In 2007, Hans Clevers and his colleagues uncovered a new type of stem cell. After nearly a decade of searching, they’d identified a stem-cell population in the mouse intestine that refreshes the organ’s lining every few days (1). Now they needed to figure out how to grow the cells in a dish.
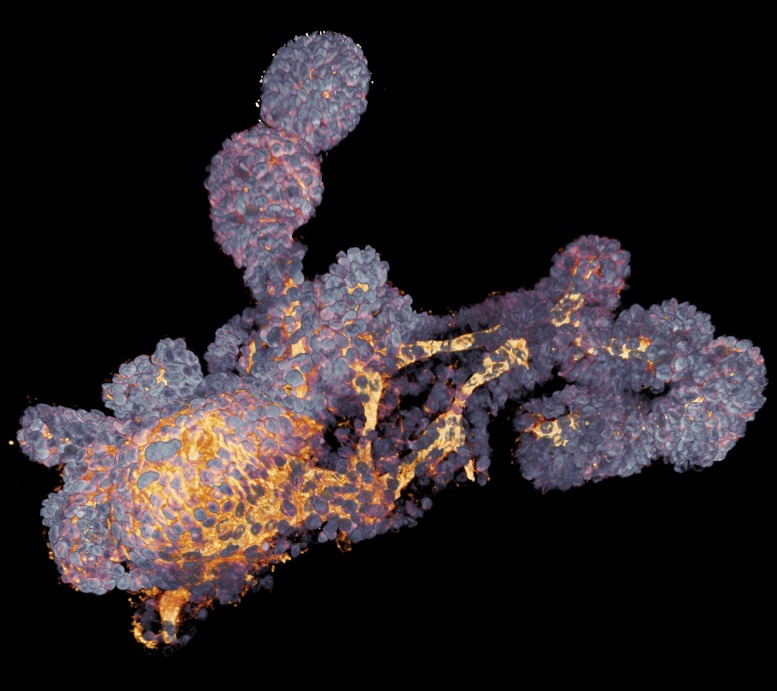
Derived from stem cells, lab-grown organoids mimic many cell types and structures in real organs. This colonic organoid, imaged with a confocal microscope, was derived from a cystic fibrosis patient. Image courtesy of Florijn Dekkers (Hubrecht Institute, Utrecht, The Netherlands) and Anne Rios (Prinses Máxima Centrum voor Kinderoncologie, Utrecht, The Netherlands).
Toshiro Sato, then a postdoctoral researcher in Clevers’ lab at the Hubrecht Institute in Utrecht, The Netherlands, tried bathing the stem cells with various growth factor cocktails. One recipe yielded a bizarre result. “Rather than just making more stem cells, we made these structures, and they looked like mini-guts,” says Clevers. “We were totally surprised.” When incubated with this mix of biochemicals, single stem cells grew into tiny multicellular balls measuring a few millimeters in diameter. Inside, the researchers found a hollow cavity surrounded by what looked like the intestinal lining: an intricate array of nutrient-absorbing protrusions, interleaved with valleys (2). The discovery, published in 2009, would become a landmark event in the fast-growing field of organoids, or miniature laboratory-grown versions of organs.
Because they mimic the genetics, cellular makeup, and structure of their source material, organoids made from human stem cells could help model human development and disease—especially conditions not well replicated in experimental animals. Mice, for example, lack a well-developed prefrontal cortex, which is thought to enable higher cognition and play a role in many psychiatric disorders. Animal models also can’t capture the entire genetic makeup of a human patient, making it especially hard to model disorders with complex genetic underpinnings. Organoids also provide a path to more personalized medicine, serving as a potential platform for testing how drugs will interact with each patient’s biology.
The possibilities have researchers flocking to the field. “There’s been an explosion of papers on organoids in the past few years,” says developmental biologist Jürgen Knoblich at the Institute of Molecular Biotechnology in Vienna. Today’s organoids are only rudimentary versions of their real-life counterparts, but with continued cell-culture method improvements, researchers hope to create larger and more elaborate models.
An Embryonic Field
Researchers have long recognized the capacity for mammalian cells to self-organize in culture; scientists have pursued organoid work of various forms since at least the 1970s. In 1989, Mina Bissell at Lawrence Berkeley National Laboratory in California laid some of the foundational work for modern organoids, publishing a method for growing mouse mammary cells into 3D structures that made milk and resembled the cellular structure of mammary alveoli—milk-producing sacs inside the mammary gland (3).
But the organoid field remained relatively small until the late 2000s. High-profile papers by Clevers’ group and researchers in Japan sparked today’s organoid boom, enabled by converging advances in stem-cell technology and developmental biology. Biologists were discovering new, more efficient ways of purifying and growing stem cells from human and animal tissue. At the same time, researchers had unraveled many of the molecular pathways that guide organ development—providing detailed roadmaps for turning stem cells into complex structures. “It was an alignment of the planets,” says developmental biologist James Wells at Cincinnati Children’s Hospital Medical Center.
In 2008, Yoshiki Sasai and his colleagues at the RIKEN Center for Developmental Biology in Kobe reported coaxing mouse and human embryonic stem cells to form layered tissue resembling pieces of cerebral cortex (4). Later, Sasai’s group developed an even more sophisticated organoid—a cup-shaped precursor to the eye, layered with all the major retinal cell types including photoreceptors, ganglion cells, and bipolar cells (5). “I would credit him with being one of the true masters of organoid technology, especially when it comes to the brain and the eye,” says neuroscientist Arnold Kriegstein at the University of California, San Francisco. In 2009, Clevers published his research on mini-guts, grown from stem cells
“I predict that this will very significantly change the entire disease drug discovery pipeline.”
—Jürgen Knoblich
in the small intestine. The organoids’ gene expression patterns closely matched those of the real small intestine and were distinct from those of the colon, a closely related tissue (2).
Together, these studies opened two major research avenues, which have since spawned miniatures of several organs, including the liver, stomach, and kidney. One approach, led by Clevers, starts with adult stem cells that live in tissues such as the intestine, which they continually help renew or repair. In the dish, these cells can be differentiated to mimic their source organ but cannot be rerouted to form a different organ.
The other tack, pioneered by Sasai, harnesses pluripotent stem cells, which include embryonic stem cells or skin and blood cells reverted to an embryonic-like state. These stem cells can develop into any tissue type with the right combination and sequence of growth factors. However, the resulting organoids only mimic the very beginnings of development, producing embryonic or fetal tissues.
In the past few years, researchers have leveraged these complementary systems to model various diseases. Organoids made from pluripotent stem cells offer a chance to study how human brain malformations and other birth defects arise in early development. Organoids derived from adult stem cells, on the other hand, are poised to reveal the mechanisms behind cancer and other diseases affecting mature tissues.
Disease in a Dish
Clevers’ lab has developed an organoid model of cystic fibrosis, which causes thick, sticky mucus buildup in the lungs, gastrointestinal tract, and other organs. Cystic fibrosis can be caused by thousands of different mutations, all disrupting the CFTR gene, which encodes an ion channel that moves ions and water across cell membranes. Starting with a relatively painless rectal biopsy from a cystic fibrosis patient, Clevers can make personalized mini-guts that mimic the patient’s version of the disease.
In a 2013 study, Clevers’ group showed that healthy gut organoids swell in response to the drug forskolin—a result of the CFTR channel drawing in fluid. Cystic fibrosis patients produce organoids that do not swell because of their faulty CFTR gene, which disrupts the channel. But compounds that increase CFTR activity restore this swelling response in the patients’ organoids. This response could help indicate which drugs will be effective against a person’s particular CFTR defect (6).
Organoids have also helped in the race to understand the link between Zika virus and microcephaly, a developmental condition that stunts head and brain growth. In 2013, Knoblich’s group developed a new cerebral organoid, made from skin-derived pluripotent stem cells, which contained molecular signatures of multiple brain regions including the hippocampus and prefrontal cortex. Larger and more complex than Sasai’s cortical chunks, this organoid allowed the researchers to recreate a version of microcephaly (7). Within a few years, cerebral organoids would pop up around the world and play a key role in researchers’ response to the Zika outbreak. “At least 10–12 labs exploited the mini-brains,” says Clevers. “That has been a big deal.” Together with other techniques, organoids helped researchers see how Zika infiltrates the developing brain and how it hinders growth by targeting neural progenitor cells (8).
Because organoids match the genetic makeup of their human donors, mini-organs could help tackle some forms of cancer as well. Tumors often arise from an accumulation of mutations, resulting in a complex genetic background that most animal models can’t capture. At the University of Cambridge in England, Meritxell Huch is growing organoids from cancerous liver tissue removed from patients. In a 2017 study, she found that these organoids faithfully preserve the tissue structure and gene expression patterns of their parent tumors, even maintaining differences across cancer subtypes. Huch’s group discovered 11 genes, never before linked to liver cancer, that were highly expressed in cancer patients’ liver organoids compared to healthy organoids. By examining a public cancer genome database, Huch further discovered that overexpression of four of those genes predicted poor prognoses (9). “This is a new capability for organoid technology—identifying potential genes involved in liver cancer,” she says.
Along with disease insights, organoid researchers are hoping for drug discoveries or better ways to test drugs for individual patients. In the next few years, others see potential for organoids to provide tissues for patients with damaged or defective organs.
Not the Real Thing
But researchers still have to grapple with several limitations of the system. For one, organoids are imperfect copies of the real thing. In the case of liver organoids, Huch notes that cells often fail to fully mature, likely because current culturing methods don’t recreate the body’s complete biochemical and physical environment. For example, organoids floating in media outside of an animal’s body lack the normal directional cues that precisely organize the body’s cells from top to bottom, left to right. As a result, when cerebral organoids self-assemble, different brain regions can end up in random locations relative to each other. And as Kriegstein’s preliminary data suggest, cerebral organoid cells sometimes show subtle gene expression differences from the cells they’re meant to imitate. “More and more people will have to take a closer look at what they’re making,” he cautions.
Another major limitation: researchers can’t grow organoids containing blood vessels. The lack of circulation limits organoid size and complexity. For now, Wells has experimented with transplanting human intestinal organoids into mice. As the animal’s blood supply enters and feeds the organoids, they become bigger and more mature than they would in a dish (10). “It’s a short-term solution,” says Wells.
In the case of cerebral organoids in particular, researchers say additional work is needed to more faithfully reproduce the brain's complex cellular makeup, including different neuron types and a variety of glial cells. Some neuroscientists are also working toward transplanting brain organoids into rodents. Such transplants could, for example, provide models for studying how to repair the damaged brain, but this line of research has raised weighty ethical concerns (11).
In 2013, Clevers helped found the nonprofit Hubrecht Organoid Technology (HUB), which maintains an organoid biobank for academic and industry researchers. The repository currently includes hundreds of cystic fibrosis and cancer organoids. HUB also works with pharmaceutical companies to develop and test drugs on organoids. Building on promising initial results from Clevers and his collaborators (6, 12), Vertex Pharmaceuticals in Boston is working with HUB on two clinical trials to test if organoids can predict whether and how much individual patients will respond to their cystic fibrosis drugs.
As the field grows and matures, many researchers are eager to combine organoids with another rising technology: gene editing. By modifying organoids with techniques such as CRISPR, researchers hope to see precisely how a patient’s tissues behave with and without a specific mutation. Such discoveries could help pinpoint disease-causing mutations and mechanisms—potentially leading to new drug targets. “I predict that this will very significantly change the entire disease drug discovery pipeline,” says Knoblich. Others envision using gene editing to correct a patient’s mutations in organoids, then transplanting that healthy tissue to replace or repair the patient’s damaged organs. “That,” says Clevers, “would be my ultimate dream.”
References
- 1.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eiraku M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 6.Dekkers JF, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster MA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian X, Nguyen HN, Jacob F, Song H, Ming GL. Using brain organoids to understand Zika virus-induced microcephaly. Development. 2017;144:952–957. doi: 10.1242/dev.140707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broutier L, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson CL, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–1314. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begley S. November 6, 2017 Tiny human brain organoids implanted into rodents, triggering ethical concerns. STAT. https://www.statnews.com/2017/11/06/human-brain-organoids-ethics/. Accessed March 2, 2018.
- 12.Dekkers JF, et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 2016;8:344ra84. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]