Significance
We demonstrate that the number of mature neurons in the human amygdala increases from childhood into adulthood. This trajectory may be due to the incorporation of immature neurons from the paralaminar nucleus in the ventral amygdala. In contrast, individuals with autism spectrum disorder (ASD) show an initial excess of mature neurons followed by a decline into adulthood. Our results suggest a degenerative component in ASD and highlight the need for a more comprehensive understanding of the protracted cellular development of the human amygdala for multiple psychiatric disorders.
Keywords: autism, amygdala, stereology, neuroanatomy, neuronal maturation
Abstract
Remarkably little is known about the postnatal cellular development of the human amygdala. It plays a central role in mediating emotional behavior and has an unusually protracted development well into adulthood, increasing in size by 40% from youth to adulthood. Variation from this typical neurodevelopmental trajectory could have profound implications on normal emotional development. We report the results of a stereological analysis of the number of neurons in amygdala nuclei of 52 human brains ranging from 2 to 48 years of age [24 neurotypical and 28 autism spectrum disorder (ASD)]. In neurotypical development, the number of mature neurons in the basal and accessory basal nuclei increases from childhood to adulthood, coinciding with a decrease of immature neurons within the paralaminar nucleus. Individuals with ASD, in contrast, show an initial excess of amygdala neurons during childhood, followed by a reduction in adulthood across nuclei. We propose that there is a long-term contribution of mature neurons from the paralaminar nucleus to other nuclei of the neurotypical human amygdala and that this growth trajectory may be altered in ASD, potentially underlying the volumetric changes detected in ASD and other neurodevelopmental or neuropsychiatric disorders.
The human amygdala comprises a cluster of 13 nuclei in the rostral temporal lobe which play a critical role in fear, emotion, and social behavior (1–3). The typical human and nonhuman primate amygdala undergoes a remarkable 40% volumetric growth into early adulthood, despite little growth of the cerebral cortex (4, 5). Prolonged amygdala maturation likely underlies the increasing functional integration of this structure as it modulates responses to an ever-changing environment. Early perturbations in amygdala cellular development could lead to a cascade of maladaptive neurodevelopmental events that affect the entire trajectory of maturation. Revealing the underlying neurobiology of this prolonged growth is critical not only for a fundamental understanding of neurotypical development but also for pinpointing when these processes deviate in disorders such as autism spectrum disorder (ASD) and other neuropsychiatric disorders (6, 7).
Although the primate amygdala is formed early in gestation and is well developed at birth (8–11), structural and functional changes extend well into adulthood (12, 13). A number of factors likely contribute to the dramatic increase in postnatal amygdala volume, including dendritic enlargement, synaptogenesis, and gliogenesis. We propose that other neuronal factors may contribute, such as (i) the maturation of a large population of immature neurons within the paralaminar nucleus (6, 14–17) and/or (ii) the migration of postnatally generated neurons (18, 19). Immature neurons have been identified in both monkey and human amygdala using immunohistochemistry with protein markers such as doublecortin (DCX) and B cell lymphoma 2 (bcl-2) (14, 16–18, 20, 21). The protracted maturational trajectory of the amygdala well beyond the perinatal period allows it to continually be shaped by external stimuli. In fact, the immature neurons within the paralaminar nucleus may develop in an activity-dependent manner (20, 22). This lengthened maturational process, however, may also make the amygdala more susceptible to developmental or environmental insults.
ASD is characterized by impairments in social communication combined with restricted interests and behaviors. Alterations in amygdala growth can be detected as early as 2 y of age (23–26) and persist into late childhood (5, 27). The severity of the individual’s social and communicative symptoms positively correlates with amygdala enlargement, suggesting a potential structure–function relationship (23). Individuals with ASD also show atypical amygdala activation during socioemotional tasks (28, 29). Microanatomical alterations to the cellular structure of the amygdala were first noted by Bauman and Kemper (30) and subsequently by Schumann and Amaral (31) and Wegiel et al. (32). These studies found a general reduction of neurons in the amygdala of adults with ASD. However, an examination of younger subjects with either neurotypical development or ASD has not yet been performed. The present study aimed to carry out a large systematic evaluation of the developmental trajectory of neuron number from youth to adulthood in the human amygdala in both neurotypical individuals and in those diagnosed with ASD. In addition, we examined the presence of immature neurons in the amygdala and evaluated whether differences in this population across the life span may contribute to the gradual decreases in neuron number we have observed in our previous studies of adults with ASD.
Results
Case Information.
Subject-specific information from all 52 subjects is presented in Table S1. Briefly, the neurotypical-subject (i.e., NT) group contained 24 subjects (mean age, 20.17 ± 13.28 y; range, 2 to 48 y), 5 of whom were female. The ASD group contained 28 subjects (mean age, 17.46 ± 11.25 y; range, 4 to 44 y), 4 of whom were female and 11 of whom had seizure disorder (n = 2 overlap). The groups did not statistically differ on age. While there is not sufficient statistical power to systematically examine sex or seizure status, excluding these subjects from the analysis did not change the pattern of results (Table 1).
Table 1.
Neuron numbers across each age group suggest a reduction of neurons in autism across all amygdala subregions examined
| Region | Age group | No. of neuronsa | F tests,b partial η2 | |
| ASD | NT | |||
| Total amygdala | Child | 12.40 (1.18)c,** | 11.46 (1.29) | (All) F(2,41) = 5.69, P = 0.007, η2 = 0.22 |
| Adolescent | 11.32 (0.91) | 12.22 (0.75) | (− Girls) F(2,33) = 4.35, P = 0.021 | |
| Adult | 10.64 (1.67)d,* | 12.75 (1.58) | (− Seizure) F(2,31) = 8.54, P = 0.001 | |
| Lateral | Child | 3.74 (0.45) | 3.81 (0.52) | (All) F(1,39) = 5.24, P = 0.028e, η2 = 0.12 |
| Adolescent | 3.46 (0.46) | 3.82 (0.60) | (− Girls) F(1,32) = 6.91, P = 0.013e | |
| Adult | 3.29 (0.61)d,** | 3.91 (0.32) | (− Seizure) F(1,29) = 6.90, P = 0.014e | |
| Basal | Child | 3.14 (0.54)c,*,f,* | 2.62 (0.27) | (All) F(2,41) = 7.13, P = 0.002, η2 = 0.26 |
| Adolescent | 3.00 (0.36) | 3.00 (0.36) | (− Girls) F(2,33) = 4.44, P = 0.020 | |
| Adult | 2.65 (0.52)d,** | 3.42 (0.70)g,** | (− Seizure) F(2,31) = 5.08, P = 0.012 | |
| Accessory basal | Child | 1.21 (0.16)c,** | 1.07 (0.14) | (All) F(2,41) = 6.85, P = 0.003, η2 = 0.25 |
| Adolescent | 1.11 (0.21) | 1.20 (0.27) | (− Girls) F(2,33) = 7.23, P = 0.002 | |
| Adult | 0.94 (0.20)d,*** | 1.25 (0.14)g,* | (− Seizure) F(2,31) = 6.85, P = 0.003 | |
| Central | Child | 0.39 (0.04)c,*,f,* | 0.31 (0.08) | (All) F(2,39) = 4.23, P = 0.022, η2 = 0.18 |
| Adolescent | 0.36 (0.07) | 0.38 (0.01) | (− Girls) F(2,31) = 7.58, P = 0.002 | |
| Adult | 0.32 (0.05) | 0.36 (0.04) | (− Seizure) F(2,30) = 3.59, P = 0.040 | |
Mean (SD) number of neurons in millions.
F tests reveal a significant interaction between age category and diagnosis among all regions, except for the lateral nucleus, which showed a main effect of diagnosis. Excluding girls (− Girls) or individuals with seizure disorder (− Seizure) did not change the pattern of results.
Child ASD > adult ASD.
P < 0.01. Post hoc results regarding adolescents are omitted for clarity.
Adult ASD < adult NT.
P < 0.05.
Main effect of diagnosis on F test; all others are age group × diagnosis interactions.
Post hoc: child ASD > child NT.
Child NT < adult NT.
P < 0.001.
Developmental Trajectory of Neuronal Numbers in the Neurotypical Amygdala.
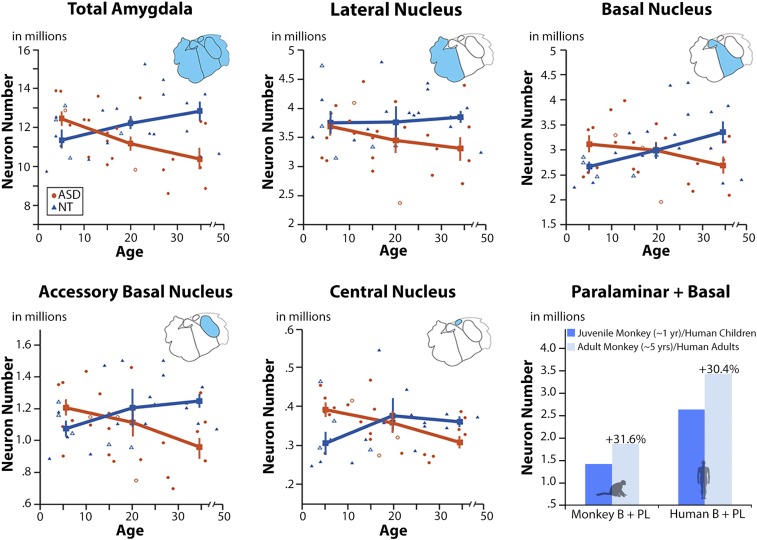
Mean estimates of neuron number and SDs across each region of interest are presented in Fig. 1 and Table 1. The univariate two-way ANOVA results are presented in Table 1. There is a significant (P < 0.05) age group × diagnosis interaction in total amygdala, basal, accessory basal, and central nuclei. These large effects (partial η2 range = 0.12 to 0.25) were further analyzed using a Fisher least significant difference (LSD) post hoc comparison (Table 1). Within the NT group, average total amygdala neuron number gradually increases by ∼11% with increasing age from youth to adulthood. This overall trend is primarily driven by a significant 30% increase in basal nucleus neurons (P = 0.003) and 17% increase in accessory basal nucleus neurons (P = 0.042). There is no significant increase of neuron number with age in the lateral or central nuclei.
Fig. 1.
Mature neuron number across amygdala nuclei between ASD and NT subjects. Young subjects with ASD show an increased number of mature neurons relative to NT subjects (in total amygdala, basal, accessory basal, and central nuclei). By adulthood, the number of mature neurons in ASD is well below the adult NT average in every nucleus examined (17%). Error bars ±1 SEM. When considering the monkey basal + paralaminar nuclei (6) as a single unit (as we have done with the human basal nucleus, Lower Right), there is a comparable increase of mature neurons (∼32%) across life between the two species.
Differences in Amygdala Neuron Number in ASD Compared with NT Cases.
The pattern of age-related change in amygdala neuron number is very different in individuals with ASD, in that total amygdala neuron number gradually decreases with increasing age (Fig. 1 and Table 1). By adulthood, on average, there are ∼17% fewer amygdala neurons in adults with ASD compared with children with ASD (P = 0.007). This same pattern was observed across all nuclei examined: basal, −16% (P = 0.042); accessory basal, −22% (P = 0.003); central, −18% (P = 0.017); and lateral, −12% (P = 0.065).
When comparing the two groups, children with ASD have significantly more neurons compared with neurotypical children in the basal (P = 0.047) and central (P = 0.021) nuclei. Adults with ASD have significantly fewer neurons relative to neurotypical adults in total amygdala (P = 0.001), lateral (P = 0.009), basal (P = 0.002), and accessory basal nuclei (P = 0.001). Omitting females [n = 9 (4 ASD, 5 NT)] or cases with seizure disorder (n = 11, all ASD) did not influence the pattern of results (Table 1).
Bcl-2 Immunohistochemistry of Immature Neurons.
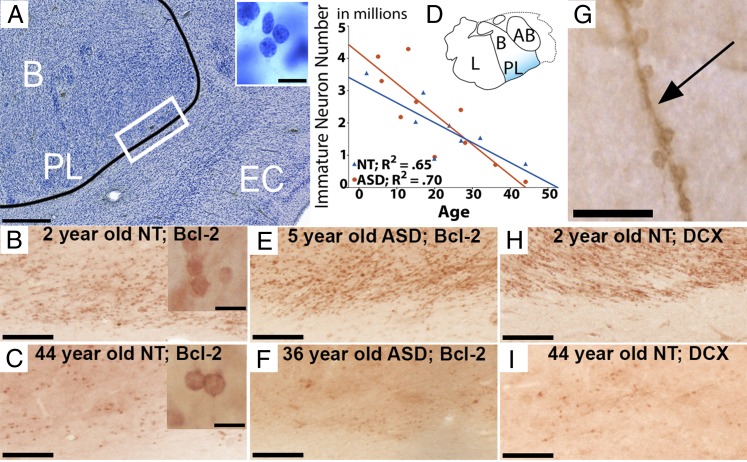
Bcl-2+ cells in the amygdala have a small, round morphology that is roughly 5 µm in diameter (Fig. 2) (6, 15–17, 33). The highest concentration of these immature neurons occurs in the most ventral aspects of the amygdala bordering subjacent white matter in the paralaminar nucleus near the emergence of the temporal horn of the lateral ventricle (Fig. S1). We observed an age-related decline of immature (bcl-2+) neurons within the paralaminar nucleus across the age range examined in both NT (β = −0.80, P = 0.016; Fig. 2 B and C) and ASD (β = −0.84, P = 0.003; Fig. 2 E and F) cases. There is also intense bcl-2+ immunoreactivity on fibers (Fig. 2G) upon which these immature cells may migrate dorsally from the paralaminar nucleus to the overlying amygdala in accordance with other anatomical reports (14, 16). Immunohistochemistry for DCX using adjacent tissue sections reveals an almost identical staining pattern to that of bcl-2 in age-related decline of immature neuron numbers (Fig. 2 H and I).
Fig. 2.
The paralaminar nucleus in a Nissl-stained section (A) relative to a bcl-2–stained section from a 2-y-old NT subject (B) and a 44-y-old NT subject (C) showing age-related differences. The immature neuron morphology for each is shown in their respective Insets. (D) Stereological assessment of bcl-2+ between ASD and NT groups from individuals aged 2 to 44 y showing an age-related decline of bcl-2+ cells in both groups across the life span. (E and F) Bcl-2 sections from a 5-y-old ASD subject (E) and a 36-y-old ASD subject (F) showing similar staining patterns relative to NT subjects. (G) A bcl-2–stained fiber upon which immature neurons appear to migrate. (H and I) DCX-stained sections from the same two subjects as in B and C showing similar patterns of immunoreactivity with bcl-2. (Scale bars: A = 500 µm, Inset = 10 µm; B and C = 100 µm, Insets = 10 µm; E, F, H, and I = 100 µm; G = 25 µm.)
Discussion
This extensive stereological study of the amygdala from youth to adulthood in 52 human brains led to three major findings. First, the number of mature neurons in the amygdala of typically developing humans increases, on average, by ∼11% from youth to adulthood (from 2 to 48 y in the present study). This increase is primarily driven by a 30% increase in the number of neurons in the basal nucleus over this age range. Second, the number of amygdala neurons in children with ASD is initially greater than age-matched neurotypical children, but by adulthood, there are fewer amygdala neurons in individuals with ASD. The number of neurons in the amygdala of ASD cases appears to decline from childhood to adulthood by about 17% and is significantly reduced in all regions we examined. Third, in the amygdala from both neurotypical and ASD cases, there is a large pool of bcl-2+ immature neurons in the paralaminar nucleus, which declines with age from childhood into adulthood.
Together, our data indicate that as the number of mature neurons increases in the neurotypical amygdala across life, the number of immature neurons in the paralaminar nucleus decreases. Therefore, it is possible that a gradual maturation and migration of immature neurons from the paralaminar nucleus contributes to the increasing number of mature neurons in the neurotypical amygdala, particularly in the basal and accessory basal nuclei. Individuals with ASD demonstrate a similar decrease in immature neurons in the paralaminar nucleus with age, yet the net number of mature neurons does not increase with age. Rather, cases of ASD show a decline in mature neuron number across the life span, suggesting a substantial loss of amygdala neurons throughout life in individuals with ASD. We discuss the rationale for this hypothesis below.
Typical Pattern of Amygdala Neuronal Development from Youth Through Adulthood.
Structural MRI studies have demonstrated that the amygdala in neurotypical individuals continues to grow in size by ∼40% throughout adolescence into adulthood. This is in contrast to most of the cerebral cortex, which ceases to grow appreciably after 6 y of age (4, 5, 12, 13). There was little or no understanding of the neurobiological substrate for this postnatal increase in volumetric growth of the human amygdala. We now report that the number of mature neurons in the amygdala increases by ∼11% from early childhood into adulthood. However, the extent of neuronal increase varies across the different subregions of the amygdala, where it was greatest in the basal nucleus (∼30%), more modest in the accessory basal nucleus (∼17%), and much less so in the lateral nucleus (∼3%).
One hypothesis to account for this increase in neuron number is the slow maturation and migration of a large pool of immature neurons within in the paralaminar nucleus produced prenatally. Here, we document a marked reduction in the number of immature (bcl-2+) cells in the human paralaminar nucleus, while mature basal nucleus neuron numbers increase from youth to adulthood. Bcl-2 immunoreactivity has been used to identify immature neurons in both human (16, 17) and nonhuman primate amygdala (6, 15, 18). A variety of other immature and migratory neuronal markers, including DCX, polysialylated-neural cell adhesion molecule, and class III β-tubulin (14), confirm their presence. Although the majority of bcl-2+ and DCX+ cells are found in the paralaminar nucleus ventral to the parvicellular portion of the basal nucleus, the paralaminar nucleus has a broad expanse, wrapping around the rostral amygdala. We also observed bcl-2+ and DCX+ cells in the ventral lateral nucleus, periamygdaloid cortex, and intercalated islands (15–17, 21, 33). Our data and others’ indicate that immature bcl-2 immunoreactive cells likely undergo a protracted maturational period well into adulthood (14), with numbers declining during normal aging in nonhuman primates (17, 21) in the paralaminar nucleus. However, the origin of these cells is debatable. In macaque monkeys, Chareyron et al. (6) found that immature paralaminar neurons, defined morphologically in Nissl-stained series, decreased in number from infancy to adulthood coincident with an increase in mature paralaminar neurons. Because the sum of these populations remained constant with age, they suggested that the paralaminar nucleus may contain a reservoir of immature neurons that mature over time. When we add together neuron numbers in basal and paralaminar nuclei in the monkey from Chareyron et al. (6) to more directly relate to our human measure, we find that macaque numbers increase by ∼32% from juveniles to adults, similar to humans (Fig. 1). This suggests a developmental mechanism shared by both species.
A second hypothesis is that postnatally generated neurons drive the neuronal increase (18). The paralaminar nucleus may represent the remnants of the fetal ganglionic eminence, making it plausible that this region retains neurogenic properties postnatally (14). Alternatively, immature neurons could migrate to the amygdala from a nearby neurogenic zone. In adult squirrel monkeys, Bernier et al. (18) observed a stream of pulse-labeled BrdU+ cells near the presumptive subventricular zone of the temporal horn of the lateral ventricle that they interpreted as newly generated neurons. The hypothesis is that newly born neurons migrate into the ventral amygdala, including the paralaminar region, where there is a high density of neurons that express the markers of immaturity previously described. We observed many bcl-2+ or DCX+ cells directly contacting immunoreactive fibers that extend dorsally into the basal nucleus, suggesting a substrate for immature neuronal migration from the paralaminar region into the amygdala (Fig. 2). It is important to note that regardless of the mechanism driving mature neuron increase in primates, similar age effects have not been observed in rats, and the presence of the paralaminar nucleus is debated in this species (34, 35). Thus, this developmental pattern may be unique to, or more extensive in, primates. However, more detailed comparative research is needed to assess the evolutionary and translational significance of these data and relevance for human susceptibility to diverse mental disorders presenting across the life span.
Atypical Pattern of Amygdala Neuronal Development Across the Life Span in ASD.
The trajectory of neuronal development in the amygdala of individuals with ASD is substantially altered from neurotypical development and suggests that processes that govern postnatal cellular maturation are dysregulated in individuals with ASD. Initially, the number of neurons in the amygdala is elevated in young children with ASD relative to neurotypical children, a finding also observed in the prefrontal cortex (36). However, in adults with ASD, amygdala neuron numbers are reduced relative to neurotypical adults. This altered developmental trajectory mirrors findings from MRI studies in which the amygdala is initially larger in childhood, but then does not undergo the corresponding age-related increase in size that occurs during neurotypical adolescence (23, 27, 37). The remainder of this discussion is divided into two developmental periods: childhood, followed by adolescence to adulthood.
Childhood—ASD.
Alterations in prenatal neurodevelopmental processes, such as disruptions to neuron proliferation, migration, maturation, and/or apoptosis, may cause this initial increase of amygdala neuron numbers during childhood in ASD. The nuclei-specific pattern of excessive neurons in children with ASD may be explained by the developmental origins of the individual amygdala nuclei. During fetal development, the medial and lateral ganglionic eminences differentially contribute to the formation of amygdala nuclei in a spatiotemporal manner (8–10). Specifically, the medial eminence is diencephalic, emerges first, and forms most of the amygdala nuclei; the lateral eminence is telencephalic and forms the lateral nucleus (38). We report here that the amygdala lateral nucleus does not show increased neuron numbers in children with ASD. Thus, the initial increases in neuron numbers in other nuclei may be explained by excessive contributions prenatally from the medial ganglionic eminence.
The increased amygdala neuron number in childhood ASD that we observed may also occur through disruptions to postnatal neurodevelopmental processes, namely those involving the paralaminar nucleus. The present study examined the possibility that this population of immature (bcl-2+) neurons within the paralaminar nucleus matures at an accelerated rate in autism, which might explain our observed increase of mature amygdala neuron number in childhood ASD. However, we actually observed a similar age-related decline of bcl-2+ cells with age in both groups, suggesting that this maturational process may be intact in individuals with ASD. At this time, we are unable to exclude other disruptions involving the paralaminar nucleus and its immature neurons. For example, while this age-related decline of bcl-2+ cells is present in ASD, it is possible that these neurons never actually mature and/or migrate into the dorsal amygdala. One limitation of the present study is that the youngest age evaluated was 2 y in the neurotypical group and 5 y in the ASD group. It remains possible that the postnatal changes in the number and maturational trajectories of bcl-2+ immature neurons are occurring earlier in postnatal development.
Another postnatal factor that may explain the increase of mature neuron number in the amygdala of individuals with ASD involves the activity-dependent maturation of immature amygdala neurons. Previous work has shown that neurons within the paralaminar nucleus may mature in an experience-dependent way (22). A recent report demonstrated that hippocampal-lesioned animals show an increased number of mature and immature neurons in the amygdala, suggesting that the lesions might not only drive neuronal differentiation but also stimulate the production of immature neurons in the nearby subventricular zone (20). It is possible that the hyperactivity of the amygdala frequently observed in individuals with ASD is in some way comparable to the stimulatory effects of these hippocampal lesions. In both instances, insults to the development and connectivity of the amygdala could conceivably lead to excessive neuronal differentiation and production.
Adolescence to adulthood—ASD.
During the time when the number of mature neurons within the amygdala is gradually increasing in neurotypical development, adolescents with ASD begin showing a reduction of neurons. The substantial decline of neuron numbers by adulthood in ASD may be driven by prolonged hyperactivation of the amygdala throughout life. There is some support for this hypothesis in other disorders, such as depression, where prolonged overactivation could result in decreases to amygdala and hippocampal volume, potentially through excessive glucocorticoid activity leading to excitotoxic neuron loss (39, 40). Similarly, this excitotoxicity may be occurring in ASD, which frequently cooccurs with anxiety such that by adulthood, the number of amygdala neurons in ASD is reduced relative to both adult-aged neurotypical and pediatric-aged ASD individuals. This excitatory/inhibitory imbalance of the amygdala in ASD may be caused by a number of factors, including a lack of habituation to sensory stimuli (41, 42), a lack of inhibitory control from frontal cortical areas, and changes to cellular and synaptic function. It is also plausible that the decline of neuron numbers in the amygdala of individuals with ASD may also be influenced by glia–neuron interactions or other immune factors. Specifically, microglia have been shown to regulate cell proliferation during the late stages of neurogenesis through phagocytosis of neural progenitors (43). Their role continues in the mature brain where they are responsible for the removal of apoptotic cells. Abnormal microglial presence and activation has been reported in ASD in the dorsolateral prefrontal cortex (44) and in the amygdala (45); however, these alterations were present only in a subset of ASD cases and unrelated to decreases in neuron number.
Together, our results suggest that there is an atypical developmental trajectory in the amygdala of individuals with ASD that results in substantial neuron loss by adulthood. Our findings are in accordance with other neuroanatomical studies that have observed increased numbers of prefrontal cortical neurons in children with ASD (36), as well as reduced neuron numbers in the fusiform gyrus and lateral nucleus of the amygdala (32, 46). However, the findings presented here may not be specific to autism and have extensive implications for a number of neurodevelopmental and neuropsychiatric disorders (7). Amygdala dysfunction has traditionally been implicated in a broad range of neuropsychiatric illnesses, including anxiety, mood disorders, posttraumatic stress disorder, and schizophrenia (47, 48). However, the precise contribution of amygdala dysfunction in these diseases and the developmental time course are not well understood. Future studies will need to address the origin, timing, plasticity, and neuronal fate of these immature neurons to further elucidate their role in neurological diseases and how they may be used as a potential therapeutic target for mental health.
Conclusion
We found that the number of mature neurons in the typical human amygdala increases over a protracted postnatal period, extending into at least late adolescence. We propose that this is due to the maturation and migration of immature neurons that are located initially in the paralaminar nucleus of the amygdala. The stimulus for this slow accretion of mature neurons is currently unclear but appears to be a unique feature of the postnatal development of the amygdala. The trajectory of neuronal development in the amygdala of individuals who had ASD during life drastically deviates from the typical developmental trajectory. There are ∼11% more neurons in the amygdala in very young individuals with ASD, but ∼20% fewer neurons in adults with ASD. This altered growth pattern may lead to altered amygdala function, manifesting in increased anxiety and further contributing to social impairments. Hyperactivity of the amygdala may also lead to neuron loss through excitotoxicity, especially in instances where the amygdala and its neurons are particularly vulnerable to stress. Understanding the regulation of neuron number at a fundamental level in this brain region will be important for interpreting the normal role of the human amygdala during life and may provide insight into some of the neural disturbances that contribute to the behavioral pathology of multiple neurodevelopmental and psychiatric disorders.
Experimental Procedures
Brain Samples.
Tissue series from 52 individuals, aged 2 to 48 y at death, were used for stereological quantification and contained the entire rostrocaudal extent of the amygdala (28 ASD, 24 NT; Table S1). All subjects were included from two cohorts, which differed in tissue-processing protocol. Cohort 1 included 19 ASD and 15 neurotypical brains processed in our laboratory obtained from the NIH NeuroBioBank or Autism BrainNet (formerly Autism Tissue Program). Partial data and diagnostic information from a subset of cohort 1 (n = 19) have previously been published by Schumann and Amaral (31). Cohort 2 included nine ASD and nine neurotypical brains from the Autism Celloidin Library distributed by Autism BrainNet. Cohorts 1 and 2 were combined for neuron number estimates and separated for cell volumetric analyses to avoid potential confounding factors due to variation in processing protocols. This study was exempt from Institutional Review Board approval.
Tissue Processing.
Detailed descriptions of tissue processing are available in previous studies from cohort 1 (31) and cohort 2 (49, 50) and in SI Experimental Procedures.
Stereological Design.
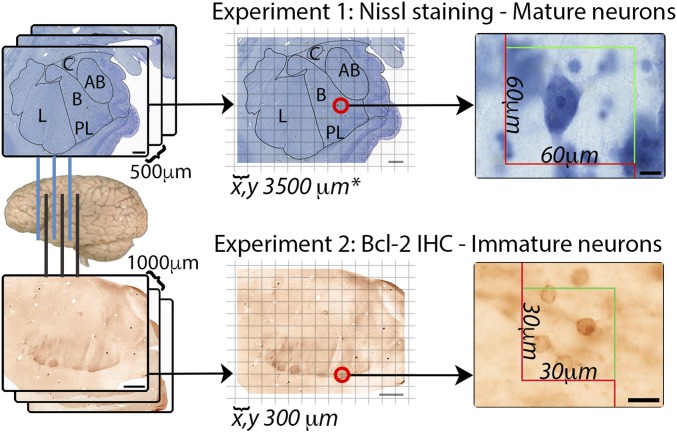
The amygdala and its lateral, basal, accessory basal, and central nuclei (Fig. S2) were delineated using previously published criteria (51). The paralaminar nucleus was identified using adjacent Nissl-stained sections. We sampled mature (Nissl) and immature (bcl-2+) neurons (100× objective, N.A. 1.3) through the entire structure of interest to derive numerical estimates using the optical fractionator as in our prior publications (52, 53). Stereological design is summarized in Fig. 3. Sampling parameters are presented in Table S2.
Fig. 3.
A methodological summary of our stereological approach. The entire rostrocaudal extent of the amygdala is sectioned. For experiment 1, we used Nissl-stained sections at a 1/5 sampling interval, and for experiment 2, we used alternating bcl-2–stained sections at a 1/10 sampling interval from the same brains. Each section has a virtual grid overlaid on top which designates physical locations to place sampling boxes. The numbers of objects are counted in each sampling box, and an estimate is extrapolated based on the size of the sampling box, the density of the sampling grid, the number of sections examined, and tissue thickness. Stereological parameters are presented in Table S2. (Scale bars: Left four, 2 mm; Right two, 10 µm.)
Statistical Analysis.
Subjects were classified a priori into three age groups: pediatric [2 to 13 y old; n = 17 (10 ASD, 7 NT)]; adolescent [14 to 20 y old; n = 11 (6 ASD, 5 NT)]; and adult [21+ y old; n = 19 (9 ASD, 10 NT)]. Univariate two-way ANOVA was used to test for effects of age group and diagnosis on neuron number, region volume, and neuron somal or nuclear volume within each amygdala nuclei. Significant age group × diagnosis interactions were further analyzed using a Fisher LSD post hoc test. The data met parametric assumptions for normality using a Shapiro–Wilk test (P > 0.05), and for homogeneity of variance using Levene’s test for equality of variance (P > 0.05).
Supplementary Material
Acknowledgments
We are grateful to the families and brain donors for their invaluable contribution. We thank Alicja Omanska for her technical and laboratory assistance and Carolyn Komich Hare and Susan Bacalman for Autism Diagnostic Interview-Revised and diagnostic collection. We also thank Dr. Sandra Taylor and the Biostatistics, Bioinformatics, and Research Design core; the MIND Institute; and the Intellectual and Developmental Disabilities Research Center at University of California, Davis (NIH Grant 1U54HD079125), for assistance in statistical analyses, and Steve Dana and Jeffrey Bennett for assistance in figure design. Tissue samples were provided by Autism BrainNet, supported by the Simons Foundation (formerly the Autism Tissue Program with Dr. Jane Pickett, supported by Autism Speaks) and the NIH NeuroBioBank at the University of Maryland Brain and Tissue Bank with Dr. Ron Zielke. This work was supported by NIH Grants MH41479, MH097236, and MH073124.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801912115/-/DCSupplemental.
References
- 1.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox AS, Oler JA, Tromp PM, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral DG. The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 4.Giedd JN, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Schumann CM, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chareyron LJ, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the amygdala: A stereological study in macaque monkeys. J Comp Neurol. 2012;520:1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey T. The development of the human amygdala during early embryonic life. J Comp Neurol. 1968;132:135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey T. The Neurobiology of the Amygdala: The Proceedings of a Symposium on the Neurobiology of the Amygdala, Bar Harbor, Maine, June 6–17, 1971. Springer; Boston: 1972. The development of the human amygdaloid complex; pp. 21–80. [Google Scholar]
- 10.Müller F, O’Rahilly R. The amygdaloid complex and the medial and lateral ventricular eminences in staged human embryos. J Anat. 2006;208:547–564. doi: 10.1111/j.1469-7580.2006.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Ann N Y Acad Sci. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 12.Østby Y, et al. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uematsu A, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.deCampo DM, Fudge JL. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci Biobehav Rev. 2012;36:520–535. doi: 10.1016/j.neubiorev.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fudge JL. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martí-Mengual U, Varea E, Crespo C, Blasco-Ibáñez JM, Nacher J. Cells expressing markers of immature neurons in the amygdala of adult humans. Eur J Neurosci. 2013;37:10–22. doi: 10.1111/ejn.12016. [DOI] [PubMed] [Google Scholar]
- 17.Yachnis AT, Roper SN, Love A, Fancey JT, Muir D. Bcl-2 immunoreactive cells with immature neuronal phenotype exist in the nonepileptic adult human brain. J Neuropathol Exp Neurol. 2000;59:113–119. doi: 10.1093/jnen/59.2.113. [DOI] [PubMed] [Google Scholar]
- 18.Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 20.Chareyron LJ, Amaral DG, Lavenex P. Selective lesion of the hippocampus increases the differentiation of immature neurons in the monkey amygdala. Proc Natl Acad Sci USA. 2016;113:14420–14425. doi: 10.1073/pnas.1604288113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernier PJ, Parent A. Bcl-2 protein as a marker of neuronal immaturity in postnatal primate brain. J Neurosci. 1998;18:2486–2497. doi: 10.1523/JNEUROSCI.18-07-02486.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fudge JL, deCampo DM, Becoats KT. Revisiting the hippocampal-amygdala pathway in primates: Association with immature-appearing neurons. Neuroscience. 2012;212:104–119. doi: 10.1016/j.neuroscience.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JE, et al. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Arch Gen Psychiatry. 2010;67:1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- 25.Mosconi MW, et al. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks BF, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 27.Nordahl CW, et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: A longitudinal study. Arch Gen Psychiatry. 2012;69:53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalton KM, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. J Neurosci. 2012;32:9469–9476. doi: 10.1523/JNEUROSCI.5294-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- 31.Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegiel J, et al. Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathol Commun. 2014;2:141. doi: 10.1186/s40478-014-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XM, et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front Neuroanat. 2009;3:17. doi: 10.3389/neuro.05.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chareyron LJ, Lavenex PB, Lavenex P. Postnatal development of the amygdala: A stereological study in rats. J Comp Neurol. 2012;520:3745–3763. doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- 35.Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: A stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courchesne E, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 37.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 38.O’Rahilly R, Müller F. Significant features in the early prenatal development of the human brain. Ann Anat. 2008;190:105–118. doi: 10.1016/j.aanat.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 41.Green SA, et al. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015;72:778–786. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleinhans NM, Richards T, Greenson J, Dawson G, Aylward E. Altered dynamics of the fMRI response to faces in individuals with autism. J Autism Dev Disord. 2016;46:232–241. doi: 10.1007/s10803-015-2565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan JT, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 45.Morgan JT, Barger N, Amaral DG, Schumann CM. Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder. PLoS One. 2014;9:e110356. doi: 10.1371/journal.pone.0110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Kooten IAJ, et al. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131:987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 49.Barger N, Sheley MF, Schumann CM. Stereological study of pyramidal neurons in the human superior temporal gyrus from childhood to adulthood. J Comp Neurol. 2015;523:1054–1072. doi: 10.1002/cne.23707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heinsen H, Arzberger T, Schmitz C. Celloidin mounting (embedding without infiltration)–A new, simple and reliable method for producing serial sections of high thickness through complete human brains and its application to stereological and immunohistochemical investigations. J Chem Neuroanat. 2000;20:49–59. doi: 10.1016/s0891-0618(00)00067-3. [DOI] [PubMed] [Google Scholar]
- 51.Schumann CM, Amaral DG. Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol. 2005;491:320–329. doi: 10.1002/cne.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 53.Lord C, Rutter M, Le Couteur A. Autism Ddiagnostic Iinterview-Rrevised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 54.Gundersen HJG, et al. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.