Abstract
Background:
Previous studies have suggested that BRCA1 dysregulation has been shown to have a role in triple-negative phenotypic manifestation. However, differences of BRCA1 expression, as a tumor suppressor gene, have rarely been investigated between luminal and triple-negative breast tumors. Therefore, the present study attempted to compare the BRCA1 expression in triple-negative with luminal breast tumors and its association with the clinicopathologic characteristics of patients.
Methods:
BRCA1 expression was evaluated by real-time PCR in 26 triple-negative and 27 luminal breast tumors.
Results:
The results revealed that there is a high frequency of BRCA1 underexpression in both triple-negative and luminal breast tumors. The BRCA1 underexpression was related to young age at diagnosis, lymph node involvement, and grade III tumors.
Conclusion:
The observations suggest that decreased BRCA1 expression, regardless of tumor subtype, has a general role in breast malignancy and associated with poor prognostic features in breast tumors.
Keywords: Breast cancer, BRCA1, Triple-negative breast neoplasm
INTRODUCTION
Breast cancer is the most common cancer among women worldwide[1]. In Iran, breast malignancy is the fifth most common cause of death with a fast-rising trend[2]. Breast tumors show different molecular features and can be divided into at least four main molecular subtypes: luminal A and B as well as triple-negative and HER2-overexpressing tumors[3]. Luminal tumors are estrogen receptor positive (ER+), progesterone receptor-positive (PR+), and positive or negative for human epidermal growth factor receptor 2 (HER2+ or HER2-). These tumors have a good prognosis and respond to targeted therapies such as tamoxifen. On the other hand, triple-negative tumors (ER-/PR-/HER-) show aggressive behavior and a worse prognosis in comparison with other subtypes[4].
BRCA1 is one of the genes that involves in breast cancer. The protein product of the BRCA1 gene is a 220-kD nuclear phosphoprotein with 1863 amino acids and different important cellular functions. BRCA1 protein helps to repair DNA double-strand breaks and plays a critical role in maintaining the genomic stability, cell cycle regulation, and apoptosis[5,6]. Accordingly, BRCA1 deficiency can activate the tumorogenesis process. The association between germline mutations of BRCA1 and hereditary form of breast cancers is well known[7,8].
Previous investigations have shown that the majority of BRCA1-mutated breast tumors (over 80%) are categorized as triple-negative subtype[9]. Furthermore, several studies have demonstrated that the reduced levels of BRCA1 expression, due to promoter hypermethylation or somatic mutation, may take a part in sporadic breast cancers[10-12]. Interestingly, the sporadic BRCA1-deficient breast tumors often show similar histological characteristics with the BRCA1-related hereditary breast cancers[13-15]. Therefore, it seems that the dysfunctional BRCA1 pathway has a function in the manifestation of the triple-negative phenotype in breast tumors[16-18]. However, the comparison of BRCA1 mRNA expression between different subtypes of breast tumors is rarely available. It is unclear that BRCA1 down-regulation is a prominent feature of triple-negative breast tumors, or it must be noticed as a more general molecular alteration in breast cancer regardless of tumor subtype. Therefore, the aim of the present investigation was to compare BRCA1 expression in the setting of triple-negative and luminal tumors and to study the association of BRCA1 expression with clinicopathological features in Iranian breast cancer patients.
MATERIALS AND METHODS
Patients and tissue collection
A total of 53 surgically resected breast tumors were obtained from the Iran National Tumor Bank (INTB) of the Cancer Institute at Imam Khomeini Hospital Complex, Tehran, Iran. As calibrator samples, four normal breast tissues were acquired from women who were undergoing mammoplasty. Tissues were placed in liquid nitrogen immediately after resection and stored at -80 °C for later use. None of the patients were under chemotherapy or radiotherapy before surgery. Clinico-pathological features of the patients (age, tumor size, ER/PR/HER2 status based on immunohistochemistry results, axillary lymph node involvement, and grade) were collected from their medical records in INTB. Informed consents were obtained from all participants, and the study was approved by the local ethical committee at Tehran University of Medical Sciences, Iran.
RNA extraction and cDNA synthesis
Total RNA was isolated from tissues using Hybrid-R™ kit from GeneAll Biotechnology Company (Korea) according to the manufacturer’s instructions. The purity and quantity of extracted RNA were checked by NanoDrop 2000 Spectrophotometer (Thermo Scientific, USA). Hyperscript™ kit (GeneAll Biotechnology Co., Korea) was applied to synthesize first-strand cDNA.
Gene expression study
Real-time quantitative RT-PCR of the BRCA1 gene was performed using RealQ Plus 2× Master Mix Green (Ampliqon, Denmark) following the manufacturer’s instructions. The primers sequence for the BRCA1 mRNA expression assay were: forward 5´-CCCTCAA GGAACCAGGGATG-3´ and reverse 5´-GCTGCA CGCTTCTCAGTGGT-3´. BRCA1 expression levels were normalized against PUM1 (Pumilio RNA-binding family member 1), as a housekeeping gene. The primers for PUM1 mRNA expression assay were: forward 5´-AGTGGGGGACTAGGCGTTAG-3´ and reverse 5´-GTTTTCATCACTGTCTGCATCC-3´.
The real-time PCR reaction mix consisted of 10 μL SYBR Green master mix, 0.5 μL of each forward and reverse primers (primer concentration: 5 pmol), 1 μL target cDNA, and 8 μL sterile water in a total volume of 20 μL. The PCR conditions were as follows: initial denaturation at 95 °C for 15 minutes, followed by 40 cycles of 95 °C for 15 seconds, and 59 °C for 60 seconds. Four normal breast tissues were used as the calibrator for obtaining relative expression between breast tumors and normal breast tissues (2-ΔΔCT method)[19]. As the range of BRCA1 expression values in four normal breast tissues was 0.51 to 2.38, the values of ≥2.5 and ≤0.4 were considered overexpression and underexpression status, respectively, in breast tumors.
Statistical analysis
Statistical calculations were performed using SPSS 21 statistical software. Data were presented with mean and 95% CI for numerical data or percentage for qualitative data. Student’s t-test was performed to analyzedifference in BRCA1 expression between luminal and triple-negative tumors. The relationship between BRCA1 relative expression and clinicopathologic factors were assessed by t-test or ANOVA and alternative non-parametric tests. Differences were considered significant when p < 0.05 was obtained.
RESULTS
Clinicopathological characteristics
In a total of 53 samples, 26 and 27 breast cancer patients were classified as triple-negative and luminal (types A or B) subtypes, respectively. The tumors were divided into different groups according to age (<50 years: 31 tumors; ≥ 50 years: 22 tumors), size (≤2 cm: 3 tumors; 2-5 cm: 44 tumors; >5 cm: 6 tumors), grade (I, II: 26 tumors; III: 27 tumors), and nodal status (positive: 19 tumors; negative: 34 tumors).
Expression status of BRCA1 mRNA in breast tumors
As the expression of BRCA1 mRNA values for all four normal breast samples were between 0.51-2.38, values of ≥2.5 were considered as the overexpression t status and those of ≤0.4 as underexpression status in breast tumors. The frequency of different statuses of BRCA1 mRNA expression in luminal and triple-negative tumors is indicated in Table 1.
Table 1.
BRCA1 mRNA expression in breast tumors
| BRCA1 mRNA expression status | Total (%) (n = 53) |
Triple-negative tumors (%) (n = 26) |
Luminal tumors (%) (n = 27) |
|---|---|---|---|
| Underexpression | 67.9 | 73.1 | 63.0 |
| Normal expression | 28.3 | 26.9 | 29.6 |
| Overexpression | 3.8 | 0.0 | 7.4 |
Comparison of BRCA1 expression between triple-negative and luminal subtypes
Independent samples t-test showed that the means of BRCA1 mRNA relative expression were not significantly different (p = 0.065) between luminal and triple-negative subtypes (Fig. 1). Also, in triple-negative tumors, BRCA1 relative expression (mean mean ± SD: 0.26 ± 0.32 showed a decreased level in comparison with luminal tumors (mean ± SD: 1.3 ± 1.5).
Fig. 1.
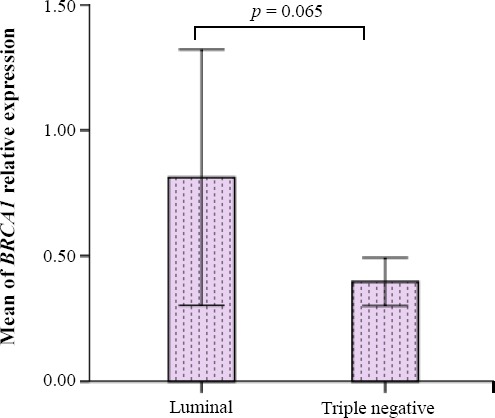
Differences in theexpression of BRCA1 in luminal and triple-negative tumors. Data were normalized to Pumilio RNA-binding family member 1expression, as a housekeeping gene (error bars: 95% CI).
BRCA1 mRNA expression and its clinico-pathological significance
Independent samples t-test in 53 breast tumors indicated that decreased expression of BRCA1 significantly related to young age at diagnosis (< 50 years, p = 0.028), lymph node involvement (p = 0.04), and grade III (p = 0.04) in breast tumor samples, but it did not significantly associate with tumor size and ER/PR/HER2 status of the studied population.
DISCUSSION
The dysfunctional BRCA1 pathway is involved in the pathogenesis of both hereditary and sporadic breast cancers. The BRCA1-related hereditary breast cancers show a trend toward triple-negative phenotype[9]. Furthermore, decreased BRCA1 expression, due to promoter hypermethylation or somatic mutations, have been reported in sporadic breast cancers, regardless of breast tumor subtypes[11,12]. As BRCA1-deficient sporadic triple-negative tumors show the same histological characteristics as BRCA1-related hereditary breast cancers[13,15], it has been suggested that BRCA1-deficiency has a role in inducing the triple-negative phenotype. However, the difference in BRCA1 expression levels, based on the tumor subtypes, has rarely been reported. Accordingly, in the current study, the BRCA1 mRNA expression was compared in the setting of triple-negative and luminal tumors, and clinicopathological significance of BRCA1 expression was evaluated in Iranian breast cancer patients.
The results of this study demonstrated that the BRCA1 underexpression is slightly different between triple-negative and luminal tumors (73.1% and 63%, respectively). This observation suggests that decreased BRCA1 expression is frequent not only in triple-negative but also in luminal breast cancer tumors. Consequently, BRCA1 deficiency has possibly a key role in breast malignancy process, apart from tumor subtypes. Interestingly, in our studied patients, BRCA1 overexpression was observed in two luminal tumors, which belonged to patients with older age at diagnosis (60 and 81 years) and low-grade breast tumors.
Our study revealed that BRCA1 expression is not significantly different between triple-negative and luminal tumors, though triple-negative tumors overally show a trend to more decrease in BRCA1 expression as compared to luminal tumors (p = 0.065). An investigation on Japanese patients indicated that BRCA1 mRNA expression is significantly decreased in triple-negative rather than luminal tumors[20].
In this study, decreased expression of BRCA1 significantly associated with young age at diagnosis, high grade, and lymph node-positive tumors. It seems that the decrease in BRCA1 expression, whether due to germline mutations in hereditary breast cancers or hypermethylation in sporadic breast cancers, could increase the risk of breast cancer in women at younger ages[21,22]. In several previous studies, it has been demonstrated that the lower level of BRCA1 expression, as a tumor suppressor gene, was associated with poor prognostic features[21-25]. On the other hand, some studies did not find any association between BRCA1 mRNA expression and clinicopathological characteristics[11,26-28], suggesting a more complex molecular association. For instance, Egawa et al.[26] suggested that decreased BRCA1 expression alone might not be enough for the development of poor prognostic features, and additional genetic alterations such as p53 abnormality might be necessary.
In conclusion, in the present study, the decreased levels of BRCA1 mRNA expression in the majority of triple-negative and luminal tumors compared to normal breast tissues indicates the involvement of BRCA1 even in luminal subtype, though down-regulation of BRCA1 expression was more remarkable in triple-negative tumors.
ACKNOWLEDGMENTS
This study was a part of a M.Sc thesis supported by Tehran University of Medical Sciences (Tehran, Iran; grant No. 30182). Tumor samples and their clinico-pathological data were provided by the Iran National Tumor Bank, which is funded by the Cancer Institute of Tehran University, for cancer research.
Footnotes
CONFLICT OF INTEREST. None declared.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Rafiemanesh H, Salehiniya H, Lotfi Z. Breast cancer in Iranian woman:Incidence by age group, morphology and trends. Asian Pacific journal of cancer prevention. 2016;17(3):1393–1397. doi: 10.7314/apjcp.2016.17.3.1393. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proceedings of the national academy of sciences. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blows FM1, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkilä P, Heikkinen T, Nevanlinna H, Akslen LA, Bégin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, García-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival:a collaborative analysis of data for 10,159 cases from 12 studies. PLoS medicine. 2010;7(5):e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson JW. BRCA1:a review of structure and putative functions. Disease markers. 1998;13(4):261–274. doi: 10.1155/1998/298530. [DOI] [PubMed] [Google Scholar]
- 6.Deng CX. BRCA1:cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic acids research. 2006;34(5):1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krainer M, Silva-Arrieta S, FitzGerald MG, Shimada A, Ishioka C, Kanamaru R, MacDonald DJ, Unsal H, Finkelstein DM, Bowcock A, Isselbacher KJ, Haber DA. Differential contributions of BRCA1 and BRCA2 to early-onset breast cancer. New England journal of medicine. 1997;336(20):1416–1422. doi: 10.1056/NEJM199705153362003. [DOI] [PubMed] [Google Scholar]
- 8.Szabo CI, King M-C. Population genetics of BRCA1 and BRCA2. American journal of human genetics. 1997;60(5):10–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Lakhani SR, van de Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Osin P, McGuffog L, Easton D. The pathology of familial breast cancer:predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. Journal of clinical oncology. 2002;20(9):2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Signori E, Bagni C, Papa S, Primerano B, Rinaldi M, Amaldi F, Fazio VM. A somatic mutation in the 5'UTR of BRCA1 gene in sporadic breast cancer causes down-modulation of translation efficiency. Oncogene. 2001;20(33):4596–4600. doi: 10.1038/sj.onc.1204620. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Wei W, Jiang Y, Yang H, Liu J. Promoter methylation and expression changes of BRCA1 in cancerous tissues of patients with sporadic breast cancer. Oncology letters. 2015;9(4):1807–1813. doi: 10.3892/ol.2015.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saelee P, Chaiwerawattana A, Ogawa K, Cho YM, Tiwawech D, Suktangman V. Clinicopathological significance of BRCA1 promoter hypermethylation in Thai breast cancer patients. Asian Pacific journal of cancer prevention. 2014;15(24):10585–10589. doi: 10.7314/apjcp.2014.15.24.10585. [DOI] [PubMed] [Google Scholar]
- 13.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, Tutt AN. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26(14):2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 14.Joosse SA, Brandwijk KI, Mulder L, Wesseling J, Hannemann J, Nederlof PM. Genomic signature of BRCA1 deficiency in sporadic basal-like breast tumors. Genes, chromosomes and cancer. 2011;50(2):71–81. doi: 10.1002/gcc.20833. [DOI] [PubMed] [Google Scholar]
- 15.Lips EH, Mulder L, Oonk A, van der Kolk LE, Hogervorst FB, Imholz AL, Wesseling J, Rodenhuis S, Nederlof PM. Triple-negative breast cancer:BRCAness and concordance of clinical features with BRCA1-mutation carriers. British journal of cancer. 2013;108(10):2172–2177. doi: 10.1038/bjc.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner N, Tutt A, Ashworth A. Hallmarks of'BRCAness'in sporadic cancers. Nature reviews cancer. 2004;4(10):814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 17.Foulkes WD, Stefansson IM, Chappuis PO, Bégin LR, Goffin JR, Wong N, Trudel M, Akslen LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. Journal of the national cancer institute. 2003;95(19):1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Fang C, Xu X, Li A, Cai Q, Long X. Androgen receptor, EGFR, and BRCA1 as biomarkers in triple-negative breast cancer:a meta-analysis. Biomed research international. 2015;2015:357485. doi: 10.1155/2015/357485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Toyama T, Yamashita H, Kondo N, Okuda K, Takahashi S, Sasaki H, Sugiura H, Iwase H, Fujii Y. Frequently increased epidermal growth factor receptor (EGFR) copy numbers and decreased BRCA1 mRNA expression in Japanese triple-negative breast cancers. BMC cancer. 2008;8:309. doi: 10.1186/1471-2407-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast cancer research. 2006;8(4):R38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben Gacem R, Hachana M, Ziadi S, Amara K, Ksia F, Mokni M, Trimeche M. Contribution of epigenetic alteration of BRCA1 and BRCA2 genes in breast carcinomas in Tunisian patients. Cancer epidemiology. 2012;36(2):190–197. doi: 10.1016/j.canep.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Rakha EA, El-Sheikh SE, Kandil MA, El-Sayed ME, Green AR, Ellis IO. Expression of BRCA1 protein in breast cancer and its prognostic significance. Human pathology. 2008;39(6):857–865. doi: 10.1016/j.humpath.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Hedau S, Batra M, Singh UR, Bharti AC, Ray A, Das BC. Expression of BRCA1 and BRCA2 proteins and their correlation with clinical staging in breast cancer. Journal of cancer research and therapeutics. 2015;11(1):158–163. doi: 10.4103/0973-1482.140985. [DOI] [PubMed] [Google Scholar]
- 25.Ansquer Y, Mandelbrot L, Lehy T, Salomon L, Dhainaut C, Madelenat P, Feldmann G, Walker F. Expression of BRCA1, HER-1 (EGFR) and HER-2 in sporadic breast cancer and relationships to other clinicopathological prognostic features. Anticancer research. 2005;25(6C):4535–4541. [PubMed] [Google Scholar]
- 26.Egawa C, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Quantitative analysis of BRCA1 and BRCA2 mRNA expression in sporadic breast carcinomas and its relationship with clinicopathological characteristics. Japanese journal of cancer research. 2001;92(6):624–630. doi: 10.1111/j.1349-7006.2001.tb01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matros E, Wang ZC, Lodeiro G, Miron A, Iglehart JD, Richardson AL. BRCA1 promoter methylation in sporadic breast tumors:relationship to gene expression profiles. Breast cancer research and treatment. 2005;91(2):179–186. doi: 10.1007/s10549-004-7603-8. [DOI] [PubMed] [Google Scholar]
- 28.Alkam Y, Mitomi H, Nakai K, Himuro T, Saito T, Takahashi M, Atsushi Arakawa Yao T, Saito M. Protein expression and methylation of DNA repair genes hMLH1, hMSH2, MGMT and BRCA1 and their correlation with clinicopathological parameters and prognosis in basal-like breast cancer. Histopathology. 2013;63(5):713–725. doi: 10.1111/his.12220. [DOI] [PubMed] [Google Scholar]


