Significance
Unlike another effector protein for algal nonphotochemical quenching (NPQ)—LIGHT HARVESTING COMPLEX II STRESS RELATED PROTEIN 3 (LHCSR3)—the role of LHCSR1 in NPQ has been very limited. In this report, we studied the fluorescence quenching event occurring in the presence and the absence of LHCSR1 and demonstrated that there is a significant excitation energy transfer from Light-harvesting complex II (LHCII) to Photosystem I (PSI), and not only to Photosystem II, upon activation of LHCSR1 by low pH. The results suggest another layer of photoprotection mechanism based on this UV-inducible protein LHCSR1.
Keywords: photosynthesis, algae, stress, light, fluorescence
Abstract
Photosynthetic organisms are frequently exposed to light intensities that surpass the photosynthetic electron transport capacity. Under these conditions, the excess absorbed energy can be transferred from excited chlorophyll in the triplet state (3Chl*) to molecular O2, which leads to the production of harmful reactive oxygen species. To avoid this photooxidative stress, photosynthetic organisms must respond to excess light. In the green alga Chlamydomonas reinhardtii, the fastest response to high light is nonphotochemical quenching, a process that allows safe dissipation of the excess energy as heat. The two proteins, UV-inducible LHCSR1 and blue light-inducible LHCSR3, appear to be responsible for this function. While the LHCSR3 protein has been intensively studied, the role of LHCSR1 has been only partially elucidated. To investigate the molecular functions of LHCSR1 in C. reinhardtii, we performed biochemical and spectroscopic experiments and found that the protein mediates excitation energy transfer from light-harvesting complexes for Photosystem II (LHCII) to Photosystem I (PSI), rather than Photosystem II, at a low pH. This altered excitation transfer allows remarkable fluorescence quenching under high light. Our findings suggest that there is a PSI-dependent photoprotection mechanism that is facilitated by LHCSR1.
Solar energy is essential for photosynthetic organisms, but the amount of light frequently exceeds the capacity of photochemical reactions, leading to potentially serious photodamage to the photosystems (1). To minimize the harmful effects of excess light-dependent reactions, photosynthetic organisms have established protection mechanisms referred to as nonphotochemical quenching (NPQ) (2). One of the NPQ mechanisms, energy-dependent quenching, can be activated rapidly (within a minute) under high-intensity light conditions to safely convert light energy into thermal energy (3). Utilizing this mechanism, photosynthetic organisms, including land plants and aquatic algae, can survive in natural light environments.
Certain key molecules, PSBS and LHCSRs, are responsible for NPQ (2). These molecules represent a family of light-harvesting complexes (LHCs) that are used in photosynthesis, while other LHCs (LHCI and LHCII) function as antennae for the photosystems (PSI and PSII). PSBS and LHCSRs have been identified in land plants (4, 5), mosses (6), and eukaryotic algae (7). Mutants deficient in these proteins are highly stressed under high light. In contrast to land plants, which constitutively express PSBS (4), the model green alga Chlamydomonas reinhardtii inducibly expresses LHCSRs (LHCSR3 and LHCSR1) when exposed to specific colors of light (8). Interestingly, although LHCSR3 and LHCSR1 are induced by different colors of light, they behave similarly to NPQ effectors under high-light conditions in C. reinhardtii (9, 10).
The molecular functions and physiological role of LHCSR3 in C. reinhardtii have been extensively studied during the past decade. Bonente et al. (11) suggested that LHCSR3 is itself a quencher and does not require interaction with other photosynthetic protein complexes. Reconstituted LHCSR3 isolated from Escherichia coli is capable of quenching light energy under conditions similar to high-light conditions (low-pH buffer). This previous report also showed that there are photosynthetic pigments (chlorophylls, xanthophylls) associated with the protein, strongly suggesting that the protein itself can be a direct energy quencher. We previously reported that LHCSR3 can associate with PSII−LHCII supercomplexes (12, 13), likely mediated by the PSII subunit PSBR (14), thereby contributing to low-pH-inducible energy quenching in PSII. LHCSR3 is also known to be protonated due to high light-dependent thylakoid luminal acidification as well as other light-harvesting proteins (11, 12). Ballottari et al. (15) reported that mutants with modified amino acid residues in LHCSR3 were incapable of efficient NPQ and showed that protonation of three residues exposed to the thylakoid lumen side are essential for quenching.
Although LHCSR1, a paralog of LHCSR3, significantly contributes to the NPQ process (16, 17), this protein has not been sufficiently investigated to date. In addition to characterizing recombinant LHCSR3, Bonente et al. (11) investigated the LHCSR1 protein expressed in E. coli; however, the obtained yields were insufficient for further characterization. The induction conditions for this gene were discovered very recently (9), and there is a report suggesting that LHCSR1 triggers pH-dependent quenching in vivo (18). In this reported study, Croce and coworkers (18) used a vitamin repressor system to completely eliminate photosystem core components (PSII and PSI) while maintaining the LHCs (and LHCSR1), allowing functional analysis of LHCSR1 in vivo. When the authors measured chlorophyll fluorescence, they found that the cells containing both LHCs and LHCSR1 without the photosystems exhibited low-pH-inducible NPQ. Thus, they concluded that the excitation energy quenching triggered by LHCSR1 occurs in free (non-photosystem-associated) LHCIIs.
The LHCSR1 sequence of the moss Physcomitrella patens does not directly correspond to LHCSR1 in C. reinhardtii but is equally related to LHCSR1 and LHCSR3 in green algae (19). Recently, Kondo et al. (20) reported a reconstituted P. patens LHCSR1 protein, as revealed via single-molecule spectroscopy. They showed that the protein exhibits both pH-dependent and carotenoid-dependent energy dissipative states and therefore concluded that the protein itself is capable of controlling quenching dynamics during photoprotective energy dissipation. These findings suggested molecular functions of the LHCSR1 found in moss, but the moss protein is clearly distinct from LHCSR1 in C. reinhardtii. Therefore, the molecular functions of LHCSR1 in the green alga remain unclear.
The molecular mechanism of LHCSR1-dependent NPQ induction in C. reinhardtii is still poorly understood. To elucidate how LHCSR1 activates NPQ, we characterized excitation energy dynamics in thylakoid membranes isolated from C. reinhardtii. Our results show that, at low pH, there is energy transfer from LHCII to PSI, mediated by LHCSR1. Time-resolved chlorophyll fluorescence analysis of mutants lacking the photosystems revealed remarkable activation of LHCSR1-dependent fluorescence quenching by PSI. We propose that LHCSR1 in C. reinhardtii activates PSI-dependent fluorescence quenching in addition to dissipating excitation energy in LHCIIs to avoid photooxidative stress under excess light.
Results
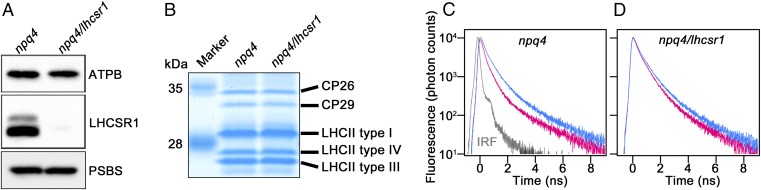
To evaluate the amplitude of LHCSR1-dependent fluorescence quenching in vitro, we first attempted to isolate the thylakoid membranes from LHCSR1-expressing C. reinhardtii strains. UV treatment is one of the most effective methods for inducing LHCSR1 (9). Therefore, we applied UV treatment before thylakoid membrane isolation from four different strains: WT, lhcsr1 (LHCSR1-lacking mutant), npq4 (LHCSR3-lacking mutant), and npq4/lhcsr1. Consistent with a previous report (9), the WT and lhcsr1 strains showed clear accumulation of the LHCSR3 protein, as the UV treatment was also effective for inducing LHCSR3 expression (Fig. S1). In contrast, neither the npq4 nor the npq4/lhcsr1 strain, which lack the LHCSR3 gene (7), showed an LHCSR3 signal (Fig. 1A and Fig. S1). Moreover, the accumulations of PSBS and the other LHCIIs between npq4 and npq4/lhcsr1 strains were comparable (Fig. 1 A and B), suggesting that the difference in NPQ (Fig. S2) between these two strains is largely based on the presence or absence of LHCSR1 protein expression. To avoid the contribution of LHCSR3 in further analyses, we focused on the npq4 and npq4/lhcsr1 strains.
Fig. 1.
Time-resolved fluorescence analysis of the isolated thylakoid membranes. (A) Immunoblotting analysis of purified thylakoid membranes from UV-treated cells using antibodies against either ATPB or LHCSRs, or PSBS. (B) Thylakoid membrane samples were analyzed by SDS/PAGE stained by Coomassie Brilliant Blue G-250. The LHCII bands were indicated as CP26 (Lhcb5), CP29 (Lhcb4), LHCII type I (LhcbM3, LhcbM 4, LhcbM 6, LhcbM 8, LhcbM 9); LHCII type III (LhcbM2, -7), or LHCII Type IV (LhcbM1). (C and D) The time-correlated single-photon counting of fluorescence for the thylakoids of (C) npq4 and (D) npq4/lhcsr1 were recorded at 682 nm (slit = 8 nm) at pH 5.5 (red) and 7.5 (blue). Instrumental response function (IRF) is shown as gray line in C. The samples, normalized to 1 μg Chl/mL, were excited at 463 nm.
To investigate pH-inducible fluorescence quenching in the isolated thylakoid membranes from the npq4 and npq4/lhcsr1 mutants, we performed room-temperature fluorescence decay analysis. The isolated thylakoids from npq4 showed a drastic decrease in the fluorescence lifetime when treated with acidic buffers (pH 5.5 in Fig. 1C). The npq4/lhcsr1 thylakoids, on the other hand, showed only a small decrease in fluorescence decay from pH 7.5 to pH 5.5 (Fig. 1D). These data suggest that the function of the LHCSR1 protein (i.e., low-pH-dependent energy quenching) can be observed not only in vivo (Fig. S2) but also in isolated thylakoid membranes.
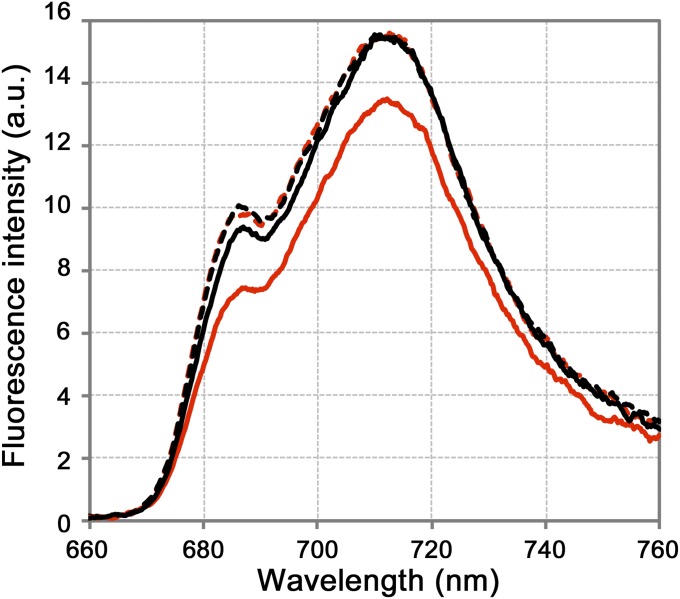
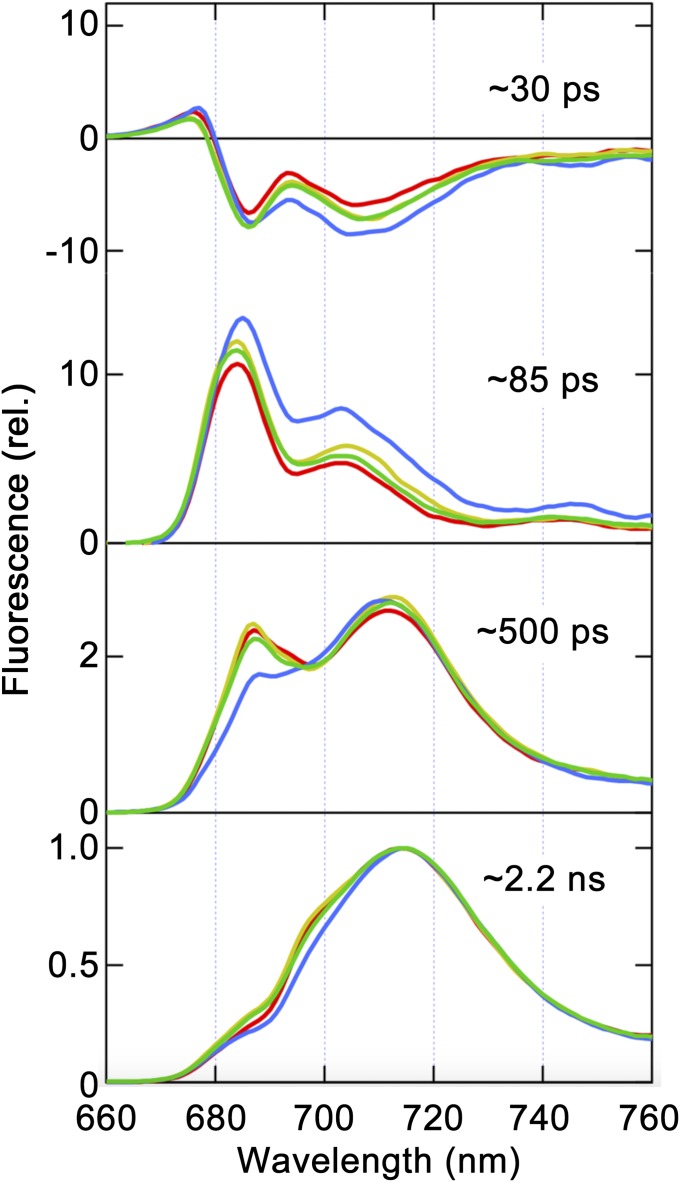
Since isolated npq4 thylakoids exhibited LHCSR1-dependent quenching at low pH, we next attempted to characterize the quenching mechanism via both steady-state and time-resolved fluorescence spectra analyses at low temperature (77 K). Consistent with the changes in room-temperature fluorescence decay observed using buffers with different pH levels (shown in Fig. 1C), the measurement of 77 K steady-state fluorescence spectra revealed that npq4 thylakoids showed a lower fluorescence intensity when exposed to low-pH buffer (Fig. 2, solid red line), whereas the npq4/lhcsr1 thylakoids did not show lower fluorescence (Fig. 2, dashed red line). Although we observed LHCSR1-mediated fluorescence quenching in thylakoid membranes, as shown above, the details of the excitation energy transfer dynamics in thylakoid membranes still need to be characterized. To characterize excitation energy dynamics, fluorescence decay kinetics at wavelengths of 660 nm to 760 nm were measured upon excitation at 459 nm, which predominantly excited LHCII, and then subjected to a global fitting analysis to identify the decay components [fluorescence decay associated spectra (FDAS) analysis shown in Fig. 3, Fig. S4, and Table S1; see Materials and Methods]. When we treated the thylakoid membranes with acidic buffer, a remarkable expansion of the negative peak around 710 nm to 720 nm was observed in the first FDAS (τ = ∼30 ps) of npq4 (Fig. 3, blue line), indicating greater energy transfer to PSI and/or energy dissipation in LHCIIs under acidic conditions. In addition, the relative amplitudes of PSII fluorescence in the third FDAS decreased, indicating faster excitation energy quenching around PSII in npq4 under low pH. Although the fluorescence lifetime components showed similar lifetimes (Table S1), the shortening of the average lifetime for npq4 at pH 5.5 (Fig. S4, solid red line) was expressed as a combination of increases in amplitudes of the 85-ps component and decreases in those of the 500-ps component (second and third FDAS in Fig. 3, blue line). Indeed, in the fourth FDAS (∼2.2 ns), which is assigned to fluorescence from the final energy traps in PSII (685 nm to 700 nm) and PSI, the PSII amplitude was reduced relative to that of PSI in npq4 (Fig. 3, blue line). This finding implies that both excitation energy dissipation at LHCII and excitation energy transfer from LHCII to PSI become more active under lower pH in the presence of the LHCSR1 protein (npq4) but not in the absence of the LHCSR1 protein (npq4/lhcsr1). The latter excitation dynamics may reflect the increase in excitation energy transfer from LHCII to PSI, rather than to PSII. These observations led us to hypothesize that LHCSR1-dependent fluorescence quenching is specifically correlated with excitation energy transfer from LHCII to PSI.
Fig. 2.
Low-temperature absolute fluorescence spectra of isolated thylakoid membranes. Fluorescence spectra of the isolated thylakoid membranes from npq4 (solid line) and npq4/lhcsr1 (dashed line). The membranes were treated with either pH 7.5 (black) or pH 5.5 (red) buffers. The fluorescence spectra were recorded with an integration sphere to obtain the absolute fluorescence photon counts for the samples. Samples normalized to 8 μg Chl/mL were excited at 480 nm.
Fig. 3.
Time-resolved fluorescence decay-associated spectrum analysis of isolated thylakoid membranes at 77 K. FDAS were derived from the time-resolved fluorescence profiles of thylakoid membranes obtained via excitation at 459 nm. The colored lines represent npq4 at pH 7.5 (green) and pH 5.5 (blue) and npq4/lhcsr1 at pH 7.5 (yellow) and pH 5.5 (red). The spectra were normalized to the maximum intensity of the slowest component (∼2.2 ns).
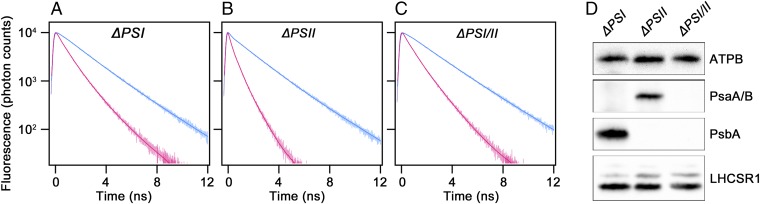
The marked excitation energy transfer from LHCII to PSI observed under low pH implies a contribution of PSI to LHCSR1-dependent fluorescence quenching. To obtain direct evidence that PSI contributes to quenching, we used photosystem mutants and conducted further spectroscopic measurements. Because visible light (photosynthetically active radiation) is not required for LHCSR1 expression (9), UV treatment of these strains successfully induced LHCSR1 protein expression, even though the strains are incapable of photosynthesis (Fig. 4). On the other hand, the photosystem-lacking mutants cannot form ΔpH, a trigger for energy quenching, due to a deficiency of the light-driven proton flux. We therefore applied the previously reported pH adjustment method (18) as follows. After UV treatment for LHCSR1 protein expression, the pH of all strains was adjusted to 5.5 or 7.5, using acetic acid or sodium hydroxide, respectively. All strains showed low-pH (acetic acid)-inducible energy quenching, as demonstrated by rapid fluorescence decay (Fig. 4 A–C). The mutants lacking PSI (ΔPSI and ΔPSI/II) exhibited relatively small changes in the average fluorescence lifetime (τave) between different pH levels compared with the difference observed in ΔPSII (Table 1). The amplitudes of these τave changes in the ΔPSI and ΔPSI/II strains were also calculated as pH-inducible quenching, and ∼46% and ∼51% of chlorophyll fluorescence was shown to be quenched at low-pH in the ΔPSI and ΔPSI/II strains, respectively. These quenching amplitudes are comparable to the amplitude observed for the LHCII+LHCSR1 cells in a previous study (∼50% in ref. 18), implying that energy dissipation occurs at LHCII and is mediated by LHCSR1 in both the ΔPSI and ΔPSI/II strains. In contrast, the ΔPSII strain showed a remarkable amplitude of low-pH-inducible quenching (∼74%, Table 1), although the protein expression levels of both LHCSR1 and LHCIIs in the mutant were almost identical to the levels found in other mutants (Fig. 4D and Fig. S6). In addition, the ΔPSII strain showed relatively less expression of LHCSR3 among the mutants (Fig. S5), strongly implying that the large fluorescence quenching observed in the mutant (ΔPSII in Fig. 4 and Table 1) is not significantly contributed by the LHCSR3 protein. FDAS of the ΔPSII strain also indicates that the PSI-related peak at around 710 nm in the first component (20 ps to 30 ps) became larger in the ΔPSII at low pH, while the other strains (ΔPSI and ΔPSI/II) showed little change in this region (Fig. S3, ΔPSII). Following the fast component, positive fluorescence peaks at 710 nm in the second (120 ps) and the third (500 ps) components increased only in the ΔPSII. These results support an efficient excitation energy transfer to PSI from LHCII at low pH. Taking into account that only the ΔPSII strain harbored PSI (Fig. 4D, PsaA/B signals), the large quenching observed in this strain was most likely dependent on the PSI machinery. We also estimated the NPQ capability of the strains, which was calculated with τave in both pH environments [Table 1, NPQcalc = τave (pH 7.5)/τave (pH 5.5) − 1]. These values showed that the ΔPSII strain exhibited a large degree of quenching (NPQcalc = ∼2.8) compared with the other strains (NPQcalc = ∼0.85 in ΔPSI and ∼1.0 in ΔPSI/II). Based on these results, we conclude that LHCSR1-mediated fluorescence quenching under acidic conditions is stimulated by excitation energy dissipation among LHCIIs and efficient excitation energy transfer from LHCII to PSI in C. reinhardtii.
Fig. 4.
In vivo characterization of photosystem mutants. (A−C) The time-correlated single-photon counting of the fluorescence of (A) ΔPSI, (B) ΔPSII, and (C) ΔPSI/II cells after 6 h of UV treatment were recorded at 682 nm (slit = 8 nm) at pH 5.5 (red) and 7.5 (blue). The samples, normalized to 2 μg Chl/mL, were excited at 480 nm. (D) UV-treated cells (2 μg Chl) were subjected to immunoblotting analysis with antibodies specific to ATPB, PsaA/B (PSI), PsbA (D1), and LHCSR1.
Table 1.
Estimated pH-inducible quenching in vivo
| Average fluorescence lifetime (τave), ns | ||||
| Strain name | At pH 7.5 | At pH 5.5 | pH-inducible quenching, % | NPQcalc |
| ∆PSI | 2.21 ± 0.22 | 1.19 ± 0.04 | 45.8 ± 0.05 | 0.85 ± 0.18 |
| ∆PSII | 2.29 ± 0.15 | 0.60 ± 0.08 | 73.9 ± 0.02 | 2.84 ± 0.27 |
| ∆PSI/II | 2.39 ± 0.13 | 1.17 ± 0.05 | 50.8 ± 0.03 | 1.04 ± 0.12 |
The efficiency of pH-inducible energy quenching was calculated as 1 − τave (pH 5.5)/τave (pH 7.5) (%). NPQcalc = τave (pH 7.5)/τave (pH 5.5) − 1; n = 3 biological replicates, mean ± SE.
Discussion
NPQ is the mechanism of feedback regulation for excess photosystem excitation, which functions by dissipating absorbed light energy as heat (21). This mechanism is based on the contributions of two stress-related LHC proteins, LHCSR1 and LHCSR3, in C. reinhardtii (7, 9, 16). Although LHCSR3 has been well studied, information about the molecular functions of LHCSR1 in this alga is limited. In addition to the energy dissipation at LHCII as reported previously (18), we provide evidence that LHCSR1-dependent fluorescence quenching is mediated by excitation energy transfer from LHCII to PSI.
Acidic pH conditions in the thylakoid lumen triggered fluorescence quenching in the double mutant lacking both photosystems (see ΔPSI/II in Fig. 4 and Table 1). To estimate the potential amplitude of NPQ in the mutants, we calculated NPQcalc using the average fluorescence lifetime obtained at pH 5.5 and 7.5 (Table 1, NPQcalc). The ΔPSI/II mutant showed a similar quenching ability (NPQcalc near to 1.0) to that observed by Dinc et al. (18) in the LHCII+LHCSR1 cells. These results strongly suggest that LHCSR1 activates quenching at LHCIIs, even in the absence of photosystems, which is consistent with a previous report proposing that LHCSR1-mediated quenching occurs at free LHCII (18).
In addition to the quenching of free LHCIIs described above, the results of time-resolved FDAS measurements implied that C. reinhardtii controls the transfer of excitation energy from LHCII to PSI via UV-inducible LHCSR1 at low pH (Fig. 2 and Fig. S3). Although this excitation energy transfer from LHCII to PSI contributes to additional layer of fluorescence quenching, its underlying mechanism is not energy dissipation but PSI charge separation (Fig. 3, Fig. S4, and Table S1).
In our study, we observed even a larger quenching when both PSI and LHCSR1 present in the cells (NPQcalc = 2.8 in ΔPSII, Table 1), suggesting PSI-dependent fluorescence quenching in C. reinhardtii. FDAS of the npq4 and ΔPSII strains at low pH shows a clear increase of the PSI fluorescence (700 nm to 710 nm) after excitation energy transfer from LHCII to PSI (see the second and third component of FDAS in Fig. 3 and Fig. S3). The observed lifetimes are similar to those reported previously, representing charge separation at PSI (22, 23). It is well known that, compared with PSII, PSI exhibits very low chlorophyll fluorescence emission at room temperature, due to its efficient energy excitation−relaxation turnover (24, 25). This phenomenon indicates that PSI exhibits shorter lifetime of excited singlet chlorophyll (1Chl*) and lower frequencies of conversion to a triplet chlorophyll (3Chl*) state, which leads to harmful singlet oxygen (1O2*) formation (26). In other words, it is reasonable to use PSI as a quencher when excess light energy accumulates around PSII.
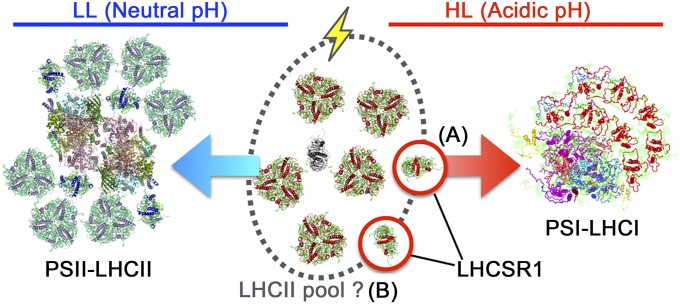
Based on our findings, we propose a tentative model of LHCSR1-mediated NPQ in C. reinhardtii (Fig. 5). When the thylakoid lumen becomes acidified under a high light intensity, LHCSR1 and LHCSR3 may sense the change in pH (15, 18). LHCSR1 plays two distinct roles in transferring excitation energy to PSI−LHCI supercomplexes (Fig. 5 process A and this study) or free LHCII (Fig. 5B process B, ref. 18, and this study). As a result, excess light energy harvested by LHCIIs is safely trapped by PSI and/or dissipated at free LHCIIs, if any. Although we present a fluorescence quenching mechanism mediated by LHCSR1, it is still unclear whether the LHCSR1 protein associates with photosynthetic pigments such as chlorophylls and/or carotenoids. It is also not clear where it localizes within the thylakoid membranes, and it is unknown whether the protein itself exhibits quenching ability. To answer these questions, more specific biochemical techniques using both native and recombinant LHCSR1 protein complexes will be required, as in Bonente et al. (11).
Fig. 5.
Tentative model of LHCSR1-dependent energy quenching in C. reinhardtii. The light captured by LHCIIs is transferred to the PSII−LHCII or PSI−LHCI supercomplexes under LL (representing neutral pH in the lumen, blue arrow) or HL (representing acidic pH in the lumen, red arrow), respectively. When the lumen is acidified, LHCSR1 (process A) mediates excitation energy transfer from LHCIIs in the pool to PSI-LHCI (this study) and/or (process B) triggers energy-dependent quenching in the LHCII pool (18). Icons surrounded by a red line represent LHCSR1.
Recently, an NPQ effector zeaxanthin was modeled at an atomic resolution in PSI of land plants (27, 28). Although there has been debate about the contribution of zeaxanthin to PSI quenching, the detailed molecular mechanisms of the fluorescence quenching in land plants have been reported (29, 30). Direct excitation energy quenching by LHCSRs surrounding PSI has also been observed in moss (31), implying that the quenching around PSI could be conserved in the green lineage. Our findings also show fluorescence quenching via excitation energy transferred from LHCII to PSI (Fig. 3 and Fig. S3), and the LHCSR1-mediated mechanisms thus can reduce the excitation of PSII at the cost of increasing PSI excitation. Taken together with the PSI quenching established in land plants and mosses, it is plausible that LHCSR1-mediated fluorescence quenching by PSI in green algae is the primitive photoprotection mechanism of green photosynthetic eukaryotes.
Materials and Methods
Culture Conditions.
The C. reinhardtii strain 137c (mt+) was obtained from the Chlamydomonas Center (https://www.chlamycollection.org/) and was used as the WT strain. The mutant strains npq4 and npq4/lhcsr1 were isolated in previous reports (7, 9, 15, 16) and were then backcrossed with the WT strain at least three times. The ΔPSI (ΔPsaA) and ΔPSII (Fud7 as ΔPsbA) strains were obtained as described previously (32). The ΔPSI/II mutant was generated in a previous study (33). All strains were grown in Tris-acetate-phosphate medium (34) under dim light (<20 μE⋅m−2⋅s−1) at 23 °C until they reached the midlog growth phase. The culture medium was subsequently exchanged with high-salt (HS) minimal medium (35) before experimental light treatment. The strains in HS medium were treated with fluorescent light including UV. A T8 ReptiSun 5.0 UVB fluorescent bulb (Zoo Med Laboratories) providing 30 μE⋅m−2⋅s−1 was used to induce LHCSR1 protein expression.
Isolation of Thylakoid Membranes and Immunoblotting Analysis.
Thylakoid membranes from the npq4 and npq4/lhcsr1 strains after UV light treatment were isolated as previously reported (12). Immunoblotting analysis was performed as described previously (32, 36), except that antibodies against either LHCSR1 (AS14 2819) or LHCSR3 (AS14 2766) were used. The antibodies were obtained from Agrisera AB and were premixed before probing.
Spectroscopic Fluorescence Measurements.
NPQ measurements were performed as in a previous study (10). Time-resolved fluorescence decays at room temperature (23 °C) were obtained via time-correlated single-photon counting of fluorescence using a FluoroCube (HORIBA Jobin-Yvon) as described previously. A picosecond pulse diode laser (DD-470L; HORIBA Jobin-Yvon) was used to excite chlorophylls at 463 nm with a 10-MHz repetition rate (1.64 pJ per pulse), and emission was detected at 682 nm through a monochromatic detector (bandwidth = 8 nm). The reversibility of fluorescence quenching was confirmed by pH treatment performed twice in the same sample (Fig. S7). Absolute intensity measurements of low-temperature (77 K) fluorescence emission spectra were conducted using a fluorometer equipped with an integrating sphere (FP-6600/ILFC-543L; JASCO) as described previously (37). To construct FDAS, time-resolved fluorescence decays at 77 K were obtained through time-correlated single-photon counting of fluorescence using an SPC-630 (Becker and Hickl, GmbH). A picosecond pulse diode laser (PiL047X; Advanced Laser Diode Systems) was used to excite chlorophylls at 459 nm with a 3-MHz repetition rate (≤0.1 nJ per pulse), and emission was detected in a wavelength range of 660 nm to 760 nm, with an interval of 1 nm (bandwidth = 2.5 nm). Fluorescence decay curves were analyzed using common lifetimes (38).
Supplementary Material
Acknowledgments
The npq4 and npq4/lhcsr1 strains were kindly provided by Drs. Thuy Truong and Krishna K. Niyogi (University of California, Berkley). We thank Dr. Konomi Kamada and Mrs. Tamaka Kadowaki for technical help with genetic crossing of the alga. We are grateful to Dr. Krishna K. Niyogi for valuable discussions. We also gratefully acknowledge Dr. Peter Jahns for providing the PSBS antibody. Drs. Raymond Burton-Smith and Kenji Takizawa are thanked for critical reading of the manuscript. This work was supported by Grants-in-Aid from Japan Society for the Promotion of Science JP16H06553 (to J.M., R.T., S.A., and E.K.), JP26251033 (to J.M. and R.T.), JP15H05599 (to R.T.), and JP16F16087 (to E.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720574115/-/DCSupplemental.
References
- 1.Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 2.Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol. 2013;16:307–314. doi: 10.1016/j.pbi.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Derks A, Schaven K, Bruce D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim Biophys Acta. 2015;1847:468–485. doi: 10.1016/j.bbabio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Li XP, et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403:391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 5.Li XP, Muller-Moule P, Gilmore AM, Niyogi KK. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA. 2002;99:15222–15227. doi: 10.1073/pnas.232447699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alboresi A, Gerotto C, Giacometti GM, Bassi R, Morosinotto T. Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc Natl Acad Sci USA. 2010;107:11128–11133. doi: 10.1073/pnas.1002873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peers G, et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature. 2009;462:518–521. doi: 10.1038/nature08587. [DOI] [PubMed] [Google Scholar]
- 8.Allorent G, Petroutsos D. Photoreceptor-dependent regulation of photoprotection. Curr Opin Plant Biol. 2017;37:102–108. doi: 10.1016/j.pbi.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Allorent G, et al. UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2016;113:14864–14869. doi: 10.1073/pnas.1607695114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petroutsos D, et al. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature. 2016;537:563–566. doi: 10.1038/nature19358. [DOI] [PubMed] [Google Scholar]
- 11.Bonente G, et al. Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol. 2011;9:e1000577. doi: 10.1371/journal.pbio.1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokutsu R, Minagawa J. Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2013;110:10016–10021. doi: 10.1073/pnas.1222606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim E, Akimoto S, Tokutsu R, Yokono M, Minagawa J. Fluorescence lifetime analyses reveal how the high light-responsive protein LHCSR3 transforms PSII light-harvesting complexes into an energy-dissipative state. J Biol Chem. 2017;292:18951–18960. doi: 10.1074/jbc.M117.805192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue H, et al. PHOTOSYSTEM II SUBUNIT R is required for efficient binding of LIGHT-HARVESTING COMPLEX STRESS-RELATED PROTEIN3 to photosystem II-light-harvesting supercomplexes in Chlamydomonas reinhardtii. Plant Physiol. 2015;167:1566–1578. doi: 10.1104/pp.15.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballottari M, et al. Identification of pH-sensing sites in the light harvesting complex stress-related 3 protein essential for triggering non-photochemical quenching in Chlamydomonas reinhardtii. J Biol Chem. 2016;291:7334–7346. doi: 10.1074/jbc.M115.704601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truong TB. 2011. Investigating the role(s) of LHCSRs in Chlamydomonas reinhardtii. Doctoral dissertation (Univ California, Berkeley, CA)
- 17.Berteotti S, Ballottari M, Bassi R. Increased biomass productivity in green algae by tuning non-photochemical quenching. Sci Rep. 2016;6:21339. doi: 10.1038/srep21339. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Dinc E, et al. LHCSR1 induces a fast and reversible pH-dependent fluorescence quenching in LHCII in Chlamydomonas reinhardtii cells. Proc Natl Acad Sci USA. 2016;113:7673–7678. doi: 10.1073/pnas.1605380113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alboresi A, Caffarri S, Nogue F, Bassi R, Morosinotto T. In silico and biochemical analysis of Physcomitrella patens photosynthetic antenna: Identification of subunits which evolved upon land adaptation. PLoS One. 2008;3:e2033. doi: 10.1371/journal.pone.0002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo T, et al. Single-molecule spectroscopy of LHCSR1 protein dynamics identifies two distinct states responsible for multi-timescale photosynthetic photoprotection. Nat Chem. 2017;9:772–778. doi: 10.1038/nchem.2818. [DOI] [PubMed] [Google Scholar]
- 21.Horton P, Ruban A. Molecular design of the photosystem II light-harvesting antenna: Photosynthesis and photoprotection. J Exp Bot. 2005;56:365–373. doi: 10.1093/jxb/eri023. [DOI] [PubMed] [Google Scholar]
- 22.Gobets B, van Grondelle R. Energy transfer and trapping in photosystem I. Biochim Biophys Acta. 2001;1507:80–99. doi: 10.1016/s0005-2728(01)00203-1. [DOI] [PubMed] [Google Scholar]
- 23.van Amerongen H, van Grondelle R, Valkunas L. Photosynthetic Excitons. World Sci; Singapore: 2000. Excitation energy transfer and trapping: Experiments; pp. 449–478. [Google Scholar]
- 24.Savikhin A. Photosystem I: The Light-Driven Plastocyanin. Ferredoxin Oxidoreductase. Vol 24. Springer; Dordrecht, The Netherlands: 2006. Ultrafast optical spectroscopy of photosystem I; pp. 155–175. [Google Scholar]
- 25.Croce R, van Amerongen H. Light-harvesting in photosystem I. Photosynth Res. 2013;116:153–166. doi: 10.1007/s11120-013-9838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger-Liszkay A. Singlet oxygen production in photosynthesis. J Exp Bot. 2005;56:337–346. doi: 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- 27.Mazor Y, Borovikova A, Nelson N. The structure of plant photosystem I super-complex at 2.8 Å resolution. eLife. 2015;4:e07433. doi: 10.7554/eLife.07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin X, Suga M, Kuang T, Shen JR. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science. 2015;348:989–995. doi: 10.1126/science.aab0214. [DOI] [PubMed] [Google Scholar]
- 29.Ballottari M, et al. Regulation of photosystem I light harvesting by zeaxanthin. Proc Natl Acad Sci USA. 2014;111:E2431–E2438. doi: 10.1073/pnas.1404377111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian L, Xu P, Chukhutsina VU, Holzwarth AR, Croce R. Zeaxanthin-dependent nonphotochemical quenching does not occur in photosystem I in the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA. 2017;114:4828–4832. doi: 10.1073/pnas.1621051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinnola A, et al. Light-harvesting complex stress-related proteins catalyze excess energy dissipation in both photosystems of Physcomitrella patens. Plant Cell. 2015;27:3213–3227. doi: 10.1105/tpc.15.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokutsu R, Kato N, Bui KH, Ishikawa T, Minagawa J. Revisiting the supramolecular organization of photosystem II in Chlamydomonas reinhardtii. J Biol Chem. 2012;287:31574–31581. doi: 10.1074/jbc.M111.331991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwai M, Yokono M, Inada N, Minagawa J. Live-cell imaging of photosystem II antenna dissociation during state transitions. Proc Natl Acad Sci USA. 2010;107:2337–2342. doi: 10.1073/pnas.0908808107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorman DS, Levine RP. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sueoka N. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1960;46:83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno Y, Aikawa S, Kondo A, Akimoto S. Light adaptation of the unicellular red alga, Cyanidioschyzon merolae, probed by time-resolved fluorescence spectroscopy. Photosynth Res. 2015;125:211–218. doi: 10.1007/s11120-015-0078-0. [DOI] [PubMed] [Google Scholar]
- 37.Correa-Galvis V, et al. Photosystem II subunit PsbS is involved in the induction of LHCSR protein-dependent energy dissipation in Chlamydomonas reinhardtii. J Biol Chem. 2016;291:17478–17487. doi: 10.1074/jbc.M116.737312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akimoto S, et al. Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta. 2012;1817:1483–1489. doi: 10.1016/j.bbabio.2012.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.