Significance
The energy barrier for a classical heterogeneous crystal nucleation can be controlled by the energy contributions from the substrate/nucleus interface and local supersaturation. Exerting control over crystal growth thus requires modifying either one of these terms. We here introduce a strategy to modulate the contributions of both parameters simultaneously using substrates containing different crystal structures of calcium carbonate. Based on a theoretical analysis, we program both the positioning and growth direction of carbonate salts on preselected polymorphs. These findings may hold relevance for understanding, mimicking, and ultimately expanding upon nature’s mineralization strategies and for developing functional microscale materials.
Keywords: crystal growth, nucleation, polymorphism, biomineralization, calcium carbonate
Abstract
Controlling nucleation and growth is crucial in biological and artificial mineralization and self-assembly processes. The nucleation barrier is determined by the chemistry of the interfaces at which crystallization occurs and local supersaturation. Although chemically tailored substrates and lattice mismatches are routinely used to modify energy landscape at the substrate/nucleus interface and thereby steer heterogeneous nucleation, strategies to combine this with control over local supersaturations have remained virtually unexplored. Here we demonstrate simultaneous control over both parameters to direct the positioning and growth direction of mineralizing compounds on preselected polymorphic substrates. We exploit the polymorphic nature of calcium carbonate (CaCO3) to locally manipulate the carbonate concentration and lattice mismatch between the nucleus and substrate, such that barium carbonate (BaCO3) and strontium carbonate (SrCO3) nucleate only on specific CaCO3 polymorphs. Based on this approach we position different materials and shapes on predetermined CaCO3 polymorphs in sequential steps, and guide the growth direction using locally created supersaturations. These results shed light on nature’s remarkable mineralization capabilities and outline fabrication strategies for advanced materials, such as ceramics, photonic structures, and semiconductors.
Spurred by the recent advancement in techniques such as microfabrication and electron microscopy, there has been tremendous advancement in understanding and controlling both biological and artificial mineralization processes (1–4). Unlike homogeneous nucleation, heterogeneous nucleation allows for an effective strategy to control the local nucleation energy barrier by modifying the chemical and structural characteristics between the nascent nucleus and substrates (5–12). Alternatively, a local increase of the supersaturation can onset nucleation (13–15)––an approach broadly utilized by natural systems through ion channels that deliver crystallizing components to the specific site where nucleation should occur––but this approach has hardly been explored in artificial systems. Only recently, such local supersaturations were created using an ion-binding biomimetic matrix (4). However, little or no work has been done to develop principles that allow for simultaneous control over the local supersaturation and interfacial free energy for directing the nucleation position, let alone the growth (15).
While virtually all previous studies have been aimed at avoiding polymorphism (16), we here use the ability of compounds to organize in different crystal forms to rationally control both local supersaturations in the vicinities of different polymorphs and subtle variations in the lattice mismatch between the substrate and the nucleus. An ideal model system to test this strategy is the crystallization of BaCO3 selectively on top of different polymorphs of CaCO3. CaCO3 is the most abundant biomineral and can be found in three anhydrous polymorphs that all occur in living organisms (17–23). At room temperature calcite is the most stable polymorph, followed by aragonite and finally vaterite as the least stable structure. To demonstrate the ability to tune the positioning of BaCO3 on top of these polymorphs and its growth direction, we analyze and exploit local variations of supersaturations induced by the difference in the dissolution rate of CaCO3 polymorphs. Additionally, the crystal structures of calcite, aragonite, and vaterite are different from each other, and only aragonite resembles that of BaCO3 (23). This variation in crystal structures allows for direct evaluation of the effects of the lattice mismatch on templated nucleation of BaCO3 on selected CaCO3 polymorphs modulated by the local supersaturation gradients.
Results and Discussion
To understand the unique interplay between the interfacial free energy that arises from the lattice mismatch and supersaturation in this system, we analyze a solution containing Ba2+ ions in which an influx of CO32− causes the templated nucleation of BaCO3 on a CaCO3 substrate with crystal structure x (Fig. 1). According to classical nucleation theory (13–15), the energy barrier to form a critical nucleus, Δgn, can be described as a function of the local supersaturation σ and the interfacial free energy α according to
| [1] |
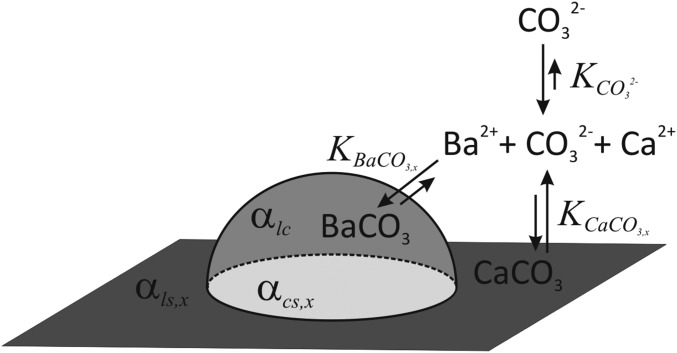
Fig. 1.
Nucleation of BaCO3 on a CaCO3 substrate. The energy barrier for nucleation is determined by (i) the contributions of the interfacial free energies arising from the lattice mismatch between liquid-crystal αlc, liquid-substrate αls,x, and substrate-crystal αcs,x, and (ii) the local supersaturation, which depends on the concentration of ions in the solution, the influx of CO32−, the crystallization rate of BaCO3, and dissolution rate of CaCO3, kBaCO3, and kCaCO3,x, respectively, for a specific crystal structure x. By controlling the influx of the CO32−, the balance can be shifted from the major contribution from the lattice mismatch at high [CO32−]bulk to a local supersaturation-dominated process controlled by the differences in the solubility of CaCO3 polymorphs at low [CO32−]bulk. Note that the hemispherical representation is chosen for illustrative purposes and will depend on the specific nucleation conditions.
The lattice mismatch between the BaCO3 nucleus and the underlying substrate directly contributes to the interfacial energy penalties, which include an energy contribution due to the difference in chemical bonding, and a strain-energy contribution from the interface and the nucleate volume that arises from the incoherent ordering at the interface. Although analytical expressions of each energy contribution are hard to achieve due to the complexity of the system, both energy contributions should decrease as the substrate/nucleus interface becomes more crystallographically aligned and coherent.
The energy gain resulting from the chemical potential energy is directly related to the supersaturation of BaCO3. In case the substrate has a higher solubility than the nucleating compound, and the rate of dissolution of the substrate is higher than the rate of growth of the nucleating crystal (Fig. 1), the supersaturation in BaCO3 can be expressed as
| [2] |
with kBaCO3 the crystallization rate constant of BaCO3, [CO32−]bulk the concentration of carbonate in the bulk solution, [Ca2+]s the Ca2+ concentration close to the surface of the CaCO3 crystal, kx the dissolution rate constant of CaCO3 for a specific polymorph x, and kx′ = kCaCO3,x/Ksp_CaCO3,x where Ksp_CaCO3,x is the solubility product of a specific polymorph x of CaCO3 (see SI Appendix and refs. 24–27). We assume kvaterite > karagonite > kcalcite and Ksp,vaterite > Ksp,aragonite > Ksp,calcite, which implies that both the dissolution rate and solubility of vaterite are higher than those for aragonite and calcite. In case of ample availability of Ba2+, the free-energy barrier Δgn for nucleation on a specific polymorph of CaCO3 is controlled by the interplay of: (i) the concentration of carbonate in the bulk solution, (ii) the polymorph-dependent lattice mismatch with the CaCO3 substrate, and (iii) the substrate solubility equilibrium. We therefore expect that the choice of the positioning of the nucleation can be tuned by the subtle changes in relative contributions of these parameters. Below we consider various scenarios where the impact of these parameters is analyzed and exploited to direct the nucleation and growth of BaCO3.
To evaluate the effects of lattice mismatch in the carbonate system studied here, we characterized the crystallographic relationships of BaCO3 grown on top of calcite, aragonite, and vaterite as substrate crystals. The overgrown BaCO3 crystals can be easily differentiated from the underlying CaCO3 crystals in backscatter electron microscopy due to their electron density difference (Fig. 2 A, i and ii). Through focused ion-beam (FIB) milling, electron-transparent transmission electron microscopy (TEM) samples were prepared at the interfaces of nucleus and substrates, from which the morphological and crystallographic characteristics were evaluated (Fig. 2 A, iii–v). BaCO3 exhibit good alignment with calcite and aragonite due to sharing the same c axes, while vaterite showed no crystallographic alignment (Fig. 2A and SI Appendix). This lack of orientation matching thus indicates a higher interfacial free energy for nucleation on vaterite compared with calcite and aragonite. A qualitative estimation of the lattice mismatch shows that BaCO3 has a slightly smaller lattice mismatch in both a and b directions on calcite as the substrate in comparison with aragonite as the substrate (SI Appendix, section 8) (12). Therefore, when all three polymorphs are present as crystallization substrates, BaCO3 will selectively first nucleate on calcite, followed by aragonite, but not on vaterite for the same supersaturation levels. On the other hand, substrate solubility should favor the nucleation of BaCO3 on vaterite over crystallization on aragonite, and least on calcite, due to significant local supersaturation occurring on the surfaces of the dissolving vaterite at low values of bulk carbonate content. The relative contributions of these two factors can be easily tuned by varying [CO32−]bulk, according to Eqs. 1 and 2. For high values of the [CO32−]bulk the lattice mismatch is expected to dominate the crystallization position, whereas for low values of [CO32−]bulk the solubility equilibrium of the substrate will increase the supersaturation in the vicinity of the least stable polymorphs.
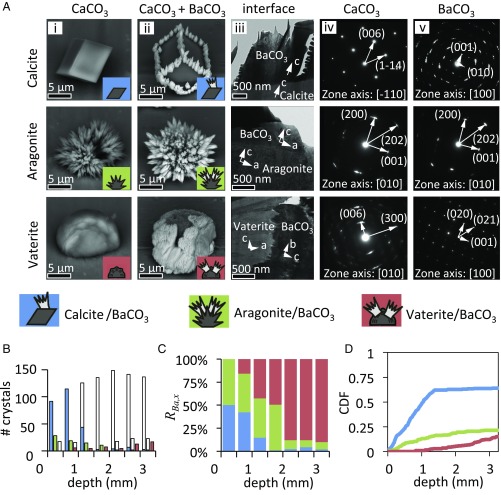
Fig. 2.
Polymorph-selective nucleation of BaCO3 on different CaCO3 polymorphs. (A) Backscatter electron microscopy images were used to highlight the difference between BaCO3 (appearing light) on different polymorphs of CaCO3 (appearing dark). The lattice mismatches were estimated by measuring the crystallographic alignment of BaCO3 on calcite, aragonite, and vaterite (SI Appendix). (A, i) Different polymorphs of CaCO3. (A, ii) BaCO3 crystals on top of the different polymorphs of CaCO3. (A, iii–v) TEM imaging and corresponding selected-area electron diffraction at the interfaces revealed crystallographic alignment of BaCO3 on top of calcite and aragonite, whereas no crystallographic alignment was found on vaterite. (B–D) Three representations of statistical data of CaCO3 overgrowth. (B) Total number of various polymorphs of CaCO3 crystals as a function of depth: calcite (left bar), aragonite (middle bar), and vaterite (right bar). The number of the overgrown CaCO3 crystals in each category are shown as filled bars: calcite-BaCO3 (blue), aragonite-BaCO3 (green), and vaterite-BaCO3 (red). (C) Polymorph ratio based on data in Fig. 2B, highlighting that close to the meniscus BaCO3 nucleation only occurs on calcite and aragonite, and deeper in the solution––only on vaterite. (D) CDF of the overgrown crystals as a function of depth.
These differences in lattice mismatches, in combination with external control over the local supersaturation, allow us to selectively nucleate BaCO3 on a predetermined polymorph of CaCO3.
To probe the influence of [CO32−]bulk, we developed a method to fabricate mixed polymorphic substrates containing all three polymorphs of CaCO3 (typically, calcite 79 ± 8%, aragonite 14 ± 5%, and vaterite 7 ± 3%) by crystallizing CaCO3 on an aluminum plate (see SI Appendix for details and setup). We vertically positioned the substrate containing all three CaCO3 polymorphs in the reaction solution. As carbonate from the air entered the solution from the top, a [CO32−] gradient was created along the substrate, thus allowing for a continuous combinatorial screening of the influence of the [CO32−]bulk on the overgrowth of the CaCO3 polymorphs. To quantify the results, we manually counted each polymorph as a function of the depth (Fig. 2B). We define the polymorph overgrowth ratio RBa,x for a specific CaCO3 polymorph x as RBa,x = (nBa,x/nx)/Σx′(nBa,x′/nx′), with nx denoting the total number of CaCO3 crystals of a specific polymorph x; nBa,x the number of CaCO3 crystals of this polymorph that have been overgrown with BaCO3, and the sum in the nominator running over all possible polymorphs (Fig. 2C). Additionally, we compute the cumulative distribution function (CDF) for each overgrown polymorph as a function of the depth in the solution (Fig. 2D).
Close to the meniscus (0.0–0.5 mm), the [CO32−]bulk is the highest, which minimizes the effect of the local carbonate supersaturation due to polymorph dissolution. The lattice mismatch thus dominates the nucleation position, and BaCO3 only nucleates on calcite and aragonite, with no nucleation on vaterite, which is expected as the lack of crystallographic alignment on vaterite results in a disadvantageous lattice mismatch. Deeper in the solution (0.5–2.0 mm), the lower [CO32−]bulk decreases the local supersaturation around the calcite and aragonite crystals, and fewer of them become overgrown with BaCO3. The lower [CO32−]bulk also increases the dissolution of the least stable polymorph following Eq. 2, thus locally increasing the supersaturation around the vaterite crystals. Since the solubility of BaCO3 is approximately 10-fold lower than that of CaCO3 polymorphs (Ksp_BaCO3 << Ksp_CaCO3,x), the increase in CO32− concentration around the dissolving vaterite crystals onsets the nucleation of BaCO3. Below 2.0 mm the supersaturation is too low for nucleation on either calcite or aragonite crystals even though the lattice mismatch is favorable for these substrates. The stability-dependent dissolution completely dominates the nucleation and all vaterite crystals become overgrown. Dissolving vaterite crystals even increases the local CO32− concentration to such an extent that crystallization of BaCO3 may occur on an accidental calcite and aragonite crystal grown in close proximity to vaterite (SI Appendix, Fig. S2B). It should be noted that a similar trend is found for the overgrowth of CaCO3 polymorphs with SrCO3 instead of BaCO3 (SI Appendix, Fig. S3), demonstrating that these principles can readily be extended to other systems. Furthermore, precise control over the spatial location and polymorphism of the CaCO3 substrates may be gained using patterned self-assembled monolayers (11).
To create more complex shapes beyond the simple morphology of BaCO3 crystals, we introduce silica to the reaction. The resulting BaCO3/SiO2 structures can straightforwardly be sculpted in a rich palette of microshapes such as vases, spirals, and corals by externally modulating the reaction conditions, such as pH and temperature (28–31). The formation of these various shapes and their orientation is highly sensitive to and nearly fully determined by the concentrations of the reacting species in the vicinity of the developing structures, and can therefore act as an ideal model system to test the sensitivity of the nucleation events described above. Recently, we have also shown that precise control of the shape could lead to functional shapes such as those that can be used in photonics (31). In particular, by integrating a fluorescent dye as a light source in the nucleating barium carbonate seed crystal, effective waveguiding and beamsplitting through the rationally designed microarchitectures were achieved. While in this previous work no control over the position of these structures was obtained, the present study can introduce the ability to precisely control the location of the nucleation of these microstructures. Moreover, we anticipated that akin to chemotaxis, the creation of the local gradient in supersaturation induced by specific polymorphs of CaCO3 that are placed in the immediate vicinity of the nucleating microstructures may be used to steer the growth of these structures in predetermined directions.
The precipitation of BaCO3/SiO2 structures starts with the formation of a BaCO3 crystal, which to a good approximation is not affected by the SiO2. Hence we can assign the nucleation position in a manner analogous to how we directed the nucleation of BaCO3 (Fig. 3 A and B). Indeed, we find the same trend: preferred nucleation on calcite and aragonite at low immersion depths, whereas nucleation on vaterite only occurs deeper in the solution. Importantly, the ratio between the overgrown aragonite and calcite crystals in the top of the substrate gradually increases, with aragonite crystals being selectively overgrown at 0.75–1.5 mm (Fig. 3B), likely due to the growing contribution of the increase in local supersaturation from the dissolving calcium carbonate in the BaCO3/aragonite system over the BaCO3/calcite, as the bulk concentration in CO2 goes down. It should be noted that the silica hampers the nucleation of BaCO3. As a result, we observe a zone (2.0–5.0 mm) where the local supersaturation is insufficient to grow BaCO3/SiO2 structures and only below 5.0 mm is the local supersaturation sufficiently high to induce nucleation on vaterite. More complex shapes can be grown by rationally adjusting the reaction conditions, as exemplified by the SrCO3/SiO2 stems in Fig. 3C that selectively nucleated on calcite and aragonite and were opened into vases using a CO2 pulse and subsequently decorated with serrated edges using temperature modulations.
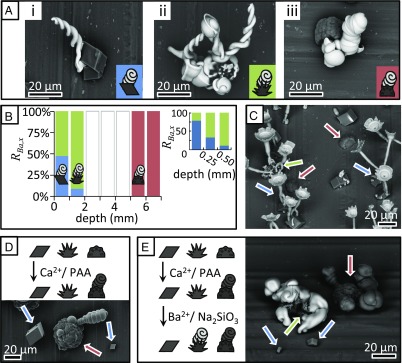
Fig. 3.
Polymorph-selective nucleation of BaCO3/SiO2 and CaCO3/PAA microstructures. (A) Backscatter SEM images of BaCO3/SiO2 grown on (A, i) calcite, (A, ii) aragonite, and (A, iii) vaterite. (B) Polymorph ratio showing that BaCO3/SiO2 structures can selectively nucleate on CaCO3 polymorphs by controlling the depth of immersion (see also SI Appendix). (Inset, Top Right) A more detailed polymorph ratio for the depth of 0–0.75 mm. (C) SrCO3/SiO2 stems that were opened into vases and decorated with serrated edges that selectively nucleated on calcite (blue arrow) and aragonite (green arrow) while vaterite (red arrow) is left empty. (D) CaCO3/PAA spirals selectively nucleate on vaterite crystals. (E) Sequential nucleation steps can be used to first grow CaCO3/PAA spirals (dark) on vaterite (dark) and subsequently place BaCO3 spirals (light) on aragonite while leaving calcite empty.
The ability to control nucleation on preselected polymorphs can also be used for positioning different materials on assigned polymorphs in sequential steps. We demonstrated this by first growing CaCO3 spirals in the presence of polyaspartic acid (PAA) (32, 33). As these spirals are composed of vaterite nanocrystals, they selectively form on vaterite in the presence of all three polymorphs independent of the depth (Fig. 3D). With both the calcite and aragonite crystals still available, we subsequently positioned BaCO3/SiO2 spirals on the aragonite crystals while leaving the calcite crystals bare (Fig. 3E) at a depth of 1.5 mm.
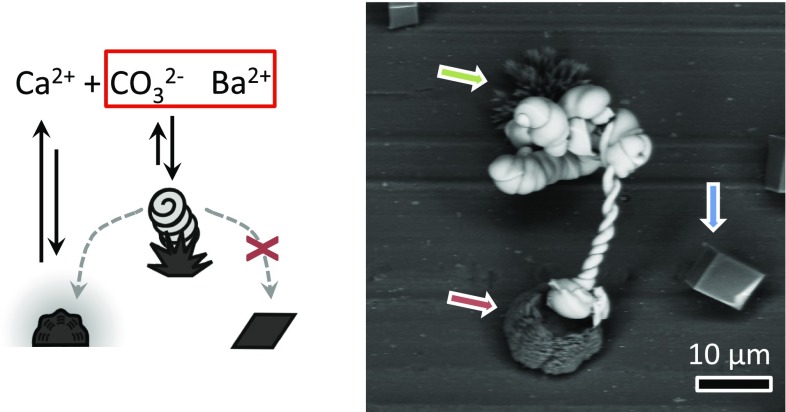
Due the sensitivity of the BaCO3/SiO2 coprecipitation to local gradients, one can further define the growth direction of these sophisticated structures, e.g., spirals, toward locally positioned carbonate sources. We ascertained this by growing BaCO3/SiO2 spirals on a substrate containing the mixture of CaCO3 polymorphs at a depth of ∼1.5 mm. According to Fig. 3B, at this depth the nucleation of BaCO3 preferentially occurs on aragonite whereas neighboring vaterite gradually dissolves, thus causing a local increase in [CO32−] that could attract the growth front of the BaCO3/SiO2 structure and induce its directional chemotaxis. Indeed, we observed that BaCO3/SiO2 spirals first formed on aragonite, and subsequently grew only toward nearby dissolving vaterite, while not being influenced by neighboring calcite (Fig. 4). It should be noted that in the absence of silica, we also found that dissolving vaterite crystals could induce sufficient carbonate concentrations to induce nucleation on nearby calcite and vaterite crystals (SI Appendix, Fig. S2B). Selectively lowering the relative energy barrier for nucleation on one polymorph and creating a nearby local supersaturation by dissolving another polymorph thus provides simultaneous control over both the nucleation on preselected polymorphs and growth direction.
Fig. 4.
Polymorph-specific nucleation and oriented, directional growth. The balance between the local supersaturation and lattice mismatch can be controlled by the influx of CO2 such that nucleation of a BaCO3/SiO2 microstructure first occurs at aragonite (green arrow) and subsequently grows toward the carbonate source released from the nearby dissolving vaterite (red arrow) while leaving nearby calcite unaffected (blue arrow). The dashed arrow in the schematic on the Left represents the direction of growth.
Summary and Conclusions
In conclusion, we have introduced a strategy to control the polymorph-specific positioning of mineralizing compounds and their directional growth using substrates with polymorphic mixtures as crystallization templates. The power of our approach is that merely the influx of carbonate allows us to manipulate the local concentrations of the crystallizing ions within a landscape of different interfacial energies. Therefore, by simultaneously controlling the concentration of carbonate from the bulk solution, the polymorph-dependent interfacial energy contribution from the lattice mismatch, and the substrate solubility, we are able to elicit unusual mineralization pathways unachievable by separate control over these individual parameters. In contrast with commonly used nucleation techniques that are based on lowering of the interfacial free energy only, we can therefore also nucleate selectively on substrates that have an unfavorable crystallographic alignment. Additionally, we can sequentially position different materials on assigned polymorphs and steer the growth of these structures in directions predetermined by the location of neighboring dissolving species. These concepts can perfect our ability to control nucleation and growth of nanostructured materials and may elucidate mechanisms that regulate nano- and microscale phenomena in biomineralization processes.
Methods
Crystallization of CaCO3 Polymorphic Substrates.
For the crystallization of mixed polymorphs of CaCO3, 0.032 g of CaCl2 was dissolved in 15 mL water in a 50- or 100-mL beaker. An aluminum slide (∼15 × 15 × 1 mm), or aluminum-coated microscope glass slide, was vertically positioned in the solution and the beaker was placed in a closed desiccator with freshly ground (NH4)2CO3 following the method developed by Addadi et al. (6). After ∼30–45 min, the slide was removed from the solution, washed twice with water, and then washed with acetone and dried in the air. For details see SI Appendix, section 1.
Overgrowth of a CaCO3 Polymorph Mixture with BaCO3.
In a 50- or 100-mL beaker, we dissolved 0.074 g BaCl2 in 15 mL water and adjusted the pH to 11.9 using NaOH. A previously prepared slide with a mixture of calcite, aragonite, and vaterite (see above) was positioned at a 90° angle in the solution such that a half of the previously overgrown area (shown schematically as a green line in SI Appendix, Fig. S2A, Left) was submerged. A Petri dish was loosely placed on the beaker, allowing for the CO2 from the air to enter the beaker. After ∼30–45 min, the slide was removed from the solution, washed twice with water, and then washed with acetone and subsequently dried. Optical microscopy and SEM were used to analyze the slide and quantify the crystallization of the BaCO3 on the CaCO3 mixture. For details see SI Appendix, section 2 and for overgrowth with SrCO3 see SI Appendix, section 3.
Overgrowth of a CaCO3 Polymorph Mixture with BaCO3/SiO2 Microstructures.
In a 50- or 100-mL beaker, we dissolved 0.074 g of BaCl2 and 0.016 g of Na2SiO3 in 15 mL water. To grow stem, vase, and coral-shaped structures, the pH was adjusted to pH 11.9 whereas for growth helices the pH was adjusted to 11.1 (30). A previously prepared slide with a mixture of calcite, aragonite, and vaterite (see above) was positioned at 90° in the solution such that half of the previously overgrown area was submerged in the solution. A Petri dish was loosely placed on the beaker such that CO2 from the air could enter the beaker. After ∼45–120 min, the slide was removed from the solution, washed twice with water, and then washed with acetone and dried in the air. Optical microscopy and SEM were used to analyze the results.
Overgrowth of a CaCO3 Polymorph Mixture with CaCO3/PAA Microstructures.
In a 50- or 100-mL beaker, we dissolved 0.032 g of CaCl2 and between 60 and 120 μg/mL poly (α,l-aspartate) (Sigma-Aldrich) in 15 mL water. A previously prepared slide with a mixture of calcite, aragonite, and vaterite (see above) was positioned at 90° in the solution such that half of the previously overgrown area was submerged. The beaker was placed in a closed desiccator with freshly ground (NH4)2CO3 and placed in an oven at 45 °C. After ∼45–120 min, the slide was removed from the solution, washed twice with water, and then washed with acetone and dried in the air. Optical microscopy, Raman microscopy, and SEM were used to analyze the slide.
FIB/TEM Procedure.
Samples were coated with Au (∼5 nm) to reduce charging effects before FIB milling with a Helios Nanolab 660 dual-beam electron microscope (FEI). Typical TEM sample preparation procedure was as follows: (i) a platinum protective layer (∼0.5 μm) was first laid down on top of the desired structure; (ii) another platinum protective layer (∼1.5 μm) was further deposited on top of the rectangular region where the TEM slab was to be milled out; (iii) two sides of the coated structure were milled away by FIB, leaving the slab of specimen (thickness: ∼1.5 μm); (iv) the slab was then cut through by FIB and transferred to a copper TEM grid by an Omniprobe and welded securely with platinum deposition; (v) the lift-out lamellar of specimen was sequentially thinned by FIB at 30-, 16-, 5-, and 2-kV ion beam voltages. Final cleaning at 2 kV and 28 pA is important to obtain a clean surface and minimize damage. TEM imaging was carried out using a JEOL 2011 operated at 120 kV. See SI Appendix, sections 6–8 for detailed figures, analysis of the crystal structure, and calculation of the crystallographic mismatch.
Supplementary Material
Acknowledgments
Dr. Liesbeth Janssen and Prof. Pieter Rein ter Wolde are kindly acknowledged for help with the manuscript. This research was supported by the NSF Designing Materials to Revolutionize and Engineer our Future under Award 15-33985 and the Harvard Materials Research Science and Engineering Centers under Award DMR 14-20570. W.L.N. thanks the Netherlands Organization for Scientific Research (NWO) for financial support from a VENI grant. L.L. thanks the Department of Mechanical Engineering at Virginia Polytechnic Institute and State University for support. Electron microscopy and FIB milling was performed at the Center for Nanoscale Systems at Harvard University, supported by the NSF under Award ECS-0335765, and at the Amsterdam nanoCenter, which was financially supported by NWO.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712911115/-/DCSupplemental.
References
- 1.De Yoreo JJ, et al. CRYSTAL GROWTH. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science. 2015;349:aaa6760. doi: 10.1126/science.aaa6760. [DOI] [PubMed] [Google Scholar]
- 2.Wegst UGK, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nat Mater. 2015;14:23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen MH, Aloni S, De Yoreo JJ. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science. 2014;345:1158–1162. doi: 10.1126/science.1254051. [DOI] [PubMed] [Google Scholar]
- 4.Smeets PJM, Cho KR, Kempen RGE, Sommerdijk NAJM, De Yoreo JJ. Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nat Mater. 2015;14:394–399. doi: 10.1038/nmat4193. [DOI] [PubMed] [Google Scholar]
- 5.Addadi L, Weiner S. Interactions between acidic proteins and crystals: Stereochemical requirements in biomineralization. Proc Natl Acad Sci USA. 1985;82:4110–4114. doi: 10.1073/pnas.82.12.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addadi L, Moradian J, Shay E, Maroudas NG, Weiner S. A chemical model for the cooperation of sulfates and carboxylates in calcite crystal nucleation: Relevance to biomineralization. Proc Natl Acad Sci USA. 1987;84:2732–2736. doi: 10.1073/pnas.84.9.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heywood BR, Mann S. Template-directed nucleation and growth of inorganic materials. Adv Mater. 1994;6:9–20. [Google Scholar]
- 8.Berman A, et al. Total alignment of calcite at acidic polydiacetylene films: Cooperativity at the organic-inorganic interface. Science. 1995;269:515–518. doi: 10.1126/science.269.5223.515. [DOI] [PubMed] [Google Scholar]
- 9.Litvin AL, Valiyaveettil S, Kaplan DL, Mann S. Template-directed synthesis of aragonite under supramolecular hydrogen-bonded Langmuir monolayers. Adv Mater. 1997;9:124–127. [Google Scholar]
- 10.Aizenberg J, Black AJ, Whitesides GM. Control of crystal nucleation by patterned self-assembled monolayers. Nature. 1999;398:495–498. [Google Scholar]
- 11.Aizenberg J. Crystallization in patterns: A bio-inspired approach. Adv Mater. 2004;16:1295–1302. [Google Scholar]
- 12.Pokroy B, Zolotoyabko E. 2005. Aragonite growth on single-crystal substrates displaying a threefold axis. Chem Commun (Camb) 2140–2142.
- 13.Gibbs JW. On the equilibrium of heterogeneous substances. Trans Conn Acad Arts Sci. 1876;3:108–248. [Google Scholar]
- 14.Chernov AA. Modern Crystallography III: Crystal Growth. Springer; Berlin: 1984. pp. 48–103. [Google Scholar]
- 15.De Yoreo JJ, Vekilov PG, Dove PM, Weiner S, editors. Biomineralization. Mineralogical Society of America; Washington, DC: 2003. Principles of crystal nucleation and growth; pp. 57–93. [Google Scholar]
- 16.Bernstein J. Polymorphism in Molecular Crystals. Oxford Univ Press; Oxford: 2007. [Google Scholar]
- 17.Lowenstam HA, Weiner S. On Biomineralization. Oxford Univ Press; Oxford: 1989. [Google Scholar]
- 18.Mann S, Ozin GA. Synthesis of inorganic materials with complex form. Nature. 1996;382:313–318. [Google Scholar]
- 19.De Yoreo JJ, Vekilov PG. Principles of crystal nucleation and growth. Rev Mineral Geochem. 2003;54:57–93. [Google Scholar]
- 20.Mann S. Biomineralization. Oxford Univ Press; Oxford: 2001. [Google Scholar]
- 21.Sommerdijk NAJM, de With G. Biomimetic CaCO3 mineralization using designer molecules and interfaces. Chem Rev. 2008;108:4499–4550. doi: 10.1021/cr078259o. [DOI] [PubMed] [Google Scholar]
- 22.Kabalah-Amitai L, et al. Vaterite crystals contain two interspersed crystal structures. Science. 2013;340:454–457. doi: 10.1126/science.1232139. [DOI] [PubMed] [Google Scholar]
- 23.Kim IW, Robertson RE, Zand R. Selected polymorphs of CaCO3 through epitaxy with inorganic substrates aligned with an electric field. Adv Mater. 2003;15:709–712. [Google Scholar]
- 24.Plummer LN, Wigley TML, Parkhurst DL. The kinetics of calcite dissolution in CO2 -water systems at 5 degrees to 60 degrees C and 0.0 to 1.0 atm CO2. Am J Sci. 1978;278:179–216. [Google Scholar]
- 25.Chou L, Garrels RM, Wollast R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem Geol. 1989;78:269–282. [Google Scholar]
- 26.Compton RG, Pritchard KL. The dissolution of calcite at pH > 7: Kinetics and mechanism. Philos Trans R Soc A. 1990;330:47–70. [Google Scholar]
- 27.Inskeep WP, Bloom PR. An evaluation of rate equations for calcite precipitation kinetics at pCO2 less than 0.01 atm and pH greater than 8. Geochim Cosmochim Acta. 1985;49:2165–2180. [Google Scholar]
- 28.García-Ruiz JM, Amoros JL. Morphological aspects of some symmetrical aggregates grown by silica gel technique. J Cryst Growth. 1981;55:379–383. [Google Scholar]
- 29.García-Ruiz JM, et al. Self-assembled silica-carbonate structures and detection of ancient microfossils. Science. 2003;302:1194–1197. doi: 10.1126/science.1090163. [DOI] [PubMed] [Google Scholar]
- 30.Noorduin WL, Grinthal A, Mahadevan L, Aizenberg J. Rationally designed complex, hierarchical microarchitectures. Science. 2013;340:832–837. doi: 10.1126/science.1234621. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan CN, et al. Controlled growth and form of precipitating microsculptures. Science. 2017;355:1395–1399. doi: 10.1126/science.aah6350. [DOI] [PubMed] [Google Scholar]
- 32.Gower LA, Tirrell DA. Calcium carbonate films and helices grown in solutions of poly(aspartate) J Cryst Growth. 1998;191:153–160. [Google Scholar]
- 33.Sims SD, Didymus JM, Mann S. Habit modification in synthetic crystals of aragonite and vaterite. J Chem Soc Chem Commun. 1995;10:1031–1032. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.