Significance
Escherichia coli has been engineered toward an archaebacterium with an unprecedented high level of archaeal ether phospholipids. The obtained cells stably maintain a mixed heterochiral membrane. This finding challenges theories that assume that intrinsic instability of mixed membranes led to the “lipid divide” and the subsequent differentiation of bacteria and archaea. Furthermore, this study paves the way for future membrane engineering of industrial production organisms with improved robustness.
Keywords: lipid biosynthesis, ether lipids, hybrid membranes, bacteria, archaea
Abstract
One of the main differences between bacteria and archaea concerns their membrane composition. Whereas bacterial membranes are made up of glycerol-3-phosphate ester lipids, archaeal membranes are composed of glycerol-1-phosphate ether lipids. Here, we report the construction of a stable hybrid heterochiral membrane through lipid engineering of the bacterium Escherichia coli. By boosting isoprenoid biosynthesis and heterologous expression of archaeal ether lipid biosynthesis genes, we obtained a viable E. coli strain of which the membranes contain archaeal lipids with the expected stereochemistry. It has been found that the archaeal lipid biosynthesis enzymes are relatively promiscuous with respect to their glycerol phosphate backbone and that E. coli has the unexpected potential to generate glycerol-1-phosphate. The unprecedented level of 20–30% archaeal lipids in a bacterial cell has allowed for analyzing the effect on the mixed-membrane cell’s phenotype. Interestingly, growth rates are unchanged, whereas the robustness of cells with a hybrid heterochiral membrane appeared slightly increased. The implications of these findings for evolutionary scenarios are discussed.
A frequently quoted hypothesis on the origin of the three domains of life (Archaea, Bacteria, and Eukarya) assumes the existence of a common living ancestor, known as cenancestor or LUCA (last universal common ancestor), from which the archaea and bacteria have diverged. Based on comparative genomics analyses, predictions have been made about the organization of the transcriptional and translational machinery present in LUCA during the early stages of evolution (1). The cell membrane of LUCA has attracted particular attention because of the major structural differences of phospholipids in bacterial and archaeal membranes. Archaeal lipids are composed of branched isoprenoids that are ether-linked to a glycerol-1-phosphate (G1P) backbone, whereas the lipids in bacteria and eukarya are based on straight-chain fatty acids that are ester-linked to an enantiomeric glycerol-3-phosphate (G3P) backbone. This striking difference has led to the hypothesis of a noncellular LUCA, which lacked any membrane-like structure (2, 3), contained simple single-chain lipids (4, 5), or made use of compartmentalization by nonbiological iron sulphide structures (6). Alternatively, a LUCA with a defined cellular membrane has been postulated, assuming that such a membrane is a prerequisite for compartmentalization of cellular processes and self-replication (7, 8). The existence of a phospholipid-based membrane in the ancestor cell is further supported by phylogenomic studies that revealed high conservation of the mevalonate pathway for the synthesis of the isoprenoid building blocks in archaea, eukarya, and some bacteria (9–11). Also, the presence in almost all bacteria and archaea of several conserved membrane proteins, such as ATP synthase (12), some respiratory proteins (13), and proteins involved in polypeptide secretion (14), seems to be most compatible with a LUCA that is surrounded by a phospholipid-based cellular membrane. Assuming that membrane synthesis in proto-cells was catalyzed by both abiotic catalysis and enzymes with poor stereoselectivity (15), the membranes might have consisted of a mixture of both G1P and G3P lipids. However, ancestral proto-cells with such hybrid heterochiral membranes are assumed to be relatively unstable and would have experienced selection pressure to evolve more stable, homochiral membranes after the evolution of stereospecific enzymes (2, 15). At this stage, the “lipid divide,” a crucial step in the differentiation between archaea and bacteria, would have occurred. In contrast to these scenarios, in vitro experiments with liposomes composed of a mixture of archaeal and bacterial lipids showed a higher stability than liposomes composed of only archaeal (16) or only bacterial lipids (17, 18). Although small quantities of ether lipids and fatty acid-based ester lipids are found in some bacteria and some archaea (19, 20), respectively, no consistent evidence for the coexistence of substantial amounts of the two enantiomeric forms of the ester- and ether-based phospholipids has been observed in the membrane of any living cell so far. Some previous studies attempted to reproduce an in vivo heterochiral mixed membrane by introducing the partial (21–23) or almost entire (24) ether lipid biosynthetic pathway into the bacterium Escherichia coli, but the levels of ether lipids produced were too low (<1% of the total lipids) to expect any physiological consequence.
Here we report the engineering of E. coli by introducing a membrane that is a hybrid of ether-linked isoprenoids and ester-linked fatty acids and that is heterochiral as these lipids consist of either a G1P or a G3P backbone. Via the up-regulated production of the isoprenoid building blocks and the coexpression of genes encoding the archaeal lipid biosynthetic pathway, archaeal lipids with the G1P configuration were produced to an unprecedented fraction (up to 30%) of the total phospholipids. Cells were perfectly viable with growth rates comparable to the wild type. Remarkably, engineered cells showed an increase in robustness toward high temperature, butanol, and freezing.
Results
Lipid Biosynthesis Engineering.
To reproduce a hybrid heterochiral membrane in E. coli, a composite pathway was constructed that consisted of both bacterial and archaeal enzymes (SI Appendix, Fig. S1) to yield unsaturated archaetidylglycerol (AG) and archaetidylethanolamine (AE), counterparts of bacterial phosphatidylglycerol (PG) and phosphatidylethanolamine (PE), respectively. To achieve high amounts of ether lipids, the endogenous MEP-DOXP pathway, responsible for isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) synthesis in E. coli, was up-regulated. This was done by integrating the native genes at the “ori” macrodomain of the chromosome (25, 26) (SI Appendix, Fig. S2A), yielding strains containing either the idi gene (IDI+) or the entire operon idi, ispD, ispF, and dxs (MEP/DOXP+) (SI Appendix, Table S1). The effect on isoprenoid production was tested in combination with the introduced ether lipid (EL) genes. Using a system of two compatible vectors (SI Appendix, Fig. S2A), we introduced up to six ether lipid genes (GGPP synthase from Pantoea ananatis, G1P dehydrogenase from Bacillus subtilis, GGGP synthase from Methanococcus maripaludis, DGGGP synthase from M. maripaludis, CDP-archaeol synthase from Archaeoglobus fulgidus and phosphatidylserine synthase from B. subtilis) (23, 24) (SI Appendix, Table S2) into E. coli, yielding strains IDI+EL+ and MEP/DOXP+EL+. For polar head group attachment, the E. coli endogenous enzymes Psd, PgsA, and PgpA, as well as B. subtilis PssA, were used to recognize the archaeol derivatives (24) (SI Appendix, Fig. S1). Increased IPP and DMAPP production dramatically stimulated the synthesis of AG (SI Appendix, Fig. S2B). Only low amounts of AE were detected. Attempts to improve AE synthesis by using different ribosome-binding sites for pssA or addition of L-serine to the growth medium were unsuccessful. A likely explanation is that overproduction of B. subtilis PssA, which has been reported to synthesize PE in addition to AE, impairs cell growth (27). It is thus likely that these attempts caused elevated levels of the nonbilayer lipid PE, which are toxic to the cells (28).
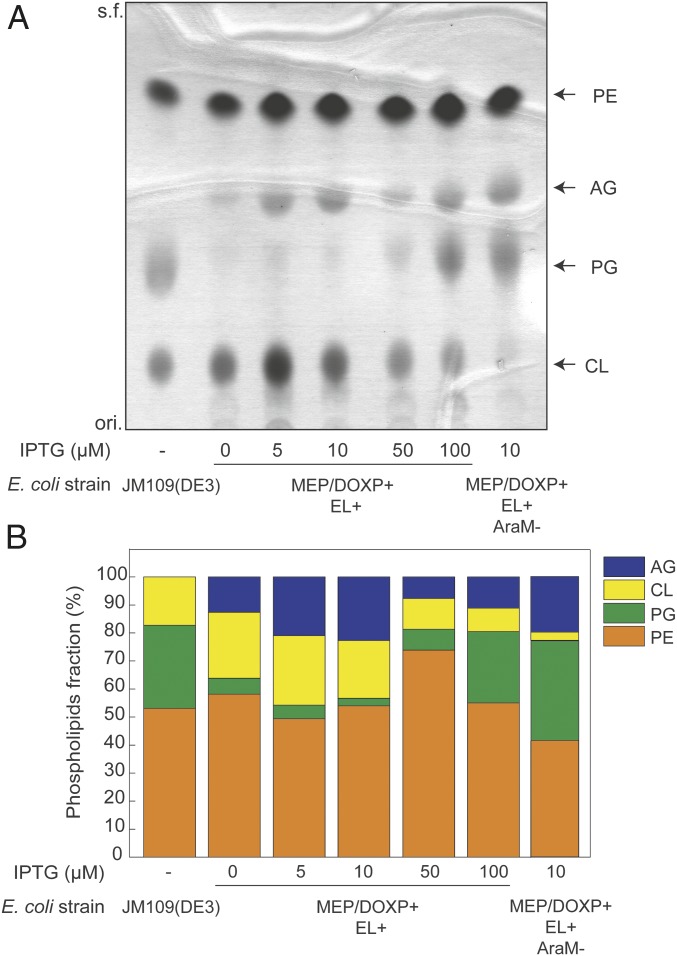
The E. coli MEP/DOXP+EL+ strain was further optimized for growth and induction to achieve the highest amounts of AG possible. Increased AG production was observed when cells were grown in statistically optimized OPT1 medium compared with LB medium (SI Appendix, Fig. S3A) and when cells were induced early during growth (SI Appendix, Fig. S3B). OPT1 medium contains glycerol and KH2PO3, which have been identified as the most dominant factors increasing lycopene production (29) through elevated isoprenoid synthesis. The various lipid species were assessed by liquid chromatography–mass spectrometry (LC–MS) (24), TLC, and phosphorous-based lipid quantitation. Tuning of the isopropyl-β-d-1-thiogalactopyranoside (IPTG) concentration, controlling heterologous gene expression, showed that lower amounts of IPTG resulted in a higher AG lipid fraction. The native PG content decreased from 30% in the wild type to 3% when the strain was induced with 10 μM IPTG (Fig. 1B), while cardiolipin (CL) levels increased (Fig. 1A). Concomitantly, the AG content increased to up to 23% of the total phospholipid fraction. Higher amounts of IPTG (50–100 μM) did not result in a further increased AG lipid fraction, but caused increased overall lipid synthesis (SI Appendix, Fig. S3C). The remarkable decrease of the PG content in favor of newly synthesized AG demonstrates the functional integration of the ether lipid biosynthetic pathway in E. coli. Importantly, the hybrid heterochiral membrane strain remained stable after serial transfer for at least 6 d, since the strain still produced ether lipids in the presence of 10 µM IPTG. Notably, the amount of AG produced even increased up to 30% of the total bacterial lipidome compared with the noninduced strain where the level of AG remained approximately constant (SI Appendix, Fig. S4).
Fig. 1.
TLC-based quantitation of in vivo archaeal lipid synthesis. (A) TLC of lipid extracts from wild-type E. coli [JM109 (DE3)] heterochiral mixed membrane E. coli (MEP/DOXP+EL+) induced early during growth (OD600 = 0.0) with different IPTG concentrations and incubated until stationary phase, and the E. coli strain harboring the entire ether lipid pathway but lacking the araM gene (MEP/DOXP+EL+AraM−) treated similarly. (B) Relative quantitation of the spots detected in the TLC. AG, archaetidylglycerol; CL, cardiolipin; PE, phosphatidylethanolamine; PG, phosphatidylglycerol.
Lipid Chirality and Enzyme Promiscuity.
The configuration of the glycerophosphate backbone represents one of the most distinctive differences between bacterial and archaeal lipids (30). The enzymes G3PDH and G1PDH, involved in the synthesis of G3P and G1P, respectively, are members of evolutionarily unrelated protein families (30). To ascertain that the correct archaeal lipid stereochemistry is realized, a strain was constructed that lacks the G1PDH due to an araM gene deletion (E. coli MEP/DOXP+EL+AraM−). Surprisingly, in the absence of G1PDH, still substantial levels of AG were detected (18%), slightly less than the strain bearing araM (23%) (Fig. 1B, last column on right). This implies that E. coli either produces G1P by itself or the composite ether lipid pathway contains nonstereoselective enzymes able to use G3P.
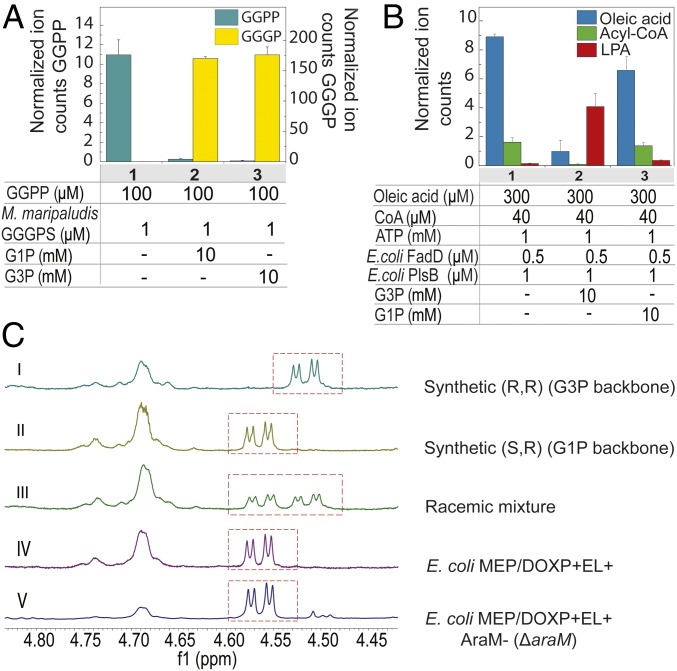
G3P acyltransferase (PlsB) in E. coli catalyzes the attachment of the glycerophosphate backbone to a fatty acid chain (31). This enzyme was tested for its stereoselectivity toward G1P and G3P. E. coli FadD (32) was used for the in vitro condensation of oleic acid and CoA to yield acyl-CoA (Fig. 2B, lanes 1), which was subsequently linked with glycerol phosphate by purified PlsB, resulting in the production of lyso-phosphatidic acid (LPA). LPA synthesis was observed only in the presence of G3P (Fig. 2B, lanes 2 versus lanes 3), demonstrating a strict stereoselectivity of PlsB. The archaeal counterpart of the bacterial PlsB system is GGGPS, which generates an ether linkage between the isoprenoid chain GGPP to G1P. Biochemical analysis of purified GGGPS from M. maripaludis indicates that the enzyme catalyzes the condensation of GGPP (100 µM) with either G1P or G3P as substrate (Fig. 2A, bars 2 and 3). Nevertheless, GGGPS has an eightfold preference for G1P (Km = 5.8 ± 1.6 µM) over G3P (Km = 46.7 ± 6 µM) (SI Appendix, Fig. S5 A and B). The weaker stereoselectivity of GGGPS toward glycerol phosphates could potentially account for G3P-based AG formation in the absence of G1PDH.
Fig. 2.
Stereochemistry of the ether lipid biosynthesis in E. coli and stereoselectivity of the archaeal GGGPS. Specificity of archaeal M. maripaludis GGGPS (A) and the bacterial E. coli PlsB (B) enzymes toward G1P and G3P. Total ion counts are normalized using n-dodecyl-β-d-maltoside (DDM) detergent as internal standard. Results are the averages of two experiments ±SEM. (C) NMR spectra of Mosher’s ester derivatized AG. Synthetic AG with G3P configuration (I), synthetic AG with G1P configuration (II), a mixture of both (III), AG from the E. coli strain expressing the whole ether lipid biosynthetic pathway (IV), and from the E. coli strain harboring the AraM gene deletion (V). The dashed red boxes highlight the diagnostic signals.
To conclusively establish the chirality of the diether lipids in the engineered E. coli strains, both enantiomers of AG were prepared chemically (33) (Fig. 2 C, I and II) and compared with AG produced in E. coli. In short, saponification of the total lipid extract allowed for the subsequent purification of the ether lipids by chromatography on silica. Samples were converted into their corresponding Mosher’s ester and analyzed by 1H- and 19F-NMR (34). Readily distinguishable diastereotopic shifts in the 1H-NMR showed unambiguously that the AG produced by the engineered E. coli strains, both with G1PDH (Fig. 2 C, IV) and without G1PDH (Fig. 2 C, V), have the archaeal G1P configuration. This demonstrates a high in vivo selectivity of used ether lipid enzymes for G1P and proves that E. coli is capable of producing G1P by a yet-unknown enzyme.
Growth, Cell Morphology, and Robustness.
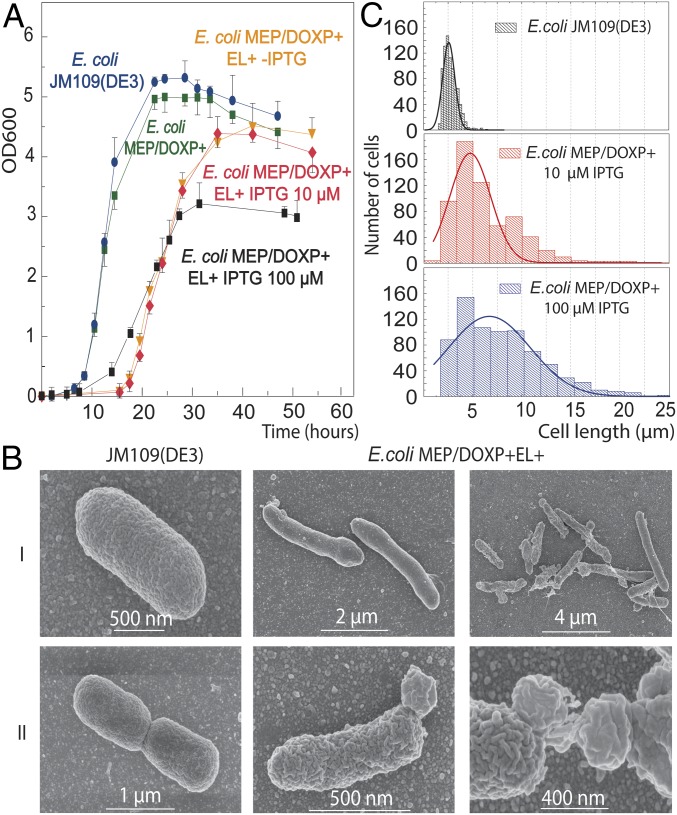
A major question is whether a hybrid heterochiral membrane affects cell characteristics. Due to the high level incorporation of AG, we were able to investigate the effects of a mixed membrane on growth, cell viability, and stability. E. coli MEP/DOXP+EL+ showed a long lag phase of ∼16 h before growth commenced with a growth rate similar to the parental strain (Fig. 3A). Both noninduced and induced (10 µM IPTG) cells showed a similar growth behavior, while the presence of AG in noninduced cells (Fig. 1B and SI Appendix, Fig. S4) is most likely caused by leakage of the lac promoter used to control expression of the archaeal lipid genes (35). Genome sequencing of the induced and adapted strain revealed 10 mutations (eight substitutions, and two deletions at seven different loci); however, none appeared in central metabolism or phospholipid synthesis, nor did similar mutations occur in sequenced duplicates (SI Appendix, Table S3). The lag phase could therefore be a result of a metabolic adaptation of the bacterial strain and/or adjustment of the expression of heterologous enzymes for the viable production of archaeal ether lipids.
Fig. 3.
Growth and cell morphology analysis of the heterochiral mixed membrane strains. (A) Growth of the E. coli MEP/DOXP+EL+ strain with all of the ether lipid enzymes [not induced (orange)], induced with 10 μM (red), and induced with 100 μM (black) of IPTG added early during growth (OD600 = 0.0) compared with two negative control strains: E. coli JM109(DE3) wild-type (blue) and E. coli MEP/DOXP+ strain with the integrated MEP-DOXP operon (green). The data are the averages of three biological replicates ±SEM. (B) SEM of wild-type E. coli and the heterochiral mixed membrane strain induced at a late (0.3 OD600) and early (0.03 OD600) growth phase using 100 μM of IPTG. (I) Altered cell shape and length. (II) aberrant cell division and formation of bulges and shreds. (C) Statistical analysis of the cell length of the E. coli JM109(DE3) (Top), E. coli MEP/DOXP+EL+ induced with 10 μM IPTG (Middle), and 100 μM IPTG (Bottom).
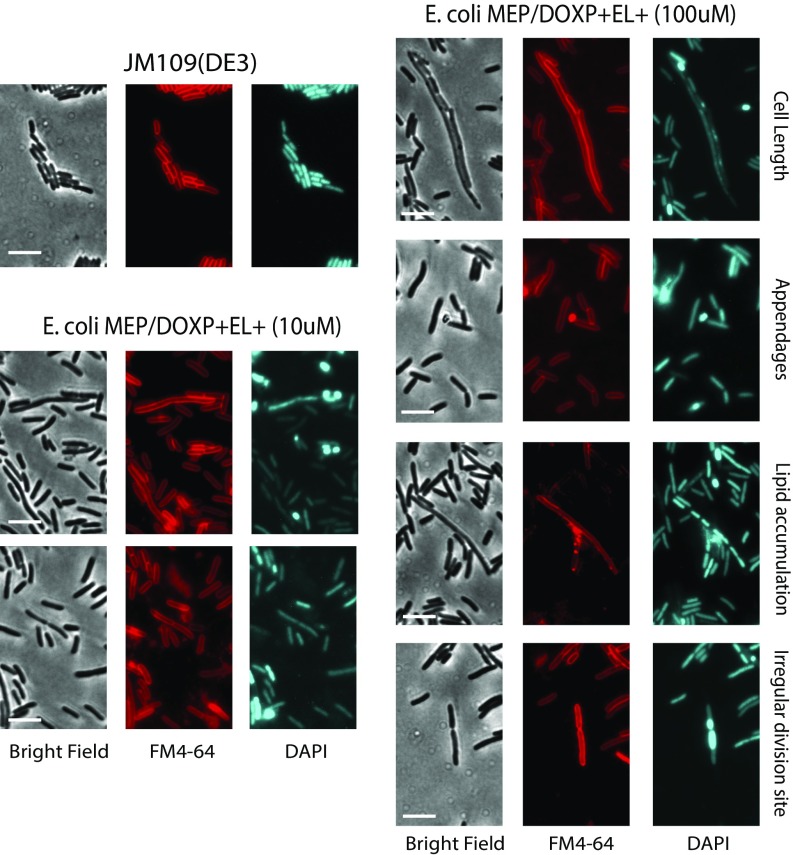
Analysis of the cells by scanning electron microscopy and bright-field microscopy revealed that the introduction of the archaeal lipid biosynthetic pathway caused an elongated cell length (Fig. 3 B, I). Compared with control cells exhibiting a relatively constant cell length of 2–2.5 µm, E. coli MEP/DOXP+EL+ cells induced with 10 µM IPTG showed lengths of 2–15 µm (Fig. 3C). Induced cells were slightly thinner with an average cell width of 0.60 μm ± 0.057 compared with control cells, 0.75 μm ± 0.037. These differences in shape may also explain the lower overall optical densities reached, as cell size is known to affect OD values. Strong induction (50–100 µM IPTG) of the archaeal lipid pathway, however, slowed down growth and caused major cellular morphology changes with formation of lobular appendages (100–500 nm in diameter) that eventually are released from the cells (Fig. 3 B, II). Lipid analysis of isolated bulges revealed the presence of a mixture of archaeal and bacterial lipids comparable to the mother cell although the fraction of AG was somewhat lower (SI Appendix, Fig. S3D). Thus, immiscibility and segregation of archaeal lipids from the endogenous bacterial lipid pool do not seem to be major factors in forming these extrusions (SI Appendix, Fig. S3D). The extrusions also contain genetic material, as indicated by double staining with the lipophilic dye FM4-64 [N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide] (36) and 4′,6-diamidino-2-phenylindole (DAPI) to monitor lipids and DNA, respectively (Fig. 4). The extrusions likely originate from nonsymmetrical cell division caused by high-level lipid biosynthesis, consistent with an important role of lipids in cell division (37–39). The lipid staining also signified the presence of intense membrane-associated spots in the highly induced cells (Fig. 4) that possibly correspond to accumulation of anionic lipids. We speculate that the abundant shredding that occurs at high induction is the result of the high level of lipid overproduction that does not keep pace with other processes of cellular growth, causing physiological disturbance including irregular division sites. Therefore, in the remainder of the study, cells were induced with 10 μM IPTG to examine the physiological consequences independently from the aberrant cell morphology.
Fig. 4.
Effect of mixed heterochiral membranes on E. coli cells detected by double staining with FM4-64 and DAPI. Lipid staining showing elongated and thinner cells in the engineered strain compared with the control, the presence of membrane associated spots in the engineered strain. Double staining with FM4-64 and DAPI showing the presence of appendages surrounded by a lipid layer and the presence of DNA. Presence of irregular division sites in engineered cells compared with the symmetrical division septum present in the wild-type cells. (Scale bar: 5 μm.)
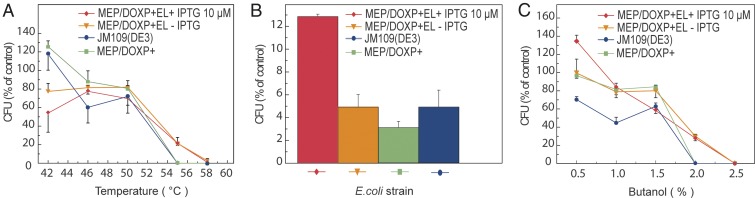
Even though not all Archaea are extremophiles, archaeal ether lipids have been associated with extremophilicity and robustness (40). Therefore, the impact of heat and cold shock on survival of the strains with hybrid heterochiral membranes was tested. Wild-type and two engineered E. coli strains [JM109 (DE3), MEP/DOXP+, MEP/DOXP+EL+] were exposed to elevated temperatures for 2 min and allowed to recover for 1 h at 37 °C. The noninduced and induced (10 μM IPTG) E. coli MEP/DOXP+EL+ strain showed an elevated overall survival upon exposure to 55 °C and 58 °C compared with the two control strains JM109(DE3) and E. coli MEP/DOXP+ that do not survive when exposed to temperatures above 50 °C (Fig. 5A). Cells were also exposed to freezing and thawing (−80 °C). Cells induced with 10 μM IPTG were remarkably more tolerant to this treatment than the controls (Fig. 5B) as evidenced by the higher cfu count. Finally, tolerance to the organic solvent l-butanol was tested by exposing the strains for 1 h to different concentrations. A higher resistance was observed in the noninduced and induced (10 μM IPTG) E. coli MEP/DOXP+EL+ strain harboring the entire archaeal lipid biosynthetic pathway compared with the controls (Fig. 5C). This was most notable when the cells were treated with 2% of l-butanol. Importantly, we show that, at low induction levels, AG lipid production is not toxic to the bacterial cell, but renders cells more resistant to different types of chemical/physical stresses.
Fig. 5.
Robustness of E. coli with a heterochiral mixed membrane. The E. coli strain with all ether lipid enzymes. MEP/DOXP+EL+ [not induced, orange; induced with 10 μM IPTG at early growth phase (OD600 = 0.0, red)] was compared with the wild-type strain JM109(DE3) (blue) and the strain harboring the integrated MEP-DOXP operon MEP/DOXP+ (green) for survival against exposure to different environmental stresses. (A) Heat shock. (B) Freezing at −80 °C. (C) l-butanol tolerance. The data were normalized against the cfu of untreated samples. The results are the averages of four biological replicates ±SEM.
Discussion
According to the discordant hypothesis, the instability of a hybrid heterochiral mixed membrane of the LUCA has triggered the segregation of archaea and bacteria toward a more stable homochiral membrane, resulting in the lipid divide that separates Bacteria and Archaea (17, 41). However, it is inherently difficult to test such a hypothesis in vivo, as the conditions of early evolution would need to be replicated and one would need a microorganism with a mixed membrane. In vitro analyses using pure lipid liposomes failed to demonstrate the assumed instability, but rather indicated that heterochiral membranes composed of both ether isoprenoids and ester fatty acids were more stable than homochiral ones (16–18). However, these pure lipid liposomes are the most simple model systems, and as such the results are considered not conclusive as they do not take the membrane protein content into account.
Here we describe the engineering of a viable bacterial cell with a heterochiral mixed membrane composed of bacterial and archaeal lipids through the introduction of the archaeal ether lipid biosynthetic pathway into E. coli. Such a heterochiral mixed membrane may serve as a biological model for the coexistence of these two phospholipid species in a prokaryotic membrane. We have previously reported on the introduction of a fully functional ether lipid pathway into the bacterium E. coli and the synthesis of the two archaeal lipids AG and AE (24). However, synthesis of the ether lipids was very low compared with the bacterial lipidome (<1%), comparable to results obtained in other studies (22, 23). In the present study, however, a higher level of isoprenoid units (IPP and DMAPP) was accomplished by a combination of the chromosomal integration of an inducible MEP-DOXP pathway (42) and the use of a statistically optimized medium (29). Further strain optimization yielded an engineered bacterial strain in which nearly the complete PG pool is replaced by the archaeal AG (up to 30% of total phospholipids). Strikingly, most of the PG was converted into CL. Overall, our data demonstrate a functional integration of ether lipids into the isolated membranes of these cells. This is supported mainly by the decrease of PG in favor of AG, but also by the steady increase of AG content during prolonged growth by serial transfers. Moreover, differential centrifugation to isolate released extrusions also confirmed a comparable mixed-lipid composition, excluding the formation of selective archaeal lipid droplets.
A critical element of the introduced pathway and the generation of a mixed heterochiral membrane is the validation of the proper stereochemical configuration of the introduced ether lipids. With no exception, bacterial membranes are characterized by G3P-based lipids while archaea have G1P-based lipids (17). The enzymes G3PDH and G1PDH, involved in the synthesis of G3P and G1P, respectively, do not share any structural or functional homology as they are members of evolutionarily unrelated protein families (30). Moreover, since there is no mechanism known in E. coli for the production of G1P, the engineered E. coli strain was anticipated to rely on G1PDH (AraM) for producing archaeal lipids. Surprisingly, however, the araM gene was found to be redundant, which could be explained by assuming the synthesis of G3P-based ether lipids due to poor stereoselectivity of the archaeal enzymes. Biochemical analysis using purified GGGPS from M. maripaludis reveals only a modest (eightfold) preference for G1P over G3P. Despite this weak stereoselectivity, however, the in vivo-synthesized archaeal lipids from the engineered E. coli strains were found to have a G1P configuration, indicating that the used set of ether lipid biosynthesis enzymes select G1P as backbone. Since this also occurs in the absence of the G1PDH (AraM), this implies that E. coli unexpectedly possesses a native mechanism of G1P formation, a feature that has not been reported before. Potential conversions that could lead to G1P formation include either the phosphorylation of glycerol by glycerol kinase or the phosphorylation and reduction of dihydroxyacetone by DHA kinase and glycerol phosphate dehydrogenase. Although these enzymes are known to generate G3P, the stereoselectivity of these enzymes has not been examined in detail yet.
The engineered membrane with an unprecedented high percentage of AG enabled us to study the effect of such a hybrid membrane on growth rate, cell viability, and robustness. The engineered bacterial strains show a long lag phase of ∼16 h before growth commences, but eventually their growth rates are comparable with the wild-type strain. Genome sequencing of the induced and adapted strain revealed no mutations involved in the central metabolism or phospholipid synthesis, and similar missense mutations did not occur in the sequenced duplicates (SI Appendix, Table S3). The lag phase could therefore be a result of a metabolic adaptation of the bacterial strain and/or an adjustment of the expression of heterologous enzymes for the viable production of archaeal ether lipids. On the other hand, strong induction of the archaeal lipid biosynthesis pathway caused severe cell stress as growth slows down and the cell morphology changes dramatically. Whereas the majority of the engineered cells show elongated and thinner cells compared with the wild-type strain, high induction resulted in the formation of lobular appendages that are eventually released from the cells. Lipid analysis of isolated extrusions revealed the presence of a mixture of archaeal and bacterial lipids much akin to the mother cell, excluding the possibility that immiscibility with the endogenous bacterial lipids and segregation of the archaeal lipids caused this phenomenon. The extrusions also contain genetic material and likely originate from nonsymmetrical cell division caused by the high level of lipid biosynthesis, which would be consistent with an important role of lipids in cell division (37, 43). As the introduced ether lipid biosynthetic pathway is not fully integrated in the cellular and phospholipid homeostasis, we speculate that the abundant shredding as seen under conditions of high induction is the result of the high level of lipid overproduction that does not keep pace with other processes of cellular growth, resulting, for example, in the formation of irregular division sites.
Importantly, we showed that a moderate induction leads to significant levels of AG lipid production, indicating that the presence of archaeal lipids was not toxic to the bacterial cell. Since the archaeal ability to survive under extreme conditions such as high temperatures has been related to archaeal membrane lipid composition (44), one may expect that incorporation of archaeal lipids into a bacterial cell membrane could partially confer this ability. Indeed, a higher tolerance to heat treatment compared with control strains was observed. It should be mentioned that the archaeal lipids are unsaturated; as such, it may be that saturation will further enhance survival under heat stress. Cells were also found to be more tolerant to freezing at −80 °C and subsequent thawing to room temperature, a feature that can be attributed to the presence of the high concentration of unsaturated archaeal lipids that confers increased membrane fluidity needed to survive this transition (45–47). Moreover, the engineered cells exhibited a higher tolerance against the organic solvent l-butanol. Although the observed differences in robustness are subtle, they are significant. Moreover, they demonstrate that, under certain conditions, bacteria may have an improved fitness because of the presence of archaeal lipids.
The work described in the present study represents a unique approach to address a possible coexistence of archaeal and bacterial phospholipids as a hybrid heterochiral membrane in a living bacterial cell. Despite the fact that bacterial integral membrane proteins have evolved to function in an ester-based phospholipid membrane, introduction of high levels of ether-based AG resulted in viable cells with growth rates comparable to that of the parental strain. As such, our findings contrast the hypothesis of the thermodynamic instability of mixed membranes (2). Additionally, it is of interest to explore E. coli strains with archaeal phospholipids for the functional overproduction of archaeal membrane proteins. Another application of the strategy described here may be for industrial microorganisms. Like the case described here of E. coli, heterochiral hybrid membranes may render production organisms more robust with a higher tolerance to organic solvents or toxic by-products without loss of productivity in bio-industrial processes. We anticipate that this study will stimulate future analyses for biotechnological applications and study of the early evolution of cellular life.
Materials and Methods
A detailed description of the materials and methods concerning operon integration and cloning procedures, bacterial strains and growth conditions, TLC, expression and purification of GGGPS, FadD, and PlsB enzymes, in vitro enzyme reactions, lipid analysis, LC-MS analysis, NMR and ether phospholipid stereochemical analysis, scanning electron microscopy and bright-field microscopy, and robustness tests can be found in SI Appendix.
Statistical Information.
All reported data obtained by in vitro and in vivo experiments are expressed as the mean of three biological replicates ±SEM. The statistical analysis was performed using Microsoft Excel. The normal distribution function was used to determine the average cell length and width in a population of more than 600 cells. The bin size was calculated using the following formula: 3.49 * SD.p * n−1/3 according to the method described by D. W. Scott (48). The statistical analysis was performed using OriginPro.
SI Appendix.
SI Appendix includes supplementary methods, five supplementary figures, five supplementary tables, and supplementary references.
Data Availability.
The authors declare that the data supporting the findings of this study are available within the paper and its SI Appendix files.
Supplementary Material
Acknowledgments
We thank Tiny Franssen-Verheijen (Wageningen Electron Microscopy Centre) for providing the electron micrographs; Teunke van Rossum for providing the Lox-KanR-lox integration cassette; Anne-Bart Seinen for graphic assistance and the development of colony-counting software; and Anabela de Sousa Borges for assistance with bright-field microscopy. This work was carried out within the research program of the biotechnology based, ecologically balanced, sustainable industrial consortium (BE-Basic).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721604115/-/DCSupplemental.
References
- 1.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koga Y, Kyuragi T, Nishihara M, Sone N. Did archaeal and bacterial cells arise independently from noncellular precursors? A hypothesis stating that the advent of membrane phospholipid with enantiomeric glycerophosphate backbones caused the separation of the two lines of descent. J Mol Evol. 1998;46:54–63. doi: 10.1007/pl00006283. [DOI] [PubMed] [Google Scholar]
- 3.Koonin EV, Martin W. On the origin of genomes and cells within inorganic compartments. Trends Genet. 2005;21:647–654. doi: 10.1016/j.tig.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer SE, Deamer DW, Boncella JM, Monnard PA. Chemical evolution of amphiphiles: glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology. 2009;9:979–987. doi: 10.1089/ast.2009.0384. [DOI] [PubMed] [Google Scholar]
- 5.Pozzi G, et al. Single-chain polyprenyl phosphates form “primitive” membranes. Angew Chem Int Ed Engl. 1996;35:177–180. [Google Scholar]
- 6.Martin W, Russell MJ. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans R Soc Lond B Biol Sci. 2003;358:59–83, discussion 83–85. doi: 10.1098/rstb.2002.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 8.Peretó J, López-García P, Moreira D. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem Sci. 2004;29:469–477. doi: 10.1016/j.tibs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Boucher Y, Kamekura M, Doolittle WF. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol Microbiol. 2004;52:515–527. doi: 10.1111/j.1365-2958.2004.03992.x. [DOI] [PubMed] [Google Scholar]
- 10.Lombard J, Moreira D. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Mol Biol Evol. 2011;28:87–99. doi: 10.1093/molbev/msq177. [DOI] [PubMed] [Google Scholar]
- 11.Matsumi R, Atomi H, Driessen AJM, van der Oost J. Isoprenoid biosynthesis in Archaea–biochemical and evolutionary implications. Res Microbiol. 2011;162:39–52. doi: 10.1016/j.resmic.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Gogarten JP, et al. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc Natl Acad Sci USA. 1989;86:6661–6665. doi: 10.1073/pnas.86.17.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baymann F, et al. The redox protein construction kit: pre-last universal common ancestor evolution of energy-conserving enzymes. Philos Trans R Soc Lond B Biol Sci. 2003;358:267–274. doi: 10.1098/rstb.2002.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao TB, Saier MH., Jr The general protein secretory pathway: phylogenetic analyses leading to evolutionary conclusions. Biochim Biophys Acta. 2003;1609:115–125. doi: 10.1016/s0005-2736(02)00662-4. [DOI] [PubMed] [Google Scholar]
- 15.Wächtershäuser G. From pre-cells to Eukarya–a tale of two lipids. Mol Microbiol. 2003;47:13–22. doi: 10.1046/j.1365-2958.2003.03267.x. [DOI] [PubMed] [Google Scholar]
- 16.Fan Q, Relini A, Cassinadri D, Gambacorta A, Gliozzi A. Stability against temperature and external agents of vesicles composed of archael bolaform lipids and egg PC. Biochim Biophys Acta. 1995;1240:83–88. doi: 10.1016/0005-2736(95)00157-x. [DOI] [PubMed] [Google Scholar]
- 17.Shimada H, Yamagishi A. Stability of heterochiral hybrid membrane made of bacterial sn-G3P lipids and archaeal sn-G1P lipids. Biochemistry. 2011;50:4114–4120. doi: 10.1021/bi200172d. [DOI] [PubMed] [Google Scholar]
- 18.Sprott G, Dicaire C, Fleming L, Patel G. Stability of liposomes prepared from archaeobacterial lipids and phosphatidylcholine mixtures. Cells and Materials. 1996;6:143–155. [Google Scholar]
- 19.Hamerly T, et al. Characterization of fatty acids in crenarchaeota by GC-MS and NMR. Archaea. 2015;2015:472726. doi: 10.1155/2015/472726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dibrova DV, Galperin MY, Mulkidjanian AY. Phylogenomic reconstruction of archaeal fatty acid metabolism. Environ Microbiol. 2014;16:907–918. doi: 10.1111/1462-2920.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai D, et al. Reconstruction of the archaeal isoprenoid ether lipid biosynthesis pathway in Escherichia coli through digeranylgeranylglyceryl phosphate. Metab Eng. 2009;11:184–191. doi: 10.1016/j.ymben.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isobe K, et al. Geranylgeranyl reductase and ferredoxin from Methanosarcina acetivorans are required for the synthesis of fully reduced archaeal membrane lipid in Escherichia coli cells. J Bacteriol. 2014;196:417–423. doi: 10.1128/JB.00927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoi T, Isobe K, Yoshimura T, Hemmi H. Archaeal phospholipid biosynthetic pathway reconstructed in Escherichia coli. Archaea. 2012;2012:438931. doi: 10.1155/2012/438931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caforio A, et al. Formation of the ether lipids archaetidylglycerol and archaetidylethanolamine in Escherichia coli. Biochem J. 2015;470:343–355. doi: 10.1042/BJ20150626. [DOI] [PubMed] [Google Scholar]
- 25.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha SK, Furukawa Y, Matsuzaki H, Shibuya I, Matsumoto K. Directed mutagenesis, Ser-56 to Pro, of Bacillus subtilis phosphatidylserine synthase drastically lowers enzymatic activity and relieves amplification toxicity in Escherichia coli. Biosci Biotechnol Biochem. 1996;60:630–633. doi: 10.1271/bbb.60.630. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K. Phosphatidylserine synthase from bacteria. Biochim Biophys Acta. 1997;1348:214–227. doi: 10.1016/s0005-2760(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, et al. Combining genotype improvement and statistical media optimization for isoprenoid production in E. coli. PLoS One. 2013;8:e75164. doi: 10.1371/journal.pone.0075164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga Y, Morii H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol Mol Biol Rev. 2007;71:97–120. doi: 10.1128/MMBR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green PR, Merrill AH, Jr, Bell RM. Membrane phospholipid synthesis in Escherichia coli. Purification, reconstitution, and characterization of sn-glycerol-3-phosphate acyltransferase. J Biol Chem. 1981;256:11151–11159. [PubMed] [Google Scholar]
- 32.Kameda K, Nunn WD. Purification and characterization of acyl coenzyme A synthetase from Escherichia coli. J Biol Chem. 1981;256:5702–5707. [PubMed] [Google Scholar]
- 33.Jain S, et al. Identification of CDP-archaeol synthase, a missing link of ether lipid biosynthesis in Archaea. Chem Biol. 2014;21:1392–1401. doi: 10.1016/j.chembiol.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Yamagishi M, Kakinuma K. Confirmation of the absolute stereochemistry of sn-2,3-Di-O-phytanyl glycerol, the unit lipid of the cell membrane of halophilic archaebacteria Halobacterium halobium. Agric Biol Chem. 1989;53:867–868. [Google Scholar]
- 35.Zhang Z, et al. High-level production of membrane proteins in E. coli BL21(DE3) by omitting the inducer IPTG. Microb Cell Fact. 2015;14:142. doi: 10.1186/s12934-015-0328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fishov I, Woldringh CL. Visualization of membrane domains in Escherichia coli. Mol Microbiol. 1999;32:1166–1172. doi: 10.1046/j.1365-2958.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 37.Mileykovskaya E, Dowhan W. Role of membrane lipids in bacterial division-site selection. Curr Opin Microbiol. 2005;8:135–142. doi: 10.1016/j.mib.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Mercier R, Domínguez-Cuevas P, Errington J. Crucial role for membrane fluidity in proliferation of primitive cells. Cell Reports. 2012;1:417–423. doi: 10.1016/j.celrep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Mercier R, Kawai Y, Errington J. Excess membrane synthesis drives a primitive mode of cell proliferation. Cell. 2013;152:997–1007. doi: 10.1016/j.cell.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 40.DeLong EF. Everything in moderation: archaea as ‘non-extremophiles’. Curr Opin Genet Dev. 1998;8:649–654. doi: 10.1016/s0959-437x(98)80032-4. [DOI] [PubMed] [Google Scholar]
- 41.Lombard J, López-García P, Moreira D. The early evolution of lipid membranes and the three domains of life. Nat Rev Microbiol. 2012;10:507–515. doi: 10.1038/nrmicro2815. [DOI] [PubMed] [Google Scholar]
- 42.Ajikumar PK, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atilla-Gokcumen GE, et al. Dividing cells regulate their lipid composition and localization. Cell. 2014;156:428–439. doi: 10.1016/j.cell.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koga Y. Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea. 2012;2012:789652. doi: 10.1155/2012/789652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichols DS, et al. Cold adaptation in the Antarctic Archaeon Methanococcoides burtonii involves membrane lipid unsaturation. J Bacteriol. 2004;186:8508–8515. doi: 10.1128/JB.186.24.8508-8515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto T, Murata N. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr Opin Microbiol. 2002;5:208–210. doi: 10.1016/s1369-5274(02)00306-5. [DOI] [PubMed] [Google Scholar]
- 47.Gibson JA, et al. Unsaturated diether lipids in the psychrotrophic archaeon Halorubrum lacusprofundi. Syst Appl Microbiol. 2005;28:19–26. doi: 10.1016/j.syapm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Scott DW. On optimal and data-based histograms. Biometrika. 1979;66:605–610. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its SI Appendix files.