Abstract
Background
Anti-angiogenesis Tyrosine kinase inhibitors (TKIs) have been proved to show promising effects on prolonging progression-free survival (PFS) for advanced sarcoma after failure of standard multimodal Therapy. Methylsulfonic apatinib is one of those TKIs which specifically inhibits VEGFR-2. This paper summarizes the experience of three Peking University affiliated hospitals in off-label use of apatinib in the treatment of extensively pre-treated sarcoma.
Methods
We retrospectively analysed files of patients with advanced sarcoma not amenable to curative treatment, who were receiving an apatinib-containing regimen between June 1, 2015 and December 1, 2016. Fifty-six patients were included: 22 osteosarcoma, 10 Ewing’s sarcoma, 3 chondrosarcoma and 21 soft tissue sarcoma.
Results
With median follow-up time of 6 months (range, 0.7–18.0 m), thirty-five (62.5%) patients had partial response, and disease was stable in 11 (19.6%). The 4-month and 6-month progression-free survival rates were 46.3 and 36.5%, respectively. The median duration of response was 3.8 months (95% CI 1.9–5.6 m), with much variability among disease subtypes. The median overall survival was 9.9 months (95% CI 7.6–12.2 m). Grade 3 and 4 toxicities were observed in 8 (14.3%) patients, the most common being hypertension, pneumothorax, wound-healing problems, anorexia, and rash or desquamation.
Conclusions
Apatinib might be effective, with a high objective response rate, in an off-label study of sarcoma patients with advanced, previously treated disease. The duration of response was consistent with reports in different subtypes of sarcomas. Prospective trials of apatinib in the treatment of selected subtypes of sarcomas are needed.
Trial registration
Retrospectively registered in the Medical Ethics Committee of Peking University People’s Hospital, Peking University Shougang Hospital and Peking University International Hospital. The trial registration number is 2017PHB176–03 and the date of registration is January 20th 2017.
Keywords: Apatinib, Tyrosine-kinase inhibitor, Osteosarcoma, Chondrosarcoma, Soft-tissue sarcoma, Ewing sarcoma
Background
Sarcomas are a rare, heterogeneous family of mesenchymal tumors, consisting mostly of bone tumors and soft tissue sarcoma (STS) [1, 2]. Use of traditional chemotherapeutic treatments has been limited by poor response rates in patients with relapsed or advanced disease. Nowadays more and more attention are paid to anti-angiogenesis tyrosine kinase inhibitors (TKIs), especially in the field of advanced osteosarcoma and soft tissue sarcoma. However, no or little progress has been made in treatment of these tumors since Grignani et al. [3] reported landmark phase II cohort trials of sorafenib or sorafenib combined with everolimus [4] in advanced refractory osteosarcoma.. PALETTE study proved that pazopanib could obviously prolong the progression-free survival (PFS) by 3 months but the partial response rate was only 6%. [5]
China has many patients with advanced sarcoma who need to be treated and managed properly. However, the country lacks resources necessary for participation in large multi-center trials. Thus, based on the results of prospective trials abroad, patients with advanced and refractory metastatic disease here are often treated with apatinib off-label, which is also an anti-angiogenesis TKIs domestically made and highly selectively inhibitor on VEGFR-2.. The IC50 of apatinib is 2 nM for VEGFR-2, 70 nM for VEGFR-1, 420 nM for c-kit and 537 nM for PDGFR-β [6, 7].
This report aims to describe objectively the use, efficacy, and safety of apatinib in advanced sarcoma patients who have been previously treated in the orthopedic oncology departments of three affiliated hospitals of Peking University,in China: Peking University People’s Hospital, Peking University Shougang Hospital, and Peking University International Hospital. A determination will be made whether apatinib warrants further investigations for sarcoma patients.
Methods
From June 1st 2015 to December 1st 2016, patients who met the following criteria were included: 1) histologically confirmed high-grade sarcoma; 2) initial treatment in the orthopedic oncology departments of the three affiliated hospitals of Peking University; 3) tumors not amenable to curative treatment or inclusion in clinical trials; 4) unresectable local advanced lesions or multiple metastatic lesions that could not be cured by local therapy; 5) measurable lesions according to Response Evaluation Criteria for Solid Tumors (RECIST1.1) [8]; 6) Eastern Cooperative Oncology Group performance status 0 or 1 [9]; and 7) acceptable hematologic, hepatic, and renal function.
All patients or children’s legal parent had ever signed informed consent for data collection and use for research purpose. The study was approved by the Institutional Review Board of Peking University People’s Hospital, Peking University Shougang Hospital, and Peking University International Hospital Ethics Committee for Clinical Investigation.
Because of various characteristics of diseases, we usually gave patients the following treatment before apatinib. Osteosarcoma patients usually progressed through four drugs, including doxorubicin, cisplatin, high-dose methotrexate, ifosfamide Ewing’s sarcoma patients usually progressed through at least two lines of chemotherapy, including VDC-IE (vincristine, doxorubicin, cyclophosphamide sequenced with ifosfamide and etoposide) and VTI (vincristine, temozolomide, irinotecan). For soft tissue sarcoma, patients usually progressed through at least doxorubicin and ifosfamide. But sometimes apatinib together with GT, which was gemcitabine 1000 mg/m2 d1,8 and docetaxel 75 mg/m2 d8 once every 21 day, were given to some initial ASPS and epithelioid sarcoma patients because of their poor response to conventional chemotherapy (Tables 1 and 3).
Table 1.
population characteristics
| Characteristics | Number of patients | Percentage & range | P (Cox analysis for PFS) |
|---|---|---|---|
| Gender | 56 | 100% | 0.050 |
| Male | 33 | 58.9% | |
| Female | 23 | 41.1% | |
| Age at diagnosis | 0.982 | ||
| Median (min–max) (year) | 24.5 | 9–63 | |
| Pathological diagnosis | 56 | 100% | 0.087 |
| Osteosarcoma | 22 | 39.3% | |
| Ewing sarcoma | 10 | 17.9% | |
| Synovial sarcoma | 6 | 10.7% | |
| MPNSTa | 3 | 5.4% | |
| Epithelioid sarcoma | 2 | 3.6% | |
| UPSb | 4 | 7.1% | |
| Fibrosarcoma | 1 | 1.8% | |
| Chondrosarcoma | 3 | 5.4% | |
| ASPSc | 3 | 5.4% | |
| Othersd | 2 | 3.6% | |
| Tumor grade | |||
| Grade III | 56 | 100% | |
| Location of primary disease | 56 | 100% | 0.374 |
| Axial skeleton | 17 | 30.3% | |
| Extremities | 37 | 66.1% | |
| Otherse | 2 | 3.6% | |
| Localization of relapse | 56 | 100% | 0.541 |
| Localized | 3 | 5.6% | |
| Metastatic | 41 | 73.2% | |
| Both | 12 | 21.4% | |
| Type of metastasis | 53 | 94.6% | 0.197 |
| Lung only | 40 | 71.4% | |
| Bone only | 3 | 5.4% | |
| Both | 5 | 8.9% | |
| Othere | 5 | 8.9% | |
| Time interval from initial chemotherapy to using apatinib | 0.584 | ||
| Median (min–max) (month) | 15.6 | 0.9–373.9 | |
| Number of previous treatment lines | 56 | 100% | 0.231 |
| 0 | 5 | 8.9% | |
| 1 | 37 | 66.1% | |
| 2 | 12 | 21.4% | |
| > 2 | 2 | 3.6% | |
| Follow-up time | |||
| Median (min–max) (month) | 6.0 | 0.7–18.0 | |
aMPNST: malignant peripheral nerve sheath tumor
bUPS: undifferentiated pleomorphic sarcoma
cASPS: alveolar soft part sarcoma
dothers including extraskeletal osteosarcoma one case and mucinous type liposarcoma one case
eothers including mediastinum and soft tissue of the backside
fothers including lymph nodes metastasis or intravenous tumor emboli as well as liver, brain metastasis
Table 3.
Different disease and duration of response
| Pathological diagnosis | Target therapy protocol | Patients number (N) | Best responsea | Median (average) DR (months) |
|---|---|---|---|---|
| Osteosarcoma | Apatinib alone | 22 | PR | 3.1 (3.7) |
| Ewing sarcoma | Apatinib + everolimus & apatinib alone | 10 | PR | 1.5 (3.3) |
| Synovial sarcoma | Apatinib alone | 6 | PR | 5.2 (5.8) |
| MPNSTc | Apatinib alone | 3 | PR | 8.8 (10.1) |
| Epithelioid sarcoma | Apatinib + GTb | 2 | PR | (4.7) |
| UPSd | Apatinib alone | 4 | PR | 5.6 (5.0) |
| Fibrosarcoma | Apatinib alone | 1 | PR | 2.7 |
| Chondrosarcoma | Apatinib alone | 3 | PR | (7.4) |
| ASPSe | Apatinib + GTb | 3 | PR | (7.4) |
| Extraskeletal osteosarcoma | Apatinib alone | 1 | SD | 6.6 |
| Mucinous type liposarcoma | Apatinib alone | 1 | PD | 1.0 |
aPR partial response, SD stable disease according to RECIST 1.1
bGT chemo-protocol combined with gemcitabine 1000 mg/m2 d1,8 and docetaxel 75 mg/m2 d8
cMPNST malignant peripheral nerve sheath tumor
dUPS undifferentiated pleomorphic sarcoma
eASPS alveolar soft part sarcoma
In the phase I trial, apatinib (Jiangsu Hengrui Medicine, Lianyungang, China) had good oral bioavailability at a dose of 850 mg a day, the maximum-tolerated dose [10]. Our patients were mostly given 750 mg apatinib orally once daily for body surface area (BSA) > 1.5, and 500 mg daily for BSA < 1.5. If the patient was less than 10 years of age, we usually used 250 mg directly. If treatment interruptions occurred because of grade 3 hematologic or grade 2 non-hematologic toxicities, doses were reduced, and supportive care was given for the management of adverse events (AEs).
The primary objective of this study was to summarize our experience on the efficacy of off-label use of apatinib in sarcoma patients. Our main concern was the objective response rate (CR + PR) and progression-free-survival (PFS) for each protocol as described containing apatinib according to RECIST 1.1. Together with that, overall survival (OS), duration of response (DR) and the characterization of toxicities were also described. In our retrospective study, PFS was defined as time from the start of using apatinib until disease progression or death, whichever occurred first. The time from appearance of response or stable disease to progression or death was thus considered the DR.
PFS and OS were estimated by use of the Kaplan Meier method, with 95% confidence interval (CI), and comparisons were made with a log-rank test in the IBM SPSS 22.0 software. Safety evaluation was based on the frequency and severity of toxicities, graded according to the Common Terminology Criteria for Adverse Events [11]. Quantitative variables and categorical variables were analyzed with Cox univariate analysis. All statistical analyses were two-sided, and significance was set at P < 0.05 or at the 95% CI for the results of statistical tests. The database was locked for statistical analysis in January 2017, and this is a descriptive analysis.
Results
Patients’ characteristics
From June 1st 2015 to December 1st 2016, 63 consecutive advanced sarcoma patients were registered. Median follow-up time was 6.0 months (range, 0.7–18.0 m). Five patients were lost to follow up; 1 patient stopped using apatinib because of toxicity and another dropped out for another reason. Finally, 56 patients were enrolled: 22 osteosarcoma, 10 Ewing’s sarcoma, 6 synovial sarcoma, 3 malignant peripheral nerve sheath tumors (MPNST), 2 epithelioid sarcoma, 4 undifferentiated pleomorphic sarcoma (UPS), 1 fibrosarcoma, 3 chondrosarcoma, 3 alveolar soft part sarcoma (ASPS), 1 extraskeletal osteosarcoma, and 1 mucinous-type liposarcoma (Table 1). Seventeen (30.3%) of the sarcomas originated primarily from the axial skeleton; 37 (66.1%) from extremities; 2 (3.5%) were soft tissue sarcomas originated from the mediastinum or back side. Forty (71.4%) patients had only multiple pulmonary metastasis; 3 (5.4%) had only multiple bone lesions; 5 (8.9%) had metastasis of both lung and bone; and 5 (8.9%) had metastases to other sites. Table 1 illustrates that none of the clinicopathological factors examined (gender, age, pathological subtypes, location of primary disease, localization of relapse, type of metastasis, time interval from initial chemotherapy to starting using apatinib, number of previous treatment lines) had an evident influence on progression-free survival (PFS) (P ≥ .05).
Before treatment with apatinib, a median of 1.5 lines of chemotherapy (range 1–4) was administrated. Five (8.9%) patients received no chemotherapy before using target therapy, 3 of whom had ASPS and 2 had epithelioid sarcoma. Thirty-seven (66.1%) patients (mostly osteosarcoma) had progressed through 1 line of chemotherapy before using apatinib, while 14 (25%) had been through 2 or more than 2 lines of chemotherapy (Table 1).
Treatment protocols
Forty-four of the 56 (78.6%) patients received only apatinib (oral administration); 7 (12.5%) received apatinib and everolimus in combination; and 5 (8.9%) received apatinib with gemcitabine and docetaxel (Table 2).
Table 2.
Different treatment combination and median duration of response
| Target therapy | Patient number (N) | Best responsea | Median (average) DR (months) |
|---|---|---|---|
| Apatinib alone | 44 (78.6%) | PR | 3.8 (5.4) |
| Apatinib+everolimus | 7 (12.5%) | PR | 8.5 (7.3) |
| Apatinib+GTb | 5 (8.9%) | PR | 8.5 (7.3) |
aPR partial response, SD stable disease according to RECIST 1.1
bGT chemo-protocol combined with gemcitabine 1000 mg/m2 d1,8 and docetaxel 75 mg/m2 d8 once every 21 days
Most of our patients were conventionally evaluated by their doctors at clinic every 2 months with at least chest CT and imaging of tumor lesions at other sites. If some of them could not go to clinic because of poor health status, our medical secretaries would call the patients for updates. However at last information collection, 5 patients were lost to follow-up (we usually defined as no information update for at least three months). Eventually we reviewed all their radiographs and pathological materials for this study.
Efficacy of apatinib-included therapies
As of the most recent follow up, 35 (62.5%) patients had partial responses and 11 (19.6%) had stable disease (Fig. 1). The 4-month and 6-month PFS rates were 46.3 and 36.5%, respectively. The median duration of response (DR) was 3.8 months (95% CI,; 1.9–5.6 m; which varied among pathological subtypes: 3.1 m (95% CI; 2.7–4.1 m) for osteosarcoma, 2.0 m (95% CI; 1.3–2.7 m) for Ewing’s sarcoma, 5.2 m (95% CI; 0.9–9.5 m) for synovial sarcoma, 8.8 m (95% CI; 4.3–11.5 m) for MPNST, and 5.6 m (95% CI, 1.3–9.8 m) for UPS (Table 3).
Fig. 1.
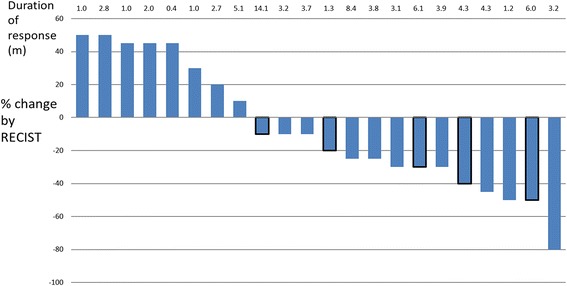
Waterfall plot of best change from baseline for 22 osteosarcoma patients. Patients’ clinical evaluations are indicated on the vertical graph as total volume increase or decrease. The numbers on the horizontal graph indicate the number of months of duration response. Strips with black frame indicate follow-up not yet at end point, and the patients’ status might continue unchanged for some while
The response conditions are illustrated in in Figs. 1, 2 and 3. The objective response rate (CR + PR according to RECIST 1.1) was 40.9% (9/22) for osteosarcoma, 70% (7/10) for Ewing’s sarcoma, 100% (3/3 cases) for chondrosarcoma, and 71.4% (15/21) for soft tissue sarcoma.
Fig. 2.
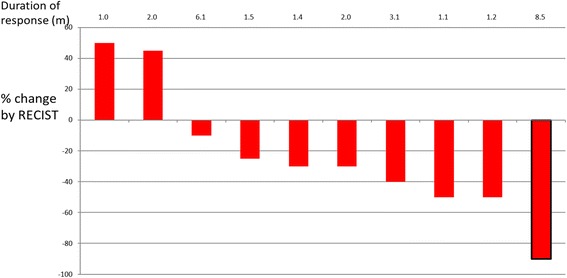
Waterfall plot of best change from baseline for 10 Ewing sarcoma patients
Fig. 3.
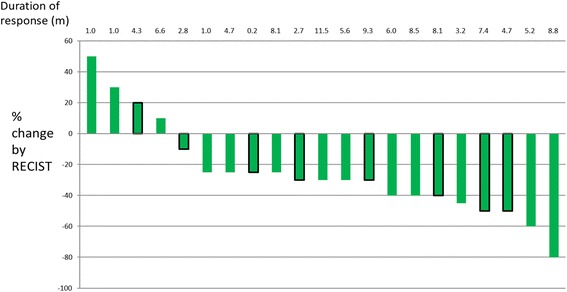
Waterfall plot of best change from baseline for 21 soft tissue sarcoma patients
Toxicity and safety
Treatment was interrupted in 10/56 (18.0%) cases because of disease progression. A 16-year-old female osteosarcoma patient died of cancer because of embolism of pulmonary venous tumor into the middle cerebral artery, and a 21-year-old male osteosarcoma patient had a seizure-like attack after taking apatinib 750 mg once daily for 3 days; the patient recovered gradually after stopping the drug. We had no explanation for this event except for a 3-week interval between stopping ifosfamide chemotherapy and starting apatinib; this rare scenario did not occur again in our series.
The adverse events are summarized in Table 4. Twenty-six Grade 3 or 4 events were recorded. Although the daily dose of apatinib we used was lower than that used in the phase II of apatinib treatment of metastatic gastric cancer [12], adverse events were not fewer, although the main kinds of adverse events were slightly different: most Grade 3 and 4 toxicities were hypertension, pneumothorax, wound-healing problems, anorexia, and rash or desquamation.
Table 4.
Adverse Events
| Total N(%) | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3–4 | ||
| Apatinib alonea | 45 (100%) | |||
| Fatigue | 8(17.8%) | 5 | 2 | 1 |
| Hypertension | 35(77.8%) | 27 | 3 | 5 |
| Proteinuria | 4(8.9%) | 3 | 1 | |
| Hand-foot syndrome | 12(26.7%) | 10 | 2 | |
| Diarrhea | 9(20%) | 5 | 3 | 1 |
| Weight loss | 19(42.2%) | 17 | 2 | |
| Hair hypopigmentation | 25(55.6%) | 20 | 5 | |
| Anorexia | 17(37.8%) | 10 | 4 | 3 |
| Rash or desquamation | 26(57.8%) | 15 | 9 | 2 |
| Mucositis | 2(4.4%) | 2 | ||
| Pneumothorax | 9(20%) | 3 | 6 | |
| Wound-healing problems | 6(13.3%) | 1 | 5 | |
| Elevated Aminotransferase or bilirubin | 3(6.7%) | 2 | 1 | |
| Thrombocytopenia | 7(15.6%) | 5 | 1 | 1 |
| Seizure-like attack | 1(2.3%) | 1 | ||
| Pancreatitis | 1(2.2%) | 1 | ||
| Anemia | 2(4.4%) | 2 | ||
| Cranial neuritis | 1(2.3%) | 1 | ||
| Apatinib + everolimusb | 7 (100%) | |||
| Mucositis | 7(100%) | 2 | 4 | 1 |
| Hypertension | 4(57.1%) | 2 | 2 | |
| Rash or desquamation | 5(71.4%) | 2 | 3 | |
| Gastrointestinal uncomfort | 1(14.3%) | 1 | ||
| Apatinib + GTc | 5 (100%) | |||
| Hypertension | 1(20%) | 1 | ||
| Rash or desquamation | 2(40%) | 2 | ||
| Wound-healing problems | 1(20%) | 1 | ||
| Thrombocytopenia | 2(40%) | 1 | 1 | |
aApatinib alone: apatinib 500-750 mg/d according to the patient’s weight
bApatinib + everolimus: apatinib 250–500 mg/d + everolimus 5 mg/d according to the patient’s weight
cGT chemo-protocol combined with gemcitabine 1000 mg/m2 d1,8 and docetaxel 75 mg/m2 d8 once every 21 days
Discussion
In this study, we found an objective response rate with apatinib used off-label in refractory relapsed sarcoma (40.9%(9/22) for osteosarcoma, 70%(7/10) for Ewing’s sarcoma, and 71.4%(15/21) for soft tissue sarcoma). Also, except for osteosarcoma, the DR of other sarcomas was not inferior to that reported with other TKIs therapy, as shown in Table 5 [3, 4, 13–15]. In comparison with different combination of therapies, PFS seemed superior for apatinib together with sirolimus, but there was no statistically significant difference (P = 0.12). To determine apatinib’s effectiveness, the drug should be evaluated separately in treatment of various types of tumors.
Table 5.
Previous studies about target therapy on sarcoma
| Drug | Combined with chemotherapy | The first author’s last name | Year of publication | Trial Sponsor | Number of patients (N) | Clinical outcome |
|---|---|---|---|---|---|---|
| osteosarcoma | ||||||
| GT | Elizabeth Fox | 2012 | SARTCSf | 14 | ORR 7.1%; | |
| Sorafenib | no | Grignani | 2011 | Italian Sarcoma Group | 35 | 4 m-PFSa 46%; DRd 4 m; ORRc14%; |
| Trastuzumab | Cytotoxic Chemotherapy | Ebb | 2012 | COGe | 41 | 30 m-EFS 32%; 30 m-OSb 50%; without significant difference comparing with control group |
| Sirolimus | Cyclophosphamide | Schuetze | 2012 | Michigan University | 5 | ORR 0%; 4 m-PFS 30% (combined with other sarcoma) |
| Cixutumumab and Temsirolimus | no | Schwartz | 2013 | MSKCCg fund | 24 | ORR 13%; median PFS 6w |
| Cixutumumab | no | Weigel | 2014 | COG | 11 | ORR 0%; 1/11 SD for 140 d |
| R1507 | no | Pappo | 2014 | SARTCSf | 38 | ORR 2.5%; DR: 12w; 12w-PFS 17% |
| Sorafenib; Everolimus | no | Grignani | 2015 | Italian Sarcoma Group | 38 | 6 m-PFS 45%; DR 5 m; ORR 10% |
| Cixutumumab; Temsirolimus | no | Wagner | 2015 | COG | 11 | ORR 0%; |
| Dasatinib | no | Schuetze | 2016 | SARTCS | 46 | ORR 6.5%; DR 5.7 m; 2y-OS 15% |
| Apatinib | no | Present study | 2017 | 22 | ORR 40.9%;median PFS 3.1 m; 4 m PFS 24.1%; 6 m PFS 18.1% | |
| Ewing sarcoma | ||||||
| GT | Elizabeth Fox | 2012 | SARTCS | 14 | ORR 14.3%; | |
| R1507 | no | Pappo | 2011 | SARTCS | 115 | ORR 9.6%; median PFS 1.3 m; median OS 7.6 m |
| Figitumumab | no | Juergens | 2011 | European organization | 106 | ORR 14.2%; median PFS 1.9 m; median OS 8.9 m |
| Cixutumumab + temsirolimus | no | Schwartz | 2013 | MSKCC | 27 | ORR 14.8%; median PFS 7.5w; median OS 16.2 m |
| Olaparib | no | Choy E | 2014 | MGHh | 12 | ORR 0%; DR 5.7w; |
| Cixutumumab + temsirolimus | no | Wagner LM | 2015 | COG | 43 | ORR 0%; 12w-PFS 16%; |
| Apatinib+everolimus & apatinib alone | no | Present study | 2017 | 10 | ORR 70%; median PFS 2.0 m; 12w-PFS 22.5% | |
| Soft tissue sarcoma | ||||||
| Topotecan +carboplatin | Bochennek K | 2013 | CSTSGi | 34 | ORR 38%; | |
| Pazopanib | no | Winette T A | 2012 | EORTCj and the PALETTE study group | 246 | ORR 6%; median PFS 4.6 m; median OS 12.5 m |
| Olaratumab | Doxorubicin | William D Tap | 2016 | MSKCC and 16 clinical sites in US | 15 in IB trial and 67 in II trial | ORR 18.2%; median PFS 6.6 m; median OS 26.5 m |
| Regorafenib | no | Thomas Brodowicz | 2015 | International trial (France, Austria, Germany) | 82 | Median PFS 5.6 m for SS and 2.9 m for none specific; |
| Apatinib alone & apatinib+everolimus | Sometimes accompanied with GTk | Present study | 2017 | 21 | ORR 71.4%; median PFS 6.6 m; 4 m-PFS 71.4%; median OS 9.9 m | |
| chondrosarcoma | ||||||
| GT | Elizabeth Fox | 2012 | SARTCS | 25 | ORR 8%; | |
| GDC-0449 | no | A. Italiano | 2013 | French Sarcoma Group/US; French National Cancer Institute | 39 | ORR 0%; median PFS 3.5 m; 6-m PFS 28.2%; 1-y PFS 19.2% |
| Imatinib | no | Grignani | 2011 | Italian Sarcoma Group | 26 | ORR 0%; 4 m-PFS 31%; median OS 11 m; |
| Sirolimus | cyclophosphamide | Bernstein-Molho R | 2012 | Israel | 10 | ORR 10%; 60% SD for more than 6 m; median PFS 13.4 m |
| IDHl inhibitor | no |
NCT02273739; NCT02481154; NCT02073994; NCT02496741 |
2016–2017 | Under investigations | ||
| Apatinib alone | no | Present study | 2017 | 3 | ORR 100%; median PFS 7.37 | |
aPFS: progression-free survival
bOS: overall survival
cORR: overall response rate, defined as complete responses (CRs) + partial responses (PRs) + MRs;
dDR: Duration of response
eCOG: Children’s Oncology Group
fSARTCS: Sarcoma Alliance for Research Through Collaboration Study
gMSKCC: Memorial Sloan-Kettering Cancer Center
hMGH: Massachusetts General Hospital
iCSTSG: Cooperative Soft Tissue Sarcoma Group
jEORTC: European Organization for Research and Treatment of Cancer
kGT: chemo-protocol combined with gemcitabine 1000 mg/m2 d1,8 and docetaxel 75 mg/m2 d8
lIDH: isocitrate dehydrogenase
Osteosarcoma patients whose disease relapses after failing standard chemotherapy present a challenging treatment dilemma. Some patients, through aggressive surgical resection of all gross disease, may have long-term survival [16]. The choice of second-line chemotherapy and the use of investigational drugs are not standardized, and the outcomes are dismal [17]. Maldegem et al. [15] summarized phase I/II clinical trials conducted between 1990 and 2010 in osteosarcoma and Ewing’s sarcoma; results were disappointing: only 8% CR, 2.8% PR, and 4% SD. Many active agents have been tested also in small series for treatment of osteosarcoma. Most anti-angiogenesis TKIs can only keep the tumor stable but not make it shrink [18]. The greatest progress in phase II trials belongs to the Italian Sarcoma Group; they have held 2 cohort phase II trials with advanced osteosarcoma patients and found an objective response rate (ORR) of 14 and 10% [3, 4]. However, 45% 6-month PFS (combination therapy) was less than the pre-specified threshold of activity deemed worthy of a phase III trial (6-month PFS of 50% or greater). In Table 5, apatinib had a higher rate of response than did sorafenib, but the duration of response seemed to be shorter.
In this study, we did notice this phenomenon that sometimes most or some patients’ lesions shrunk or remained stable during observation, while one or two lesions progressed. And this is especially common phenomenon happened during the third month after using apatinib for osteosarcoma. Patients might still get benefit from this VEGFR-2 highly selective drug with help of local therapy for those advanced lesion because of tumor heterogeneity. However as we use the criteria of RECIST 1.1, the duration of response seemed to be short. From our 56 patients, 4 osteosarcoma patients and one synovial sarcoma patient were in these circumstances.
Ewing’s sarcoma is genetically characterized by chromosomal translocation involving the Ewing’s sarcoma breakpoint region 1 (EWSR1) gene.. In this study we have 10 advanced Ewing’s sarcoma cases. Table 5 illustrates that for refractory Ewing’s sarcoma, the objective response rate was only 0 to 14.8% [15, 19, 20]. Nevertheless, the DR for Ewing’s sarcoma in various reports has been short compared with that of other sarcomas, with a median time of 5.7 weeks to less than 2 months [21–23]. In our study, more than half the Ewing’s sarcoma patients took apatinib together with everolimus, whereas the remainder took apatinib alone. Seventy percent of all these patients, who had apatinib containing protocol, had partial response, which seemed to indicate that apatinib was the most effective in these trials, and that anti-VEGFR2 target therapy might be another promising approach for treating Ewing’s sarcoma although with limited duration of response.
Soft tissue sarcoma is another huge group of sarcomas with diverse biological behaviors. For advanced cases, the only truly new treatment approved for sarcoma failing standard therapy is trabectedin, which has been approved by the European Medicines Agency 2007 [24]. Gemcitabine with dacarbazine or docetaxel [13, 25] and paclitaxel for treatment of angiosarcoma [26] seemed to improve PFS and OS in non-randomized and adaptively randomized trials. Targeted therapies, such as imatinib and sunitinib, have activity against gastrointestinal stromal tumors [27, 28]. Generally, anti-angiogenesis TKIs therapy with pazopanib has been a hallmark for all non-adipocytic soft tissue sarcoma after phase II and III trial verification, with median PFS 4.6 months (3.7–4.8 m; 95% CI) and best overall objective response rate of 6% (14/246) [14, 29]. Thomas et al. [30] reported that regorafenib, which is an inhibitor of VEGFR-1, − 2 and − 3 and tumor cell signaling kinases (RET, KIT, PDGFR, and Raf), yielded median PFS of 4.6 months in advanced sarcoma patients, which was almost the same as with pazopanib. Recently, the US Food and Drug Administration approved olaratumab [31], a human antiplatelet-derived growth factor receptor α monoclonal antibody, together with doxorubicin as first-line therapy for unresectable or metastatic soft tissue sarcoma. The approval was based on the drug having significant improvement in median OS (11.8 months), however this is for initially treated soft tissue sarcoma not for refractory cases. We used various combinations of therapy, including apatinib, achieving ORR of 71.4%, which is an astonishing result compared with other therapies [14, 24, 31] for treatment of advanced sarcoma. Although there were only 21 cases, we compared the subtype constitution in Table 3 and believed that it did not have obvious selective bias. In comparison with those agents, apatinib seemed to be more effective. The drug needs to be tested against other types of soft tissue sarcoma, such as MPNST and ASPS. We had 3 MPNST patients treated with apatinib, two of whom attained PR, and the PFS was 18.0 and 10.2 months. The other MPNST patient manifested as SD, and until last follow-up, at 4.3 months, her disease was stable. For target therapy for ASPS [29], objective response has rarely been reported, perhaps because the disease is indolent, progressing over decades, and few drugs have caused shrinkage of the tumors. However, with apatinib, which is a highly selective inhibitor of VEGFR-2, 2 of our 3 ASPS patients had PR, which seems a notable response. However, these PR cases firstly manifested as SD for 2 or 3 months and then started to shrink. However, the median OS with apatinib-containing therapy is shorter than that with pazopanib (9.9 m vs and 12.5 m). We suppose that this difference may be because patients with secondary resistance to apatinib might quickly die of the disease without much more efficient treatment options.
Our experience with toxicity associated with apatinib (Table 4) seemed to be more severe from that described in clinical trials [7]. One patient had to stop using apatinib because of neuro-toxicity. Three patients had so serious anorexia and weight loss that they stopped using the agent. Nine of our patients had treatment-related pneumothorax and six patients had wound healing problems.
We acknowledge this study’s limitations. First, it is a retrospective study that some patients might have some combination therapy which made this study not so suitable for comparion with other drugs. Second, because of the rarity of some types of sarcoma, we had insufficient numbers to permit subset analyses, which could have reduced the statistical power. Third, the study is off-label; hence, it may not have been as rigorously controlled as are prospective trials. Finally, 5 patients were lost to follow-up, which may have affected data accuracy.
Conclusions
Apatinib might be with a high objective response rate, in an off-label study of sarcoma patients who had tumors not amenable to curative treatment or inclusion in clinical trials. The duration of response were consistent with responses reported in clinical trials with other anti-angiogenesis TKIs. Investigation of apatinib in the treatment of some special subtypes of sarcoma, for example metastatic MPNST and ASPS, in prospective trials is needed.
Acknowledgements
We thank Dr. Shen Danhua and Dr. Sun Kunkun for reviewing all the pathological slides of these sarcoma patients and Dr. Li Yuan for doing clinical PET/CT evaluation for all our patients.
Funding
One of the authors (TJ) has received research support funding from the National Natural Science Foundation of China (No. 81402382). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the patients’ database of Peking University People’s Hospital, Peking University Shougang Hospital and Peking University International Hospital separately but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request and with permission of Peking University People’s Hospital, Peking University Shougang Hospital and Peking University International Hospital.
Abbreviations
- 95% CI
95% confidence interval
- AEs
Adverse events
- ASPS
Alveolar soft part sarcoma
- BSA
Body surface area
- CS
Chondrosarcomas
- DR
Duration of response
- EFS
Event-free survival
- ES
Ewing’s sarcoma
- ESFT
Ewing’s sarcoma family tumors
- GT
Gemcitabine and docetaxel
- IDH
Isocitrate dehydrogenase
- IGF-1R
Insulin-like growth factor 1 receptor
- MPNST
Malignant peripheral nerve sheath tumors
- mTOR
Mammalian target of rapamycin
- ORR
Object response rate
- OS
Overall survival
- PDGFR
Platelet-derived growth factor receptor
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response Evaluation Criteria for Solid Tumors
- SD
Stable disease
- STS
Soft tissue sarcomas
- TKIs
Tyrosine Kinase Inhibitors
- UPS
Undifferentiated pleomorphic sarcoma
- VDC-IE
Vincristine, doxorubicin, cyclophosphamide sequenced with ifosfamide, etoposide
- VEGFR
Vascular endothelial growth factor receptor
- VTI
Vincristine, temozolomide, irinotecan
Authors’ contributions
WG, YW and TY designed and developed this retrospective study. LX and JX collected all the patients’ basic information. LX analyzed and interpreted patients’ data and was a major contributor in writing the manuscript. TJ did statistical analysis and revised the article separately. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the institutional review board, Peking University People’s Hospital, Peking University Shougang Hospital and Peking University International Hospital Ethics Committee for Clinical Investigation. All those patients had signed informed consent forms for data collection and use for research purpose, of which children patients’ informed forms had be signed by their legal parents. The ethical approval reference number was IRBK-2017-019-01.
Consent for publication
All those patients had signed informed consent forms for data collection and publication, of which children patients’ informed forms had been signed by their legal parents.
Competing interests
The authors declare that they have no competing interests in this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lu Xie, Email: sweetdoctor@163.com.
Wei Guo, Email: bonetumor@163.com.
Ye Wang, Email: wangye198169@163.com.
Taiqiang Yan, Email: Yantqzh@163.com.
Tao Ji, Email: jitaomd@163.com.
Jie Xu, Email: xujie_pkuph@sina.com.
References
- 1.Paulussen M, Bielack S, Jurgens H, Casali PG, Group EGW Ewing's sarcoma of the bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):140–142. doi: 10.1093/annonc/mdp155. [DOI] [PubMed] [Google Scholar]
- 2.Jo VY, Doyle LA. Refinements in sarcoma classification in the current 2013 World Health Organization classification of Tumours of soft tissue and bone. Surg Oncol Clin N Am. 2016;25(4):621–643. doi: 10.1016/j.soc.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Grignani G, Palmerini E, Dileo P, Asaftei SD, D'Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F, Casali PG, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian sarcoma group study. Ann Oncol. 2012;23(2):508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 4.Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y, Sangiolo D, Marchesi E, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16(1):98–107. doi: 10.1016/S1470-2045(14)71136-2. [DOI] [PubMed] [Google Scholar]
- 5.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schoffski P, Aglietta M, Staddon AP, Beppu Y, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, double-blind, placebo-controlled phase III trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 8.Ding Q, Cheng X, Yang L, Zhang Q, Chen J, Li T, Shi H. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST) J Thorac Dis. 2014;6(6):677–683. doi: 10.3978/j.issn.2072-1439.2014.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the eastern cooperative oncology group scoring scale and vice versa? Eur J Cancer. 1992;28A(8–9):1328–1330. doi: 10.1016/0959-8049(92)90510-9. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, Yv K, Wang F, Li C, Qian J, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. doi: 10.1186/1471-2407-10-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirillo M, Venturini M, Ciccarelli L, Coati F, Bortolami O, Verlato G. Clinician versus nurse symptom reporting using the National Cancer Institute-common terminology criteria for adverse events during chemotherapy: results of a comparison based on patient's self-reported questionnaire. Ann Oncol. 2009;20(12):1929–1935. doi: 10.1093/annonc/mdp287. [DOI] [PubMed] [Google Scholar]
- 12.Fornaro L, Vasile E, Falcone A. Apatinib in advanced gastric Cancer: a doubtful step forward. J Clin Oncol. 2016;34(31):3822-3823. 10.1200/JCO.2016.68.6931. [DOI] [PMC free article] [PubMed]
- 13.Fox E, Patel S, Wathen JK, Schuetze S, Chawla S, Harmon D, Reinke D, Chugh R, Benjamin RS, Helman LJ. Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of sarcoma alliance for research through collaboration study 003. Oncologist. 2012;17(3):321. doi: 10.1634/theoncologist.2010-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schoffski P, Collin F, Pandite L, Marreaud S, De Brauwer A, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27(19):3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 15.van Maldegem AM, Bhosale A, Gelderblom HJ, Hogendoorn PC, Hassan AB. Comprehensive analysis of published phase I/II clinical trials between 1990-2010 in osteosarcoma and Ewing sarcoma confirms limited outcomes and need for translational investment. Clin Sarcoma Res. 2012;2(1):5. doi: 10.1186/2045-3329-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, Gobel U, Helmke K, Jundt G, Kabisch H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the cooperative osteosarcoma study group (COSS) J Clin Oncol. 2005;23(3):559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 17.Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O, 3rd, Widemann BC, Zwerdling T, Bomgaars L, Langevin AM, et al. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through Children's Cancer group, pediatric oncology group, and Children's oncology group: learning from the past to move forward. J Clin Oncol. 2016;34(25):3031–3038. doi: 10.1200/JCO.2015.65.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox E, Aplenc R, Bagatell R, Chuk MK, Dombi E, Goodspeed W, Goodwin A, Kromplewski M, Jayaprakash N, Marotti M, et al. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan-vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28(35):5174–5181. doi: 10.1200/JCO.2010.30.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, Craft AW. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European intergroup cooperative Ewing's sarcoma study group. J Clin Oncol. 2000;18(17):3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 20.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hematol Oncol. 2008;30(6):425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 21.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13(18 Pt 2):5549s–5555s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 22.Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, Ludwig J, Chen HX, Doyle LA, Kurzrock R. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors. Clin Cancer Res. 2012;18(9):2625–2631. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappo AS, Vassal G, Crowley JJ, Bolejack V, Hogendoorn PC, Chugh R, Ladanyi M, Grippo JF, Dall G, Staddon AP, et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a sarcoma alliance for research through collaboration study. Cancer. 2014;120(16):2448–2456. doi: 10.1002/cncr.28728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Broto J, Pousa AL, de Las Penas R, Garcia Del Muro X, Gutierrez A, Martinez-Trufero J, Cruz J, Alvarez R, Cubedo R, Redondo A, et al. Randomized phase II study of Trabectedin and doxorubicin compared with doxorubicin alone as first-line treatment in patients with advanced soft tissue sarcomas: a Spanish Group for Research on sarcoma study. J Clin Oncol. 2016;34(19):2294–2302. doi: 10.1200/JCO.2015.65.3329. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Del-Muro X, Lopez-Pousa A, Maurel J, Martin J, Martinez-Trufero J, Casado A, Gomez-Espana A, Fra J, Cruz J, Poveda A, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on sarcomas study. J Clin Oncol. 2011;29(18):2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]
- 26.Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, Kerbrat P, Fournier C, Taieb S, Jimenez M, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;26(32):5269–5274. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Kang H, Zhao H, Hu J, Zhang H, Li X, Du N, Huang Y. Sunitinib for patients with locally advanced or distantly metastatic dermatofibrosarcoma protuberans but resistant to imatinib. Int J Clin Exp Med. 2015;8(5):8288–8294. [PMC free article] [PubMed] [Google Scholar]
- 28.Patrikidou A, Domont J, Chabaud S, Ray-Coquard I, Coindre JM, Bui-Nguyen B, Adenis A, Rios M, Bertucci F, Duffaud F, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French sarcoma group. Eur J Cancer. 2016;52:173–180. doi: 10.1016/j.ejca.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 29.Bains R, Magdum A, Bhat W, Roy A, Platt A, Stanley P. Soft tissue sarcoma - a review of presentation, management and outcomes in 110 patients. Surgeon. 2016;14(3):129–135. doi: 10.1016/j.surge.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Brodowicz T, Liegl-Atzwager B, Tresch E, Taieb S, Kramar A, Gruenwald V, Vanseymortier M, Clisant S, Blay JY, Le Cesne A, et al. Study protocol of REGOSARC trial: activity and safety of regorafenib in advanced soft tissue sarcoma: a multinational, randomized, placebo-controlled, phase II trial. BMC Cancer. 2015;15:127. doi: 10.1186/s12885-015-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, Agulnik M, Cooney MM, Livingston MB, Pennock G, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the patients’ database of Peking University People’s Hospital, Peking University Shougang Hospital and Peking University International Hospital separately but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request and with permission of Peking University People’s Hospital, Peking University Shougang Hospital and Peking University International Hospital.


