Abstract
Background:
The majority of male patients with spinal cord injury (SCI) suffer from infertility. Nucleotide-binding oligomerization domain-like receptors NOD-like receptors (NLRs) are a kind of receptors that corporate in the inflammasome complex. Recent studies have introduced the inflammasome as the responsible agent for secreting cytokines in semen. Reactive oxygen species (ROS) is one of the elements that trigger inflammasome activation. Genital infections in SCI can lead to ROS generation. We investigated the relation between lipid peroxidation and inflammasome complex activity in testicular tissue of SCI rats.
Methods:
Adult male rats (n=20), weighting 200-250 g, were included and divided into four groups: three experimental groups, including SCI1, SCI3, and SCI7, i.e. the rats were subjected to SCI procedure and sacrificed after one, three, and seven days, respectively and a control group. We performed a moderate, midline spinal contusion injury at thoracic level 10. The animals were anesthetized, and testes were collected for measurement of gene expression by real-time PCR. Caudal parts of epididymis were collected for malondialdehyde (MDA) measurement.
Results:
No NLRP1a mRNA overexpression was seen in the testes of control and SCI groups. After seven days from SCI surgery, NLRP3 mRNA expression was significantly increased in SCI7 animals (p ≤ 0.05). There was a significant difference in MDA level in SCI7 versus control group, as well as SCI1 and SCI3 animals (p ≤ 0.05).
Conclusion:
NLRP3 overexpression occurs due to the increased ROS production in testis tissue of SCI rats
Keywords: Infertility, Lipid peroxidation, Testis, Spinal cord injuries
INTRODUCTION
Every year, spinal cord injury (SCI) caused by war and accidents afflicts numerous individuals. The mean frequency of this injury is annually 10,000 persons/year in the United States[1] and approximately 2000 persons/year in Iran[2]. Most commonly, the victims include young men in the prime of their fertility, but only 10% of these patients may procreate without the help of assisted reproductive technologies. Therefore, the issue of fertility in men with SCI constitutes a major source of psychological tension and stress in these patients[3-7]. The most important causes of infertility secondary to cord injury include disorders of erection, ejaculation, and semen abnormalities[8]. Although the electroejaculator technology may overcome, to a great extent, the erectile and ejaculatory dysfunctions, semen samples collected in this way are of poor quality. Leukocytospermia, teratozoospermia, elevated number of immature sperms, poor motility, necrospermia, increased sperm DNA fragility, and high semen viscosity are common problems in these samples[9]. Moreover, DNA damage is one of the main effects of SCI on spermatozoa[10].
Different etiologies have been proposed to account for the poor quality of semen following SCI, including changes in urination pattern[11], scrotal hyper-thermia[12], hormonal disorders[13], and changes in sperm transportation and retention due to stasis[14,15]. None of these, however, have been proven conclusively[7,9].
Recent studies have indicated that increase in inflammatory cytokines, such as IL-1β and IL-18, in seminal plasma of SCI patients is toxic to sperm and affects semen quality[16]. The association between increased inflammatory cytokines in semen and poor sperm quality implicates the innate immune system and inflammasome complex as important factors contributing to infertility in SCI[17,18].
The innate immune system initiates the defense response and activates the adaptive immune system. A key element of the innate immune system is responding through NOD-like receptors (NLRs), which are able to identify noxious stimuli within cytoplasm[19-21]. NLRs contribute to the formation of a cytoplasmic complex, known as inflammasome, which is composed of a multiprotein complex to activate caspase 1 and subsequently, IL-1β and IL-18. Caspase 1 is a cysteine protease that initiates and performs pyroptosis−a form of programmed cell death[22,23]. NLRP1a was the first gene to be identified in association with this complex; nevertheless, little is known about its activators. Its best known activation element is lethal antrax toxin[23]. NLRP3 is another well-known gene of the inflammasome complex that is activated in various diseases[24-27]. It has recently been recognized as a factor contributing to testicular tissue disruption in the ischemia reperfusion model[28]. Oxidative stress in cells is a major activator of the inflammasome complex[29]. A common event following SCI is testicular and epididymal inflammation[30], which paves the way for infiltration of leukocytes or their mediators[20,31]. Leukocytes constitute the most important source of reactive oxygen species (ROS) production, in order to control infection via these biochemically active molecules[32]. On the other hand, production of a large number of ROS creates oxidative stress inside the cells, contributes to inflammasome complex activation[33].
So far, two studies have been conducted on the presence of inflammasome components in the ejaculatory fluid; their results indicated that inhibiting these components in patients with SCI improves sperm motility[17,18]. Considering the fact that inside the testes, spermatogonial stem cells are exposed to pathogens as well as to inflammatory cells and their mediators, testicular infection, and inflammation will pose a greater risk to the reproductive function, as compared to afflictions of the accessory glands[20,31]. Previous studies have paid less attention to the inflammasome complex in the testis and its activators following SCI. Regarding the crucial role of inflammation and oxidative stress in disrupting testicular function, this study aimed to investigate the expression of inflammasome complex genes in testicular tissue and its association with oxidative stress at three time points during the acute phase of injury. The findings may contribute to development of new treatment interventions for patients with SCI.
MATERIALS AND METHODS
Animal and housing conditions
In this study, we used 20 male adult Wistar rats weighing 200-250 g. In accordance with the regulations of the ethics committee of Tehran University of Medical Sciences (Iran), the animals were housed in groups of two in special cages with food and water provided ad libitum. Their environment followed standard 12-hour dark and 12-hour light cycles, without sound pollution, regularly monitored and preserved at 22 ± 2 °C. Starting on the day after surgery, the rats were administered enrofloxacin (1 mg/kg) for 10 days and dextrose serum for 7 days subcutaneously. After surgery, the bladder was emptied on a daily basis or twice daily, if necessary. Over the period of seven days, cage beddings were renewed daily, and the animals were assessed for bed sores every day.
Laminectomy and surgery for induction of SCI
The entire process of laminectomy was performed under sterile conditions. In order to develop a model of spinal cord contusion, the animal was first anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). After confirming anesthesia, the animal’s back was thoroughly shaved. Since our lab model of SCI was set at the level of T10, this vertebra was identified by palpation[34-36]. Then using a sharp scalpel, a 3-cm incision was made on the skin; connective tissue and muscles on both sides of the vertebral column were pushed aside and held in place with retractors. The lamina of T10 vertebra was removed to expose a surface proportionate to the rod of the impactor device. Subsequently, the animal was transported to the NYU MASCIS (New York University Multicenter Animal Spinal Cord Injury Study) impactor. After fixing vertebrae 9 and 11 using spinous process clamps and after confirming the alignment of the rod over exposed spinal cord, the 10-g rod was dropped from a height of 25 mm. After 1 to 3 minutes, cord contusion was prepared (Fig. 1). Once the rod is dropped, three events may confirm the development of contusion: first, bruising of the spinal without disruption of the dura at the impact site (Fig. 1), second, wagging of tail intensively, and third, bruising and flaccidity of the animal’s both plants. Finally, the animal was sutured: initially, the connective tissue was closed using 3-0 absorbable filament, and then skin was repaired with 4-0 non-absorbable filament. The animal was placed on a warm plate (37 °C) to regain consciousness. After surgery, enrofloxacin and normal saline (5 mL) were administered subcutaneously.
Fig. 1.
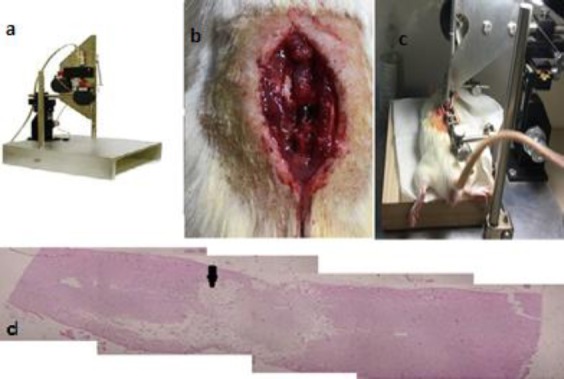
Tools needed to create a spinal cord injury model and model confirmation. (a) NYU MASCIS (New York University, Multicenter Animal Spinal Cord Injury Study) apparatus; (b) cord bruising following the lesion; (c) the animal’s tail reflex immediately after the impact of rod on spinal cord; (d) longitudinal section of the spinal cord depicting glial scar and cavity at the level of T10 and confirming the contusion/compression model.
Categorization
The rats were randomly categorized into four groups (five animals per group) as follows: SCI1, sacrificed on the day after surgery; SCI3, sacrificed three days after surgery; SCI7, sacrificed seven days after surgery; the control group which did not undergo surgery
Behavioral test
Twenty-four hours after the surgery, the animals were evaluated using the Basso, Beattie, Bresnahan Locomotor Rating Scale (BBB)[37]. Animals with grade 0 BBB results entered the study. In order to confirm the model of spinal injury, slides of T10 with Hematoxylin and Eosin (H&E) staining were prepared from some animals with grade 0 BBB.
Removing testis and epididymis
Rats were anesthetized with high-dose ketamine/xylazine. A small incision was made on the scrotum, and the epididymis was extracted and preserved in Ham’s F10 (Gibco, USA) solution in an incubator at 37 °C for subsequent studies. Next, the animal’s chest was opened, and perfusion was performed with 1000 cc normal saline containing 1 cc heparin until the testes turned completely white, and the tissue became void of blood cells. The testes were then extracted.
RNA extraction and cDNA synthesis
Total RNA was extracted from testicular fragments using TRIzol solution as described by manufacturer (Sigma-Aldrich, St Louis, MO, USA). Qualitative evaluation of the extracted RNA was performed using a NanoDrop instrument[38]. cDNA synthesis was carried out by SCRIPT cDNA synthesis kit (prime synthesis kit, TAKARA, Japan). All the steps were performed in a thermal cycler (Eppendorf, Germany), and the final product was kept at -20 °C.
Primer preparation
In this study, we used the β-actin housekeeping gene, as the internal control. Initially, the primers were designed using Perl Primer V1.1.21. The NCBI/Primer-BLAST was used to confirm their specificity for the template (Table 1)
Table 1.
Primers sequences
| Gene | Primer sequence (5’→3’) | Accession number |
|---|---|---|
| NLRP1a | Forward: CTGCCGACTAAAGACCTTGTG | NM_001145755.2 |
| Reverse: GTCTAGTTCAGCCAACCTTGAG | ||
| NLRP3 | Forward: AGCCAACCATCTCTTCTTCC |
NM_001309432.1 |
| Reverse: AAGTCATGCTGTAGCCAACC |
Real-time PCR
Real-time PCR was used for quantitative assessment of inflammasome genes. The reaction was performed using SYBR Green and at a total volume of 20 µL in each well. The reaction mixture was prepared by mixing 5 µl SYBER Green 5, 1 µl forward primer, 1 µl reverse primer, 2 µl cDNA, and 11 µl water. Then the thermal protocol was implemented as follows: first denaturation, 10 minutes at 95 °C, primer annealing and extension for each cycle, as well as fluorescence reading at the end of each cycle. Data were analyzed with the 7000 Applied Biosystem SDS software (version 201) using CTΔΔ method.
Quantifying lipid peroxidation
The concentration of epididymal sperm malondialdehyde (MDA) was measured spectro-photometrically using the thiobarbituric acid (TBA) method. Initially, the epididymis tail was placed in 0.5 mL previously warmed Ham’s F10 (Gibco, USA) solution containing FBS. The epididymis tail was perforated in several spots to allow sperms to enter the solution. Using computer-assisted sperm analysis[39], the number of sperms in each sample was determined. Then a volume was acquired to achieve the equal numbers of sperms in all samples. Also Ham’s F10 solution was added to the acquired sperm specimen to obtain a final volume of 1 mL. Subsequently, this solution was place in boiling water (95 °C) bath for 15 minutes with 2 mL TBA mixture (1 : 2) containing 15% chloroacetoacetic acid, TBA, and 0.25 normal hydrochloric acid; all ingredients were procured from Sigma Alderich, Germany. Afterwards, the solution was cooled with running water. Eventually, after centrifugation at 1789 ×g for 20 minutes, the supernatant was removed and its light absorption at 532 nm was read. MDA concentration was calculated using an extinction coefficient of 1.56 × 105 mol-1.L.cm-1 for the MDA-TBA complex and expressed in μM[38].
Light microscopy
For routine histological preparations, the tissue samples were fixed and dehydrated in ascending grades of ethanol, cleared in xylene and embedded in paraffin wax. The sections (5 µm) were cut on a microtome and stained with Harris H&E[40].
Statistical analysis
All statistical analyses were performed using SPSS software version 22. The results were expressed as means ± SEM. Parametric variables were analyzed using one-way analysis of variance (ANOVA), t-test, and Tukey’s test. Non-parametric variables were analyzed using Kruskall-Wallis test and Dunn’s multiple comparisons for post test. P values <0.05 were considered statistically significant.
RESULTS
Expression of NLRP1a and NLRP3
In this study, we assessed the expression of NLRP1a and NLRP3 genes in testis tissue. Our results indicated no significant difference in the expression of NLRP1a between the days studied (Fig. 2A). Nevertheless, the expression of NLRP3 was significantly different between the SCI7 group and the other groups (p < 0.05; Fig. 2B).
Fig. 2.
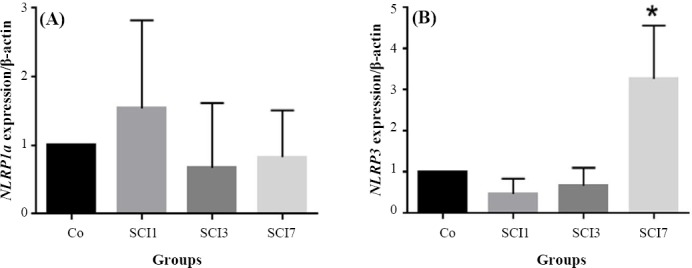
Evaluating the expression of NRPL1a and NRPL3 in rat testes after induction of SCI. Co, control group; SCI1, group sacrificed on day one after induction of SCI; SCI3, group sacrificed on day three after induction of SCI; SCI7, group sacrificed on day seven after induction of SCI. * p < 0.05 compared to the control group
MDA quantification
In this study, testicular tissue MDA was assessed and, based on the findings, a significant difference in MDA concentration between the group SCI7 and the others was observed (p < 0.05; Fig. 3). There was a significant correlation between MDA and NLRP3 expression in rat testes after the induction of SCI (p = 0.0001, r = 0.803, Fig. 4).
Fig. 3.
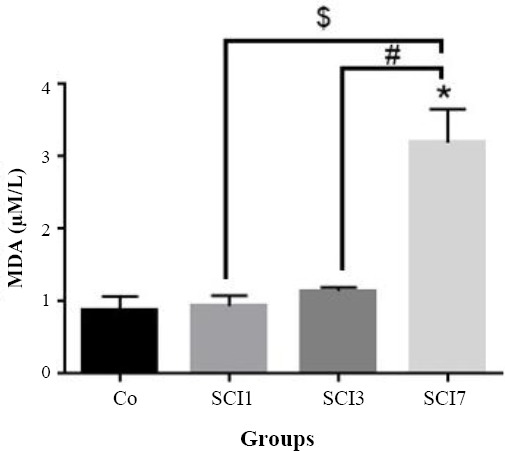
Quantification of malondialdehyde (MDA) in rat testes after induction of SCI. Co, control group; SCI1, group sacrificed on day one after induction of SCI; SCI3, group sacrificed on day three after induction of SCI; SCI7, group sacrificed on day seven after induction of SCI. * p < 0.05 compared to the control group; # p < 0.05 compared to SCI group, $ p < 0.05 compared to SCI7 group
Fig. 4.
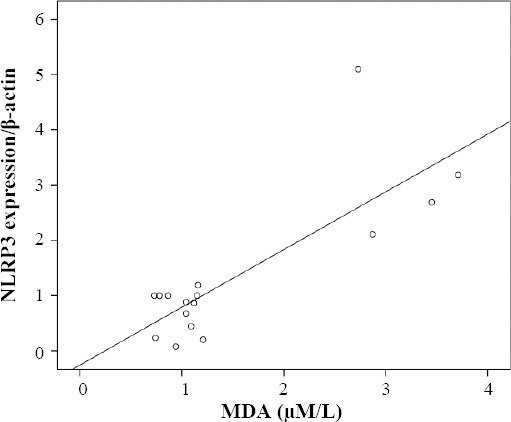
The correlation between malondialdehyde (MDA) and NLRP3 expression in rat testes after induction of SCI (p = 0.0001, r = 0.803). By increasing the MDA, the expression of NLRP3 was enhanced.
H&E staining
Based on Figure 5, there was no significant difference in spermatogenesis between the control as well as SCI1 and SCI7 groups. A moderate regression was seen in spermatogenesis in the testicular tissue of SCI7 rats.
Fig. 5.
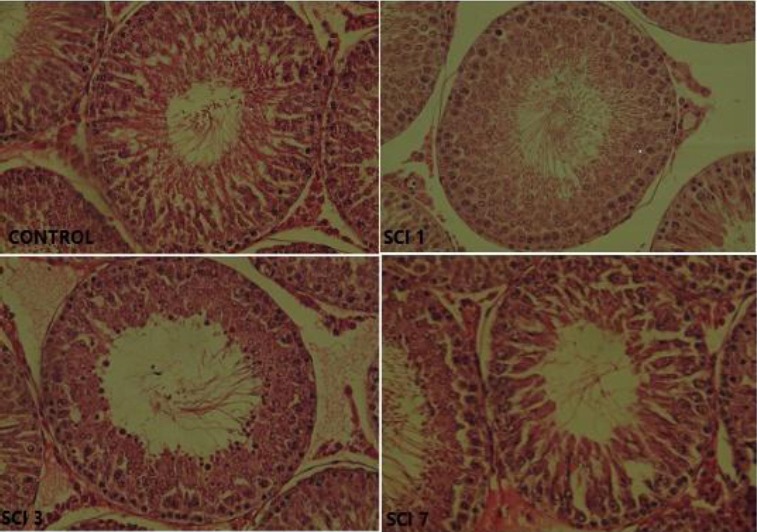
H&E staining from testis transverse sections (400× magnification). Control, control group; SCI1, group sacrificed on day one after induction of SCI; SCI3, group sacrificed on day three after induction of SCI; SCI7, group sacrificed on day seven after induction of SCI
DISCUSSION
In this study, we investigated lipid peroxidation and its role in the expression of inflammasomes complex (NLRP3 and NRPL1a genes) in male rat testicular tissue following SCI. Our results showed that seven days after SCI, the MDA concentration and NLRP3 expression level were significantly increased in parallel.
Infertility is one of the complications of SCI, caused by erection and ejaculation disorders as well as disorders of the semen[4] such as asthenospermia, necrospermia, and DNA fragmentation, which occur extensively since the first days after injury[7,32]. Different etiologies have been proposed for these disorders, including scrotum hyperthermia, hormonal deficits, disorders of the hypothalamus-pituitary axis, and disorders of the blood-testis barrier; however, none of which have been proven unequivocally.
In 1990, Hirsch et al.[41] was the first one who mentioned the crucial role of the immune system in poor sperm motility. He reported that the role of the immune system must be addressed when treating infertility in these patients. The findings of a study in 1996 indicated that following SCI, the high level of inflammatory cytokines in semen makes it toxic for sperms[42]. Ever since, activation of the immune system has been accepted as a major contributor to infertility after SCI.
In 2011, Dulin et al.[43] reported that 72 hours after SCI in rats, the integrity of the blood-testis barrier is disrupted, with a considerable increase in the apoptosis of germinal cells and IL-1β. They attributed these findings to intense immune response in the testes, although they could not provide an exact mechanism. Two recent studies have addressed an important component of the immune system, namely the inflammasome complex, as a contributing factor to infertility following SCI. In the first study, the presence of IL-1β and IL-18 in semen from patients with SCI was attributed to the activation of the inflammasome complex. In the subsequent study, some components of the complex were identified in sperms, and administering antibodies against them was reported to improve sperm motility[17,18]. These two studies failed to exactly demonstrate whether the testes or accessory glands are the source of the inflammasome complex in semen. Most investigations attribute seminal leukocytes to the prostate and seminal vesicles[44]. Therefore, it may be presumable that the abundant leukocytes present in semen after SCI might secrete the components of inflammasome into semen. The current study aimed to evaluate the expression of two genes, which are the upstream of the inflammasome complex, in testes where the sensitive spermatogonia begin the process of spermatogenesis. The findings will reveal the role of testes alone in expression of inflammasome genes and infertility following SCI.
Inflammation of the testis and epididymis is a common problem of the reproductive system after spinal cord lesions[32]. This problem will give rise to the condition in which immune cells infiltrate the testis and epididymis to produce considerable oxidative stress with high level of oxygen consumption[20,31]. In our study, we observed high levels of MDA production in epididymal sperm 72 hours and one week after
the SCI, indicating lipid peroxidation. The findings demonstrate that seven days after the cord injury, simultaneously with elevated oxidative stress, the expression of NLRP3 was significantly increased. As mentioned in a previous study, reactive ROSs constitute an activator of the inflammasome complex[45], which is corroborated by our findings. In a study conducted by Iremashvili et al.[7] in 2012, white blood cells were incriminated as the source of IL-1β in semen. On the other hand, our findings suggest that following SCI, testicular cells with activated inflammasome complex may also contribute to the elevated seminal level of IL-1β.
Numerous studies have reported extensive apoptosis of sperm and testicular cells after SCI[46,47]. Talebi et al.[10] investigated the integrity of nuclear DNA in epididymal spermatozoa following chronic SCI and showed a disruption in sperm parameters following chronic SCI in rats, including DNA integrity of sperms. It is known that the high level of unsaturated fatty acids in the cell membrane of sperms renders them susceptible to lipid peroxidation and production of MDA[4]. Combination of MDA with DNA[45] may trigger apoptosis or pyroptosis resulting from inflammasome activation, which can manifest as TUNEL-positive cells or necrospermia. As a corroboration for this assumption, the MDA produced by monosodium urate has been shown to result in DNA alterations in dendritic cells and induction of NLRP3 activation[45]. As mentioned above, activation of the inflammasome complex eventually leads to a form of programmed cell death, known as pyroptosis. Despite the difference in cell types, the findings are consistent with our results regarding the mechanism of NLRP3 activation. In 2015, a study by Minutoli[28] on a testicular ischemia/reperfusion model showed that 24 and 72 hours after surgery, the expression of downstream genes of the inflammasome complex was significantly increased in testes of mice with suppressed NLRP3 gene compared to the normal model. This is the only study to explicitly address the inflammasome complex in testis and reports the high level of ROS as the major factor in the signaling cascade, leading to the disruption of spermatogenesis and activation of the inflammasome complex. Based on the mentioned study, a significant increase in expression of downstream genes of inflammasome was observed after 24 hours of the injury, whereas our findings did not indicate any increase during the first 72 hours. This discrepancy may reflect the difference in methodologies. While the study by Minutoli[28] inflicted a direct change on the testes to develop a model of ischemia, we chose to create the lesion at the level of T10, which does not affect testicular innervation and perfusion directly. Therefore, we attempted to develop a model to study the impact of SCI on the testes in a more precise manner. In one of the first studies addressing this issue, Huang et al.[48] reported apparent abnormalities in spermatogenesis of rats within one week after SCI. In another studies, the qualitative and quantitative impairment of spermato-genesis in rats occured during the acute phase of SCI[48,49]. In addition, Choobineh et al.[28] observed that the greatest reduction in the amount of testosterone occurs during the first week after SCI. Considering these findings as well as those of Dulin et al.[43] mentioned above, we designed this study to address the acute phase during the first week after SCI.
The expression of NLPR1α was not increased significantly after SCI. Various reasons may account for this findings; for instance, the oxidative stress may not act as an inducer for this gene. Furthermore, our study only covered the acute phase of injury, and it is possible that increased expression may occur after one week.
Our study appears to be the first to address the expression of inflammasome genes in testes in a model of SCI at three time points during the acute and sub-acute phases. Further studies are required to determine exactly which cells express this gene in the testes. It seems that the concomitant expression of NLRP3 and increased production of quantifying lipid peroxidation may be considered as a key target for treating infertility in such patients. Creating a balance between oxidants and antioxidants in these patients may minimize the activity of inflammasome and might improve their fertility and the quality of their life.
ACKNOWLEDGEMENTS
This study was supported by research grant No. 29381 from Tehran University of Medical Sciences (Iran). We would like to thank Royan Institute (the Embryology Department and the Electrophysiology Laboratory) for the technical assistance.
Footnotes
CONFLICT OF INTEREST. None declared.
REFERENCES
- 1.Taylor Z, Molloy D, Hill V, Harrison K. Contribution of the assisted reproductive technologies to fertility in males suffering spinal cord injury. Australian and New Zealand journal of obstetrics and gynaecology. 1999;39(1):84–87. doi: 10.1111/j.1479-828x.1999.tb03451.x. [DOI] [PubMed] [Google Scholar]
- 2.Habibi P, Kesmani M. Sperm functional asset in spinal cord injury by electroejaculation. Medical bulletin. 1993;4:7–12. [Google Scholar]
- 3.Brown DJ, Hill ST, Baker HW. Male fertility and sexual function after spinal cord injury. Progress in brain research. 2006;152:427–439. doi: 10.1016/S0079-6123(05)52029-6. [DOI] [PubMed] [Google Scholar]
- 4.Brackett NL, Lynne CM, Ibrahim E, Ohl DA, Sonksen J. Treatment of infertility in men with spinal cord injury. Nature reviews urology. 2010;7(3):162–172. doi: 10.1038/nrurol.2010.7. [DOI] [PubMed] [Google Scholar]
- 5.Deforge D, Blackmer J, Garritty C, Yazdi F, Cronin V, Barrowman N, Fang M, Mamaladze V, Zhang L, Sampson M, Moher D. Male erectile dysfunction following spinal cord injury:a systematic review. Spinal cord. 2006;44(8):465–473. doi: 10.1038/sj.sc.3101880. [DOI] [PubMed] [Google Scholar]
- 6.Kafetsoulis A, Brackett NL, Ibrahim E, Attia GR, Lynne CM. Current trends in the treatment of infertility in men with spinal cord injury. Fertility and sterility. 2006;86(4):781–789. doi: 10.1016/j.fertnstert.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Iremashvili V, Brackett NL, Lynne CM. Impact of Spinal Cord Injury. In: Parekattil SJ, Agarwal A, editors. Male Infertility:Contemporary Clinical Approaches, Andrology, ART & Antioxidants. New York: Springer; 2012. pp. 337–348. [Google Scholar]
- 8.DeForge D, Blackmer J, Garritty C, Yazdi F, Cronin V, Barrowman N, Fang M, Mamaladze V, Zhang L, Sampson M, Moher D. Fertility following spinal cord injury:a systematic review. Spinal cord. 2005;43(12):693–703. doi: 10.1038/sj.sc.3101769. [DOI] [PubMed] [Google Scholar]
- 9.Brackett NL, Nash MS, Lynne CM. Male fertility following spinal cord injury:facts and fiction. Physical therapy. 1996;76(11):1221–1231. doi: 10.1093/ptj/76.11.1221. [DOI] [PubMed] [Google Scholar]
- 10.Talebi AR, Khalili MA, Hossaini A. Assessment of nuclear DNA integrity of epididymal spermatozoa following experimental chronic spinal cord injury in the rat. International journal of andrology. 2007;30(3):163–169. doi: 10.1111/j.1365-2605.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohl DA, Denil J, Fitzgerald-Shelton K, McCabe M, McGuire EJ, Menge AC, Randolph JF. Fertility of spinal cord injured males:effect of genitourinary infection and bladder management on results of electroejaculation. The journal of the American paraplegia society. 1992;15(2):53–59. doi: 10.1080/01952307.1992.11735862. [DOI] [PubMed] [Google Scholar]
- 12.Wang YH, Huang TS, Lin MC, Yeh CS, Lien IN. Scrotal temperature in spinal cord injury. American journal of physical medicine and rehabilitation. 1993;72(1):6–9. doi: 10.1097/00002060-199302000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Wang YH, Huang TS, Lien IN. Hormone changes in men with spinal cord injuries. American journal of physical medicine and rehabilitation. 1992;71(6):328–332. doi: 10.1097/00002060-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Beretta G, Chelo E, Zanollo A. Reproductive aspects in spinal cord injured males. Paraplegia. 1989;27(2):113–118. doi: 10.1038/sc.1989.17. [DOI] [PubMed] [Google Scholar]
- 15.Ohl DA, Menge AC, Jarow JP. Seminal vesicle aspiration in spinal cord injured men:insight into poor sperm quality. Journal of urology. 1999;162(6):2048–2051. doi: 10.1016/S0022-5347(05)68097-4. [DOI] [PubMed] [Google Scholar]
- 16.Basu S, Aballa TC, Ferrell SM, Lynne CM, Brackett NL. Inflammatory cytokine concentrations are elevated in seminal plasma of men with spinal cord injuries. Journal of andrology. 2004;25(2):250–254. doi: 10.1002/j.1939-4640.2004.tb02785.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Ibrahim E, de Rivero Vaccari JP, Lotocki G, Aballa TC, Dietrich WD, Keane RW, Lynne CM, Brackett NL. Involvement of the inflammasome in abnormal semen quality of men with spinal cord injury. Fertil steril. 2013;99(1):118–124. doi: 10.1016/j.fertnstert.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim E, Castle SM, Aballa TC, Keane RW, de Rivero Vaccari JP, Lynne CM, Brackett Nl. Neutralization of ASC improves sperm motility in men with spinal cord injury. Human reproduction. 2014;29(11):2368–2373. doi: 10.1093/humrep/deu230. [DOI] [PubMed] [Google Scholar]
- 19.Mankan AK, Kubarenko A, Hornung V. Immunology in clinic review series;focus on autoinflammatory diseases:inflammasomes:mechanisms of activation. Clinical and experimental immunology. 2012;167(3):369–381. doi: 10.1111/j.1365-2249.2011.04534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittington K, Ford WC. Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions. International journal of andrology. 1999;22(4):229–235. doi: 10.1046/j.1365-2605.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 21.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proceedings of the national academy of sciences. 2011;108(Suppl 1):4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Martinon F, Burns K, Tschopp J. The inflammasome:a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 24.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150(3):606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocasai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 27.Zendedel A, Johann S, Mehrabi S, Joghataei MT, Hassanzadeh G, Kipp M, Beyer C. Activation and regulation of NLRP3 inflammasome by intrathecal application of SDF-1a in a spinal cord injury model. Molecular neurobiology. 2016;53(5):3063–3075. doi: 10.1007/s12035-015-9203-5. [DOI] [PubMed] [Google Scholar]
- 28.Minutoli L, Antonuccio P, Irrera N, Rinaldi M, Bitto A, Marini H, Pizzino G, Romeo C, Pisani A, Santoro G, Puzzolo D, Mango C, Squadrito F, Micali A, Altavilla D. NLRP3 inflammasome involvement in the organ damage and impaired spermatogenesis induced by testicular ischemia and reperfusion in mice. Journal of pharmacology and experimental therapeutics. 2015;355(3):370–380. doi: 10.1124/jpet.115.226936. [DOI] [PubMed] [Google Scholar]
- 29.Tschopp J, Schroder K. NLRP3 inflammasome activation:The convergence of multiple signalling pathways on ROS production? Nature reviews immunology. 2010;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 30.Lim TC, Mallidis C, Hill ST, Skinner DJ, Carter PD, Brown DJ, Baker HW. A simple technique to prevent retrograde ejaculation during assisted ejaculation. Paraplegia. 1994;32(3):142–149. doi: 10.1038/sc.1994.27. [DOI] [PubMed] [Google Scholar]
- 31.Fraczek M, Kurpisz M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. Journal of andrology. 2007;28(2):325–333. doi: 10.2164/jandrol.106.001149. [DOI] [PubMed] [Google Scholar]
- 32.Patki P, Woodhouse J, Hamid R, Craggs M, Shah J. Effects of spinal cord injury on semen parameters. The journal of spinal cord medicine. 2008;31(1):27–32. doi: 10.1080/10790268.2008.11753977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. The journal of biological chemistry. 2007;282(5):2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choobineh H, Sadighi Gilani MA, Pasalar P, Jahanzad I, Ghorbani R, Hassanzadeh G. The effects of testosterone on oxidative stress markers in mice with spinal cord injuries. International journal of fertility and sterility. 2016;10(1):87–93. doi: 10.22074/ijfs.2016.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amini Pishva A, Akbari M, Farahabadi A, Arabkheradmand A, Beyer C, Dashti N, Moradi F, Hassanzadeh G. Effect of estrogen therapy on TNF-alpha and iNOS gene expression in spinal cord injury model. Acta medica Iranica. 2016;54(5):296–301. [PubMed] [Google Scholar]
- 36.Farahabadi A, Akbari M, Amini Pishva A, Zendedel A, Arabkheradmand A, Beyer C, Dashti N, Hassanzadeh G. Effect of progesterone therapy on TNF-alpha and iNOS gene expression in spinal cord injury model. Acta medica Iranica. 2016;54(6):345–351. [PubMed] [Google Scholar]
- 37.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 38.Hajiaghalou S, Ebrahimi B, Shahverdi A, Sharbatoghli M, Beigi Boroujeni N. Comparison of apoptosis pathway following the use of two protocols for vitrification of immature mouse testicular tissue. Theriogenology. 2016;86(8):2073–2082. doi: 10.1016/j.theriogenology.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Chung PH, Verkauf BS, Eichberg RD, Casady L, Sanford EJ, Maroulis GB. Electroejaculation and assisted reproductive techniques for anejaculatory infertility. Obstetrics and gynecology. 1996;87(1):22–26. doi: 10.1016/0029-7844(95)00335-5. [DOI] [PubMed] [Google Scholar]
- 40.Ramzan F, Qureshi IZ. Intraperitoneal kisspeptin-10 administration induces dose-dependent degenerative changes in maturing rat testes. Life sciences. 2011;88(5-6):246–256. doi: 10.1016/j.lfs.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Hirsch IH, Sedor J, Callahan HJ, Staas WE., Jr Systemic sperm autoimmunity in spinal-cord injured men. Archives of andrology. 1990;25(1):69–73. doi: 10.3109/01485019008987596. [DOI] [PubMed] [Google Scholar]
- 42.Brackett NL, Davi RC, Padron OF, Lynne CM. Seminal plasma of spinal cord injured men inhibits sperm motility of normal men. The Journal of urology. 1996;155(5):1632–1635. [PubMed] [Google Scholar]
- 43.Dulin JN, Moore ML, Gates KW, Queen JH, Grill RJ. Spinal cord injury causes sustained disruption of the blood-testis barrier in the rat. PloS one. 2011;6(1):e16456. doi: 10.1371/journal.pone.0016456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pentyala S, Lee J, Annam S, Alvarez J, Veerraju A, Yadlapalli N, Khan SA. Current perspectives on pyospermia:a review. Asian journal of andrology. 2007;9(5):593–600. doi: 10.1111/j.1745-7262.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 45.Licandro G, Ling Khor H, Beretta O, Lai J, Derks H, Laudisi F, Conforti-Andreoni C, Liang Qian H, Teng GC, Ricciardi-Castagnoli P, Mortellaro A. The NLRP3 inflammasome affects DNA damage responses after oxidative and genotoxic stress in dendritic cells. European journal of immunology. 2013;43(8):2126–2137. doi: 10.1002/eji.201242918. [DOI] [PubMed] [Google Scholar]
- 46.Talebi AR, Khalili MA, Vahidi S, Ghasemzadeh J, Tabibnejad N. Sperm chromatin condensation, DNA integrity, and apoptosis in men with spinal cord injury. The journal of spinal cord medicine. 2013;36(2):140–146. doi: 10.1179/2045772312Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang HF, Linsenmeyer TA, Li MT, Giglio W, Anesetti R, von Hagen J, Ottenweller JE, Serenas C, Pogach L. Acute effects of spinal cord injury on the pituitary-testicular hormone axis and Sertoli cell functions:a time course study. Journal of andrology. 1995;16(2):148–157. [PubMed] [Google Scholar]
- 48.Linsenmeyer TA, Pogach LM, Ottenweller JE, Huang HF. Spermatogenesis and the pituitary-testicular hormone axis in rats during the acute phase of spinal cord injury. The journal of urology. 1994;152(4):1302–1307. doi: 10.1016/s0022-5347(17)32572-7. [DOI] [PubMed] [Google Scholar]


