullary thyroid carcinogenesis.
Keywords: Thyroid carcinoma, Genetic markers, Proto-oncogene
INTRODUCTION
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor that originates from the parafollicular cells (C cells) and produces calcitonin[1]. Approximately, a quarter of MTCs are genetic in nature; they are caused by a mutation in the rearranged during transfiction (RET) proto-oncogene, a receptor tyrosine kinase gene, which can undergo oncogenic activation through both cytogenetic rearrangement and activation of point mutation. REven though MTC is mostly sporadic (70-80%), some hereditary patterns can be seen in 20-30% of cases: these are classified as familial MTC (FMTC) with autosomal dominant trait[2-5]. High serum concentration of calcitonin and carcinoembryonic antigen is regularly regarded as MTC markers in blood[6-8]. It is common knowledge that cancer is the result of genetic changes accumulated in a manner that disturbs the normal homeostatic stability between cell proliferation and cell death[9,10]. In addition to genetic changes, epigenetic events have been considered as key indicators of carcinogenesis. Research on epigenetics has become gradually noticeable with the aim of understanding the role of epigenetic mechanisms in the abnormal events leading to cancer[11-13]. In fact, previous studies on cancer suggest that genetic and epigenetic alterations are two sides of the same coin responsible for morphological changes occurring during cancer progression[12,14-16]. Moreover, the notion that early-stage cancer is not as systematically aggressive as late-stage cancer is based on the finding that gene expression profiles is alike in early-stage cancer and fully metastatic cancer[17-19]. Thus, both genetic and epigenetic events correspond to several steps of carcinogenesis. In this review, we summarize current concepts on genetic and epigenetic changes associated with MTC and then discuss their potential relevance as biomarkers for cancer detection, diagnosis, and prognosis.
Hallmarks of genetic MTC
A mutation is a stable modification in the DNA sequence of a given gene, which may alter the normal gene function[20]. Mutations can occur anywhere, from a single DNA building block (base pair) to a large segment of a chromosome, including multiple genes[20]. Some of the mutations are heritable, i.e. they are inherited from a parent and are present throughout a person’s life in virtually every cell in the body. These mutations are called germline mutations since they are present in the parent’s egg or sperm cells (germ cells)[20,21]. Other group of mutations are acquired mutations (or somatic): These happen only at a particular time during a person’s life and are present only in certain cells in the body[20]. These mutations can be caused by environmental factors, such as ultraviolet radiation from the sun or can occur if an error takes place in DNA replication during cell division. Acquired mutations in somatic cells (cells other than sperm and egg cells) cannot be passed to the next generation[22-25].
MTC has been described in two forms: sporadic and hereditary/familial. About one-fourth of MTC patients haveone of three different syndromes, which are FMTC, multiple endocrine neoplasia type 2A (MEN 2A), or type 2B (MEN 2B). Around a half of the patients with MEN 2A or MEN 2B develop pheochromocytomas[26-30]. Moreover, 25% of patients with MEN 2A will possibly develop primary hyperparathyroidism[28,31], while patients with MEN 2B develop marfanoid habitus and mucosal/intestinal ganglioneuromatosis[32]; patients with just FMTC individually develop MTC[29,32]. In fact proto-oncogene RET germline mutations is presented in 90% of patients with hereditary MTC (FMTC, MEN 2A, or MEN 2B)[33]. Thus, the entire hereditary syndromes are attributed to the same disease-causing gene[34,35]. RET proto-oncogene is a tyrosine kinas receptor coding gene, and it is an element of the glial cell line-derived neurotrophic factor (GDNF) family which are classified as extracellular signaling molecules[36]. Human RET gene with 21 exons is localized on chromosome 10 (10q11.2)[37,38]. Like other tyrosine kinase receptors, RET is able to motivate several signaling pathways, including RAS/extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)/AKT, p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) pathways[30,39-44]. The typical splicing of the RET gene results in three different isoforms. The C-terminal region in RET51, RET43, and RET9 have 51, 43, and 9 amino acids, respectively[45]. As shown in Figure 1—which is premised on the protein data bank code 2IVT—all RET protein isoforms can be subdivided into three main domains: an N-terminal extracellular domain with four cadherin-like repeats and a cysteine-rich region, a hydrophobic transmembrane domain, and a cytoplasmic tyrosine kinase domain that is divided through the 27 amino acids insertion[36,46].
Fig. 1.
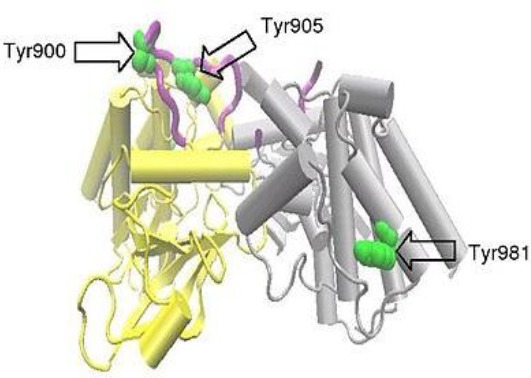
A RET dimer formed between two protein molecules, each spanning amino acids 703-1012 of the RET molecule and covering RET intracellular tyrosine kinase domain. One protein molecule, molecule A, is shown in yellow and the other, molecule B, in grey. The activation loop is colored purple and selected tyrosine residues in green. Part of the activation loop from molecule B is absent. mRET proto-oncogene with three main domains: an N-terminal extracellular domain with four cadherin-like repeats and a cysteine-rich region, a hydrophobic transmembrane domain, and a cytoplasmic tyrosine kinase domain. Phosphorylation of Tyr981 and the additional tyrosinesTyr1015, Tyr1062, and Tyr1096 are not covered by the above structure, though these have been shown to be important for initiation of the intracellular signal transduction processes[36].
Glial cell line-derived neurotrophic factor and some other related molecules like neurturin, artemin, and persephin trigger an intracellular signaling pathway through a unique multi-component receptor systems including glycosyl-phosphatidylinositol-anchored co-receptor in addition to RET tyrosine kinase[37,40,47-49]. These neurotrophic factors support the survival of many neurons, including the central motor dopamine neurons; they also cause peripheral autonomy [50-52] more than renal development and facilitate regulation of spermatogonia differentiation (Fig. 2)[53,54].
Fig. 2.
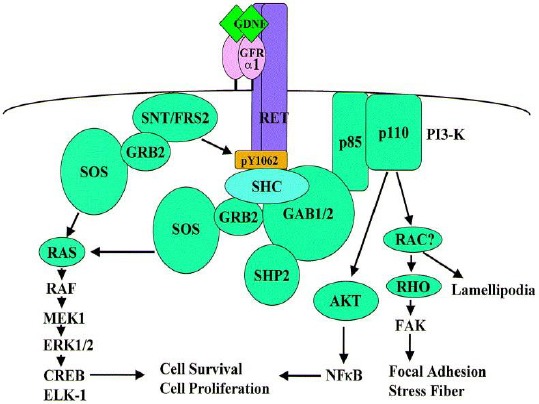
Intracellular signaling pathways mediated by activated RET[55].
Unfortunately, unlike hereditary MTC, the etiology of sporadic MTC has not been completely elucidated. This is despite the fact that 75% of all MTC patients being sporadic without any family history of MTC or incidence of any other MEN 2-specific disease. Recently, some additional mutations, such as HRAS and KRAS mutations, have been found to be more appropriate diagnostic markers than RET for MTC. This observation has been suggested on the basis of high-throughput mutation profiling study (Fig. 3)[56-59]. In few cases of sporadic MTC, a deletion of codons Glu632 and Leu633 of RET proto-oncogene was identified. These mutations activate the RET gene more effectively than the Cys634Arg missense mutation. They also induce stable dimer formation in the absence of ligand[60,61].
Fig. 3.
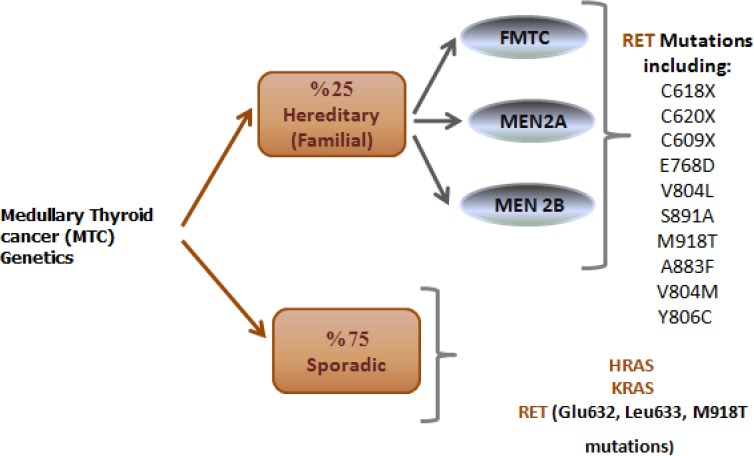
Genetics of medullary thyroid cancer.
In more aggressive phenotypes of sporadic MTC, the M918 TRET mutation has been found in around 30 to 50% of cases[62]. Actually, the somatic RET mutation (M918T) is associated with stage of the disease and persistence of the disease after total thyroidectomy because it makes the chance of recurrence and metastasis greater than before and reduces chances of free survival[62,63]. A connection between the presence of this somatic mutation with the more advanced pathological TNM stage has also been identified[64-67].
Epigenetic hallmarks of MTC
The word ‘epigenetics’ refers to covalent modification of DNA, protein, or RNA, resulting in changes in the function and/or regulation of DNA without modification of their original sequences. In some cases, epigenetic modifications can be stable and can pass on to future generations; mostly, however, they are vigorous modifications in response to environmental stimuli[68]. The major mechanisms responsible for epigenetic regulation are DNA methylation, histone modifications, and non-coding RNAs[59,69-72]. The role of epigenetics in MTC is largely defined as hypermethylation of CpG islands in the promoter region of Ras-association domain family member 1A (RASSF1A)[73,74] and telomerase reverse transcriptase (TERT) genes[75], overexpression of histone methyltransferases like EZH2 and SMYD3[76,77], and microRNAs (miRNAs) expression profile (Fig. 4)[78-84].
Fig. 4.
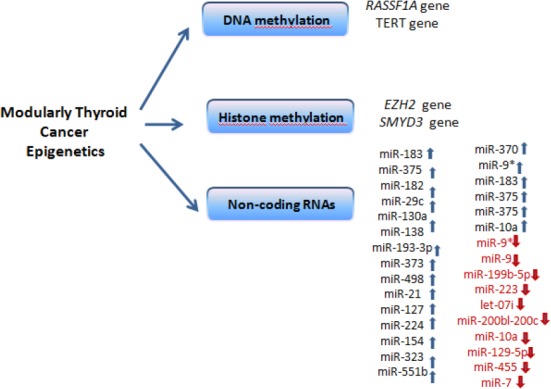
Epigenetics of medullary thyroid cancer.
In spite of the fact that RASSF1A gene promoter hypermethylation is linked to more aggressive thyroid cancers, CpG island methylation of tumor-associated genes— such as p16, TSHR, MGMT, and PTEN—have not shown any significant degree of hypermethylation in MTC[73,85-87]. Nevertheless, the existence of methylation in the promoter region of TERT gene and consequent variation of DNA copy numbers within a huge cohort study of MTC cases have been documented[75]. In fact, telomerase is a protein responsible for keeping and fixing telomeres of the chromosomes. Its activation by TERT has been increased frequently in many types of cancers, including MTC[86]. Wang and his colleagues[75] have reported that TERT gene hypermethylation is related to high DNA copy number, and MTC patients with higher TERT methylation have lower chances of survival[75].
Epigenetic control through histone methyltrasferases in more aggressive forms of MTC have recently been investigated by Sponziello et al.[76]. In fact, the platform of epigenetic regulatory factors and their mRNA levels profiling in a big cohort of MTC tissues has revealed the fact that overexpression of two histone methyltransferases, EZH2 and SMYD3, is connected with higher risk of metastases, disease consistency, and finally death of patients: These can be prognostic biomarkers for MTC[76,88]. Remarkably, gene expression profile was free of RET or RAS mutations.
The most common mutation is related to the RET mutation in genetic alteration of MTC tumorigenesis. The transcriptional activity of RET is controlled by epigenetic processes as well. It has been demonstrated that in colorectal cancer CpGisland methylation of RET gene promoter is a potential prognostic marker for stage II of the disease[89,90]. Patients with considerable hypermethylated RET have worse overall survival compared to those with unmethylated RET promoter[91]. Significantly, RET expression is regulated by a transcription factor, homeobox B5[92], which is related to the multi-species conserved sequence in the primary intron of the RET gene in addition to the higher level of RET transcription. Another regulating mechanism of RET transcription level is acethylation because in human neuroblastoma cells with a low RET mRNA level, histone deacetylase inhibitor and sodium butyrate cause hyper acetylation and increase the transcription of RET gene[93].
MiRNAs are small non-coding RNAs with the lengths of 20-23 nucleotides; they are classified as epigenetic modifiers[94]. MiRNAs are non-protein-coding RNAs that alter gene expression through mRNA translation inhibition or by means of the target molecule degrading[94,95]. In reality, mutations or abnormal expression of miRNAs are more often related to the pathogenesis of a wide range of cancers because they affect both tumor suppressors and oncogenes[96]. In spite of the fact that several studies have highlighted the role of miRNA profiling of MTC and its malignancy (Table 1), due to difficulty in obtaining normal C cells, none of the existing literature has compared miRNA profiles between MTC and normal C cells[97].
Table 1.
A list of suggesting microRNA (miRNA) in medullary thyroid cancer
| A signature of increased and decreased miRNA associated with MTC | |
|---|---|
| Increasing miRNA | miR-183, miR-375, miR-182, miR-29c, miR-130a, miR-138, miR-193-3p, miR-373, miR-498, miR-21, miR-127, miR-224, miR-154, miR-323, miR-551b, miR-370, miR-9, miR-183, miR-375, miR-375, miR-10a |
| Decreasing miRNA | miR-199b-5p, miR-223, let-07i, miR-200bl-200c, miR-10a, miR-129-5p, miR-455, and miR-7, miR9 |
Ten miRNAs were shown to have different expression pattern between sporadic MTC and
hereditary MTC[78]. In correlation with clinical outcomes, high levels of miR-183 and miR-375 were linked to the lateral lymph node and distant metastases[78]. Significantly, similarity in miRNA profiles of miR-183, miR-375, and miR-9-3p (miR-9) between primary tumor tissues and lymph node metastasis tissues was observed[79]. This was further to the role of miR-9-3pin in regulation of autophagy[80]. More than that, a comparison of miRNA profiling between primary and metastatic forms of MTC highlighted 10 deregulated miRNAs[81-83,98]. There is possibility that the constitutive activation of RET, as a crucial occurrence in MTC tumor genesis, is regulated through epigenetic mechanisms like miRNAs[81,99,100].
From genetic point of view, RET mutations in codons 609 (C609X), 618 (C618X), 620 (C620X), 786 (E768D), 804 (V804L), 819 (S891A), 918 (M918T), 833 (A883F), 804 (V804M), 806 (Y806C), 632 (Glu632), 633 (Leu633), and 918 (M918T) as well as HRAS, and KRAS mutations are the most important mutations that cause medullary thyroid carcinogenesis. From epigenetic perspective, RASSF1, TERT promoter methylations, histone methyltransferases (EZH2 and SMYD3) overexpression, and wide ranging increase and decrease of non-coding RNAs contribute to medullary thyroid carcinogenesis.
ACKNOWLEDGMENTS
This article is a part of a larger project granted by the Iranian organization of the National Institute for Medical Research Development (NIMAD, grant number: 957222).
Footnotes
CONFLICT OF INTEREST. None declared.
REFERENCES
- 1.Alford EM, Hu MI, Ahn P, Lamont JP. Cancer Management:Thyroid and Parathyroid Cancers. 13th ed. Cancer Network; 2011. [Google Scholar]
- 2.Leboulleux S, Baudin E, Travagli JP, Schlumberger M. Medullary thyroid carcinoma. Clinical endocrinology (Oxf) 2004;61(3):299–310. doi: 10.1111/j.1365-2265.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- 3.Haghpanah V, Soliemanpour B, Heshmat R, Mosavi-Jarrahi AR, Tavangar SM, Malekzadeh R, Larijani B. Endocrine cancer in Iran:based on cancer registry system. Indian journal of cancer. 2006;43(2):80–85. doi: 10.4103/0019-509x.25889. [DOI] [PubMed] [Google Scholar]
- 4.Larijani B, Mohagheghi MA, Bastanhagh MH, Mosavi-Jarrahi AR, Haghpanah V, Tavangar SM, Bandarian F, Khaleghian N. Primary thyroid malignancies in Tehran, Iran. Medical principles and practice. 2005;14(6):396–400. doi: 10.1159/000088112. [DOI] [PubMed] [Google Scholar]
- 5.Larijani Mohammad Bagher A, Shirzad M, Mohagheghi SMA, Haghpanah V, Mousavi Jarahi SAR, Tavangar SM, Vassigh AR, Hosseinnezhad A, Bandarian F, Baradar Jalili R. Epidemiologic analysis of the Tehran Cancer Institute data system registry (TCIDSR) Asian Pacific journal of cancer prevention. 2004;5(1):36–39. [PubMed] [Google Scholar]
- 6.Fragu P. Calcitonin's fantastic voyage:From hormone to marker of a genetic disorder. Gesnerus. 2007;64(1-2):69–92. [PubMed] [Google Scholar]
- 7.Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF, Group GS. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. The journal of clinical endocrinology and metabolism. 2005;90(11):6077–6084. doi: 10.1210/jc.2005-0044. [DOI] [PubMed] [Google Scholar]
- 8.Haddadi-Nezhad S, Larijani B, Tavangar SM, Nouraei SM. Comparison of fine-needle-nonaspiration with fine-needle-aspiration technique in the cytologic studies of thyroid nodules. Endocrine pathology. 2003;14(4):369–373. doi: 10.1385/ep:14:4:369. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes and development. 2001;15(1):50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nature reviews genetics. 2002;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nature reviews genetics. 2006;7(1):21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 13.Momeny M, Sabourinejad Z, Zarrinrad G, Moghaddaskho F, Eyvani H, Yousefi H, Mirshahvaladi S, Poursani EM, Barghi F, Poursheikhani A, Dardaei L, Bashash D, Ghazi-Khansari M, Tavangar SM, Dehpour AR, Yaghmaie M, Alimoghaddam K, Ghavamzadeh A, Ghaffari SH. Anti-tumour activity of tivozanib, a pan-inhibitor of VEGF receptors, in therapy-resistant ovarian carcinoma cells. Scientific reports. 2017;7:45954. doi: 10.1038/srep45954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You JS, Jones PA. Cancer genetics and epigenetics:two sides of the same coin? Cancer cell. 2012;22(1):9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zingg J-M, Jones PA. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis. 1997;18(5):869–882. doi: 10.1093/carcin/18.5.869. [DOI] [PubMed] [Google Scholar]
- 16.Nasseri-Moghaddam S, Malekzadeh R, Sotoudeh M, Tavangar M, Azimi K, Sohrabpour AA, Mostadjabi P, Fathi H, Minapoor M. Lower esophagus in dyspeptic Iranian patients:A prospective study. Journal of gastroenterology and hepatology. 2003;18(3):315–321. doi: 10.1046/j.1440-1746.2003.02969.x. [DOI] [PubMed] [Google Scholar]
- 17.Schedin P, Elias A. Multistep tumorigenesis and the microenvironment. Breast cancer research. 2004;6(2):93–101. doi: 10.1186/bcr772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavangar SM, Monajemzadeh M, Larijani B, Haghpanah V. Immunohistochemical study of oestrogen receptors in 351 human thyroid glands. Singapore medical journal. 2007;48(8):744–747. [PubMed] [Google Scholar]
- 19.Amoli MM, Yazdani N, Amiri P, Sayahzadeh F, Haghpanah V, Tavangar SM, Amirzargar A, Ghaffari H, Nikbin B, Larijani B, Mostaan LV, Bazzaz JT. HLA-DR association in papillary thyroid carcinoma. Disease markers. 2010;28(1):49–53. doi: 10.3233/DMA-2010-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loewe L. Genetic mutation. Nature education. 2008;1(1):113. [Google Scholar]
- 21.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, Edkins S, O'Meara S, Vastrik I, Schmidt EE, Avis T, Barthorpe S, Bhamra G, Buck G, Choudhury B, Clements J, Cole J, Dicks E, Forbes S, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jenkinson A, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, Webb T, West S, Widaa S, Yates A, Cahill DP, Louis DN, Goldstraw P, Nicholson AG, Brasseur F, Looijenga L, Weber BL, Chiew YE, DeFazio A, Greaves MF, Green AR, Campbell P, Birney E, Easton DF, Chenevix-Trench G, Tan MH, Khoo SK, Teh BT, Yuen ST, Leung SY, Wooster R, Futreal PA, Stratton MR. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper PS. Practical Genetic Counselling. 7th Edition. CRC Press; 2010. [Google Scholar]
- 23.Erickson RP. Somatic gene mutation and human disease other than cancer:An update. Mutation research. 2010;705(2):96–106. doi: 10.1016/j.mrrev.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Miller BF, Keane CB. Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health. 7th ed. Elsevier; 2017. [Google Scholar]
- 25.Tavangar SM, Shariftabrizi A, Soroush AR. Her-2/neu over-expression correlates with more advanced disease in Iranian colorectal cancer patients. Medical science monitor. 2005;11(3):CR123–CR126. [PubMed] [Google Scholar]
- 26.Romei C, Pardi E, Cetani F, Elisei R. Genetic and clinical features of multiple endocrine neoplasia types 1 and 2. Journal of oncology. 2012;2012 doi: 10.1155/2012/705036. Article ID 705036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells SA, Jr, Pacini F, Robinson BG, Santoro M. Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma:An Update. The Journal of clinical endocrinology and metabolism. 2013;98(8):3149–3164. doi: 10.1210/jc.2013-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melmed S, Polonsky K, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 12th ed. Elsevier; 2011. [Google Scholar]
- 29.Orth DN, Kovacs W, DeBold C. The adrenal Cortex. In: Wilson JD, Foster DW, editors. Williams Textbook of Endocrinology. Philadelphia: Saunders; 1992. [Google Scholar]
- 30.Khatami F, Tavangar SM. Current diagnostic status of pheochromocytomaand future perspective:A mini review. Iranian journal of pathology. 2017;12(3):313–322. [PMC free article] [PubMed] [Google Scholar]
- 31.Giusti F, Tonelli F, Brandi ML. Primary hyperparathyroidism in multiple endocrine neoplasia type 1:when to perform surgery? Clinics. 2012;67(Suppl 1):141–144. doi: 10.6061/clinics/2012(Sup01)23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cetin D, Unübol M, Soyder A, Güney E, Coşkun A, Ozbaş S, Unsal A, Erkuş M. Coexistence of multiple endocrine neoplasia type 2B and chilaiditi sign:A case report. Case reports in endocrinology. 2012;2012:360328. doi: 10.1155/2012/360328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lodish MB, Stratakis CA. RET oncogene in MEN2, MEN2B, MTC, and other forms of thyroid cancer:molecular genetics and therapeutic advances. Expert review of anticancer therapy. 2008;8(4):625–632. doi: 10.1586/14737140.8.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Höppener JW, van Amstel HK, Romeo G, Lips CJM, Buys HCM. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367(6461):375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- 35.Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA Jr. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Human molecular genetics. 1993;2(7):851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 36.Knowles PP, Murray-Rust J, Kjær S, Scott RP, Hanrahan S, Santoro M, Ibáñez CF, McDonald NQ. Structure and chemical inhibition of the RET tyrosine kinase domain. Journal of biological chemistry. 2006;281(44):33577–33587. doi: 10.1074/jbc.M605604200. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42(2):581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 38.Ceccherini I, Bocciardi R, Luo Y, Pasini B, Hofstra R, Takahashi M, Romeo G. Exon structure and flanking intronic sequences of the human RET proto-oncogene. Biochemical and biophysical research communications. 1993;196(3):1288–1295. doi: 10.1006/bbrc.1993.2392. [DOI] [PubMed] [Google Scholar]
- 39.Feng L, Wang CY, Jiang H, Oho C, Dugich-Djordjevic M, Mei L, Lu L. Differential signaling of glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor in cultured ventral mesencephalic neurons. Neuroscience. 1999;93(1):265–73. doi: 10.1016/s0306-4522(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 40.Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. Journal of neuroscience. 1999;19(21):9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakami H, Iwashita T, Asai N, Shimono Y, Iwata Y, Kawai K, Takahashi M. Enhanced phosphatidylinositol 3-kinase activity and high phosphorylation state of its downstream signalling molecules mediated by ret with the MEN 2B mutation. Biochemical and biophysical research communications. 1999;262(1):68–75. doi: 10.1006/bbrc.1999.1186. [DOI] [PubMed] [Google Scholar]
- 42.Latteyer S, Klein-Hitpass L, Khandanpour C, Zwanziger D, Poeppel T, Schmid K, Führer D, Moeller LC. A 6-base pair in frame germline deletion in exon 7 of RET leads to increased RET phosphorylation, ERK activation, and MEN2A. The journal of clinical endocrinology and metabolism. 2016;101(3):1016–10122. doi: 10.1210/jc.2015-2948. [DOI] [PubMed] [Google Scholar]
- 43.Hong DS, Cabanillas ME, Wheler J, Naing A, Tsimberidou AM, Ye L, Busaidy NL, Waguespack SG, Hernandez M, El Naggar AK, Bidyasar S, Wright J, Sherman SI, Kurzrock R. Inhibition of the Ras/Raf/MEK/ERK and RET kinase pathways with the combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in medullary and differentiated thyroid malignancies. The journal of clinical endocrinology and metabolism. 2011;96(4):997–1005. doi: 10.1210/jc.2010-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouyang B, Knauf JA, Smith EP, Zhang L, Ramsey T, Yusuff N, Batt D, Fagin JA. Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clinical cancer research. 2006;12(6):1785–1793. doi: 10.1158/1078-0432.CCR-05-1729. [DOI] [PubMed] [Google Scholar]
- 45.Myers SM, Eng C, Ponder BA, Mulligan LM. Characterization of RET proto-oncogene 3'splicing variants and polyadenylation sites:a novel C-terminus for RET. Oncogene. 1995;11(10):2039–2045. [PubMed] [Google Scholar]
- 46.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine and growth factor reviews. 2005;16(4):441–467. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Van Weering DH, Medema JP, Van Puijenbroek A, Burgering BM, Baas PD, Bos JL. Ret receptor tyrosine kinase activates extracellular signal-regulated kinase 2 in SK-N-MC cells. Oncogene. 1995;11(11):2207–2214. [PubMed] [Google Scholar]
- 48.Xing S, Furminger TL, Tong Q, Jhiang SM. Signal transduction pathways activated by RET oncoproteins in PC12 pheochromocytoma cells. Journal of biological chemistry. 1998;273(9):4909–4914. doi: 10.1074/jbc.273.9.4909. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, Nakao A, Takahashi M. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19(39):4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- 50.De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, Billaud M, Fusco A, Tsichlis PN, Santoro M. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer research. 2000;60(14):3727–3731. [PubMed] [Google Scholar]
- 51.Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibáñez CF. Released GFRα1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29(1):171–84. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 52.Kajbafzadeh AM, Payabvash S, Salmasi AH, Monajemzadeh M, Tavangar SM. Smooth muscle cell apoptosis and defective neural development in congenital ureteropelvic junction obstruction. Journal of urology. 2006;176(2):718–723. doi: 10.1016/j.juro.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 53.Costantini F. GDNF/Ret signaling and renal branching morphogenesis:From mesenchymal signals to epithelial cell behaviors. Organogenesis. 2010;6(4):252–262. doi: 10.4161/org.6.4.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waxman S. Molecular Neurology. 1st ed. USA: Academic Press; 2007. [Google Scholar]
- 55.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine growth factor reviews. 2001;12(4):361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 56.Simbolo M, Mian C, Barollo S, Fassan M, Mafficini A, Neves D, Scardoni M, Pennelli G, Rugge M, Pelizzo MR, Cavedon E, Fugazzola L, Scarpa A. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows archiv. 2014;465(1):73–78. doi: 10.1007/s00428-014-1589-3. [DOI] [PubMed] [Google Scholar]
- 57.Moura MM, Cavaco BM, Leite V. RAS proto-oncogene in medullary thyroid carcinoma. Endocrine-related cancer. 2015;22(5):R235–R252. doi: 10.1530/ERC-15-0070. [DOI] [PubMed] [Google Scholar]
- 58.Romei C, Cosci B, Renzini G, Bottici V, Molinaro E, Agate L, et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC) Clinical endocrinology (Oxford) 2011;74(2):241–247. doi: 10.1111/j.1365-2265.2010.03900.x. [DOI] [PubMed] [Google Scholar]
- 59.Tabriz HM, Adabi K, Lashkari A, Heshmat R, Haghpanah V, Larijani B, Tavangar SM. Immunohistochemical analysis of nm23 protein expression in thyroid papillary carcinoma and follicular neoplasm. Pathology research and practice. 2009;205(2):83–87. doi: 10.1016/j.prp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Marsh DJ, Learoyd DL, Andrew SD, Krishnan L, Pojer R, Richardson AL, Delbridge L, Eng C, Robinson BG. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clinical endocrinology (Oxford) 1996;44(3):249–257. doi: 10.1046/j.1365-2265.1996.681503.x. [DOI] [PubMed] [Google Scholar]
- 61.Bongarzone I, Vigano E, Alberti L, Mondellini P, Uggeri M, Pasini B, Borrello MG, Pierotti MA. The Glu632-Leu633 deletion in cysteine rich domain of Ret induces constitutive dimerization and alters the processing of the receptor protein. Oncogene. 1999;18(34):4833–4838. doi: 10.1038/sj.onc.1202848. [DOI] [PubMed] [Google Scholar]
- 62.Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, Miccoli P, Berti P, Pacini F, Pinchera A. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer:a 10-year follow-up study. The journal of clinical endocrinology and metabolism. 2008;93(3):682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 63.Van Veelen W, De Groot J, Acton D, Hofstra R, Höppener J, Links T, Lips CJ. Medullary thyroid carcinoma and biomarkers:past, present and future. Journal of internal medicine. 2009;266(1):126–140. doi: 10.1111/j.1365-2796.2009.02106.x. [DOI] [PubMed] [Google Scholar]
- 64.Dvorakova S, Vaclavikova E, Sykorova V, Vcelak J, Novak Z, Duskova J, Ryska A, Laco J, Cap D, Kodetova D, Kodet R, Krskova L, Vlcek P, Astl J, Vesely D, Bendlova B. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Molecular and cellular endocrinology. 2008;284(1-2):21–27. doi: 10.1016/j.mce.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 65.Saffar H, Sanii S, Emami B, Heshmat R, Panah VH, Azimi S, Tavangar SM. Evaluation of MMP2 and caspase-3 expression in 107 cases of papillary thyroid carcinoma and its association with prognostic factors. Pathology research and practice. 2013;209(3):195–199. doi: 10.1016/j.prp.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Sanii S, Saffar H, Tabriz HM, Qorbani M, Haghpanah V, Tavangar SM. Expression of matrix metalloproteinase-2, but not caspase-3, facilitates distinction between benign and malignant thyroid follicular neoplasms. Asian Pacific journal of cancer prevention. 2012;13(5):2175–2178. doi: 10.7314/apjcp.2012.13.5.2175. [DOI] [PubMed] [Google Scholar]
- 67.Attaran SY, Omrani G, Tavangar SM. Lympho-epithelial-like intrathyroidal thymic carcinoma with foci of squamous differentiation. Case report. APMIS. 1996;104(6):419–423. doi: 10.1111/j.1699-0463.1996.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 68.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–3988. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 69.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khatami F, Noorinayer B, Ghiasi S, Mohebi R, Hashemi M, Zali MR. Single nucleotide polymorphisms of DNA methyltransferase 1 gene and gastric cancer in Iranian patients:a case control study. Iranian journal of cancer prevention. 2012;1(3):111–118. [PubMed] [Google Scholar]
- 71.Khatami F, Noorinayer B, Mohebi SR, Ghiasi S, Mohebi R, Hashemi M, et al. Effects of amino acid substitution polymorphisms of two DNA methyltransferases on susceptibility to sporadic colorectal cancer. Asian Pacific journal of cancer prevention. 2009;10(6):1183–1188. [PubMed] [Google Scholar]
- 72.Khatami F, Mohebi SR, Ghiasi S, Haghighi MM, Safaee A, Hashemi M, Zali MR. Amino acid substitution polymorphisms of two DNA methyltransferases and susceptibility to sporadic colorectal cancer. Gastroenterology and hepatology from bed to bench. 2009;1(3):105–112. [Google Scholar]
- 73.Schagdarsurengin U, Gimm O, Hoang-Vu C, Dralle H, Pfeifer GP, Dammann R. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Cancer research. 2002;62(13):3698–3701. [PubMed] [Google Scholar]
- 74.Mohammadi-Asl J, Larijani B, Khorgami Z, Tavangar SM, Haghpanah V, Kheirollahi M, Mehdipour P. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Medical oncology. 2011;28(4):1123–1128. doi: 10.1007/s12032-010-9587-z. [DOI] [PubMed] [Google Scholar]
- 75.Wang N, Kjellin H, Sofiadis A, Fotouhi O, Juhlin CC, Bäckdahl M, Zedenius J, Xu D, Lehtiö J, Larsson C. Genetic and epigenetic background and protein expression profiles in relation to telomerase activation in medullary thyroid carcinoma. Oncotarget. 2016;7(16):21332–21346. doi: 10.18632/oncotarget.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sponziello M, Durante C, Boichard A, Dima M, Puppin C, Verrienti A, Tamburrano G, Di Rocco G, Redler A, Lacroix L, Bidart JM, Schlumberger M, Damante G, Russo D, Filetti S. Epigenetic-related gene expression profile in medullary thyroid cancer revealed the overexpression of the histone methyltransferases EZH2 and SMYD3 in aggressive tumours. Molecular and cellular endocrinology. 2014;392(1-2):8–13. doi: 10.1016/j.mce.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Haghpanah V, Shooshtarizadeh P, Heshmat R, Larijani B, Tavangar SM. Immunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinoma. Applied immunohistochemistry and molecular morphology. 2006;14(4):422–425. doi: 10.1097/01.pai.0000213100.88074.b8. [DOI] [PubMed] [Google Scholar]
- 78.Abraham D, Jackson N, Gundara JS, Zhao J, Gill AJ, Delbridge L, Robinson BG, Sidhu SB. MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clinical cancer research. 2011;17(14):4772–4781. doi: 10.1158/1078-0432.CCR-11-0242. [DOI] [PubMed] [Google Scholar]
- 79.Gundara JS, Zhao JT, Gill AJ, Clifton-Bligh R, Robinson BG, Delbridge L, Sidhu SB. Nodal metastasis microRNA expression correlates with the primary tumour in MTC. ANZ journal of surgery. 2014;84(4):235–239. doi: 10.1111/j.1445-2197.2012.06291.x. [DOI] [PubMed] [Google Scholar]
- 80.Gundara JS, Zhao J, Gill AJ, Lee JC, Delbridge L, Robinson BG, McLean C, Serpell J, Sidhu SB. Noncoding RNA blockade of autophagy is therapeutic in medullary thyroid cancer. Cancer medicine. 2015;4(2):174–182. doi: 10.1002/cam4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santarpia L, Calin GA, Adam L, Ye L, Fusco A, Giunti S, Thaller C, Paladini L, Zhang X, Jimenez C, Trimarchi F, El-Naggar AK, Gagel RF. A miRNA signature associated with human metastatic medullary thyroid carcinoma. Endocrine-related cancer. 2013;20(6):809–823. doi: 10.1530/ERC-13-0357. [DOI] [PubMed] [Google Scholar]
- 82.Mian C, Pennelli G, Fassan M, Balistreri M, Barollo S, Cavedon E, Galuppini F, Pizzi M, Vianello F, Pelizzo MR, Girelli ME, Rugge M, Opocher G. MicroRNA profiles in familial and sporadic medullary thyroid carcinoma:preliminary relationships with RET status and outcome. Thyroid. 2012;22(9):890–896. doi: 10.1089/thy.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hudson J, Duncavage E, Tamburrino A, Salerno P, Xi L, Raffeld M, Moley J, Chernock RD. Overexpression of miR-10a and miR-375 and downregulation of YAP1 in medullary thyroid carcinoma. Experimental and molecular pathology. 2013;95(1):62–67. doi: 10.1016/j.yexmp.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duan L, Hao X, Liu Z, Zhang Y, Zhang G. MiR-129-5p is down-regulated and involved in the growth, apoptosis and migration of medullary thyroid carcinoma cells through targeting RET. FEBS letters. 2014;588(9):1644–1651. doi: 10.1016/j.febslet.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Schagdarsurengin U, Gimm O, Dralle H, Hoang-Vu C, Dammann R. CpG island methylation of tumor-related promoters occurs preferentially in undifferentiated carcinoma. Thyroid. 2006;16(7):633–642. doi: 10.1089/thy.2006.16.633. [DOI] [PubMed] [Google Scholar]
- 86.Tavangar SM, Larijani B, Mahta A, Hosseini SMA, Mehrazine M, Bandarian F. Craniopharyngioma:A clinicopathological study of 141 cases. Endocrine pathology. 2004;15(4):339–344. doi: 10.1385/ep:15:4:339. [DOI] [PubMed] [Google Scholar]
- 87.Sarmadi S, Izadi-Mood N, Sotoudeh K, Tavangar SM. Altered PTEN expression;a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagnostic pathology. 2009;4:41. doi: 10.1186/1746-1596-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joo LJS, Zhao JT, Gild ML, Glover AR, Sidhu SB. Epigenetic regulation of RET receptor tyrosine kinase and non-coding RNAs in MTC. Molecular and cellular endocrinology. 2017 doi: 10.1016/j.mce.2017.03.014. doi:10.1016/j.mce.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 89.Munnes M, Patrone G, Schmitz B, Romeo G, Doerfler W. A 5′-CG-3′-rich region in the promoter of the transcriptionally frequently silenced RET proto-oncogene lacks methylated cytidine residues. Oncogene. 1998;17(20):2573–2583. doi: 10.1038/sj.onc.1202165. [DOI] [PubMed] [Google Scholar]
- 90.Draht MX, Smits KM, Tournier B, Jooste V, Chapusot C, Carvalho B, Cleven AH, Derks S, Wouters KA, Belt EJ, Stockmann HB, Bril H, Weijenberg MP, van den Brandt PA, de Bruïne AP, Herman JG, Meijer GA, Piard F, Melotte V, van Engeland M. Promoter CpG island methylation of RET predicts poor prognosis in stage II colorectal cancer patients. Molecular oncology. 2014;8(3):679–688. doi: 10.1016/j.molonc.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Draht MX, Smits KM, Jooste V, Tournier B, Vervoort M, Ramaekers C, Chapusot C, Weijenberg MP, van Engeland M, Melotte V. Analysis of RET promoter CpG island methylation using methylation-specific PCR (MSP), pyrosequencing, and methylation-sensitive high-resolution melting (MS-HRM):impact on stage II colon cancer patient outcome. Clinical epigenetics. 2016;8:44. doi: 10.1186/s13148-016-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu JJ, Kam MK, Garcia-Barceló MM, Tam PK, Lui VC. HOXB5 binds to multi-species conserved sequence (MCS+9.7) of RET gene and regulates RET expression. International journal of biochemistry and cell biology. 2014;51:142–149. doi: 10.1016/j.biocel.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 93.Puppo F, Griseri P, Fanelli M, Schena F, Romeo G, Pelicci P, et al. Cell-line specific chromatin acetylation at the Sox10-Pax3 enhancer site modulates the RET proto-oncogene expression. FEBS letters. 2002;523(1-3):123–127. doi: 10.1016/s0014-5793(02)02957-5. [DOI] [PubMed] [Google Scholar]
- 94.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity:microRNA biogenesis pathways and their regulation. Nature cell biology. 2009;11(3):228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 95.Peschansky VJ, Wahlestedt C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics. 2014;9(1):3–12. doi: 10.4161/epi.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh P, Soon PS, Feige JJ, Chabre O, Zhao JT, Cherradi N, LAlli E, Sidhu SB. Dysregulation of microRNAs in adrenocortical tumors. Molecular and cellular endocrinology. 2012;351(1):118–128. doi: 10.1016/j.mce.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 97.Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors:biological significance and diagnostic utility. Journal of clinical endocrinology and metabolism. 2008;93(5):1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lassalle S, Zangari J, Popa A, Ilie M, Hofman V, Long E, Patey M, Tissier F, Belléannée G, Trouette H, Catargi B, Peyrottes I, Sadoul JL, Bordone O, Bonnetaud C, Butori C, Bozec A, Guevara N, Santini J, Hénaoui IS, Lemaire G, Blanck O, Vielh P, Barbry P, Mari B, Brest P, Hofman P. MicroRNA-375/SEC23A as biomarkers of the in vitro efficacy of vandetanib. Oncotarget. 2016;7(21):30461–30467. doi: 10.18632/oncotarget.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu Y, Wang H, Chen E, Xu Z, Chen B, Lu G. Candidate microRNAs as biomarkers of thyroid carcinoma:a systematic review, meta-analysis, and experimental validation. Cancer medicine. 2016;5(9):2602–2614. doi: 10.1002/cam4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Samimi H, Zaki Dizaji M, Ghadami M, Shahzadeh F, Khashayar P, Soleimani M, Larijani B, Haghpanah V. MicroRNAs networks in thyroid cancers:focus on miRNAs related to the fascin. Journal of diabetes and metabolic disorders. 2013;12(1):31. doi: 10.1186/2251-6581-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]


