Abstract
The dual gradient energy coupling hypothesis posits that chloroplast thylakoid membranes are energized for ATP formation by either a delocalized or a localized proton gradient geometry. Localized energy coupling is characterized by sequestered domains with a buffering capacity of approximately 150 nmol H+ mg−1 chlorophyll (Chl). A total of 30 to 40 nmol mg−1 Chl of the total sequestered domain buffering capacity is contributed by lysines with anomolously low pKas, which can be covalently derivatized with acetic anhydride. We report that in thylakoid membranes treated with acetic anhydride, luminal acidification by a photosystem I (duraquinol [DQH2] to methyl viologen [MV]) proton pumping partial reaction was nearly completely inhibited, as measured by three separate assays, yet surprisingly, H+ accumulation still occurred to the significant level of more than 100 nmol H+ mg Chl−1, presumably into the sequestered domains. The treatment did not increase the observed rate constant of dark H+ efflux, nor was electron transport significantly inhibited. These data provide support for the existence of a sequestered proton translocating pathway linking the redox reaction H+ ion sources with the CF0 H+ channel. The sequestered, low-pKa Lys groups appear to have a role in the H+ diffusion process and chemically modifying them blocks the putative H+ relay system.
Photophosphorylation in plant chloroplasts is driven by the H+ electrochemical potential gradient, Δμ̃H+. Most, if not all textbooks (and no exceptions are known to the authors) show models of and/or discuss the concept that only a transmembrane Δμ̃H+ (lumen-to-stroma) is involved in energizing ATP formation. It is certainly true that much evidence supports the notion that transmembrane Δμ̃H+ gradients in thylakoids are able to generate ATP, just as bulk phase-to-bulk phase gradients can drive ATP formation by acid-base transitions in lipid vesicles containing, as the only protein, the purified CF0-CF1 complex (Fromme and Gräber, 1990). The situation is complicated in thylakoid research, however, by there being a large body of evidence consistent with the occurrence, under some circumstances, of a localized energy coupling mechanism (for reviews, compare with Westerhoff et al., 1984; Ferguson, 1985; Rottenberg, 1985; Dilley, 1991). The localized, but still chemiosmotic, proton gradient energy coupling hypothesis is not widely accepted, in part because it is not structurally clear how a localized Δμ̃H+ can be maintained. In regard to the latter point, there is a large body of data identifying sequestered acid-base groups such as Lys and carboxyl residues of thylakoid membrane proteins, and there is evidence that the low-pKa Lys groups in the sequestered domains interact directly with the H+ ions from the redox reactions as they diffuse to the CF0-CF1 in energizing ATP formation (Theg et al., 1988; Dilley and Schreiber, 1984).
The postulated sequestered domains have the following characteristics (for review, see Dilley et al., 1987; Dilley, 1991): (a) they have a proton-buffering capacity of about 150 nmol H+ mg−1 chlorophyll (Chl), of which there are 30 to 40 nmol Lys residue with anomolously low pKas (approximately 7.5), and the remaining buffering most likely is supplied by carboxyl groups (Laszlo et al., 1982); (b) the buffering groups are contained in membrane-associated proteins including the three extrinsic proteins of the photosystem II (PSII) oxygen-evolving complex, the light-harvesting proteins (LHC), the photosystem I (PSI) proteins, and subunit III of the CF0-CF1 complexes (Laszlo et al., 1984). Buried carboxyl groups associated with some of the LHC proteins have been identified by Jahns and Junge (1990), Jahns et al., (1988), and Walters et al. (1996) using [14C]dicyclohexylcarbodiimide (DCCD) as a probe for COO− groups in hydrophobic regions; (c) protons in the sequestered domains are supplied by redox turnovers of PSI and PSII. Jahns et al. (1988) showed that DCCD—probably through derivatizing LHCII carboxyl groups in hydrophobic regions—inhibited H+ movement to the lumen. In carefully prepared thylakoids kept under conditions favoring the localized gradient energy coupling mode, the sequestered protons were not in equilibrium with protons in the lumen, and ATP formation appears to be driven by the localized gradient dissipating through the CF0-CF1 (Theg et al., 1988).
A key point in the work done by this group is the facile switching from an apparent localized Δμ̃H+ gradient to a delocalized Δμ̃H+ gradient supplying energy for ATP formation (Beard and Dilley, 1986, 1988; Chiang and Dilley, 1987) under the control of Ca2+ ions interacting with the CF0H+ channel 8-kD subunit (Chiang and Dilley, 1987; Chiang et al., 1992). The conversion from an apparent localized to a delocalized energy coupling mode has the properties of a regulated event, and our studies indicate that over-energization produces sufficient acidity in the local domains to displace the CF0-bound Ca2+, the H+ gradient then equilibrates freely with the lumen, and the delocalized gradient coupling mode is established (Dilley, 1991). The energization-dependent switching of the energy coupling mode from localized to delocalized is interesting because the modulation of the lumen pH has been discussed by several groups as a key signaling element in the chloroplast photoprotective response to excess light intensity (Laasch and Weiss, 1989; Dilley, 1991; Demmig-Adams and Adams, 1992; Pfündel et al., 1994; Ruban and Horton, 1995; Gilmore, 1997). We suggest that such a physiological stress signaling/stress alleviation mechanism, by utilizing the switching from localized to delocalized energy coupling proton gradients, provides a biological reason for the existence of the dual proton gradient energy coupling modes.
Clearly, the hypothesis linking a stress signaling system with switching between localized and delocalized proton gradient-driven ATP formation requires additional testing, and one part of that endeavor is to define better the properties of the sequestered H+ buffering domains. In this report, we show that acetic anhydride treatment, which acetylates low-pKa Lys groups, blocks H+ accumulation into the lumen but does not block H+ uptake into the sequestered domain buffering pool. The data provide new evidence supporting the concept of a membrane domain H+-buffering array that is separated by significant diffusion barriers from the lumen phase.
MATERIALS AND METHODS
Thylakoid Isolation
Thylakoids were isolated from either market spinach (Spinacia oleracea) or greenhouse-grown pea (Pisum sativum cv Little Marval). Pea plants were grown in moist vermiculite in a growth chamber for 16 to 20 d as described by Pfündel et al., (1994). Plants were illuminated for a 12-h photoperiod with a photon flux density of 450 μmol m−2 s−1. The temperature was maintained at 15°C and 20°C for dark and light conditions, respectively.
Prior to chloroplast isolation, the spinach leaves or the pea plants were dark adapted for 8 to 12 h so that all zeaxanthin and antheraxanthin was converted to violaxanthin. Chloroplasts were isolated as described previously (Ort and Izawa, 1973) under reduced light. The thylakoids were resuspended in high-salt medium containing 30 mm sorbitol,5mm4-(2-hydroxyethyl)-1-piperazineethane- sulfonic acid (HEPES)-KOH (pH 7.5), 3 mm MgCl2, 100 mm KCl, and 0.5 g L−1 defatted bovine serum albumin (BSA) to yield a Chl concentration of 2 to 4 mg mL−1. The Chl concentration was determined according to the method of Arnon (1949).
Thylakoids used were either freshly isolated or from frozen stocks. Frozen thylakoids were prepared by adding 4 parts of thylakoid suspension at 3 to 4 mg Chl mL−1 to one part pure ethylene glycol as a cryoprotectant and stored in liquid nitrogen until used. Prior to the experiments, thylakoids were quickly thawed, stored on ice, and used within 2 to 3 h. The average ATP formation activity loss after the freeze-thaw cycle was 15%, as measured by the ATP yield per single-turnover flash, and over a 2- to 3-h period at ice temperature, activity declined less than 20%.
Acetic Anhydride Modification of Thylakoids
Thylakoids were modified with acetic anhydride according to Baker et al., (1981). Thylakoids equal to 80 μg Chl mL−1 were added to a reaction mixture of 100 mm sorbitol, 50 mm Tricine-KOH (pH 8.6), 3 mm MgCl2, and 3 mm KH2PO4. Thirty seconds later, 400 nm cyanide m-chlorophenylhydrazone (CCCP) was added to shift the protonated, low-pKa Lys groups to the uncharged form, which reacts with acetic anhydride (Means and Feeney, 1971). Defatted BSA (1 mg mL−1 final concentration) was added after an additional 3 min to remove the CCCP from the thylakoids and the medium (Theg et al., 1988). A final concentration (3.5 mm) of acetic anhydride was added 2 min later and allowed to react with the thylakoids for 30 s, at which time glycylglycine (pH 8.6) was added (to give a final concentration of 50 mm) to react with the free acetic anhydride. As a control, glycylglycine was added prior to the anhydride in a portion of the thylakoids. All of the above steps were performed at 20°C. The thylakoids were then centrifuged at 5,000g for 15 min (4°C). The resulting pellet was resuspended in high-salt medium (see above) at 2 to 3 mg Chl mL−1.
Baker et al. (1981) reported that acetic anhydride treatment modifies both the oxygen-evolving complex and the CF1 portion of ATP synthase. To determine the efficiency of acetic anhydride treatment, electron transport rates were measured under whole chain (water to MV) and PSI-only (DQH2 to MV plus 3-[3,4-dichlorophenyl]-1,1-dimethylurea [DCMU]) conditions prior to all other assays. Only those treated thylakoids which showed 85% to 90% (or more) inhibition of whole chain electron transport were used.
The light intensities used are listed in the figure and table legends and were adjusted so that the glycylglycine-quenched control and anhydride-treated thylakoids exhibited close to equal rates of PSI-only electron transport.
Violaxanthin Deepoxidation
Deepoxidation of violaxanthin by the luminal enzyme violaxanthin deepoxidase (VDE) (Yamamoto, 1979) to the photoprotective pigments antheraxanthin and zeaxanthin was measured spectrophotometrically and by HPLC pigment analysis. Dual-wavelength spectrophotometric measurements were done on a spectrophotometer (DW-2, Aminco International, Lake Forest, CA) using a sample wavelength of 505 nm and a reference wavelength of 560 nm according to the method of Pfündel and Dilley (1993). The thylakoids were osmotically shocked to decrease the light- scattering component of the absorbance signal. Sixty micrograms of Chl-equivalent thylakoids (either treated or untreated) were put into 1.0 mL of ice-cold distilled water for 30 s, after which time 1 mL of 2× reaction buffer was added to yield a final concentration of 100 mm Suc, 50 mm HEPES-KOH (pH 7.6), 3 mm MgCl2, and 3 mm KH2PO4. This osmotic shock protocol was tested by Pfündel et al. (1994) for its effect on ATP formation in thylakoids not treated with acetic anhydride or the glycylglycine quench, and it had no inhibitory effect.
Additional components added to the assay mixture were 100 μm MV, 100 nm nonactin, 50 mm ascorbate, 5 μm diadenosine pentaphosphate, 5 μm DCMU, ±5 mm dithiothreitol (DTT), and 500 μm DQH2. DQH2 was prepared daily by reducing tetramethyl-p-benzoquinone according to the method of Allen and Horton (1980). The cuvette temperature was 20°C. Violaxanthin deepoxidation was initiated with actinic light passed through a CuSO4 heat filter and a red filter (Corning 2–64, Corning, NY). As a control, ascorbate-driven violaxanthin deepoxidation at pH 5.6 in the dark was also measured. The reaction mixture for the pH 5.6 assays contained 100 mm Suc, 50 mm MES-KOH (pH 5.6), 3 mm MgCl2, 3 mm KH2PO4, 100 nm nonactin, 500 μm nigericin, ±5 mm DTT, and thylakoids equal to 30 μg Chl mL−1. Fifty millimolar ascorbate was added to initiate the reaction. Assays were typically run for 9 min.
Samples for pigment analysis were collected and processed for HPLC analysis as described by Pfündel and Dilley (1993). Thylakoids from the above assays were washed in 100 mm Suc, 50 mm HEPES-KOH (pH 7.5), and 3 mm MgCl2 for several minutes before being centrifuged. The resulting pellets were resuspended in 100% acetone and centrifuged. The supernatants were filtered through a microfilterfuge (0.2-mm Nylon-66 membrane filters, Rainin Instrument, Woburn, MA) and the filtrates were stored at −80°C until separated by HPLC.
Reverse-phase HPLC separation of the xanthophylls and Chl was performed on a Spheresorb ODS-1, N-capped column protected by an ODS-1 direct-connect cartridge guard column (Alltech, Deerfield, IL) according to Gilmore and Yamamoto (1991) with slight changes in the solvent A composition (65:10:3 of acetonitril:methanol:0.1 m Tris [pH 8.0]). Ten-microliter samples equal to 75 ng of Chl were loaded onto the column. Xanthophylls and Chl were separated isocratically at a flow rate of 1.5 mL min−1. A solvent of methanol and hexane (4:1, v/v, solvent B) was then used to remove carotenes from the column. The column was reequilibrated with solvent A for at least 10 min prior to subsequent separations.
An HPLC system (Dynamax, Rainin Institute, Woburn, MA) was used to run the gradient program. Peaks were determined with the detector set at 440 nm. Pigment amounts were computed from the areas under the peak using the processing program in the software provided with the HPLC and extinction coefficients provided by Gilmore and Yamamoto (1991).
Measurement of Proton Uptake
The extent of proton uptake was measured with a semimicro combination (Ag/AgCl) pH electrode (Corning Scientific Instruments, Medfield, MA) and a pH meter built by the Purdue University Department of Biological Sciences Electronics shop. The reaction mixture contained 100 mm sorbitol, 3 mm MgCl2, 5 mm KH2PO4 (pH 8.0), 100 μm MV, 100 nm nonactin, 5 μm DCMU, 500 μm DQH2, with or without 0.5 mm 4-(2-hydroxyethyl)morpholine (HEM). The pH was adjusted to 8.0 and thylakoids equal to 33 μg Chl mL−1 were added to the cuvette. After 3 min of incubation, the thylakoids were illuminated with either saturating (for the anhydride-treated thylakoids) or subsaturating light (for the control thylakoids, to bring the DQH2 → MV electron transport rate to the same rate as the anhydride-treated sample) from a 500 W projection lamp (General Electric, Cleveland). The light was passed through a 2% (w/v) CuSO4 heat filter and a red filter prior to illuminating the thylakoids. Illumination lasted 20 to 30 s. The temperature was held at 20°C. After dark relaxation of the proton gradient, the signal was calibrated with HCl.
Relative ΔpH Determination by 9-Amino-6-Chloro-2-Methoxyacridine (ACMA)
A relative ΔpH was determined by ACMA fluorescence quenching. The reaction mixture contained 100 mm sorbitol, 50 mm Tricine-KOH (pH 8.0), 3 mm MgCl2, 3 mm KH2PO4, 100 nm nonactin, 5 μm DCMU, and 500 μm DQH2. Thylakoids equal to 20 μg Chl mL−1 were added to this mixture and allowed to incubate for 3 min. The temperature was 20°C. Two micromolar ACMA was added to the reaction solution just prior to measurement of fluorescence. Quenching was measured in a fluorimeter (DMX-1000, SLM, Urbana, IL). The excitation wavelength was 405 nm, the emission wavelength was 483 nm, the excitation slit widths were 4 nm, and the emission slit widths were 8 nm. The fluorescence quenching was driven by red light intensities adjusted to give comparable rates of DQH2 to MV electron transport in the control and anhydride-treated thylakoids.
Electron Transport Rates
Electron transport was measured with a Clark-type oxygen electrode. The reaction mixture contained 100 mm sorbitol, 1 mm Tricine-KOH (pH 8.0), 3 mm MgCl2, and 3 mm KH2PO4, 100 μm MV, 5 mm NaN3, 200 units mL−1 superoxide dismutase, 100 nm nonactin, 500 μm DQH2, 5 μm DCMU, and thylakoids equal to 40 μg Chl mL−1. The temperature was 20°C. The oxygen sensitivity was calibrated by thylakoid reduction of known amounts of K3Fe(CN)6. Electron transport was driven by light as described above in the “Measurement of Proton Uptake” section. For some experiments PSII-dependent electron flow was measured by the pH change method to detect the rate of water oxidation as H+ release (using a similar assay buffer as the one for measuring H+ uptake described above).
RESULTS
Previous work had shown that a 30-s room temperature treatment with 3.5 mm acetic anhydride severely inhibits PSII water oxidation activity in spinach thylakoids but had very little effect on the PSI partial electron transport reaction, DQH2 → MV (Baker et al., 1981). The earlier work did not deal with proton uptake activity, mainly because the acetic anhydride treatment totally inhibited the CF1-dependent ATP formation activity, so there was no interest at that point in the proton gradient energization. In this work we have studied the H+ uptake reactions after anhydride treatment, concentrating on the proton pump-competent PSI partial reaction, DQH2 → MV, as the driving reaction. The unexpected result was that while a significant amount of uncoupler-sensitive H+ uptake from the medium was observed after acetic anhydride treatment of either spinach or pea thylakoids, there seemed to be little, if any, luminal acidification. First, we will provide characterization of the effects of the anhydride treatment on PSI plus PSII whole chain and the PSI partial electron transport reactions.
Acetic Anydride Effects on Electron Transport
Spinach thylakoids were used in earlier studies (Baker et al., 1981), and while spinach was mostly used here, pea thylakoids were used for some electron and proton transport experiments. Both sources of thylakoids gave similar responses to acetic anhydride in terms of electron transport and H+ uptake. Table I shows the anhydride inhibitory effect on PSII-dependent ferricyanide reduction in spinach thylakoids as near 96% of the uncoupled rate of the untreated control. The glycylglycine-quenched sample (item 2) was inhibited about 64%. There was a 2-fold uncoupler stimulation of electron transport in the glycylglycine-quenched sample but no uncoupler response in the anhydride-treated sample. In some experiments, there was a slight uncoupler stimulation of the very low rate of ferricyanide reduction in the anhydride-treated thylakoids (data not shown). Similar anhydride inhibition results were obtained with pea thylakoids for PSII-dependent electron transport (data not shown).
Table I.
Acetic anhydride inhibition of PSII-dependent ferricyanide reduction in spinach thylakoids
| Thylakoid Sample Used | Whole-Chain Electron Transport Rate
|
|
|---|---|---|
| Basal | Uncoupled | |
| μeq (mg Chl h)−1 | ||
| Untreated thylakoidsa | 192 | 630 (100%) |
| Glycylglycine-quenched controlb | 113 | 228 (36%) |
| Acetic anhydride-treatedc | 29 | 28 (4%) |
See “Materials and Methods” for the treatment reaction conditions and for the electron transport assay. Saturating light intensity was used (the same intensity for all assays). Nigericin (40 μm) was added to uncouple electron transport after a 1-min light exposure, followed by an additional 1 min of illumination. The numbers in parentheses are the percentages of the uncoupled, untreated control rates.
Untreated thylakoids were the stock suspension, after isolation, from which aliquots were taken for the glycylglycine-quenched and acetic anhydride treatments.
Glycylglycine was added before the acetic anhydride to quench the anhydride before appreciable reaction occurred with thylakoid reactive groups.
The anhydride was added to the thylakoids for the specified reaction time (compare with “Materials and Methods”) followed by the glycylglycine quench.
Table II shows the effect of the glycylglycine quench and acetic anhydride treatments on the PSI partial electron transport reaction, DQH2 → MV, in pea thylakoids. In this case the PSI uncoupled electron transport activity was inhibited by the anhydride treatment about 60% compared with the glycylglycine quenched sample at saturating light intensity. When the quenched sample was given a reduced light intensity to bring its basal rate to a similar level as the anhydride-treated sample at full light, the two samples were similarly stimulated by uncoupling (nearly 2-fold). In the thylakoids used for the Table II data, the uncoupled PSII-dependent ferricyanide reduction rates were about 90% inhibited by the anhydride treatment (data not shown).
Table II.
Effect of acetic anhydride treatment of pea thylakoids on PSI electron transport
| Thylakoid Sample Used | PSI Electron Transport Rate
|
|
|---|---|---|
| Basal | Uncoupled | |
| μeq (mg Chl h)−1 | ||
| Glycylglycine-quenched control-full light | 454 ± 46 (100%) | 1358 ± 22 (100%) |
| Glycylglycine-quenched control-reduced lighta | 310 ± 26 (68%) | 550 ± 6 (40%) |
| 3.5 mm Anhydride-full light | 296 ± 12 (65%) | 570 ± 72 (42%) |
Electron transport rates were measured on the same thylakoids used for the proton uptake assays depicted in experiment 1 of Table III. PSI-only electron transport (DQH2 → MV in the presence of DCMU) was performed as described in “Materials and Methods.” Results are expressed as means ± sd. n = 3 (in all cases). Nigericin (500 μm) was used to uncouple the thylakoids.
Electron transport in the anhydride-treated thylakoids was driven by saturating light. To obtain electron transport rates in the control thylakoids near the same level as in the anhydride-treated thylakoids, the light intensity was reduced.
Despite the roughly 35% decrease in the capacity for the DQH2 → MV basal electron flow caused by the anhydride treatment, the 2-fold stimulation of rate by the uncoupler indicates that the thylakoids were not made fully permeable to H+ ions by the acetic anhydride treatment. In fact, there was a large H+ uptake supported by the DQH2 → MV PSI partial reaction in the anhydride-treated thylakoids, but the H+ uptake had unexpected properties, as described next.
Acetic Anhydride Effects on Proton Uptake
The uncoupler stimulation of the DQH2 → MV partial reaction electron transport rate in the anhydride-treated thylakoids implies both that: (a) the acidic pH-sensitive step, which is known to control electron transport rates (Nishio and Whitmarsh, 1993), is still retained after the anhydride treatment; and (b) the uncoupler dissipated the acidic condition. Table III shows that H+ uptake occurred to a considerable extent in the anhydride-treated pea thylakoids, ranging from 90 to 165 nmol H+ (mg Chl)−1 in different thylakoid preparations. Similar results were obtained with anhydride-treated spinach thylakoids (Fig. 1) with the DQH2 → MV partial reaction. The anhydride treatment did not cause a significant increase in the proton efflux rate constant (Table IV), indicating that the treatment did not produce a H+ leak between the acidic compartment and the external suspension.
Table III.
Effect of acetic anhydride treatment on proton uptake in pea thylakoids in the presence and absence of HEM
| Thylakoid Sample Used | Extent of H+ Uptake
|
Increase | |
|---|---|---|---|
| −HEM | +HEM | ||
| nmol H+ (mg Chl)−1 | |||
| Experiment 1 | |||
| Glycylglycine-quenched control-reduced light | 126 ± 12 | 220 ± 12 | 94 |
| 3.5 mm Anhydride-treated | 91 ± 26 | 121 ± 14 | 30 |
| Experiment 2 | |||
| Glycylglycine-quenched control-reduced light | 185 ± 15 | 312 ± 39 | 127 |
| 3.5 mm Anhydride-treated | 165 ± 4 | 193 ± 11 | 28 |
| Thylakoids illuminated during acetic anhydride treatment | |||
| Glycylglycine-quenched control | 141 ± 8 | 310 ± 40 | 169 |
| 3.5 mm Anhydride-treated | 111 ± 11 | 228 ± 21 | 117 |
Acetic anhydride modification and proton uptake measurements were performed as described in “Materials and Methods.” Electron transport in the anhydride-treated thylakoids was driven by saturating white light. To obtain electron transport at the same level as in the anhydride-treated thylakoids, the light intensity for the control case was reduced to give rates close to those of the anhydride-treated samples. n = 3 (all cases). Experiment 1. The extent of proton uptake was measured on the same sets of thylakoids used for determination of the electron transport rates shown in Table II. Experiment 2. Thylakoids for this experiment were isolated and treated with anhydride on a different day than in experiment 1. For the “light protection” experiment the acetic anhydride and the control (glycylglycine added before the anhydride) treatments were illuminated 30 s prior to adding the reagents with 0.5 mm methyl viologen present to activate electron transport. Illumination continued during the 30-s treatment period. n = 3 (in all cases).
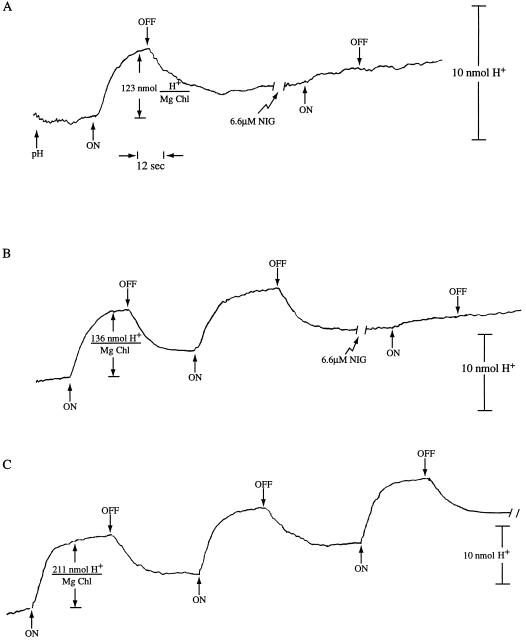
Figure 1.
Uncoupler inhibition of H+ uptake in acetic-anhydride-treated spinach thylakoids. Anhydride-treated and control thylakoids (the glycylglycine quencher added before the acetic anhydride) were prepared and proton uptake activity assayed as described in “Materials and Methods.” DQH2 → MV electron transport (with DCMU present) activated H+ pumping. A, Acetic-anhydride-treated thylakoids at 20 μg Chl mL−1 were used. A single light-dark cycle of H+ pumping was followed by the addition of 6.6 μm nigericin (NIG) and then another light-dark cycle was given. B, Acetic-anhydride-treated thylakoids as in A above were used. Two light-dark cycles were given, followed by the addition of 6.6 μm nigericin and another light-dark cycle. C, Glycylglycine-quenched control thylakoids were used at 20 μg Chl mL−1. Three light-dark cycles were given.
Table IV.
Effect of anhydride treatment on the relaxation kinetics of the proton gradient in HS-stored pea thylakoids
| Thylakoid Sample Used |
t1/2
|
|
|---|---|---|
| −HEM | +HEM | |
| s | ||
| Experiment 1 | ||
| Glycylglycine-quenched control | 5.6 ± 1.3 | 5.6 ± 1.4 |
| 3.5 mm Anhydride-treated | 5.6 ± 0.7 | 4.4 ± 1.4 |
| Experiment 2 | ||
| Glycylglycine-quenched control | 4.8 ± 0.0 | 6.8 ± 0.8 |
| 3.5 mm Anhydride-treated | 5.8 ± 0.4 | 7.4 ± 1.3 |
Despite these data, Table III indicates that the expected increase in H+ uptake due to the presence of a permeable amine (hydroxyethane morpholine, HEM) did not occur in the anhydride-treated samples. The glycylglycine-quenched thylakoids gave about a 2-fold increase in H+ uptake in the presence of HEM. Stimulation of total H+ uptake in illuminated thylakoids by weak amines is a well-known phenomenon, and it is diagnostic of luminal acidification (Nelson et al., 1971; Avron, 1972). The lack of an amine stimulation in the anhydride-treated thylakoids, given that total H+ uptake was sufficient to normally show the expected amine effect and that the dark H+ efflux first order rate constant was not increased by anhydride treatment is a remarkable and very unexpected result. To our knowledge, it is unprecedented in the chloroplast literature. This finding implies either that the anhydride treatment blocked luminal acidification but not the proton accumulation into another set of buffering groups; or that a rapid leak between the lumen and the outside was induced, with only a much slower flow of protons from the domains to the lumen. In either event, such a localized buffering array cannot be in rapid equilibrium with the lumen aqueous phase. We have previously characterized a sequestered array of low-pKa Lys groups [about 40 nmol (mg Chl)−1] and a larger amount of (likely) carboxyl groups [120–150 nmol (mg Chl)−1] (Dilley et al., 1987). The present findings support in a new way the notion that the sequestered buffering array is physically distinct from the luminal buffering groups.
The PSI-dependent H+ uptake observed in the anhydride-treated thylakoids is a typical energy-dependent active transport wherein the inward pump works against the pas-sive leak; i.e. the pH change measured by the external pH electrode cannot be attributed to a scaler H+ change unrelated to a true concentration gradient. The evidence for this, shown in Figure 1, is that the protonophore nigericin inhibited the DQH2 → MV light-dependent pH changes in spinach thylakoids (pea thylakoids gave similar results, data not shown), as is normally seen with the thylakoid H+ uptake activity. Figure 1 also shows that the DQH2 → MV H+ pump had the expected second cycle light-dark H+ uptake and release activity in the anhydride-treated sample. In all respects—except for the apparent absence of H+ ion entry into the lumen—the H+ uptake in the anhydride-treated thylakoids showed normal bioenergetic properties. Other criteria were sought to test the implication that anhydride treatment blocked luminal acidification.
Luminal Acidification as Detected by ACMA Fluorescence Quenching
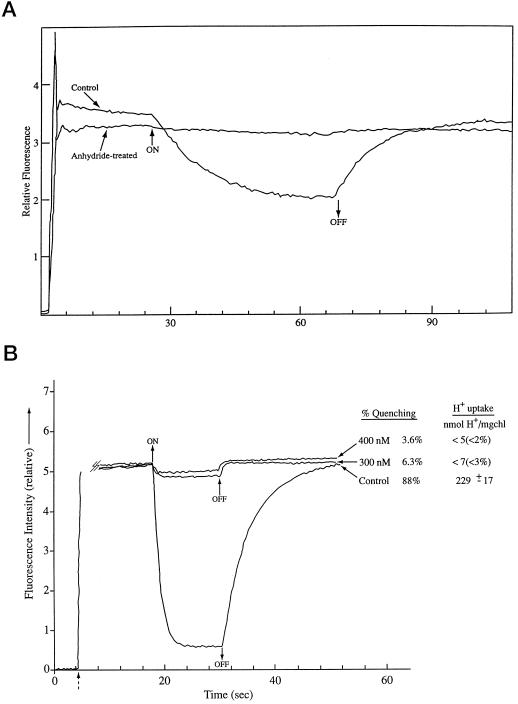
Fluorescent dyes such as ACMA and 9-aminoacridine have been widely used to estimate the ΔpH across bioenergetic membranes (Grzesiek and Dencher, 1988). These dyes give only a relative rather than an accurate ΔpH measurement (Rottenberg et al., 1972; Schuldiner et al., 1972; Benedetti and Garlaschi, 1977). Figure 2A shows that the light-induced fluorescence quenching of anhydride-treated spinach thylakoids was barely detectable compared with the signal in the glycylglycine-quenched thylakoids. One way to gain some sense of the significance of such a small quenching change is to compare it with the quenching observed in glycylglycine-quenched thylakoids in the presence of an uncoupler, and to measure concurrently the total H+ uptake. Such an experiment is shown in Figure 2B.
Figure 2.
A, ACMA fluorescence quenching in acetic-anhydride-treated and glycylglycine-quenched control spinach thylakoids. ACMA fluorescence quenching was measured under basal conditions (no ADP) as described in “Materials and Methods.” All measurements were done at 18°C. Light intensity was 40 and 180 μmol (m2 s)−1 in the control and anhydride-treated cases, respectively. The vertical line at approximately 2 s indicates the addition of the dye. B, ACMA fluorescence quenching in glycylglycine-quenched control spinach thylakoids with and without nigericin added compared with concurrent measurement of H+ uptake in parallel assays. ACMA procedures were as in Figure 1A. The dashed arrow indicates addition of the dye. For the H+ pump extent measurements, the assay conditions were the same as for the ACMA fluorescence assays except that 1 mm Tricine-KOH replaced the 50 mm Tricine-KOH to allow detection of pH changes, and 50 mm KCl was added to the pH assay mix to compensate for the lower Tricine-KOH. The photosynthetically active radiation (PAR) light intensity was measured at the rear of the cuvette compartment in the fluorimeter at full red light, and this intensity (40 μmol m−2 s−1) was reproduced at the front surface of the pH detection cuvette also using a red filter. The cuvette temperature was 18°C for both instruments. Glycylglycine (20 μg Chl mL−1)-quenched control thylakoids was used for both measurements. Duplicate measurements were made at the 300 and 400 nm nigericin levels (the same nigericin sample was used for both assays), and triplicate measurements were made for the control.
In that experiment—also using spinach thylakoids—the conditions for the ACMA quenching and for H+ uptakeusing a pH electrode were kept as closely identical as possible. Except for the ACMA measurement reaction medium with 50 mm Tricine, pH 8.0, and the pH measurement buffer with 1 mm Tricine, all other conditions were the same; light intensities at the respective cuvette surfaces were adjusted to 40 μmol (m2 s)−1, the maximum intensity achievable in the spectrofluometer cuvette holder. Corning 2–64 red filters were used, the same nigericin solution was the uncoupler source, the temperature was 18°C, the Chl concentration in the two cuvettes was 20 μg mL−1 and the measurements were done at the same time. Figure 2B shows that the 300 and 400 nm nigericin treatments inhibited the ACMA quenching 93.7% and 96.4%, respectively, in glycylglycine-quenched thylakoids. The H+ uptake measured at those nigericin concentrations was just barely detectable above the noise, <7 and <5 nmol H+ (mg Chl)−1 for the 300 and 400 nm nigericin concentrations, respectively. Those results indicate that the ACMA fluorescence quenching measured in the anhydride-treated thylakoids correspond to an extremely low H+ uptake in the compartment responsible for the acidic pH fluorescence quenching (that compartment is considered to be the lumen (Rottenberg et al., 1972; Schuldiner et al., 1972).
Violaxanthin Deepoxidase Activity as a Measure of Luminal Acidification
Violaxanthin deepoxidase activity is an intrinsic lumen pH indicator having no activity above about pH 6.3 and maximal rate at pH 5.8 (Yamamoto, 1979; Pfündel and Dilley, 1993; Günther et al., 1994; Hager and Holocher, 1994).
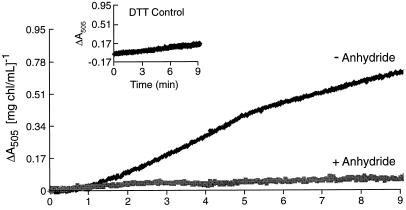
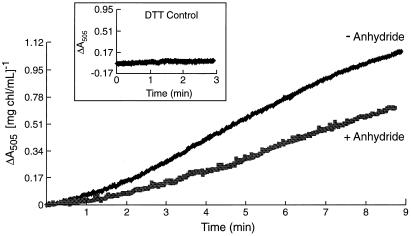
Figure 3 shows the effect of acetic anhydride treatment of spinach thylakoids on the light-dependent (external pH 7.6) ΔA505 signal. The control thylakoids (glycylglycine-quenched) showed a rate of light-dependent violaxanthin deepoxidation comparable to the rates reported by Pfündel et al. (1994) in their experiments with pea thylakoids at pH 8.0. In the experiments reported here, pea and spinach thylakoids were virtually identical in their responses to acetic anhydride treatment in the parameters of electron and proton transport, but pea thylakoids gave a smaller extent of violaxanthin deepoxidation for the untreated samples (data not shown), so spinach thylakoids were preferred. The known pH dependence of the deepoxidation reaction (Pfündel and Dilley, 1993) implies that the lumen pH fell to near or below pH 6.0 in the glycylglycine-quenched thylakoids, as expected for basal conditions. In contrast, thylakoids treated with anhydride followed by the chemical quenching and a washing step (compare with “Materials and Methods”) showed almost no change in the light-dependent ΔA505 signal. The anhydride treatment did not inhibit the enzyme activity per se, shown by the lack of inhibition when the enzyme was exposed pH 5.6 conditions in the dark. Although the VDE requires ascorbate (Siefermann and Yamamoto, 1974) and a lumen pH near or less than 6.3 for activation (Pfündel and Dilley, 1993), enzyme activity per se is not contingent upon light treatment (Günther et al., 1994). Figure 4 shows that at pH 5.6 in the dark, similar rates of violaxanthin deepoxidation occurred in both the control and anhydride-treated thylakoids (also similar to the control, light-dependent rate shown in Fig. 3), indicating that the anhydride treatment itself did not inhibit the violaxanthin deepoxidation enzyme activity (assuming that the anhydride would not selectively block enzyme action at an external pH of 7.6 while allowing activity at an external pH of 5.6). Following this logic, the availability of the substrate can likewise be presumed to be unaffected by the anhydride treatment. It is important to emphasize that in all cases, the acetic anhydride and the quenched control treatments were done at pH 8.6, the thylakoids washed free of the respective medium and prepared for either the light or dark experiments outlined above. Samples from the violaxanthin deepoxidation reactions for Figures 3 and 4 were collected, acetone extraction of lipids carried out, and xanthophylls separated isocratically by HPLC according to Pfündel and Dilley (1993). The results are shown in Figures 5 and 6 and Table I.
Figure 3.
Light-dependent deepoxidation of violaxanthin at pH 7.6 measured by ΔA505 in spinach thylakoids. Violaxanthin deepoxidation of anhydride-treated and glycylglycine-quenched spinach thylakoids was followed in the dual-wavelength mode by the ΔA505 versus ΔA560 as described in “Materials and Methods.” To obtain comparable electron transport rates in the DQH2 → MV reaction, the control thylakoids were illuminated by a reduced intensity (55 μmol m−2 s−1) and the anhydride-treated sample got the full intensity of 275 μmol m−2 s−1. Thylakoids were illuminated starting at time = 0 min and illumination continued until the end of the assay. DTT-sensitive deepoxidation was calculated from ΔA505 by subtracting the ΔA505 in the presence of DTT (the light-scattering signal) from the ΔA505 obtained in the absence of DTT. Light scattering not due to deepoxidation is shown in the inset.
Figure 4.
Ascorbate-induced deepoxidation of violaxanthin at pH 5.6 in the dark measured by ΔA505. Spinach thylakoids were from the same preparation as used for Figure 3. At t = 0, 50 mm ascorbate was added to activate the VDE. DTT-sensitive deepoxidation was calculated as described in the Figure 3 legend.
Figure 5.
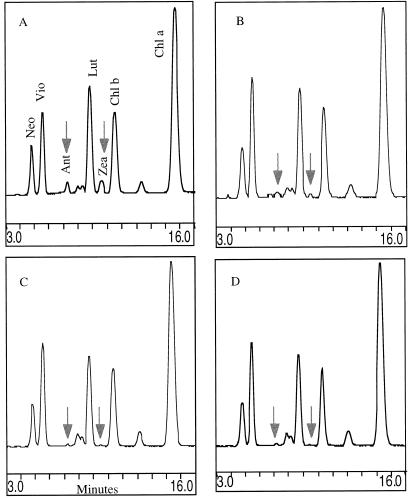
Separation of thylakoid xanthophylls by isocratic HPLC from violaxanthin deepoxidation assays performed at pH 7.6. Xanthophylls from extracts of thylakoids used in the Figure 3 experiment were separated isocratically as described in “Materials and Methods.” Chromatograms were reproduced using the “Chrom Pic” program in the Rainin Dynamax software. A, −Acetic anhydride/−DTT; B, −acetic anhydride/+DTT; C, +acetic anhydride/−DTT; D, +acetic anhydride/+DTT. Neo, Neoaxanthin; Vio, violaxanthin; Ant, anteraxanthin; Lut, lutein; Zea, zeaxanthin.
Figure 6.
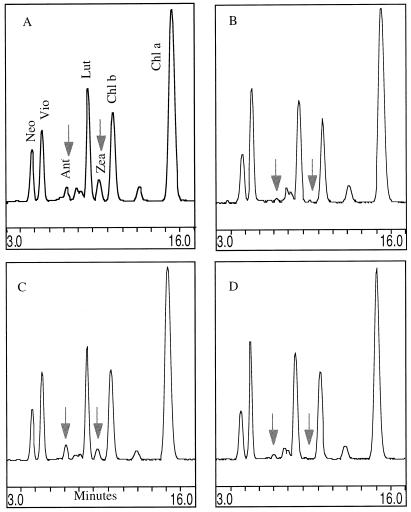
Separation of thylakoid xanthophylls by isocratic HPLC from violaxanthin deepoxidation assays performed at pH 5.6 (xanthophylls from extracts of thylakoids used in the Fig. 4 experiment). A, −Acetic anhydride/−DTT; B, −acetic anhydride/+DTT; C, +acetic anhydride/−DTT; D, +acetic anhydride/+DTT. Experimental procedure was as described in the Figure 3 legend.
Figure 5A (light-driven violaxanthin deepoxidation) shows that in the absence of DTT, the control thylakoids converted violaxanthin to antheraxanthin and zeaxanthin. The DTT control inhibited the deepoxidation of violaxanthin, as expected (Fig. 5B). Figure 5C shows that acetic anhydride treatment inhibited the deepoxidation of violaxanthin. Quantitation of the separated xanthophylls from the Figure 5 experimental treatments is shown in Table V.
Table V.
HPLC assay of xanthophyll pigments
| Treatment | DTT | V | A | Z | Sum (V + A + Z) | A + Z |
|---|---|---|---|---|---|---|
| mm | mmol (mol chl a)−1 | |||||
| Light-induced violaxanthin deepoxidation at pH 7.6 | ||||||
| Control | 0.0 | 145 ± 10 | 23 ± 4 | 34 ± 11 | 202 ± 4 | 58 ± 9 |
| Control | 5.0 | 206 ± 7 | N.D. | N.D. | 206 ± 7 | N.D. |
| 3.5 mm Anhydride | 0.0 | 198 ± 4 | N.D. | N.D. | 198 ± 4 | N.D. |
| 3.5 mm Anhydride | 5.0 | 199 ± 5 | N.D. | N.D. | 199 ± 5 | N.D. |
| Ascorbate-induced violaxanthin deepoxidation at pH 5.6 | ||||||
| Control | 0.0 | 118 ± 10 | 21 ± 4 | 55 ± 16 | 195 ± 5 | 76 ± 13 |
| Control | 5.0 | 206 ± 3 | N.D. | N.D. | 206 ± 3 | N.D. |
| 3.5 mm Anhydride | 0.0 | 145 ± 6 | 24 ± 4 | 25 ± 6 | 194 ± 4 | 50 ± 9 |
| 3.5 mm Anhydride | 5.0 | 195 ± 5 | N.D. | N.D. | 195 ± 5 | N.D. |
Xanthophyll concentration was determined from the areas under the respective peaks after isocratic separation by HPLC. Data shown is from the same samples used to generate Figures 3 and 4. Pigment concentrations are listed as mmol xanthophyll (mol Chl a)−1. Results are expressed as means ± sd. n = 3 (in all cases). V, Violaxanthin; A, antheraxanthin; Z, zeaxanthin. N.D., Not detectable.
Figure 6 (dark, pH 5.6, ascorbate-driven violaxanthin deepoxidation) shows that the acetic anhydride-treated thylakoids without DTT (Fig. 6C) converted a substantial amount of violaxanthin to antheraxanthin and zeaxanthin (in Fig. 6, compare A and C with B and D). As shown in Table V, acetic anhydride treatment did cause a decrease of violaxanthin deepoxidation (35% conversion compared to the controls) but not the total inhibition exhibited under light-driven violaxanthin deepoxidation. Therefore, the explanation for the anhydride inhibition of light-dependent VDE activity must be sought in either the inability of the co-substrate, ascorbate, to work properly or in the failure of those thylakoids to attain a sufficiently acidic lumen pH. The ascorbate entry into the lumen was not tested but it is reasonable to assume that it penetrated equally well at pH 7.6 conditions for both the control and anhydride-treated thylakoids.
Light Protection against Acetic Anhydride Inhibition
Baker et al. (1981) demonstrated that illumination of thylakoids during anhydride treatment protected against the anhydride-inhibition of PSII electron transport and decreased the derivatization of membrane protein Lys groups (measured by incorporation of radiolabel from the [3H]acetic anhydride). Their explanation for the protection of PSII activity was that the low pKa lysines in the three extrinsic proteins of the oxygen evolving complex were kept protonated by H+ ions released in the redox turnovers, making the Lys ε-amino groups less likely to react with the anhydride (Means and Feeney, 1971), thus affording protection against anhydride inhibition of PSII and giving less labeling. We employed such a “light protection” protocol in the thylakoid treatment step prior to the proton uptake assay to determine if the inhibition of luminal acidification responded in a similar way.
Table III shows that illuminating the thylakoids during the anhydride treatment did protect against the inhibitory effect of anhydride treatment on the amine-dependent component of H+ uptake into the lumen. This finding is consistent with the notion that the anhydride reaction with the critical Lys groups was largely blocked when the lysines were protonated prior to and during the treatment.
DISCUSSION
These results show the unexpected finding that acetic anhydride treatment of thylakoids—which is known to derivatize low pKa Lys groups in sequestered membrane domains (Dilley et al., 1987)—inhibits luminal acidification severely but permits a surprisingly large H+ ion accumulation into the sequestered domain buffering array, containing both the low pKa lysines and carboxyl groups. Luminal pH has been a difficult parameter to measure, but recent demonstration that the location of the VDE enzyme is restricted to the lumen space (Hager and Holocher, 1994) and that its pH optimum is near pH 5.8 (Pfündel and Dilley, 1993; Günther et al., 1994) makes this enzyme activity an excellent intrinsic lumen pH indicator. We used the VDE enzyme activity, ACMA fluorescence quenching and permeable amine effects on H+ uptake to monitor lumen acidification in the anhydride-treated and the glycylglycine-quenched control thylakoids and all three methods indicated that the lumen did not go as acidic in the anhydride-treated membranes as in the control thylakoids.
We do not have a precise value for the lumen pH, but using the known pH dependence of the VDE enzyme, we estimate that the pH did not get below about 6.6 in the anhydride-treated case (Fig. 3), but reached near pH 5.7 to 5.8 in the control thylakoids. It is important to note that the DQH2 → MV PSI partial electron transport system rates and H+ uptake into thylakoid buffering groups driven by that system were kept similar in both thylakoid samples by lowering the light intensity used in the control thylakoids. The lumen pH for the control case was estimated from the VDE activity—the intrinsic lumen pH indicator—as follows: We first compared the VDE activity at pH 5.6 in the dark for the glycylglycine-quenched control sample (Fig. 4) with the VDE rate in the light for the quenched control (Fig. 3). The rates were quite close; 0.13 ΔA(mg Chl mL)−1 min−1 for the dark, pH 5.6 case (Fig. 4) and 0.10 ΔA(mg Chl mL)−1 min−1 for the control in the light (Fig. 3). The rates are similar to those reported by Pfündel et al. (1994), whose figure 2 rate for the high salt basal case was 0.13 ΔA(mg Chl mL)−1 min−1. If we assume that the rate versus pH curve for our spinach thylakoids has a similar shape as that published for spinach (Günther et al., 1994), then there is only a 0.05 pH difference for our light-dependent VDE (Fig. 3) compared to the pH 5.6 dark sample (Fig. 4); i.e. the lumen pH in the illuminated glycylglycine-quenched control thylakoids attained a pH near 5.7. Thus, at a comparable total H+ uptake (about 100–150 nmol H+ [mg Chl]−1, Table III) there was a striking lack of H+ ions accumulated in the lumen in the anhydride-treated thylakoids.
It should be pointed out that these results support the notion, already suggested by Laasch and Weiss, (1988) that photosynthetic control—known to be an acidic pH effect on the rate of plastoquinol oxidation by the cyt b6f complex (Nishio and Whitmarsh, 1993)—is exerted in a local domain, not the lumen, although a luminal pH effect is widely, and it would now seem erroneously, assumed. The stimulation of the DQH2 → MV electron transport rate by an uncoupler in the anhydride-treated thylakoids (Table II) implies that the acidity in the vicinity of the quinol oxidation site was much more acidic than pH 6.5. This was deduced by comparison of our data to the figure 4 data of Nishio and Whitmarsh (1993) where they showed the stimulation of cyt f reduction by 2 μm nigericin; (those authors showed that only below pH 6.5 did nigericin stimulate cyt f reduction). In our anhydride-treated thylakoids, nigericin stimulated the DQH2 → MV electron transport rate about 2-fold (Table II). Relating that to the Nishio and Whitmarsh data (their figure 4) suggests that the pH in the vicinity of the rate limiting quinol oxidation should be near or less than pH 6.0. But, the VDE “pH indicator” property discussed above predicted that the lumen pH in the anhydride-treated thylakoids was close to pH 6.6. Therefore, the quinol oxidation site, and its pH sensitivity, can most simply be viewed as buried in the sequestered proton buffering domain, which can be significantly more acidic than the lumen (compare with Bamberger et al., 1973).
We need to understand how the anhydride acetylation of a group of low-pKa Lys residues, some of which are buried in sequestered domains, leads to the loss of luminal acidification, while the thylakoids maintain a quite large H+ uptake into an obviously quite acidic compartment? One possibility is that a transmembrane channel for H+ flux from the lumen to the outside becomes much more permeable. In this option, the H+ ions would first build up an acidic pool in sequestered domains and relatively slowly diffuse to the lumen, where they would rapidly leak to the outside.
Another possibility is that acetylation blocks the normal proton movement within the domains to the lumen, while allowing the localized domains to go acidic, leading to the protonation of the domain buffering groups. In this situation and in the operation of the hypothesized localized H+ gradient energy coupling model, there is the question of how the protons leak from the postulated local domains to the outside phase. Of course, during ATP formation, some portion of the accumulated domain protons (or protons from the lumen as well in the delocalized gradient mode) efflux through the CF0-CF1 complex in the energy-linked H+ efflux. But, as is well known by researchers in this area, there is a significant component of the H+ gradient—about 80 nmol H+ (mg Chl)−1 according to Hangarter and Ort (1986)—accumulated before the threshold H+ uptake energization is reached (with the electric field [Δψ] kept at zero).
After steady-state ATP formation is reached, followed by a dark period (when the Δψ is near 0), about 60 nmol H+ (mg Chl)−1 remains in the H+ gradient when ATP can no longer be formed (Gould and Izawa, 1974). These H+ accumulation levels are less than the buffering capacity of the localized domains. Therefore, in the localized coupling mode, it is our viewpoint that the efflux of 60 to 80 nmol H+ (mg Chl)−1 not attributable to ATP formation probably occurs not necessarily through the CF0-CF1 complex, but very likely the main efflux is through the background H+ leak of the thylakoid lipid bilayer. Support for this comes from the observation that the H+ efflux rate constant is not very much changed after blocking the CF0 H+ channel with dicyclohexylcarbodiimide (Bulychev et al., 1980). The (background) permeability constant (PH+) of thylakoids is surprisingly high, with some reports giving 5 × 10−5 cm s−1 (Bulychev et al., 1980) and 2 × 10−5 cm s−1 (Schönfeld and Schickler, 1984), with a somewhat greater PH+ (6 × 104−4 cm s−1) reported for isolated thylakoid lipid bilayers (Fuks and Homble, 1996). Either this background H+ permeability or a more specific H+ channel (see the comments below about the Jahns and Junge [1990] results) could shunt the domain protons to the outside when they do not go through the CF0-CF1. If this is the way the accumulated domain protons efflux back to the outside, rather than via the first possibility mentioned above, it remains a puzzle as to how the barrier to the lumen is constructed.
The localized domain model we have been testing includes the idea that the normal H+ diffusion from PSII water oxidation and the plastoquinol oxidation centers to the CF0 H+ channel utilizes a type of proton relay system involving, for example, the buried low-pKa Lys groups and perhaps other acid-base groups (carboxyls) and water associated with the buried protein surfaces (Dilley et al., 1987; Theg et al., 1988; Dilley, 1991; Renganathan and Dilley, 1994). There is a body of data (see below) supporting the notion of some type of proton accumulation and proton relay along a buried pathway involving protein -COO− groups of the LHC II proteins.
Jahns and Junge (1990) and Jahns et al., (1988) have identified buried carboxyl residues (Glu and Asp) of LHC II proteins as likely candidates for participation in a H+ ion relay system from the buried water oxidation site to the lumen. They showed that DCCD derivatization of buried carboxyl groups (DCCD only forms stable covalent adducts with carboxyl groups that are in a hydrophobic environment) blocks luminal acidification by PSII-released protons, causing them to be shunted to the external side of the membrane where the plastoquinone reduction site is located. Horton's group has also used DCCD reactivity with LHC II proteins in their studies of non-photochemical quenching of Chl fluorescence (also called high energy state or qE quenching) associated with thermal dissipation of excess absorbed light by chloroplasts (Walters et al., 1996). That group also attributes their observation of DCCD inhibition of qE quenching to blockage of a proton relay mechanism in the LHC II proteins on the luminal side of the thylakoid, resulting in less luminal acidification. Renganathan and Dilley (1994) showed that the absence of LHC II proteins in the chlorina f2 barley mutant (a mutant lacking the LHC II b and LHC II d subunits) causes the loss of the localized Δμ̃H+ gradient ATP formation allowing only the delocalized coupling mode. Those data also support the concept that LHC II proteins play a critical role in some type of proton relay through buried regions.
LHC II proteins are a major target of acetic anhydride modification (Laszlo et al., 1984) and the predicted folding of the LHC II proteins across the thylakoid membrane indicates numerous Lys residues (compare with figure 5 of Jahns and Junge, 1990) on the luminal side. The more exact LHC II structural model of Külbrandt et al. (1994) also shows four Arg and four Lys residues on the part of the LHC II protruding into the lumen. The low-pKa Lys residues identified by our pH 8.6 acetic anhydride labeling require that an Arg+ or a Lys+ cation be close to the target Lys residue to decrease the pKa by electrostatic effects, thus enhancing pH 8.6 reactivity with acetic anhydride.
The present results, together with the above-mentioned work on LHC II proteins, give supportive evidence for the existence of sequestered proton buffering domains in thylakoids. Evidence that the Lys buffering groups in the sequestered domains may be important in a proton relay mechanism feeding H+ ions into the CF0-CF1 complex comes from earlier experiments of Theg et al. (1988), who showed that in thylakoids exhibiting localized coupling, the protons involved in driving ATP formation in a train of single-turnover flashes first protonate the (previously de-protonated) low-pKa Lys residues in the domains before any are available to energize ATP formation.
The present results offer a new set of data supporting the concept that thylakoids have a sequestered proton buffering domain and a proton diffusion pathway associated with membrane proteins, which allows large-scale (up to about 150 nmol H+ [mg Chl]−1) proton buffering in a locus separate from the lumen. Yet both the lumen or the localized domain proton pools can be bioenergetically connected to the CF0CF1 ATP-forming complex (and shifting ionic conditions, particularly Ca2+ levels, can control which buffering pool is the source of protons for driving ATP formation; Dilley, 1991; Chiang et al., 1992; Wooten and Dilley, 1993). Calcium bound to the luminal side of the 8-kD CF0 subunit III (Chiang et al., 1992; Zakharov et al., 1995, 1996) can block H+ ions from the domains entering the lumen without blocking the domain Δμ̃H+ from driving ATP formation (Chiang et al., 1992).
In the reciprocal experiment using an acid-base jump for ATP formation, Ca2+ bound at the CF0 can block protons from the lumen getting out through the H+ channel (Wooten and Dilley, 1993). However, as discussed in and/or shown in the two studies referred to above, the Ca2+ bound at the gating site can be readily displaced by protons when the pH becomes acidic enough. Such a mechanism involving two different proton pools with facile Ca2+ gating of H+ fluxes into the lumen and consequent control of lumen pH may provide chloroplasts with a stress signaling system coupled to a plant's response to excess light intensity as a means of regulating the chloroplast photoprotection mechanisms (compare with Dilley, 1991; Demmig-Adams and Adams, 1992; Pfündel et al., 1994; Zakharov et al., 1995; Gilmore, 1997),
ACKNOWLEDGMENTS
The authors would like to acknowledge Runsun Pan for his excellent programming skills in data acquisition and his helpful discussions of the data, and Connie Philbrook and Wanitta Thompson for help with manuscript preparation.
Footnotes
This work was supported in part by the U.S. Department of Agriculture (grant no. 94–37100–0292 to R.A.D.).
LITERATURE CITED
- Allen JF, Horton P. Chloroplast protein phosphorylation and chlorophyll fluorescence quenching activation by tetramethyl- p-hydroquinone, an electron donor to plastoquinone. Biochim Biophys Acta. 1980;638:290–295. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidases in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M. The relation of light induced reactions of isolated chloroplasts to proton concentrations. In: Forti GM, Avron M, Melandri A, editors. Proceedings of the Second International Congress on Photosynthesis Research. Vol. 2. The Hague, The Netherlands: Dr. W Junk NV; 1972. pp. 861–871. [Google Scholar]
- Baker GM, Bhatnager D, Dilley RA. Proton release in photosynthetic water oxidation: evidence for proton movement in a restricted domain. Biochemistry. 1981;20:2307–2315. doi: 10.1021/bi00511a037. [DOI] [PubMed] [Google Scholar]
- Bamberger ES, Rottenberg H, Avron M. Internal pH, ΔpH, and the kinetics of electron transport in chloroplasts. Eur J Biochem. 1973;34:557–563. doi: 10.1111/j.1432-1033.1973.tb02795.x. [DOI] [PubMed] [Google Scholar]
- Beard WA, Dilley RA. A shift in chloroplast energy coupling by KCl from localized to bulk phase delocalized proton gradients. FEBS Lett. 1986;201:57–62. [Google Scholar]
- Beard WA, Dilley RA. ATP formation onset lag and postillumination phosphorylation initiated with single-turnover flashes. III. Characterization of the ATP formation onset lag and postillumination phosphorylation for thylakoids exhibiting localized or bulk-phase delocalized energy coupling. J Bioenerg Biomembr. 1988;20:129–154. doi: 10.1007/BF00762141. [DOI] [PubMed] [Google Scholar]
- Benedetti ED, Garlaschi FM. On the estimation of proton gradient and osmotic volume in chloroplast membranes. J Bioenerg Biomembr. 1977;9:195–201. doi: 10.1007/BF00743193. [DOI] [PubMed] [Google Scholar]
- Bulychev AA, Andrianov VK, Kurella GA. Effect of dicyclocarbodiimide on the proton conductance of thylakoid membranes in intact chloroplasts. Biochim Biophys Acta. 1980;590:300–308. doi: 10.1016/0005-2728(80)90201-7. [DOI] [PubMed] [Google Scholar]
- Chiang G, Dilley RA. Evidence for Ca++-gated proton fluxes in chloroplast thylakoid membranes: Ca++ controls a localized to delocalized proton gradient switch. Biochemistry. 1987;26:4911–4916. [Google Scholar]
- Chiang GG, Wooten DC, Dilley RA. Calcium-dependent interaction of chlorpromazine with the chloroplast 8-kilodalton CF0 protein and calcium gating of H+ fluxes between thylakoid membrane domains and the lumen. Biochemistry. 1992;31:5808–5819. doi: 10.1021/bi00140a017. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Dilley RA. Energy coupling in chloroplasts: a calcium-gated switch controls proton fluxes between localized and delocalized proton gradients. Curr Top Bioenerg. 1991;16:265–318. [Google Scholar]
- Dilley RA, Schreiber U. Correlation between membrane-localized protons and flash-driven ATP formation in chloroplast thylakoids. J Bioenerg Biomembr. 1984;16:173–193. doi: 10.1007/BF00751048. [DOI] [PubMed] [Google Scholar]
- Dilley RA, Theg SM, Beard WA. Membrane-proton interactions in chloroplast bioenergetics: Localized proton domains. Annu Rev Plant Physiol. 1987;38:348–389. [Google Scholar]
- Ferguson SJ. Fully delocalized chemiosmotic or localized proton flow pathways in energy coupling? A scrutiny of experimental evidence. Biochim Biophys Acta. 1985;811:47–95. [Google Scholar]
- Fromme P, Gräber P. Activation/inactivation and uni-site catalysis by the reconstituted ATP-synthase from chloroplasts. Biochim Biophys Acta. 1990;1016:29–42. doi: 10.1016/0005-2728(90)90003-m. [DOI] [PubMed] [Google Scholar]
- Fuks B, Homble F. Mechanism of proton permeation through chloroplast lipid membranes. Plant Physiol. 1996;112:759–766. doi: 10.1104/pp.112.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM. Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant. 1997;99:197–209. [Google Scholar]
- Gilmore AM, Yamamoto HY. Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C18 high performance liquid chromatographic column. J Chromatogr. 1991;543:137–145. [Google Scholar]
- Gould JM, Izawa S. Studies on the energy coupling sites of photophosphorylation. IV. The relation of proton fluxes to the electron transport and ATP formation associated with photosysytem II. Biochim Biophys Acta. 1974;333:509–524. doi: 10.1016/0005-2728(74)90135-2. [DOI] [PubMed] [Google Scholar]
- Grzesiek S, Dencher NA. The ΔpH-probe 9-aminoacridine: response time, binding behaviour and dimerization at the membrane. Biochim Biophys Acta. 1988;938:411–424. doi: 10.1016/0005-2736(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Günther G, Thiele A, Laasch H. A new method for the determination of the transthylakoid pH gradient in isolated chloroplasts: the pH-dependent activity of violaxanthin de-epoxidase. Plant Sci. 1994;102:19–30. [Google Scholar]
- Hager A, Holocher K. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light dependent) pH decrease. Planta. 1994;192:581–589. [Google Scholar]
- Hangarter R, Ort DR. The relationship between light-induced increases in the H+ conductivity of thylakoid membranes and activity of the coupling factor. Eur J Biochem. 1986;158:7–12. doi: 10.1111/j.1432-1033.1986.tb09713.x. [DOI] [PubMed] [Google Scholar]
- Jahns P, Junge W. Dicyclohexylcarbodiimide-binding proteins related to the short circuit of the proton-pumping activity of photosystem II identified as light-harvesting chlorophll a/b binding proteins. Eur J Biochem. 1990;193:731–736. doi: 10.1111/j.1432-1033.1990.tb19393.x. [DOI] [PubMed] [Google Scholar]
- Jahns P, Polle A, Junge W. The photosynthetic water oxidase: its proton pumping activity is short-circuited within the protein by DCCD. EMBO J. 1988;7:589–594. doi: 10.1002/j.1460-2075.1988.tb02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Külbrandt W, Wang DN, Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Laasch H, Weiss E. Differential sensitivity to dibucaine of photosynthetic control of electron transport and photophosphorylation in chloroplasts. Biochim Biophys Acta. 1988;936:99–107. [Google Scholar]
- Laasch H, Weiss E. Photosynthetic control, energy dependent quenching of chlorophyll fluorescence and photophosphorylation under influence of tertiary amines. Photosynth Res. 1989;22:137–146. doi: 10.1007/BF00035444. [DOI] [PubMed] [Google Scholar]
- Laszlo JA, Baker GA, Dilley RA. Chloroplast thylakoid membrane proteins having buried amine buffering groups. Biochim Biophys Acta. 1984;765:160–169. [Google Scholar]
- Laszlo JA, Millner PA, Dilley RA. Light-dependent chemical modification of membrane proteins with carboxyl-directed reagents. Arch Biochem Biophys. 1982;215:571–581. doi: 10.1016/0003-9861(82)90118-7. [DOI] [PubMed] [Google Scholar]
- Means GE, Feeney RE. Chemical Modification of Proteins. San Francisco: Holden-Day; 1971. [Google Scholar]
- Nelson N, Nelson H, Naim Y, Neumann J. Effect of pyridine on the light-induced pH rise and postillumination ATP synthesis in chloroplasts. Arch Biochem Biophys. 1971;145:263–267. doi: 10.1016/0003-9861(71)90035-x. [DOI] [PubMed] [Google Scholar]
- Nishio JN, Whitmarsh J. Dissipation of the proton gradient in intact chlorophasts. II. The pH gradient monitored by cytochrome f reduction kinetics. Plant Physiol. 1993;101:89–96. doi: 10.1104/pp.101.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Izawa S. Studies on the energy-coupling sites of photophosphorylation. II. Treatment of chloroplasts with NH2OH plus ethylenediaminetetracetate to inhibit water oxidation while maintaining energy-coupling efficiencies. Plant Physiol. 1973;52:595–600. doi: 10.1104/pp.52.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfündel EE, Dilley RA. The pH dependence of violaxanthin deepoxidation in isolated pea chloroplasts. Plant Physiol. 1993;101:65–71. doi: 10.1104/pp.101.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfündel EE, Renganathan M, Gilmore A, Yamamoto H, Dilley RA. Intrathylakoid pH in isolated pea chloroplasts as probed by violaxanthin deepoxidation. Plant Physiol. 1994;106:1647–1658. doi: 10.1104/pp.106.4.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Dilley RA. Evidence that the intrinsic membrane protein LNC II in thylakoids is necessary for maintaining localized ΔμH+ energy coupling. J Bioenerg Biomembr. 1994;26:117–125. doi: 10.1007/BF00763223. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Modern Cell Biology. Vol. 4. New York: Alan R. Liss; 1985. Proton-coupled energy conversion: chemiosmotic and intramembrane coupling; pp. 47–83. [Google Scholar]
- Rottenberg H, Grunwald T, Avron M. Determination of ΔpH in chloroplasts: distribution of [14C]methylamine. Eur J Biochem. 1972;25:54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Horton P. Regulation of non-photochemical quenching of chlorophyll fluorescence in plants. Aust J Plant Physiol. 1995;22:221–230. [Google Scholar]
- Schönfeld M, Schickler H. The permeability of the thylakoid membrane for protons. FEBS Lett. 1984;167:231–234. [Google Scholar]
- Schuldiner S, Rottenberg H, Avron M. Determination of ΔpH in chloroplasts: fluorescent amines as a probe for the determination of ΔpH in chloroplasts. Eur J Biochem. 1972;25:64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Siefermann D, Yamamoto HY. Light-induced de-epoxidation of violaxanthin in lettuce chloroplasts: dehydroascorbate, a link between photosynthetic electron transport and de-epoxidation. In: Avron M, editor. Proceedings of the Third International Congress on Photosynthesis. Amsterdam: Elsevier; 1974. pp. 1991–1998. [Google Scholar]
- Theg SM, Chiang G, Dilley RA. Protons in the thylakoid membrane-sequestered domains can directly pass through the coupling factor during ATP synthesis in flashing light. J Biol Chem. 1988;263:673–681. [PubMed] [Google Scholar]
- Walters RG, Ruban AV, Horton P. Identification of proton-active residues in a higher plant light-harvesting complex. Proc Natl Acad Sci USA. 1996;93:14204–14209. doi: 10.1073/pnas.93.24.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerthoff HV, Melandri BA, Venturoli G, Azoone GF, Kell DB. A minimal hypothesis for membrane-linked free-energy transduction. Biochim Biophys Acta. 1984;768:257–292. doi: 10.1016/0304-4173(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Wooten DC, Dilley RA. Calcium gating of H+ fluxes in chloroplasts affects acid-base-driven ATP formation. J Bioenerg Biomembr. 1993;25:557–567. doi: 10.1007/BF01108412. [DOI] [PubMed] [Google Scholar]
- Yamamoto HY. Biochemistry of the violaxanthin cycle in higher plants. Pure Appl Chem. 1979;51:639–648. [Google Scholar]
- Zakharov SD, Ewy R, Dilley RA. Calcium binding to the chloroplast and E. coli (CF0) F0 subunit (III) c of the ATP-synthase. Protoplasma. 1995;184:42–49. [Google Scholar]
- Zakharov SD, Li X, Red'ko T, Dilley RA. Calcium binding to the subunit c of E. coli ATP-synthase and possible functional implications in energy coupling. J Bioenerg Biomembr. 1996;28:483–494. doi: 10.1007/BF02110438. [DOI] [PubMed] [Google Scholar]