Abstract
Despite the availability of antiretroviral prophylactic treatment, pediatric human immunodeficiency virus type 1 (HIV-1) continues to be a significant risk factor in the post-cART era. The time of infection (i.e., during pregnancy, delivery or breastfeeding) may play a role in the development of neurocognitive deficits in pediatric HIV-1. HIV-1 viral protein exposure on postnatal day (P)1, preceding the postnatal brain growth spurt in rats, had deleterious effects on neurocognitive development and anatomical parameters of the hippocampus (Fitting et al., 2008a; Fitting et al., 2008b). In the present study, rats were stereotaxically injected with HIV-1 viral proteins, including Tat1–86 and gp120, on P10 to further examine the role of timing on neurocognitive development and anatomical parameters of the hippocampus (Fitting et al., 2010). The dose-dependent virotoxin effects observed across development following P10 Tat1–86 exposure were specific to spatial learning and absent from prepulse inhibition and locomotor activity. A relationship between alterations in spatial learning and/or memory and hippocampal anatomical parameters was noted. Specifically, the estimated number of neurons and astrocytes in the hilus of the dentate gyrus explained 70% of the variance of searching behavior in Morris water maze acquisition training for adolescents and 65% of the variance for adults; a brain-behavior relationship consistent with observations following P1 viral protein exposure. Collectively, late viral protein exposure (P10) results in selective alterations in neurocognitive development without modifying measures of somatic growth, preattentive processing, or locomotor activity, as characterized early viral protein exposure (P1). Thus, timing may be a critical factor in disease progression, with children infected with HIV earlier in life being more vulnerable to CNS disease.
Keywords: Tat, gp120, Pediatric HIV-1, Spatial Learning, Stereology, Hippocampus
1. INTRODUCTION
Pediatric human immunodeficiency virus type 1 (HIV-1) predominantly results from ``vertical" mother-to-child transmission (MTCT), occurring during active labor and delivery, pregnancy and/or postnatal breastfeeding (Kourtis et al., 2001; AIDSinfo, 2017). Marked decreases in the prevalence of pediatric HIV-1 were observed following the implementation of interventions aimed at preventing MTCT, including treatment with combination antiretroviral therapy (cART; Luzuriaga & Mofenson, 2016). cART is commonly prescribed to HIV-1-infected mothers during pregnancy (Suksomboon et al., 2007; Volmink et al., 2007; Townsend et al., 2008; Briand et al., 2013; Reliquet et al., 2014; Townsend et al., 2014; Wang et al., 2016, Peters et al., 2017) and HIV-1 infected newborns and/or children (Riordan & Bugembe, 2009; Chadwick et al., 2011; van der Plas et al., 2013; Bitnun et al., 2014; Mutwa et al., 2014, Shiau et al., 2017). Nevertheless, despite the availability of antiretroviral prophylactic treatment, vertical MTCT continues to be a significant risk factor in the post-cART era, with approximately 160,000 children being newly infected in 2016, most of whom live in resource-limited settings (UNAIDS, 2017).
Additionally, the advent of cART shifted the global epidemic into a new phase as HIV-1 seropositive children survive into adulthood (Sohn & Hazra, 2013; Crowell et al., 2014; Smith & Wilkins, 2015). By 2020, it is estimated that approximately 1.94 million children will be living with HIV-1 (Penazzato et al., 2014). The chronic nature of pediatric HIV-1 in the post-cART era has enormous implications for children’s neurocognition and development (Vijayan et al., 2009; Brady et al., 2010; Paramesparan et al., 2010; Sohn & Hazra, 2013; Crowell et al., 2014). Specifically, high rates of subtle to severe neurocognitive deficits have been reported in HIV-1 infected children on cART that survive to adulthood (Burns et al., 2008; Webb et al., 2009; Paramesparan et al., 2010; Crowell et al., 2014). The causes of these neurocognitive deficits, despite effective cART, are multifactorial and likely include continued viral replication in the central nervous system (CNS), ongoing neuroinflammation, irreversible CNS injury prior to cART initiation, neurotoxic effects of cART, and socioeconomic and psychosocial constraints (Crowell et al., 2014).
Pediatric HIV-1 infection presents a very different clinical picture compared to HIV-1 infection in adulthood (Sohn & Hazra, 2013; Crowell et al., 2014). In the post-cART era, HIV-1 infected children commonly exhibit high rates of chronic neurological impairment, including significant delays in cognitive development, motor skills, and language (Lindsey et al., 2007; Van Rie et al., 2008; Walker et al., 2013). The rates of disease progression within the pediatric HIV-1 population, however, have been shown to dramatically vary and are likely related to differences in viral load, strain of HIV, time of HIV infection, and/or genetic vulnerabilities (Belman, 1997; Rigardetto et al., 1999; Chearskul et al., 2002; Crowell et al., 2014). A relationship between the time of perinatal infection and the course of the disease in infants has also been suggested by other studies (Blanche et al., 1990; Blanche et al., 1994; Becquet et al., 2012) with a greater sensitivity of the immature brain to the devastating effects of HIV-1 (Meeker et al., 2004; Becquet et al., 2012). To date, however, the relative importance of timing and its effects on neurocognitive development has been relatively understudied in pediatric HIV-1.
In human pediatric HIV/AIDS cases, the time of infection is broadly defined as ‘occurring in the period shortly before and after birth’, which describes both prenatal (i.e., 20th to 28th week of gestation) and postnatal (i.e., 7 to 28 days after birth) infection (Wiley et al., 1994; Donovan & Palumbo, 2010; CDC, 2014). Technological limitations of HIV-1 testing prevent us from determining either the exact timing or degree of early postnatal transmission of HIV-1. DNA or RNA polymerase chain reactions (PCR), viral culture, and p24 antigen tests, although able to detect the virus itself, are not able to define the route of infection before the age of 2–3 months (Ogundele & Coulter, 2003; CDC, 2014). Due to experimental limitations and ethical constraints in human pediatric AIDS research, an animal model that is (A) translational to the important health issues surrounding pediatric AIDS, and (B) able to determine the timing of MTCT and its effects on the CNS in a precise and controlled manner, is of great clinical relevance in defining the different rates of progression in pediatric HIV/AIDS. In the present study, stereotaxic injections of HIV-1 viral proteins at postnatal day 10 (P10), including the transactivator of transcription (Tat1–86) and envelope glycoprotein 120 (gp120), provides an opportunity to elucidate the effect of neurotoxic proteins on neurodevelopment (Carryl et al., 2015).
Neurotoxic viral proteins, including Tat and gp120, are active in the CNS, even when peripheral immune system function is restored under cART, and may be partially responsible for the neuroanatomical alterations commonly observed in HIV-1 seropositive individuals (e.g., synaptodendritic alterations: Ellis et al., 2007; Gelman & Nguyen, 2010; Desplats et al., 2013; neuronal loss: Del Valle et al., 2000; Jones et al., 2000; Nath et al., 2000). Tat, the transactivating protein for retroviral replication (Cann et al., 1985; Li et al., 2010), is produced very early after infection, and is necessary for viral expression, cell-to-cell virus transmission and disease progression (Sodroski et al., 1985; Ensoli et al., 1993; Chang et al., 1997; Karn, 1999; Lin et al., 2003; Ensoli et al., 2006; Richter & Palu, 2006). Gp120, a structural viral gene product, is vital for viral entry into the CNS (Hao & Lyman, 1999) and may cause neurotoxicity by binding to cell surface receptors, ultimately leading to neuronal death (Lipton, 1991; Corasaniti et al., 2001a; Haughey & Mattson, 2002). Preclinical assessments, including both in vitro and in vivo studies, have extensively examined both Tat and gp120 (Brenneman et al., 1988; Lipton et al., 1995; Nath et al., 1996; Cheng et al., 1998; Aksenov et al., 2001; Corasaniti et al., 2001b; Bruce-Keller et al., 2003; Aksenov et al., 2006; Aksenova et al., 2006; Fitting et al., 2006a; Aksenov et al., 2008; Adams et al., 2010; Zhu et al., 2011; Bertrand et al., 2013; Fitting et al., 2013; Hahn et al., 2013; Bertrand et al., 2014; Bertrand et al., 2015; Marks et al., 2016), providing strong evidence for the role of HIV-1 viral proteins in neurocognitive and neuroanatomical alterations in adulthood.
Although preclinical and clinical studies have characterized HIV-1 associated neurocognitive disorders (HAND) in adults (e.g., review, Woods et al., 2009; Heaton et al., 2010), neurocognitive deficits in HIV-infected children are poorly understood (Crowell et al., 2014). A limited number of preclinical studies have focused on the effects of early exposure to HIV-1 viral toxic proteins on the development of neurocognitive disorders, independent of the virus itself (Bussiere et al., 1999; Fitting et al., 2008b; Webb et al., 2009; Moran et al., 2014a; Fitting et al., 2015; McLaurin et al., 2017a). Specifically, stereotaxic injections of the viral proteins Tat and/or gp120 on P1 revealed deleterious effects on multiple reflexive, preattentive (e.g., prepulse inhibition (PPI), and neurocognitive assessments (e.g., Morris water maze), as well as alterations in anatomical parameters of the hippocampus (Fitting et al., 2008a; Fitting et al., 2008b; Moran et al., 2014a). A histological dose-response study that injected HIV-1 proteins at P10 indicated that timing may have a differential effect on the anatomical parameters of the hippocampus in adult rats (~ 5 months) compared to the P1 study (Fitting et al., 2008a; Fitting et al., 2010). Stereotaxic injections of HIV-1 viral proteins on P1 in rats were chosen to mimic early transmission (i.e., in utero) of the virus, whereas HIV-1 protein delivery on P10 more closely resembles HIV-1 protein entry into the CNS at labor/delivery in humans.
Thus, the present study investigated the effects of perinatal P10 HIV-1 protein neurotoxicity using a series of neurocognitive assessments throughout development and adulthood to address two interrelated aims. First, dose-response functions were used to examine the short and long-term effects of Tat1–86 and gp120 on developmental milestones and neurocognition. Measures of somatic growth, including body weight and eye opening, and motor development, including locomotor activity, were used to examine developmental milestones. Neurocognitive assessments included PPI, to examine sensorimotor gating and temporal processing, and the Morris water maze, to examine spatial learning and memory. Assessments were chosen as indexes of neuropsychological assessments in humans that have been shown to be impaired in pediatric HIV-1 infection (Boivin et al., 1995; Newell et al., 2003; Jeremy et al., 2005; Lodha et al., 2005; Martin et al., 2006; Willen, 2006; Lindsey et al., 2007; Baillieu & Potterton, 2008; Van Rie et al., 2009; Webb et al., 2009; Paramesparan et al., 2010; Le Doare et al., 2012; Walker et al., 2013). Second, the relationship between neurocognitive deficits and neuroanatomical alterations (Fitting et al., 2010) was investigated. Thus, the present study provides an opportunity to determine whether the developing CNS is more vulnerable to later HIV-1 viral protein exposure period (i.e., P10) relative to the earlier published findings at P1 (Fitting et al., 2008a; Fitting et al., 2008b; Moran et al., 2014a), aiding in the understanding of the role of timing in chronic neurological impairment in pediatric HIV-1.
2. RESULTS
2.1 Somatic Growth
2.1.1 Body Weight
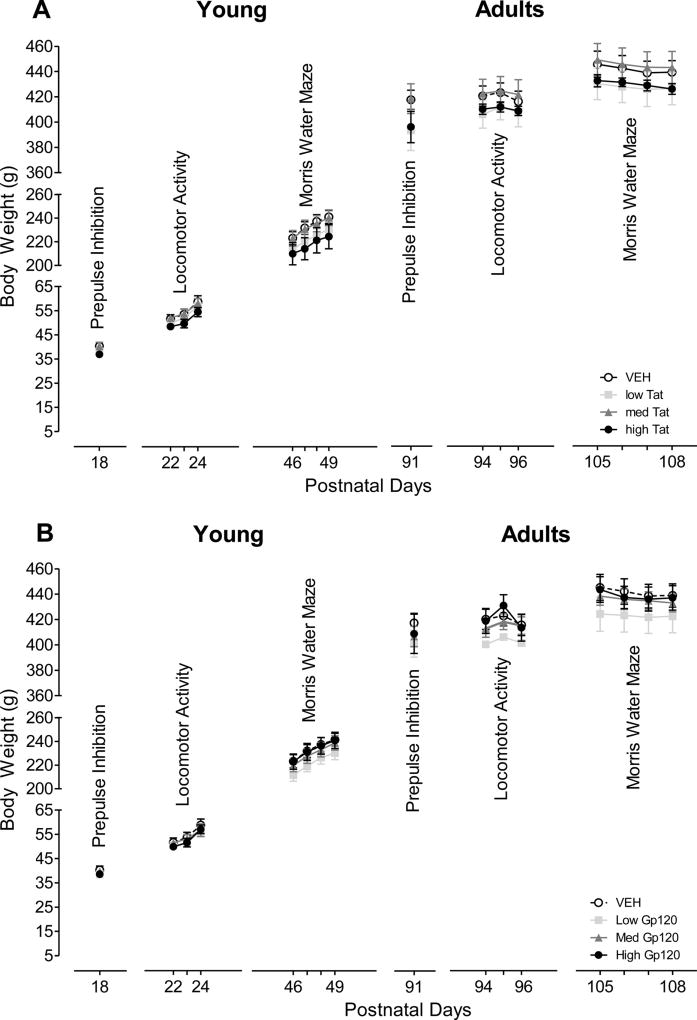
Figure 1 illustrates the mean (± S.E.M.) body weight data across different test days for each treatment group (Tat: A, Gp120: B). Body weight increased significantly with age across all groups. Separate ANOVAs were conducted for each testing set with Tat dose treatment (4 levels) or gp120 dose treatment (4 levels) as a between-subjects factor, and when appropriate, a within-subjects test day factor. For Tat or gp120, ANOVAs demonstrated no treatment effect or treatment by day interaction for any of the test sets. Planned contrast analyses for Tat or gp120 revealed no overall treatment effect, no dose-dependent treatment effect, and hence no threshold effect. Results indicated that the HIV-1 viral protein doses produced no general growth deficits when injected on P10 consistent with the findings of the previous P1 study (Fitting et al., 2008b).
Figure 1.
Mean (± S.E.M) body weight across the various test days for HIV-1 proteins (A) Tat dose treatment and (B) gp120 dose treatment. No Tat or gp120 treatment effects were noted at any test set/day.
2.1.2 Eye opening
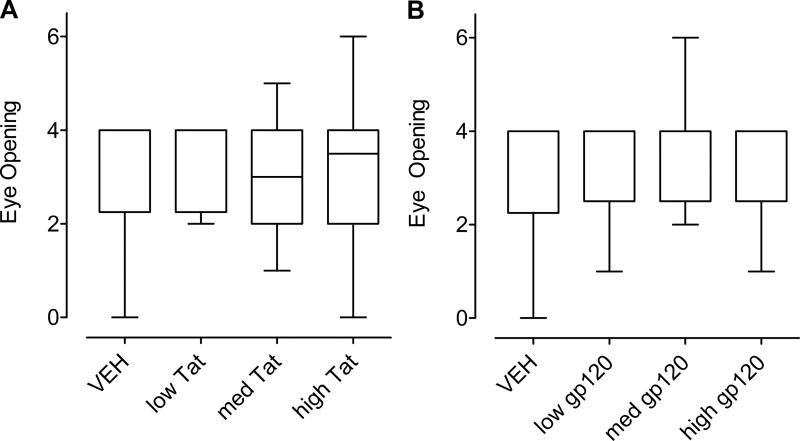
Figure 2 illustrates the median (± Interquartile Range) for eye opening collapsed across P13 – P16. Eye opening began at P13; eyes were fully open for all rats by P16 (maximum rank score was 6 and the minimum rank score was 0). For the Tat treatment, a Kruskal-Wallis test revealed no significant difference among the four dose conditions (Figure 2A). Separate Mann-Whitney U-Tests, for the overall Tat effect and each Tat dose individually compared to the VEH control group revealed no significant difference from the VEH group. Similarly, no significant treatment effects were found across the gp120 dose conditions (Figure 2B). The lack of any P10 HIV-1 protein exposure effect on eye opening contrasts the effect of P1 Tat1–72 exposure, but is consistent with the effect of P1 gp120 exposure (Fitting et al., 2008b), suggesting that an early exposure period is more vulnerable to the developing CNS system.
Figure 2.
Median (± Interquartile Range) eye opening scores collapsed across P13 – P16 for HIV-1 proteins (A) Tat dose treatment and (B) gp120dose treatment. No significant treatment effects were noted for either the gp120- or Tat treatments.
2.2 Sensorimotor Function (PPI of the ASR)
2.2.1 Control Trials (0, 4000 msec Interstimulus Interval (ISI) combined)
For preweanlings and adults, one-way ANOVAs on peak ASR amplitude, revealed no significant treatment effect for Tat or gp120 dose treatment. Planned contrast analyses for Tat or gp120 revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect, suggesting that the baseline startle response for the HIV-1 viral protein groups were comparable to the VEH condition.
2.2.2 PPI trials (8–120 msec ISI)
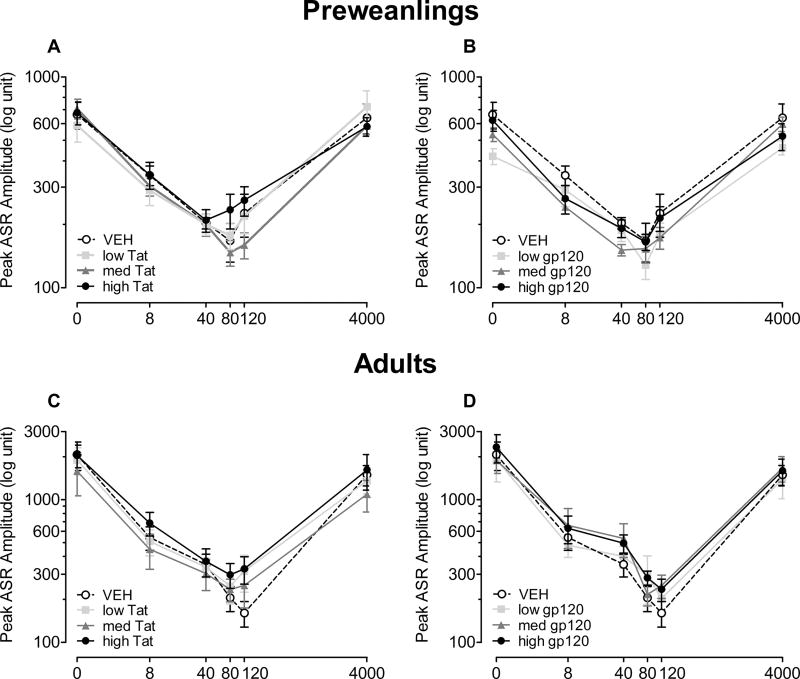
For preweanlings, at 18 days of age, Figure 3A and 3B illustrate mean peak ASR amplitude (± S.E.M.) across ISI (0–4000 msec) for the Tat dose treatment (Figure 3A) and the gp120 dose treatment (Figure 3B).
Figure 3.
Peak ASR amplitude data (± S.E.M.) across ISI (0–4000 msec) for preweanlings (Tat (A) and gp120 (B)) and adults (Tat (C) and gp120 (D)). No significant treatment effect and/or interaction was noted at either age.
For the Tat dose treatment groups a 64.4% decrease in response amplitude was found for the PPI trials compared to the ASR control trials, again, indicating effective sensorimotor gating for all four groups. A 4 (Tat) × 4 (ISIs 8, 40, 80, 120 msec) mixed-model ANOVA conducted on peak ASR amplitude demonstrated a significant ISI effect [F(3, 84) = 20.5, p ≤ 0.001] with linear and quadratic components [F(1, 28) = 15.0, p ≤ 0.001 and F(1, 28) = 44.1, p ≤ 0.001, respectively]. No treatment effect or treatment by ISI interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect. For the ISI data, there was no significant shift in the point of maximal peak inhibition for Tat dose treatment compared to the VEH-treated animals; maximal peak inhibition occurred at either the 40 msec or 80 msec ISI.
For the gp120 dose groups, there was a 63.3% decrease in response amplitude for the PPI trials compared to the ASR control trials, demonstrating effective sensorimotor gating for all four groups. A 4 (Gp120) × 4 (ISIs 8, 40, 80, 120 msec) mixed-model ANOVA conducted on peak ASR amplitude demonstrated a significant ISI effect [F(3, 84) = 23.9, p ≤ 0.001] with linear and quadratic components [F(1, 28) = 17.9, p ≤ 0.001 and F(1, 28) = 48.0, p ≤ 0.001, respectively]. No treatment effect or treatment by ISI interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect. For the ISI data, no significant shift in the point of maximal inhibition occurred for gp120 dose treatment compared to the VEH-treated animals; maximal peak inhibition occurred at either the 40 or 80 msec ISI.
For adults, at 91 days of age, Figure 3 displays (± S.E.M.) peak ASR amplitude across ISI (0–4000 msec) for Tat dose treatment (Figure 3C) and gp120 dose treatment (Figure 3D).
For the Tat dose groups, analyses of the data revealed an inhibition of the baseline ASR by 78.9%, again demonstrating effective sensorimotor gating for all four Tat dose groups. A 4 (Tat) × 4 (ISIs 8, 40, 80, 120 msec) mixed-model ANOVA conducted on peak ASR amplitude demonstrated a significant ISI effect [F(3, 78) = 30.8, p ≤ 0.001] with significant linear and quadratic components [F(1, 26) = 37.9, p ≤ 0.001 and F(1, 26) = 22.5, p ≤ 0.001, respectively]. No treatment effect or treatment by ISI interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect. For the ISI data, no significant shift in the point of maximal peak inhibition occurred for Tat dose treatment groups relative to the VEH-treated animals; maximal peak inhibition occurred at either the 80 msec or 120 msec ISI.
For the gp120 dose groups, analyses of the data revealed an inhibition of the baseline ASR by 79.3%, again demonstrating effective sensorimotor gating for all four gp120 dose groups. A 4 (Gp120) × 4 (ISIs 8, 40, 80, 120 msec) mixed-model ANOVA conducted on peak ASR amplitude demonstrated a significant ISI effect [F(3, 84) = 28.5, p ≤ 0.001] with a prominent linear component [F(1, 28) = 35.6, p ≤ 0.001]. No treatment effect or treatment by ISI interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect. For the ISI data, no significant shift of maximal peak inhibition occurred for gp120 dose treatment compared to the VEH-treated animals; maximal peak inhibition occurred at either the 80 or 120 msec ISI.
In sum, as preweanlings and as adults, effective sensorimotor gating was demonstrated by all groups. No significant treatment effect and/or interaction were noted for any of the analyses, indicating that sensorimotor function was not affected by P10 HIV-1 viral protein treatment. The previous P1 study supports the lack of any Tat1–72 effect in preweanling rats, but demonstrated significant Tat1–72 alterations in the maximum peak inhibition response in adulthood, suggesting long-lasting effects of the HIV-1 protein Tat1–72 on the process of sensorimotor gating, when injected at P1 (Fitting et al., 2008b). The findings for sensorimotor function support the conclusion that an early exposure period, specifically to Tat, appears to be more vulnerable to the developing CNS system.
2.3 Locomotor Activity
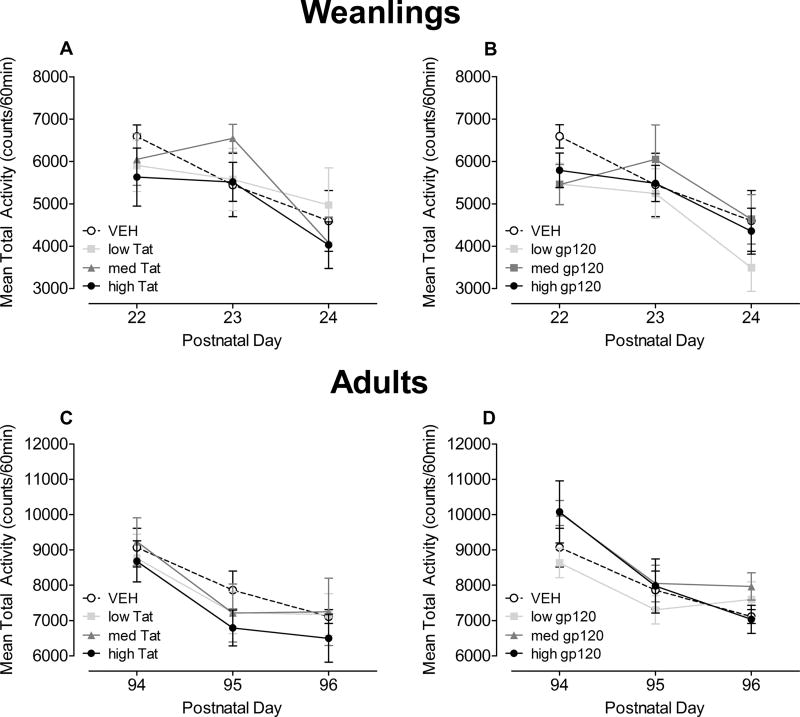
For weanlings, the mean total activity data (60 min) across P22 – P24 are illustrated in Figure 4A and 4B.
Figure 4.
Mean (± S.E.M) total activity across the three test days in weanlings at P22 – P24 (Tat (A) and gp120 (B)) and adults at P94 – P96 (Tat (C) and gp120 (D)). For both ages, neither a significant treatment nor treatment by day interaction was noted.
A 4 (Tat) × 3 (Test days) mixed-model ANOVA revealed significant habituation as expressed by a significant effect of test day [F(2, 56) = 7.3, p ≤ 0.001] with a linear component [F(1, 28) = 10.9, p ≤ 0.01] (see Figure 4A). No treatment effect or treatment by day interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect.
A 4 (Gp120) × 3 (Test days) mixed-model ANOVA also revealed significant habituation as expressed by a test day effect [F(2, 56) = 8.6, p ≤ 0.001] with a linear component [F(1, 28) = 17.8, p ≤ 0.001] (Figure 4B). No treatment effect or treatment by day interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect. Thus, neither Tat nor gp120 dose treatment had any significant effect on the activity measure in weanlings.
For adults, mean total activity data (60 min) across P94 – P96 are illustrated in Figure 4C and Figure 4D.
A 4 (Tat) × 3 (Test days) mixed-model ANOVA revealed significant habituation as expressed by a significant effect of test day [F(2, 52) = 25.3, p ≤ 0.001] with a prominent linear component [F(1, 26) = 35.9, p ≤ 0.001] (Figure 4C). No treatment effect or treatment by day interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect.
A 4 (Gp120) × 3 (Test days) mixed-model ANOVA revealed significant habituation as expressed by a significant test day effect [F(2, 56) = 31.6, p ≤ 0.001] with a prominent linear component [F(1, 28) = 44.7, p ≤ 0.001] (Figure 4D). No treatment effect or treatment by day interaction was noted. Planned contrast analyses revealed no overall treatment effect, no dose-dependent treatment effect, and no threshold effect.
Collectively, as for weanlings, neither P10 Tat or gp120 dose treatment had a significant effect on locomotor activity in adulthood, contrary to findings in the P1 study (Fitting et al., 2008b). Although no motoric dysfunction was detected, less habituation was reported for the P1 Tat-treated animals and the P1 gp120-treated animals in adulthood (Fitting et al., 2008b).
2.4 Spatial Learning and Memory
2.4.1 Adolescent Rats (P46 – P51)
2.4.1.1.Acquisition Training
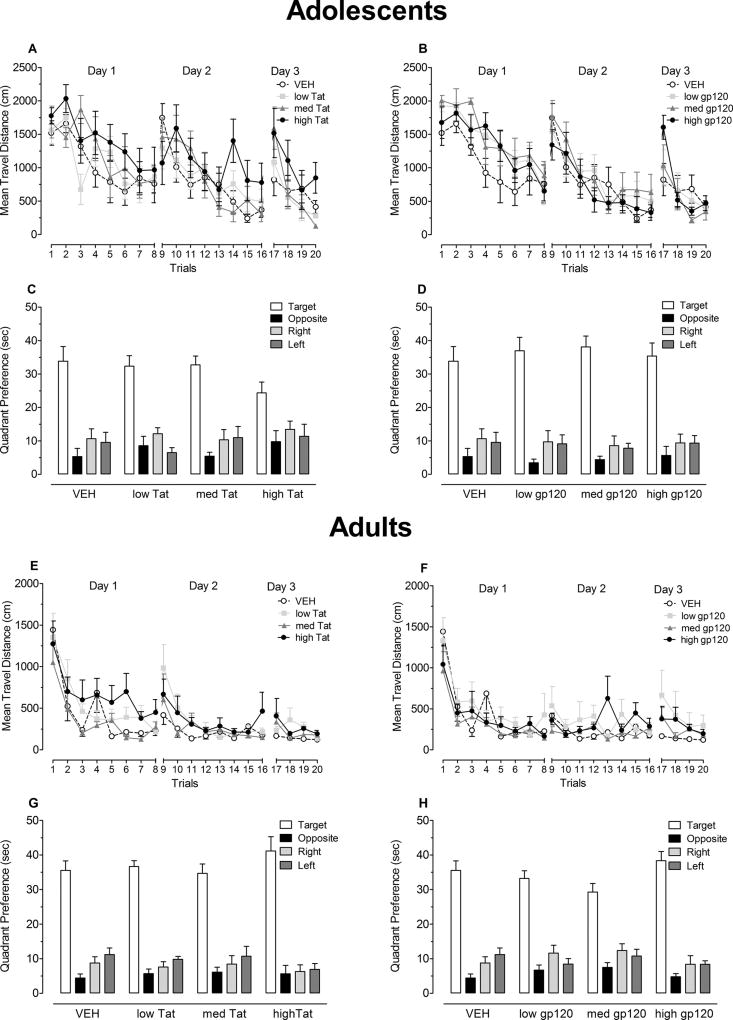
Travel distance during acquisition across the 20 training trials is shown in Figure 5A and 5B. Mixed-model ANOVAs were conducted for each day with either Tat or gp120 dose treatment as the between-subjects factor.
Figure 5.
Acquisition training and probe test assessments for adolescents, at P46 – P51 (Tat(A, C) and gp120(B, D)), and adults, at P104 – P109 ((Tat (E, G) and gp120 (F, H)). Mean (± S.E.M.) travel distance in acquisition training is illustrated in panels A and E for Tat dose treatment and panels B and F for gp120 dose treatment. Significant treatment effects were noted for Tat dose treatment on days 1 and 3 in both adolescence and adulthood. Mean (± S.E.M.) time spent in the four quadrants during the probe test is illustrated in panels C and G for Tat dose treatment and panels D and H for gp120 dose treatment. Despite significant discrimination and evasion effects, planned contrast analyses revealed no overall or dose-dependent treatment effects for either Tat or GP120 treatment.
For the Tat dose groups (see Figure 5A), on day 1, a main effect for trial was revealed [F(7, 182) = 9.2, p ≤ 0.001] with a significant linear component [F(1, 26) = 72.8, p ≤ 0.001], indicating that animals performance improved across training trials. Planned contrast analyses on day 1, revealed a linear dose-response treatment effect collapsed across trials [F(1, 26) = 5.0, p ≤ 0.05], with an increase in travel distance by Tat treatment. Planned comparisons indicated a significant deficit in search performance for the high Tat dose group compared to the VEH controls [F(1, 26) = 4.4, p ≤ 0.05]. On days 2 and 3, significant main effects of trial [F(7, 182) = 9.3, p ≤ 0.001 and F(3, 78) = 14.3, p ≤ 0.001, respectively] were characterized by a significant linear component [F(1, 26) = 63.3, p ≤ 0.001 and F(1, 26) = 34.9, p ≤ 0.001, respectively]. No Tat dose treatment effect or treatment by trial interactions were noted on day 2, suggesting that all four groups traveled similar distances to find the target platform location on their second day of acquisition training. As on day 1, planned contrast analyses on day 3 revealed a linear dose-response treatment effect collapsed across trials [F(1, 26) = 4.4, p ≤ 0.05], with an increase in travel distance by Tat treatment. Planned comparisons failed to detect a threshold effect for either the low or medium Tat dose treatment conditions. As shown in Figure 5A, continued training across days improved performance of all treatment conditions. Neonatal P10 exposure of the viral toxin indicated a dose-response Tat1–86-induced deficit in acquisition training in young rats on day 1 and day 3.
For the gp120 dose groups (see Figure 5B), on day 1, a main effect for trial was revealed [F(7, 196) = 19.0, p ≤ 0.001] with a significant linear component [F(1, 28) = 81.1, p ≤ 0.001], indicating that animals performance improved across training trials. No significant treatment effect or treatment by trial interaction was noted, suggesting that all four groups traveled similar distances to find the target platform location on their first day of acquisition. On days 2 and 3, significant main effects of trial [F(7, 196) = 17.1, p ≤ 0.001 and F(3, 196) = 14.3, p ≤ 0.001, respectively] were characterized by a prominent linear component [F(1, 28) = 120.3, p ≤ 0.001 and F(1, 28) = 31.9, p ≤ 0.001, respectively]. No gp120 dose treatment effects or treatment by trial interactions were noted on any of the three training days. Planned contrast analyses on the three training days, revealed no overall treatment effect, no dose-dependent treatment and/or interaction effects, and no threshold effect.
2.4.1.2 Probe Test
Figure 5C and 5D illustrates quadrant preference in the probe test and represents the amount of time the animal searches in each of the four quadrants when no platform was present.
For the Tat dose groups (Figure 5C), a 4 (Tat) × 4 (Quadrant) mixed-model ANOVA conducted on the time spent searching in each of the four quadrants revealed a significant quadrant effect [F(3, 78) = 41.5, p ≤ 0.001]. There was no significant treatment or treatment by quadrant interaction. Planned contrast analyses on each of the four quadrants separately, revealed no overall treatment effect, no dose-dependent treatment effects, and no threshold effect. All four groups spent the greatest time searching in the target quadrant relative to the opposite or the adjacent quadrants [discrimination effect: F(1, 26) = 72.1, p ≤ 0.001 and F(1, 26) = 85.0, p ≤ 0.001, respectively], suggesting they were able to discriminate and remember the prior platform location. A significant effect of evasion was revealed [F(1, 26) = 5.4, p ≤ 0.001], with rats receiving Tat dose treatment spending relatively more time in the opposite quadrant relative to the adjacent quadrants compared to controls. Planned contrast analyses on the discrimination and evasion measure, revealed no overall or dose-dependent treatment effects.
For the gp120 dose groups (see Figure 5D), a 4 (Gp120) × 4 (Quadrant) mixed-model ANOVA conducted on the time spent in each of the four quadrants revealed a significant quadrant effect [F(3, 84) = 73.4, p ≤ 0.001]. No treatment effect or treatment by quadrant interaction was observed. Planned contrast analyses on each of the four quadrants separately revealed no overall treatment effect, no dose-dependent treatment effects, and no threshold effect. All four groups spent the greatest time searching in the target quadrant relative to the opposite or adjacent quadrants [discrimination effect: F(1, 28) = 127.8, p ≤ 0.001 and F(1, 28) = 112.2, p ≤ 0.001, respectively], suggesting they were able to discriminate and remember the prior platform location. In addition, a significant effect of evasion was revealed [F(1, 28) = 24.2, p ≤ 0.001], with all rats receiving gp120 dose treatment spending less time in the opposite quadrant relative to the adjacent quadrants. Planned contrast analyses on the discrimination and evasion measure, revealed no overall or dose-dependent treatment effects. Thus, all groups avoided the opposite quadrant relative to the adjacent quadrants, suggesting that they had learned where not to go.
In sum, results of the probe test indicated that animals of all treatment groups developed an understanding about where to go and where not to go in the testing environment, suggesting the preservation of spatial learning and memory in the adolescents.
2.4.2 Adult Rats (P104 – P109)
2.4.2.1 Acquisition Training
Travel distance during acquisition training across the 20 training trials is shown in Figure 5E and 5F.
For Tat dose groups (see Figure 5E), mixed-model ANOVAs were conducted on travel distance for each of the three days of training. Day 1 of training revealed a significant main effect of trial [F(7, 182) = 19.6, p ≤ 0.001] with a prominent linear component [F(1, 26) = 79.9, p ≤ 0.001] and a significant treatment effect [F(3, 26) = 3.4, p ≤ 0.05]. Planned contrast analyses revealed no overall treatment effect, but a dose-dependent treatment effect with a cubic component [F(1, 26) = 6.2, p ≤ 0.05]. Planned contrasts revealed significantly longer travel distances for the high dose Tat-treated animals (M = 666.6, S.E.M. = 108.4) compared to the VEH-treated animals (M = 462.3, S.E.M. = 60.8) [F(1, 26) = 4.6, p ≤ 0.05]. No significant differences in comparison to the VEH-treated animals were noted for the low dose Tat group (M = 549.9, S.E.M. = 44.4) or the medium dose Tat group (M = 368.0, S.E.M. = 41.8). Day 2 of training indicated a significant trial effect [F(7, 182) = 7.5, p ≤ 0.001] with prominent linear and quadratic components [F(1, 26) = 10.4, p ≤ 0.01 and F(1, 26) = 12.2, p ≤ 0.01, respectively]. No treatment effect or treatment by trial interaction was noted, indicating that the Tat-treated groups approximated the performance level of the vehicle control animals on day 2. On day 3, no significant trial effect was noted. Planned contrast analyses for overall treatment revealed a significant difference between the VEH group and the overall Tat dose groups [F(1, 26) = 5.4, p ≤ 0.05]. Further, planned contrast analyses revealed no dose-dependent treatment effect. For the threshold effect, planned contrast analyses indicated that the low Tat group (M = 250.7, S.E.M. = 45.3) and the high Tat group (M = 263.3, S.E.M. = 55.2) traveled significantly longer distances compared to the VEH group (M = 140.4, S.E.M. = 10.4) [F(1, 26) = 4.5, p ≤ 0.05 and F(1, 26) = 5.2, p ≤ 0.05, respectively]. The medium Tat group (M = 208.8, S.E.M. = 29.1) was not significantly different from the VEH group.
For the gp120 dose groups (see Figure 5F), mixed-model ANOVAs on travel distance revealed a significant main effect of trial on day 1 [F(7, 196) = 23.8, p ≤ 0.001], with prominent linear and quadratic components [F(1, 28) = 86.0, p ≤ 0.001 and F(1, 28) = 9.1, p ≤ 0.01, respectively]. No treatment effect or treatment by trial interaction was noted. On day 2, no significant effects were noted. Day 3 revealed a significant main effect of trial [F(3, 84) = 4.4, p ≤ 0.05], with a significant linear component [F(1, 28) = 6.5, p ≤ 0.05]. No treatment effect or treatment by trial interaction was noted. Planned contrast analyses on the three training days, revealed no overall treatment and/or interaction effects, no dose-dependent treatment effects, and no threshold effect. All groups learned the spatial location of the platform, approximating an asymptotic performance level on day 3. No treatment effects or treatment by trial interactions were noted for any of the three training days. As illustrated in Figure 5E and 5F, all groups reached asymptotic performance as adults.
2.4.2.2 Probe Test
Figure 5G and 5H illustrates quadrant preference in the probe test, with the amount of time spent in each of the four quadrants when no platform was present.
For the Tat dose groups (Figure 5G), a 4 (Tat) × 4 (Quadrant) mixed-model ANOVA conducted on the time spent in each of the four quadrants revealed a significant quadrant effect [F(3, 78) = 141.4, p ≤ 0.001]. No treatment effect or treatment by quadrant interaction was observed. Planned contrast analyses on each of the four quadrants separately revealed no overall treatment effect, no dose-dependent treatment effects, and no threshold effect. As illustrated in Figure 5G, all animals were able to discriminate and searched primarily in the target quadrant relative to the opposite or the adjacent quadrants [F(1, 28) = 230.2, p ≤ 0.001 and F(1, 26) = 216.3, p ≤ 0.001, respectively], suggesting that all animals displayed spatial memory for the prior platform location. In addition, a significant effect of evasion was revealed [F(1, 26) = 12.5, p ≤ 0.01], with all groups searching less in the opposite quadrant relative to the adjacent quadrants. No treatment effect or interactions with treatment were noted. Planned contrast analyses on the discrimination and evasion measure, revealed no overall- or dose-dependent treatment effects.
For the gp120 dose groups (Figure 5H), a 4 (Gp120) × 4 (Quadrant) mixed-model ANOVA conducted on the time spent in each of the four quadrants revealed a significant quadrant effect [F(3, 84) = 128.2, p ≤ 0.001]. No treatment effect or treatment by quadrant interaction was observed. Planned contrast analyses on each of the four quadrants separately, revealed no overall treatment effect, no dose-dependent treatment effects, and no threshold effect. As illustrated in Figure 5H, all animals were able to discriminate and searched primarily in the target quadrant relative to the opposite or the adjacent quadrants [F(1, 28) = 292.2, p ≤ 0.001 and F(1, 28) = 182.6, p ≤ 0.001, respectively], suggesting that all gp120 dose groups displayed spatial memory for the prior platform location. In addition, a significant effect of evasion was revealed [F(1, 28) = 22.7, p ≤ 0.001], with all rats searching less in the opposite quadrant relative to the adjacent quadrants. No treatment effect or interaction with treatment was noted. Planned contrast analyses on the discrimination and evasion measure revealed no overall or dose-dependent treatment effects. Thus, all groups avoided the opposite quadrant relative to the adjacent quadrants and remembered where not to go.
In sum, results of the probe test, were consistent with the findings in adolescent animals; all treatment groups developed an understanding about where to go and where not to go in the testing environment, suggesting the preservation of spatial learning and memory in adulthood.
2.5 Relationship Between Behavior and Anatomy
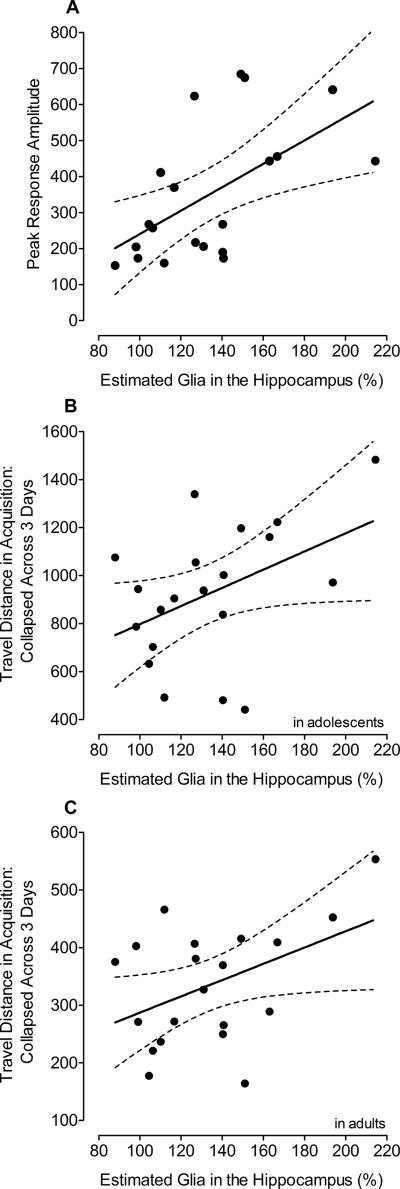
Even though no significant Tat1–86 effects were noted for most of the behavioral measures or on estimates of total neuron numbers, correlation analyses were conducted to assess brain-behavior relationships. It was hypothesized that the hippocampus plays an important role in behavioral measures that correlated with anatomical measures in the previous P1 study, such as locomotor activity in weanlings and spatial memory in adults (Fitting et al., 2008b). Table 1 depicts results of a correlation matrix (Pearson) that was derived for the estimated total number of cells in the five subdivisions of the hippocampus combined (Fitting et al., 2010), expressed as a percentage from the mean of the VEH-treated group, and the behavioral measures in preweanling, adolescent and adult rats. Significant correlations were only noted for estimated number of glia with PPI in adults, and travel distance in acquisition training in adolescent and adult rats (Table 1). The significant correlations were further assessed by separate stepwise multiple regression analyses using percent number of glia as the predictor variable. Approximately 19% – 33% of total variance in the behavioral scores, including prepulse inhibition (Figure 6A), and spatial learning in adolescence (Figure 6B) and adulthood (Figure 6C) was predicted by the anatomical measures (also summarized in Table 2).
Table 1.
Pearson Intercorrelation Matrix between the Different Behavioral Measures and Percent Cell Number from the Mean of the VEH-treated Animals for the Five Hippocampal Subfields Combined (N = 20).
| Neurons (in %) | Glia (in %) | ||
|---|---|---|---|
| Eye Opening (Ranking)+ | −0.095 | 0.015 | |
| Prepulse Inhibition (Amplitude) | young | 0.333 | 0.352 |
| adult | 0.066 | 0.573** | |
| Locomotor Activity (Counts/60 min) | young | −0.136 | −0.184 |
| adult | −0.213 | −0.344 | |
| Distance Traveled on Days 1,2, and 3 | young | 0.014 | 0.436* |
| adult | 0.240 | 0.445* | |
Distribution in searching behavior during the probe test in the MWM
 (in sec) (in sec) |
young | 0.199 | −0.121 |
| adult | −0.039 | 0.010 |
Note: MWM = Morris water maze;
p ≤ 0.01,
p ≤ 0.05,
Spearman Correlation
Figure 6.
Simple linear regression models indicating the relationship between, (A) peak response amplitude in sensorimotor gating and % glia in the hippocampus, (B) distance traveled in acquisition training collapsed across the three training days for adolescents and % glia in the hippocampus, and (C) distance traveled in acquisition training collapsed across the three training days for adults and % glia in the hippocampus.
Table 2.
Stepwise Multiple Regression Analyses Predicting Different Behavioral Measures Including Percent Neurons and Percent Glia As the Two Anatomical Predictors.
| Behavior | Predictors | Coeff. | S.E.M. | t-statistics | Model Fit | R2 |
|---|---|---|---|---|---|---|
| PPI in adults | % glia | 0.573 | 1.096 | 3.0, p ≤ 0.01 | F(1, 18) = 8.8, p ≤ 0.01 | 33% |
| Travel Distance across the 3 days in adolescents | % glia | 0.436 | 1.842 | 2.1, p ≤ 0.05 | F(1, 18) = 4.2, p ≤ 0.05 | 19% |
| Travel Distance across the 3 days in adults | % glia | 0.446 | 0.669 | 2.1, p ≤ 0.05 | F(1, 18) = 4.5, p ≤ 0.05 | 20% |
Note: MWM = Morris water maze, Coeff. = Coefficient
Further exploration of the relationships between the five hippocampal subdivisions and PPI during adulthood, the distance traveled in acquisition training in adolescence, and the distance traveled in acquisition training during adulthood was undertaken. Linear multiple regression analyses were conducted to assess how much variance of prepulse inhibition and travel distance was accounted for by the anatomical measures. Starting with the highest parameter models, we then constrained the models by seeking the lowest parameter models that did not cause a significant R2-change. PPI was examined, despite no significant Tat1–86 effects, because of the significant correlation between estimated number of glia and PPI in adults (i.e., 0.573); the most profound correlational relationship observed in the present study. Travel distance was chosen because Tat1–86 produced significant dose-response effects on first and last acquisition training day; further, the hippocampus is well known to be involved in spatial learning. Based on the previous P1 study (Fitting et al., 2008b), we used estimates of numbers of neurons and astrocytes in the DGH as predictors for distance traveled during acquisition training (collapsed across the three training days) in adolescents and adults.
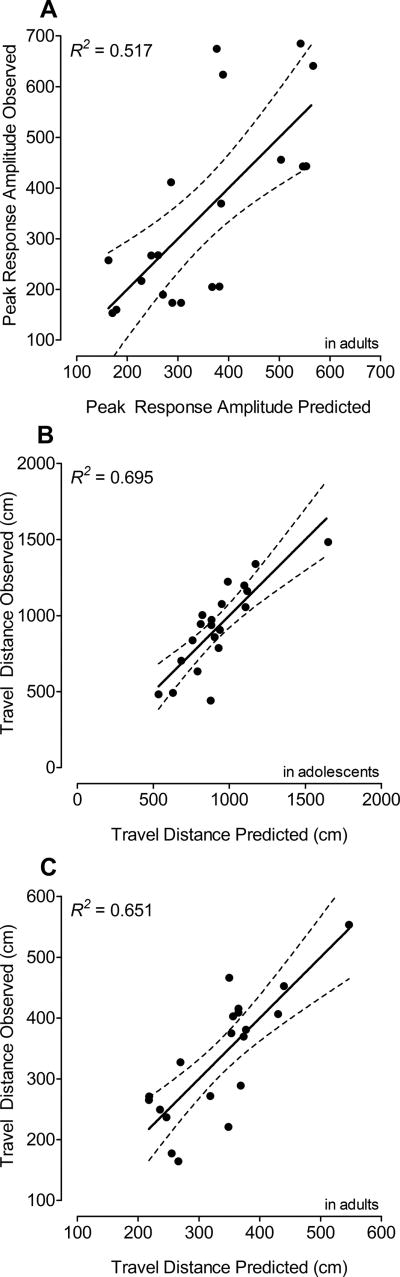
For PPI during adulthood, the final model was a 7-parameter model including three intercepts b0 that varied with condition, and four weights that depended on the Tat dose treatment condition (see Table 3 in the corresponding column). As illustrated in Figure 7A, the model provided a good fit of the peak response amplitude in PPI in adults, accounting for 52% of the variance of the data (R2 = 0.517).
Table 3.
Parameter Values and Fit Indices of the Parameter Models for Prepulse Inhibition and Travel Distance during Acquisition Training (Collapsed Across the Three Training Days) in Adolescence and Adulthood.
| Prepulse Inhibition Adult |
Travel Distance Adolescence Adulthood |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition | b0 | bDGHN | aDGHA | b0 | bDGHN | aDGHA | b0 | bDGHN | aDGHA |
| VEH | −1698.57 | 0.027 | 0.010 | 3139.4 | −0.022 | bVEH | 2252.6 | −0.026 | −0.011 |
| Low Dose Tat | −551.95 | aVEH | 0.005 | 1444.0 | bVEH | bMed Tat | 227.0 | 0.001 | bLow Tat |
| Medium Dose Tat | b0 Low Tat | aLow Tat | aVEH | −25.3 | 0.008 | bMed Tat | −527.5 | 0.007 | bMed Tat |
| High Dose Tat | 878.46 | −0.016 | aLow Tat | 190.5 | bMed Tat | bMed Tat | −24.5 | bMed Tat | bMed Tat |
Note: b0 = intercept or constant, bDGHN = weight of the total number of neurons in the DGH, bDGHA = weight of the total number of astrocytes in the DGH.
Figure 7.
Actual scores collapsed across the three assessment days illustrated on the y-axis against the predicted scores of travel distance (x-axis). (A) Peak response amplitude in sensorimotor gating in adults: Scores were derived from a 7-parameter model explaining 52% of the variance of the data. (B) Distance traveled during acquisition training in adolescents: Scores were derived from a 6-parameter model explaining 70% of the variance of the data. (C) Distance traveled during acquisition training in adults: Scores were derived from an 8-parameter model explaining 65% of the variance of the data.
For the distance traveled during adolescence, the final model version was a 6-parameter model including four intercepts b0 that varied with condition, and two weights that depended on the Tat dose treatment condition (see Table 3 in the corresponding column). As illustrated in Figure 7B, the model provided a good fit of the distance traveled during acquisition training, accounting for 70% variance of the data (R2 = 0.695).
For the distance traveled during adulthood, the final model version was an 8-parameter model including four intercepts b0 that varied with condition, and four weights that depended on the Tat dose treatment condition (see Table 3 in the corresponding column). As illustrated in Figure 7C, the model provided a good fit of the distance traveled during acquisition training, accounting for 65% variance of the data (R2 = 0.651).
3. DISCUSSION
Selective, dose-dependent, neurocognitive deficits were observed following late (i.e., P10) exposure to the HIV-1 viral proteins Tat1–86 and gp120, revealing critical information on the role of timing in pediatric HIV-1. Stereotaxic injections of viral proteins into the rat hippocampus at P10 provide a translational preclinical model to examine the effects of neurotoxic proteins on neurocognitive development in children infected during labor or delivery. No neurotoxic effects were observed following exposure to gp120. In sharp contrast, specific, dose-dependent deficits in spatial learning, assessed using the Morris water maze, were observed following exposure to Tat1–86. Neurocognitive alterations in spatial learning were primarily (65–70%) explained by an increase in gliosis in the DGH. Compared to the deleterious neurocognitive effects observed following early viral protein exposure (i.e., P1; Fitting et al., 2008b; Moran et al., 2014a), the results of the present study indicated that late viral protein exposure is best described as having task specific effects on neurocognitive development.
The only dose-dependent virotoxin effects observed in young and adult rats following P10 Tat1–86 exposure were specific to spatial learning. Specifically, stereotaxic injections of Tat1–86 increased travel distance on training days 1 and 3, suggesting a deficit in acquisition, in both adolescent and adult animals. Tat treatment, however, did not alter the animal’s spatial discriminability, evidenced by a significant evasion effect (Carman & Mactutus, 2002; Carman et al., 2003). Notably, no dose-response effects were noted for P10 Tat1–86 treatment on spatial memory in adolscence or adulthood. Deficits in early training acquisition were also reported in adolescent, but not adult, animals following stereotaxic injections of Tat1–72 on P1 (Fitting et al., 2008b). P1 exposure to Tat1–72 also altered the distribution of searching behavior in adult animals, suggesting long-lasting deficits in spatial memory (Fitting et al., 2008b); a deficit which was not observed following P10 Tat1–86 exposure despite the higher doses. Given the role of the hippocampus in spatial learning and memory (O'Keefe & Nadel, 1978), as well as the effect of HIV-1 Tat on hippocampal function and anatomy (Maragos et al., 2003; Fitting et al., 2006a; Fitting et al., 2008a; Aksenov et al., 2009; Bertrand et al., 2011; Bertrand et al., 2013; Fitting et al., 2013; Hargus & Thayer, 2013; Zucchini et al., 2013; Harricharan et al., 2015; Marks et al., 2016), alterations in spatial learning following Tat exposure are not surprising.
The long-lasting effects of Tat on spatial learning are supported by the long-term dose-response impact of P10 Tat1–86 exposure on the anatomical parameters of the hippocampus (Fitting et al., 2008a). Tat1–86 treatment increased numbers of astrocytes in the DGH and SUB and numbers of oligodendrocytes in the DGH in a linear dose-dependent manner. No significant Tat dose-response effects were noted on the estimated number of neurons in any of the five hippocampal subfields (Fitting et al., 2010). In sharp contrast, a decrease in neuron numbers in the DGH and the CA2/3 subfield were reported following Tat exposure on P1 (Fitting et al., 2008a). Similarly, a previous study in Tat transgenic mice indicated that Tat exposure later in life caused a variety of different inclusions in astrocytes characteristic of lysosomes, autophagic vacuoles, and lamellar bodies with minimal dendritic pathology and no evidence of pyramidal neuron death (Fitting et al., 2013). Astrogliosis has been frequently reported in pediatric HIV-1 infection, evidenced by the presence of viral proteins/genes in some astrocytes (Saito et al., 1994; Tornatore et al., 1994), white matter damage (Hoare et al., 2015; Ackermann et al., 2016; Cohen et al., 2016), and/or increases in macrophages and microglia (Vallat et al., 1998). Perhaps most interestingly, regression analyses revealed a significant brain-behavior relationship between virotoxin-induced alterations in spatial learning and/or memory with cell numbers in the DGH; a relationship similar to the one observed following P1 virotoxin exposure (Fitting et al., 2008b). Specifically, the present study demonstrated that the estimated number of neurons and astrocytes in the DGH explained 70% variance of the searching behavior in the MWM acquisition training for adolescents and 65% variance of the searching behavior in the MWM acquisition training for adults. HIV-1 viral protein exposure at P1 indicated that the estimated number of neurons and astrocytes in the DGH explained 81% variance of the distribution in searching behavior in the adult MWM probe test (Fitting et al., 2008b). Thus, the results from both studies suggest that spatial learning and/or memory deficits following HIV-1 viral protein exposure may be due to increased gliosis and/or neuronal loss in the DGH. Previous work has found associations between the DGH and spatial learning and/or memory in multiple disease models, (Zhang et al., 2011; Martinez-Canabal et al., 2013; Cahill et al., 2014; Piazza et al., 2014; Taylor et al., 2015; Zerwas et al., 2015), supporting the findings of the present study.
Dopamine (DA) system dysfunction, observed in both clinical (e.g., Wang et al., 2004; Chang et al., 2008; Kumar et al., 2009; Lee et al., 2014) and preclinical studies (e.g., Webb et al., 2010; Moran et al., 2012; Reid et al., 2016a), may also underlie alterations in spatial learning. Specifically, alterations in the striatal DA system have been reported in pediatric HIV-1 research (Webb et al., 2009; Fitting et al., 2015). Children with pediatric HIV-1 also exhibited improvements in motor function following treatment with levodopa, a DA agonist, suggesting the relevance of the DA system in pediatric HIV-1 (Mintz et al., 1996). The HIV-1 transgenic (Tg) rat, which expresses 7 of the 9 HIV-1 genes (a gag-pol deletion renders them noninfectious; Reid et al., 2001) has been used to examine the developmental effects of HIV-1 and its impact on the DA system (Webb et al., 2010; Moran et al., 2012). Findings revealed a significant increase in phosphorylated tyrosine hydroxylase protein expression and a decrease in dopamine transporter (DAT) mRNA, without changes in tyrosine hydroxylase or DAT protein in the HIV-1 transgenic rat midbrain, suggesting selective vulnerability of the DA system in developing brains to HIV-1 infection (Webb et al., 2010). Additionally, HIV-1 Tg rats assessed throughout the lifespan in neurocognitive tasks tapping the DA system, exhibited significant alterations in temporal processing (Moran et al., 2013a; McLaurin et al., 2016; McLaurin et al., 2017a; McLaurin et al., 2017b; McLaurin et al., 2017c; McLaurin et al., 2017d), habituation (Chang & Vigorito, 2006; Moran et al., 2013b; Reid et al., 2016b; McLaurin et al., 2017d), sustained attention (Moran et al., 2014b), working memory (Repunte-Canonigo et al., 2014) and spatial learning (Vigorito et al., 2007; Lashomb et al., 2009).
Results of the present study, following P10 HIV-1 protein delivery, are in sharp contrast to the deficits observed following P1 HIV-1 protein delivery (Fitting et al., 2008a; Fitting et al., 2008b), suggesting the importance of timing in the presence of neurocognitive impairments observed in pediatric HIV-1. Specifically, virotoxin exposure on P10 resulted in dose-dependent-induced deficits for Tat1–86 in spatial learning, while HIV-1 protein exposure on P1 resulted in significant alterations in most of the neurobehavioral and neurocognitive assessments (i.e., righting reflex, prepulse inhibition, locomotor activity, spatial memory). A comparative summary of findings in the present P10 study and the previous P1 study is illustrated in Table 4. In preweanling HIV-1 Tg rats, which express viral proteins constitutively throughout development, neurocognitive assessments revealed significant alterations in both prepulse inhibition and locomotor activity (McLaurin et al., 2017a); neurocognitive deficits which were similar to those reported following early viral protein exposure (P1; Fitting et al., 2008b; Moran et al., 2014a). Thus, collectively, these studies provide strong evidence for the effect of timing on the development of neurocognitive impairment, with early viral protein exposure (P1, HIV-1 Tg rat) having a more deleterious effects on the developing CNS than late viral protein exposure (P10).
Table 4.
Comparative Summary of Findings Following P1 Stereotaxic Injections (Fitting et al., 2008a; Fitting et al., 2008b) and P10 Stereotaxic Injections.
| Neuropsychological Deficits |
Neurobehavioral Assessments | P1 Results |
P10 Results |
|---|---|---|---|
| Somatic Growth Impairments | Body Weight | No | No |
| Eye Opening | Yes | No | |
| Early Reflex | Righting Reflex | Yes | - |
| Developmental Deficits | Negative Geotaxis | Yes | - |
| Attentional Deficits | PPI in Preweanlings | No | No |
| PPI in Adults | Tat1–72 Effect | No | |
| Motor Dysfunction | Locomotor Activity in Weanlings | Yes | No |
| Locomotor Activity in Adults | Gp120 effect | No | |
| Learning Deficits | MWM in Adolescents | Yes | Tat1–86 Dose Effect |
| MWM in Adults | No | Tat1–86 Dose Effect | |
| Memory Deficits | MWM in Adolescents | No | No |
| MWM in Adults | Tat1–72 Effect | No |
Note: PPI = Prepulse Inhibition, MWM = Morris water maze
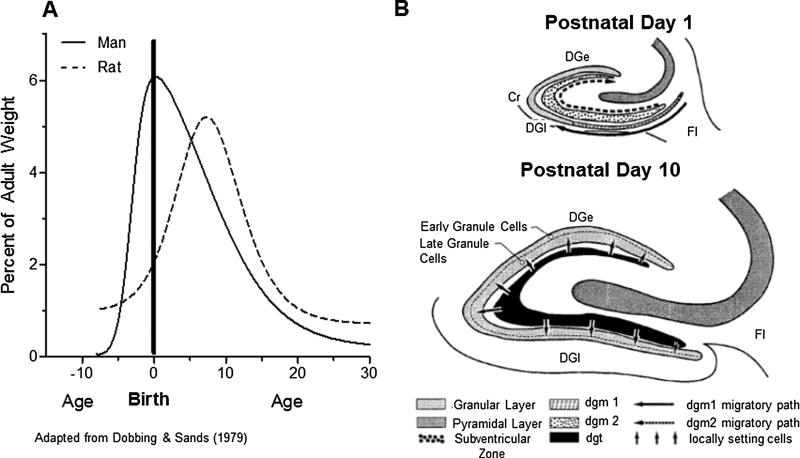
The rationale for using P1 (Fitting et al., 2008a; Fitting et al., 2008b) and P10 CNS injections as the virotoxin exposure period was based on the human brain developmental perspective, providing an opportunity to extrapolate findings to HIV-1 infected children. The period of rapid brain growth, putatively known as the ‘brain growth spurt’, reflects a period of enhanced vulnerability to nutritional and other growth restriction (Dobbing & Sands, 1979). Timing of the brain growth spurt is different in relation to birth across species and thus, is one of the major factors to be taken into account when any attempt is made to extrapolate results obtained in one species to another (Dobbing & Sands, 1979, see Figure 8). Brain growth velocity curves (Figure 8A) compare the brain growth spurt in humans and rats. Based on the rate of brain development, P10 exposure mimics clinical HIV-1 CNS infection at labor/delivery, as brain growth of P10 rats more closely matches that of human brain growth at birth (i.e., late virotoxin exposure). In sharp contrast, stereotaxic injections at P1, as in Fitting et al., 2008b, mimics clinical HIV-1 CNS infection in pregnancy. Specifically, HIV-1 viral protein exposure at P1 mimics in utero infection occurring as the placenta begins to separate from the uterine wall (i.e., early virotoxin exposure).
Figure 8.
(A) The brain growth spurts of humans and rats expressed as first-order velocity curves of the increase in weight with age. The units of time for humans are in month and for rats in days. Rates are expressed as weight gain as a percentage of adult weight for each unit of time. (B) Summary diagram of the morphogenesis of the dentate granular layer.
Further specification of construct validity may be made at the level of hippocampal development (Figure 8B). In both rats and humans the development of the hippocampus, and specifically the development of different regions of the hippocampus, occurs at different time periods in brain maturation (Bayer et al., 1993). Neurons in the subiculum and in the CA1/3 subfields are estimated to be generated from the late 6th week through the 14th week of development in humans, equivalent to embryonic days (E)15 – E20 in rats. In contrast, the granule cells in the dentate gyrus are generated at a time point exceptionally late in development. Approximately 85% of these cells are estimated to be generated from the 12th week up to birth and possibly beyond in humans (in rats: after birth, mainly during the first two postnatal weeks, P1 – P13; Bayer et al., 1993). Thus, from a hippocampal development point of view, stereotaxic injections of HIV-1 viral proteins at P1 interferes with the development of dentate granule cells. In sharp contrast, by P10, most of these cells would already be generated.
Behavioral and anatomical findings provide strong evidence for the role of timing in the development of CNS disease in HIV-1 infected children. Late viral protein exposure (P10), occurring at the peak of brain growth in rats, selectively altered spatial learning and memory in adolescence and adulthood. In sharp contrast, early viral protein exposure (P1), modeled in our previous study (Fitting et al., 2008b), preceded the postnatal increase in the rate of brain growth and had more deleterious effects to the developing CNS. Thus, timing may be a critical factor involved in disease progression, with children infected with HIV earlier in life being more vulnerable to CNS disease.
4. EXPERIMENTAL PROCEDURE
4.1 Animals
Pregnant dams (Sprague-Dawley, Harlan Laboratories Inc., Indianapolis, IN; n=8) were delivered to the animal vivarian prior to embryonic day seven. Dams were individually housed with food (Pro-Lab Rat, Mouse Hamster Chow #3000, NIH diet #31) and water available ad libitum. P0 was designated as the day pups were found in the cage. The next day (i.e., P1), litters were culled to 10 offspring with a male: female distribution of 7:3. Seven treatment conditions were used in the experimental design to allow for simultaneous dose-response assessment of both gp120 and Tat. To control for independence of observations, one rat from each litter was represented in each treatment group (i.e., seven male rats per litter). On P21, animals were weaned and separated by sex. On P28, animals were pair- or group- housed with their littermates for the duration of experimentation. Target conditions for the animal vivarium were: 20 ± 2 °C, 50 ± 10 % relative humidity and a 12 h light: 12 h dark cycle with lights on at 0700 h (EST). Guidelines established by the National Institutes of Health (NIH) were used to keep animals in AALAC-accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina, Columbia (Federal Assurance: # A3049–01).
4.2 HIV-1 Proteins
Commercially available recombinant biologically active full-length Tat1–86 (Diatheva s.r.l., Italy) was used for the Tat treatment condition. Purified gp120 LAV (T-tropic; Protein Sciences Corp., Meriden, CT) was used for the gp120 treatment condition. Doses of both Tat and gp120 were chosen based on previously established dose-response studies (Tat: Aksenov et al., 2001; gp120: Fitting et al., 2006b; Fitting et al., 2007) and to bracket those used to examine the effect of intrahippocampal injections on P1 (Fitting et al., 2008b). Thus, the design paralleled the previous P1 study and provided the opportunity to capture potential lower or higher toxicity of P10 injections.
4.3 Surgical Techniques and Protein Treatment
Tat, gp120 and vehicle treatments were delivered using standard stereotaxic surgery techniques. Modifications to standard procedures were made for neonates. At P10, pups were individually removed from the dam. Pups were anesthetized using sevofluorane (Abbott Laboratories, Inc.) in 3% oxygen and placed in a stereotaxic mold modified for surgery of neonates (Kopf, Inc). Throughout the duration of the surgery, pups were maintained in an anesthetic state using sevofluorane, which was delivered through a nose cone. A heating pad was used to maintain body temperature at ~37 °C. The skull was held in place using rubber head bars while bilateral microinjections were made directly into the hippocampus using stereotaxic coordinates for the left and right hippocampus (2.3 mm posterior to the bregma, ±1.0 mm lateral to bregma, −2.5 mm dorsal from dura) and a microsyringe (Hamilton Co., Nevada, USA). Injection coordinates used in the present study were confirmed during pilot work with Nissl-stained sections through the hippocampus. An injection volume of 2.0 µl, a higher volume dose due to solvability issues, was released over two-min after a one-min resting period that allowed the tissue to return to its original conformation. Intrahippocampal bilateral injections were made with one of the seven protein treatments: sterile buffer [vehicle (VEH); 10 mM Tris HCl, 300 nM NaCl, ph = 7.58, sterile)], one of the three gp120 doses: 20 ng (low), 100 ng (medium), 200 ng (high), or one of the three Tat doses: 5 µg (low), 25 µg (medium), 50 µg (high). The injection needle was withdrawn over two-min to prevent reflux. Surgical glue and surgical polypropylene sutures (Ethican, Inc.) were used to close skin piercings in the head. Animals recovered from anesthesia in a chamber on a heating pad (~37 °C) prior to being returned to the dam. Pups were closely monitored for any indications of rejection, which were not observed.
4.4 Experimental Design
Seven male rats per litter (n = 8) were randomly selected and assigned to their treatment groups (i.e., VEH; low-, medium-, high gp120; and low-, medium-, high Tat) and received bilateral hippocampal injections on P10. Assessments of somatic growth, including body weight and eye opening, began at P10. Specifically, all rats were weighed at each of the specific test days and eye opening was assessed on P13 – P17. Neurobehavioral assessments began on P18. All rats were tested for (1) sensorimotor gating, indexed by PPI of the acoustic startle response (ASR) prior to weaning (P18) and in adulthood (P91); (2) locomotor activity, an index of motor function, in weanling rats (P22 – P24) and in adulthood (P94 – P96); and (3) spatial learning and memory, indexed by performance in the MWM (P46 – P51 and P104 – P109). Procedures used for neurocognitive testing followed a similar procedure as reported in the previous P1 study (Fitting et al., 2008b), but will be outlined briefly in the following sections.
4.5 Somatic Growth
In the pre-cART era, HIV-1 infected children exhibited slower growth relative to HIV-1 seronegative children; differences which were exacerbated after the second year of life (Newell et al., 2003). However, findings in the post-cART era suggest that HIV-infected children on antiviral prophylaxis treatments displayed a significant improvement in growth parameters (Lodha et al., 2005; Guillen et al., 2007; Parachure et al., 2015). To examine the specific effect of HIV-1 proteins on developmental milestones, somatic growth was assessed by body weight and eye opening.
4.5.1 Body Weight
Pups were weighed on a weekly basis starting on P10, as well as on the days animals were tested.
4.5.2 Eye Opening
A second index of somatic growth, eye opening, was assessed from P13 – P16. The right and left eyes were assessed separately between 0800 – 1000 h (EST) and coded as ‘eye not open’ (0), ‘slit’ (1), ‘half-open’ (2) and ‘eye open’ (3). Rank scores, with a maximum of 6 and a minimum of 0, were calculated by collapsing across P13--P16 to examine the frequency of eye opening.
4.6 Sensorimotor Function (PPI of the ASR)
PPI of the ASR is a translational experimental technique commonly used to examine preattentive processes, which may underlie deficits in higher-order cognitive functions. Significant alterations in PPI were observed following stereotaxic injections of HIV-1 viral proteins at P1 (Fitting et al., 2008b; Moran et al., 2014a) and in preweanling and adult HIV-1 Tg rats (e.g., Moran et al., 2013a; McLaurin et al., 2017a; McLaurin et al., 2017b; McLaurin et al., 2017c; McLaurin et al., 2017d). PPI also revealed alterations in sensorimotor gating in HIV-1 seropositive adults (Minassian et al., 2013). In the present study, auditory PPI was used to determine whether timing (i.e., P10) and dose of HIV-1 viral proteins altered preattentive processes. All rats were assessed as preweanlings on P18 and as adults on P91.
4.6.1 Apparatus
A 10 cm thick double-walled, 81 × 81 × 116 cm isolation cabinet (external dimensions; Industrial Acoustic Company, Inc., Bronx, NY) enclosed the startle chamber (SR-Lab Startle Reflex System, San Diego Instruments, Inc.). All assessments were conducted individually in the dark. A high-frequency loudspeaker, which was affixed in the chamber 30 cm above the Plexiglas cylinder, delivered all auditory prepulse and startle stimuli. A Plexiglas cylinder (preweanling rats: 3.9 cm internal diameter; adult rats: 8.75 cm internal diameter), resting on a 12.5 × 20 cm Plexiglas stand, was deflected by the animal’s response to the startle stimulus. Deflections were converted into an analog signal by a piezoelectric accelerometer integral to the bottom of the cylinder. Both the startle stimuli (100 dB(A)) and auditory prepulse stimuli (85 dB(A)) were 20 msec in duration. A SR-Lab Startle Calibration System was used to calibrate acoustic stimulus intensities and response sensitivities. A sound level meter (model #2203, Bruël & Kjaer, Norcross, GA) was used to measure and calibrate sound levels with the microphone placed inside the Plexiglas cylinder. The signals were then digitized (12 bit A to D) and saved to a hard disk on a Pentium class computer.
4.6.2 Testing Procedures
Animals were assessed for auditory PPI using a 20-min test session, which began with a five-min acclimation period, including 70 dB(A) background white noise. The test session began with six adaptation trials, which included pulse only ASR-trials. Subsequently, 36 PPI trials, with interstimulus intervals (ISIs) of 0, 8, 40, 80, 120, and 4000 msec were presented according to a Latin-square design. Control trials (i.e., 0 and 4000 msec ISIs) provided the baseline ASR within the PPI test session. The dependent measures analyzed were (1) the baseline ASR indexed by control trials, (2) percent inhibition of the PPI trials (8–120 msec ISIs) relative to the baseline ASR (control trials), and (3) ISI in which the maximal peak response inhibition occurred across the PPI trials (8 – 120 msec ISIs).
4.7 Locomotor Activity
Significant alterations in motor development have been frequently reported both in the pre-(Boivin et al., 1995; Baillieu & Potterton, 2008) and post-cART eras (Foster et al., 2006; Ferguson & Jelsma, 2009; Whitehead et al., 2014). To test whether HIV-1 proteins themselves induce effects on motor activity, locomotor activity was assessed in weanlings and adults for 60-min on three consecutive days, at 21, 22, and 23 days of age and 94, 95 and 96 days of age, respectively.
4.7.1 Apparatus
The square (40 × 40 cm) activity monitors (Hamilton-Kinder Inc., Poway, CA) were converted into round (~ 40 cm diameter) compartments by adding clear perspex inserts. An infrared photocell grid (32 emitter/detector pairs), tuned by the manufacturer to handle the extra Perspex width, detected the animal’s free movement. All activity monitors were located in an isolated room. A 60-min test session was conducted between 1500 – 1700 h (EST) under dim light conditions, in the absence of direct overhead lighting (< 10 l×). The dependent variable was the animal’s total activity (i.e., the sum of the recorded basic movements and x and y ambulation).
4.8 Spatial Learning and Memory
Despite antiretroviral prophylactic treatment, HIV-1-infected children exhibit high rates of chronic neurological impairment, including neurodevelopmental delays (Franklin et al., 2005; review, Van Rie et al., 2007; Baillieu & Potterton, 2008; Paramesparan et al., 2010). Neurocognitive assessments in HIV-1-infected children have revealed significant deficits in memory, including visuospatial working memory (Boivin et al., 1995; Koekkoek et al., 2008). Previous preclinical assessments, using adult HIV-1 Tg rats, also provide evidence for alterations in spatial learning and memory (Vigorito et al., 2007; Lashomb et al., 2009). Thus, animals were tested in the Morris water maze, to assess spatial learning and memory, as both adolescents and adults over four consecutive days between 49 – 55 days of age and 113 –121 days of age, respectively.
4.8.1 Apparatus
Spatial learning and memory was assessed using the Morris water maze, an aluminum tank measuring 1.8 m in diameter by 0.60 m high. The water depth inside the Morris water maze was 0.40 m. The temperature of the water was maintained at 28 ± 1 °C throughout experimentation. A 10 × 10 cm escape platform was located 2.5 cm below the surface of the water level on a radius of 45 cm in one of the four locations within the north (N), south (S), east (E), or west (W) arbitrarily designated quadrants. Two sets of cues, including four distinct background curtains and 12 objects outside the tank perimeter, surrounded the tank, which was located in a 3 × 3 m room. The four background curtains were: solid navy (north), solid white (south), vertical navy and white stripes (7.6 cm wide; east), and horizontal navy and white stripes (7.6 cm wide; west) Four hanging objects, in a variety of sizes (i.e., from a 20 cm coffee can to a 60 cm mailbox) were suspended from the ceiling. Eight objects, including circles and quadrangles, were placed on the curtains. The starting points used were NW, NE, SE, SW and varied by using two different entry points diagonal to each other in a counterbalanced order, ABBAABBA. Swimming behavior was recorded using a closed circuit video system, which was recessed mounted in the ceiling above the center of the pool.
4.8.2 Procedure
Acquisition training consisted of a total of 20 training trials across 3 days (Day 1: 8 trials; Day 2: 8 Trials; Day 3: 4 Trials). Throughout the duration of training, the escape platform was located in a fixed location for each subject; however, platform location (N,S,E or W) was balanced across subjects. A different platform location was used for training in adolescence and adulthood. The rat was placed into the pool using one of the consecutive entry points, at which point the training trial began. Swimming behavior was recorded for 60 sec, at which point the trial was terminated. If an animal failed to find the platform within the allotted time (i.e., 60 sec), the animal was directed to the escape platform. No animal was removed directly from the pool, only from the platform. Animals were allowed to remain on the platform for 15 sec, at which point they were returned to a holding cage. Due to the high correlation between escape latency and travel distance (r = 0.96, p ≤ 0.001), only travel distance is reported.
Following the last acquisition trial on day 3, a probe test, which involved removal of the escape platform, was conducted. The visual-spatial environment was not altered during the probe test. The probe test was conducted like the acquisition training trials, with swimming behavior recorded for 60 sec prior to terminating the trial. However, a novel (i.e., not used in training) starting position was used. At the termination of the trial, the escape platform was placed in the pool. Animals were allowed to find the escape platform and remain on it for 15 sec.
For the probe test, quadrant preference (i.e., the relative distribution of swimming time in each of the quadrants) provided an index of the animal’s spatial discriminability. To analyze quadrant preference, the target and opposite quadrant areas were compared. An evasion measure (Carman & Mactutus, 2002, Carman et al., 2003) provided an additional comparison, by examining the opposite and adjacent quadrant areas. Both quadrant preference and the evasion measure provide an index of spatial discriminability. In the quadrant preference measure, discriminability is evidenced by spending more time in the target quadrant relative to the opposite quadrant (i.e., rats have learned where to go in the pool). In sharp contrast, in the evasion measure, discriminability is evidenced by animals learning where not to go in the pool. Thus, the probe test assesses the rat’s more ‘implicit’ knowledge of the escape platform location.
4.9 Stereology
Stereology methods used to quantify total neuron number and glial estimates in the five hippocampal cellular layers (i.e., granular layer (GL), DGH, cornu ammonis fields (CA)2/3. CA1, and subiculum (SUB)) were reported in Fitting et al. (2010). In brief, brain tissue was sectioned into 50 µm thick slices using a microtome cryostat (Microm HM500M, Walldorf, Germany). Cresyl violet (Nissl) staining [1 g cresyl violet acetate (Sigma, St. Lous, MO) in 400 ml distilled water (pH 4.0)] was employed on every 6th section in the series as a method of systematic-random sampling. Neurons were identified morphologically and defined by features including a larger, pale nuclei surrounded by darkly stained cytoplasm containing Nissl bodies. Astrocytes and oligodendrocytes were also identified morphologically in the DGH and SUB.
A Nikon eclipse E800 microscope (Nikon, Melville, NY) equipped with a motorized LEP MAC 5,000 XYZ stage controller (Ludl Electronic Products, NY) and StereoInvestigator software 7.0 (Microbrightfield, Williston, VT) was used for stereology. Unbiased estimates of the total number of neurons and astrocytes were made using the optical fractionator, a stereological technique (West et al., 1991; Gundersen et al., 1999). Cells were counted in a known fraction of the five different hippocampal subregions using a sampling scheme for the optical fractionator. Based on the sample from a known fraction of the entire hippocampus (fractionator sampling), an estimate of the total number of neurons and astrocytes was obtained (West et al., 1991).
4.10 Data Analysis
As appropriate, the data were expressed as mean (± S.E.M.) or cumulative frequency (%). Separate analyses were conducted for the dose-response groups of Tat and gp120. The VEH group was used as the common vehicle control group. All statistical analyses were completed using SYSTAT 11.0 (for Windows, SYSTAT Inc.). An alpha level of p ≤ 0.05 was considered significant for all statistical tests.
Analysis of variance (ANOVA) techniques were used to analyze continuous data (i.e., body weight, locomotor activity, Morris water maze). Either Tat dose treatment (4 levels) or gp120 dose treatment (4 levels) was included as the between-subjects factor. Within-subjects terms (i.e., day, trial) were included in the analyses as appropriate. Orthogonal decompositions were preferentially used to handle potential violations of sphericity (Winer, 1971). Specific planned contrasts were employed to answer three basic questions: First, whether a Tat (or gp120) effect exists (overall treatment effect)? In other words, comparison of VEH vs. Tat or gp120 dose groups. Second, whether there was a Tat (or gp120) dose-response effect (dose-dependent treatment effect)? In other words, were specific dose-dependent treatment effects for Tat or gp120 revealed by orthogonal component analyses. Third, what was the threshold dose for the Tat (or gp120) effect? To answer this question, planned contrasts were performed by comparing VEH-treated animals with each treatment condition of Tat or gp120.
Two non-parametric tests, the Kruskal-Wallis Test and the Mann-Whitney U-Test, were performed on the rank scores of the somatic growth index eye opening for the dose response groups of gp120 treatment or Tat treatment. For categorical data, such as the interstimulus interval (ISI) data in the PPI test, the Fisher’s exact test was applied.
To examine the relationship between the behavioral measures and the total number of cells in the five subregions of the rat hippocampus, we employed correlation and multiple regression analyses. The purpose of these analyses was to determine if observed changes in the estimated total number of cells in specific hippocampal areas (Fitting et al., 2010) were significantly predictive of behavioral outcome.
Highlights.
-
►
P10 viral protein exposure was best characterized as having task specific effects
-
►
Spatial learning deficits were observed following P10 HIV-1 viral protein exposure
-
►
DGH neuron and astrocyte number explained 65-70% of the variance in MWM acquisition
-
►
Timing of infection may be a critical factor in progression of pediatric HIV-1
Acknowledgments
This work was supported, in part, by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392; National Institute of Neurological Diseases and Stroke, NS100624). Portions of this manuscript were submitted by S. Fitting in partial fulfillment of the requirement of the Ph.D. at the University of South Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann C, Andronikou S, Saleh MG, Laughton B, Alhamud AA, van der Kouwe A, Kidd M, Cotton MF, Meintjes EM1. Early antiretroviral therapy in HIV-infected children is associated with diffuse white matter structural abnormality and corpus callosum sparing. Am J Neuroradiol. 2016;37:2363–2369. doi: 10.3174/ajnr.A4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM1. ER-β mediates 17β-estradiol attenuation of HIV-1 Tat-induced apoptotic signaling. Synapse. 2010;64:829–838. doi: 10.1002/syn.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AIDSinfo;1. Preventing Mother-to-Child Transmission of HIV. 2017 Available at: https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/24/50/preventing-mother-to-child-transmission-of-hiv.
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM1. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM1. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Silvers JM, Mactutus CF, Booze RM1. Different effects of selective dopamine uptake inhibitors, GBR 12909 and WIN 35428, on HIV-1 Tat toxicity in rat fetal midbrain neurons. Neurotoxicology. 2008;29:971–977. doi: 10.1016/j.neuro.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM1. Attenuated neurotoxicity of the transactivation-defective HIV-1 Tat protein in hippocampal cell cultures. Exp Neurol. 2009;219:586–590. doi: 10.1016/j.expneurol.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM1. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Baillieu N, Potterton J1. The extent of delay of language, motor, and cognitive development in HIV-positive infants. J Neurol Phys Ther. 2008;32:118–121. doi: 10.1097/NPT.0b013e3181846232. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X1. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, Essex M, Ekouevi DK, Jackson D, Coutsoudis A, Kilewo C, Leroy V, Wiktor SZ, Nduati R, Msellati P, Zaba B, Ghys PD, Newell ML, Group UCS1. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belman AL1. Infants, children, and adolescents. In: Berger JR, Levy RM, editors. AIDS and the nervous system. Lippincott-Raven; Philadelphia: 1997. pp. 223–253. [Google Scholar]
- Bertrand SJ, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM1. Endogenous amyloidogenesis in long-term rat hippocampal cell cultures. BMC neuroscience. 2011;12:38. doi: 10.1186/1471-2202-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Aksenova MV, Mactutus CF, Booze RM1. HIV-1 Tat protein variants: critical role for the cysteine region in synaptodendritic injury. Exp Neurol. 2013;248:228–235. doi: 10.1016/j.expneurol.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM1. Synaptodendritic recovery following HIV Tat exposure: neurorestoration by phytoestrogens. J Neurochem. 2014;128:140–151. doi: 10.1111/jnc.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Hu C, Aksenova MV, Mactutus CF, Booze RM1. HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front Microbiol. 2015;6:894. doi: 10.3389/fmicb.2015.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitnun A, Samson L, Chun TW, Kakkar F, Brophy J, Murray D, Justement S, Soudeyns H, Ostrowski M, Mujib S, Harrigan PR, Kim J, Sandstrom P, Read SE1. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59:1012–1019. doi: 10.1093/cid/ciu432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche S, Tardieu M, Duliege A, Rouzioux C, Le Deist F, Fukunaga K, Caniglia M, Jacomet C, Messiah A, Griscelli C1. Longitudinal study of 94 symptomatic infants with perinatally acquired human immunodeficiency virus infection. Evidence for a bimodal expression of clinical and biological symptoms. Am J Dis Child. 1990;144:1210–1215. doi: 10.1001/archpedi.1990.02150350042021. [DOI] [PubMed] [Google Scholar]
- Blanche S, Mayaux MJ, Rouzioux C, Teglas JP, Firtion G, Monpoux F, Ciraru-Vigneron N, Meier F, Tricoire J, Courpotin C1, et al. Relation of the course of HIV infection in children to the severity of the disease in their mothers at delivery. N Engl J Med. 1994;330:308–312. doi: 10.1056/NEJM199402033300502. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA1. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14:13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, Van Dyke RB1. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB1. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Briand N, Warszawski J, Mandelbrot L, Dollfus C, Pannier E, Cravello L, Nguyen R, Matheron I, Winer N, Tubiana R, Rouzioux C, Faye A, Blanche S, Group A-ECCS1. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903–914. doi: 10.1093/cid/cit374. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A1. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S, Hernandez-Reif M, Jessee P1. A review of pediatric HIV effects on neurocognitive development. Issues in comprehensive pediatric nursing. 2008;31:107–121. doi: 10.1080/01460860802272870. [DOI] [PubMed] [Google Scholar]
- Bussiere JL, Hardy LM, Peterson M, Foss JA, Garman RH, Hoberman AM, Christian MS1. Lack of developmental neurotoxicity of MN rgp 120/HIV-1 administrated subcutaneously to neonatal rats. Toxicological Science. 1999;48:90–99. doi: 10.1093/toxsci/48.1.90. [DOI] [PubMed] [Google Scholar]
- Cahill SP, Hatchard T, Abizaid A, Holahan MR1. An examination of early neural and cognitive alterations in hippocampal-spatial function of ghrelin receptor-deficient rats. Behav Brain Res. 2014;264:105–115. doi: 10.1016/j.bbr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Cann AJ, Rosenblatt JD, Wachsman W, Shah NP, Chen IS1. Identification of the gene responsible for human T-cell leukaemia virus transcriptional regulation. Nature. 1985;318:571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- Carman HM, Mactutus CF1. Proximal versus distal cue utilization in spatial navigation: the role of visual acuity? Neurobiol Learn Mem. 2002;78:332–346. doi: 10.1006/nlme.2002.4062. [DOI] [PubMed] [Google Scholar]
- Carman HM, Booze RM, Snow DM, Mactutus CF1. Proximal versus distal cue utilization in preweanling spatial localization: the influence of cue number and location. Physiol Behav. 2003;79:157–165. doi: 10.1016/s0031-9384(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Carryl H, Swang M, Lawrence J, Curtis K, Kamboj H, Van Rompay KK, De Paris K, Burke MW1. Of mice and monkeys: Can animal models be utilized to study neurological consequences of pediatric HIV-1 infection? ACS Chem;1; Neurosci. 2015;6:1276–1289. doi: 10.1021/acschemneuro.5b00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. Updated Recommendations. [Published June 27, 2014];2014 Available at http://stacks.cdc.gov/view/cdc/23447.
- Chadwick EG, Yogev R, Alvero CG, Hughes MD, Hazra R, Pinto JA, Robbins BL, Heckman BE, Palumbo PE, Capparelli EV1 International Pediatric Adolescent Clinical Trials Group, P.T. Long-term outcomes for HIV-infected infants less than 6 months of age at initiation of lopinavir/ritonavir combination antiretroviral therapy. AIDS. 2011;25:643–649. doi: 10.1097/QAD.0b013e32834403f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B1. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Chang SL, Vigorito M1. Role of HIV-1 infection in addictive behavior: A study of the HIV-1 transgenic rat model. Am J Infect Diseases. 2006;2:98–106. [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS1. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with and without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chearskul S, Chotpitayasunondh T, Simonds RJ, Wanprapar N, Waranawat N, Punpanich W, Chokephaibulkit K, Mock PA, Neeyapun K, Jetsawang B, Teeraratkul A, Supapol W, Mastro TD, Shaffer N1. Survival, disease manifestations, and early predictors of disease progression among children with perinatal human immunodeficiency virus infection in Thailand. Pediatrics. 2002;110:e25. doi: 10.1542/peds.110.2.e25. [DOI] [PubMed] [Google Scholar]
- Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS1. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience. 1998;82:97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Cohen S, Caan MW, Mutsaerts HJ, Scherpbier HJ, Kuijpers TW, Reiss P, Majoie CBLM, Pajkrt D1. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2016;86:19–27. doi: 10.1212/WNL.0000000000002209. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Piccirilli S, Paoletti A, Nistico R, Stringaro A, Malorni W, Finazzi-Agro A, Bagetta G1. Evidence that the HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci Lett. 2001a;312:67–70. doi: 10.1016/s0304-3940(01)02191-7. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Turano P, Bilotta A, Malorni W, Stringaro AR, Nistico R, Finazzi-Agro A, Bagetta G1. Evidence that increases of mitochondrial immunoreactive IL-1beta by HIV-1 gp120 implicate in situ cleavage of pro-IL-1beta in the neocortex of rat. J Neurochem. 2001b;78:611–618. doi: 10.1046/j.1471-4159.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- Crowell CS, Malee KM, Yogev R, Muller WJ1. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol. 2014;24:316–331. doi: 10.1002/rmv.1793. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K1. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E1. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80:1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J1. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Donovan M, Palumbo P1. Diagnosis of HIV: challenges and strategies for HIV prevention and detection among pregnant women and their infants. Clin Perinatol. 2010;37:751–763. doi: 10.1016/j.clp.2010.08.014. viii. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E1. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC1. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Fiorelli V, Ensoli F, Cafaro A, Titti F, Butto S, Monini P, Magnani M, Caputo A, Garaci E1. Candidate HIV-1 Tat vaccine development: from basic science to clinical trials. AIDS. 2006;20:2245–2261. doi: 10.1097/QAD.0b013e3280112cd1. [DOI] [PubMed] [Google Scholar]
- Ferguson G, Jelsma J1. The prevalence of motor delay among HIV infected children living in Cape Town. South Africa.I nt J Rehabil Res. 2009;32:108–114. doi: 10.1097/MRR.0b013e3283013b34. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF1. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006a;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF1. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006b;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF1. Neonatal intrahippocampal gp120 injection: an examination early in development. Neurotoxicology. 2007;28:101–107. doi: 10.1016/j.neuro.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF1. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2008a;18:135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF1. Neonatal intrahippocampal injection of the HIV-1 proteins gp120 and Tat: differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008b;1232:139–154. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF1. Dose-dependent long-term effects of Tat in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2010;20:469–480. doi: 10.1002/hipo.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF1. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF1. HIV-1 Proteins, Tat and gp120, Target the Developing Dopamine System. Curr HIV Res. 2015;13:21–42. doi: 10.2174/1570162x13666150121110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CJ, Biggs RL, Melvin D, Walters MD, Tudor-Williams G, Lyall EG1. Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Dev Med Child Neurol. 2006;48:677–682. doi: 10.1017/S0012162206001423. [DOI] [PubMed] [Google Scholar]
- Franklin S, Lim HJ, Rennie KM, Eastwood D, Cuene B, Havens PL1. Longitudinal intellectual assessment of children with HIV infection. J Clin Psychol Med S. 2005;12:367–376. [Google Scholar]
- Gelman BB, Nguyen TP1. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5:92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen S, Ramos JT, Resino R, Bellon JM, Munoz MA1. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26:334–338. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J1. The efficiency of systematic sampling in stereology–reconsidered. J Microsc. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE1. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct. 2013;220:605–623. doi: 10.1007/s00429-013-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HN, Lyman WD1. HIV infection of fetal human astrocytes: the potential role of a receptor-mediated endocytic pathway. Brain Res. 1999;823:24–32. doi: 10.1016/s0006-8993(98)01371-7. [DOI] [PubMed] [Google Scholar]
- Hargus NJ, Thayer SA1. Human immunodeficiency virus-1 Tat protein increases the number of inhibitory synapses between hippocampal neurons in culture. J Neurosci. 2013;33:17908–17920. doi: 10.1523/JNEUROSCI.1312-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan R, Thaver V, Russell VA, Daniels WM1. Tat-induced histopathological alterations mediate hippocampus-associated behavioural impariments in rats. Behav Brain Funct. 2015;11:3. doi: 10.1186/s12993-014-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP1. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31:S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Eillis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant 1I CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Fouche JP, Phillips N, Joska JA, Donald KA, Thomas K, Stein DJ1. Clinical associations of white matter damage in cART-treated HIV-positive children in South Africa. J Neurovirol. 2015;21:120–128. doi: 10.1007/s13365-014-0311-1. [DOI] [PubMed] [Google Scholar]
- Jeremy RJ, Kim S, Nozyce M, Nachman S, McIntosh K, Pelton SI, Yogev R, Wiznia A, Johnson GM, Krogstad P, Stanley K1. Neuropsychological functioning and viral load in stable antiretroviral therapy-experienced HIV-infected children. Pediatrics. 2005;115:380–387. doi: 10.1542/peds.2004-1108. [DOI] [PubMed] [Google Scholar]
- Jones MV, Bell JE, Nath A1. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS. 2000;14:2709–2713. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Karn J1. Tackling Tat. J Mol Biol. 1999;293:235–254. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- Koekkoek S, de Sonnevile LM, Wolfs TF, Licht R, Geelen SP1. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2008;12:290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kourtis AP, Bulterys M, Nesheim SR, Lee FK1. Understanding the timing of HIV transmission from mother to infant. JAMA. 2001;285:709–712. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M1. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15:257–274. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashomb AL, Vigorito M, Chang SL1. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2009;15:14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- Le Doare K, Bland R, Newell ML1. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:e1326–1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- Lee DE, Reid WC, Ibrahim WG, Peterson KL, Lentz MR, Maric D, Choyke PL, Jagoda EM, Hammoud DA1. Imaging dopaminergic dysfunction as a surrogate marker of neuropathology in a small-animal model of HIV. Mol Imaging. 2014;13:1–10. doi: 10.2310/7290.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JC, Yim HC, Lau AS1. Role of HIV-1 Tat in AIDS pathogenesis: its effects on cytokine dysregulation and contributions to the pathogenesis of opportunistic infection. AIDS. 2010;24:1609–1623. doi: 10.1097/QAD.0b013e32833ac6a0. [DOI] [PubMed] [Google Scholar]
- Lin X, Irwin D, Kanazawa S, Huang L, Romeo J, Yen TS, Peterlin BM1. Transcriptional profiles of latent human immunodeficiency virus in infected individuals: effects of Tat on the host and reservoir. J Virol. 2003;77:8227–8236. doi: 10.1128/JVI.77.15.8227-8236.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JC, Malee KM, Brouwers P, Hughes 1MD PACTG 219C Study Team. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119:e681–693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- Lipton SA1. HIV-related neurotoxicity. Brain Pathol. 1991;1:193–199. doi: 10.1111/j.1750-3639.1991.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Brenneman DE, Silverstein FS, Masliah E, Mucke L1. gp120 and neurotoxicity in vivo. Trends Pharmacol Sci. 1995;16:122. doi: 10.1016/s0165-6147(00)88998-1. [DOI] [PubMed] [Google Scholar]
- Lodha R, Upadhyay A, Kabra SK1. Antiretroviral therapy in HIV-1 infected children. Indian Pediatr. 2005;42:789–796. [PubMed] [Google Scholar]
- Luzuriaga K, Mofenson LM1. Challenges in the elimination of pediatric HIV-1 infection. N Engl J Med. 2016;374:761–770. doi: 10.1056/NEJMra1505256. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A1. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Marks WD, Parris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, Knapp PE, Hauser KF1. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol. 2016;22:747–762. doi: 10.1007/s13365-016-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L1. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART) Dev Neuropsychol. 2006;30:633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- Martinez-Canabal A, Wheeler AL, Sarkis D, Lerch JP, Lu WY, Buckwalter MS, Wyss-Coray T, Josselyn SA, Frankland PW1. Chronic over-expression of TGFbeta1 alters hippocampal structure and causes learning deficits. Hippocampus. 2013;23:1198–1211. doi: 10.1002/hipo.22159. [DOI] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF1. Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci Rep. 2016;6:32831. doi: 10.1038/srep32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF1. Selective developmental alterations in the HIV-1 transgenic rat: Opportunities for diagnosis of pediatric HIV-1. J Neurovirol. 2017a;23:87–98. doi: 10.1007/s13365-016-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Moran LM, Li H, Booze RM, Mactutus CF1. A gap in time: Extending our knowledge of temporal processing deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2017b;12:171–179. doi: 10.1007/s11481-016-9711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF1. Temporal processing demands in the HIV-1 transgenic rat: Amodal gating and implications for diagnostics. Int J Dev Neurosci. 2017c;57:12–20. doi: 10.1016/j.ijdevneu.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF1. Evolution of the HIV-1 transgenic rat: Utility in assessing the progression of HIV-1-associated neurocognitive disorders. J Neurovirol. 2017d doi: 10.1007/s13365-017-0544-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker RB, Boles JC, Bragg DC, Robertson K, Hall C1. Development of neuronal sensitivity to toxins in cerebrospinal fluid from HIV-type 1-infected individuals. AIDS Res Hum Retroviruses. 2004;20:1072–1086. doi: 10.1089/aid.2004.20.1072. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W1 Translational Methamphetamine AIDS Research Center (TMARC) Group. Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz M, Tardieu M, Hoyt L, McSherry G, Mendelson J, Okeske J1. Levodopa therapy improves motor function in HIV-infected children with extrapyramidal syndromes. Neurology. 1996;47:1583–1585. doi: 10.1212/wnl.47.6.1583. [DOI] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF1. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF1. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2013a;8:988–97. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF1. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013b;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Fitting S, Booze RM, Webb KM, Mactutus CF1. Neonatal intrahippocampal HIV-1 protein Tat1-86 injection: neurobehavioral alterations in the absence of increased inflammatory cytokine activation. Int J Dev Neurosci. 2014a;38:195–203. doi: 10.1016/j.ijdevneu.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF1. Modeling deficits in attention, inhibition, and flexibility in HAND. J Neuroimmune Pharmacol. 2014b;9:508–521. doi: 10.1007/s11481-014-9539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutwa PR, Boer KR, Asiimwe-Kateera B, Tuyishimire D, Muganga N, Lange JM, van de Wijgert J, Asiimwe A, Reiss P, Geelen SP1. Safety and effectiveness of combination antiretroviral therapy during the first year of treatment in HIV-1 infected Rwandan children: a prospective study. PLoS One. 2014;9:e111948. doi: 10.1371/journal.pone.0111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD1. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD1. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–194. [PubMed] [Google Scholar]
- Newell ML, Borja MC, Peckham C1. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111:e52–60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L1. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Ogundele MO, Coulter JB1. HIV transmission through breastfeeding: problems and prevention. Ann Trop Paediatr. 2003;23:91–106. doi: 10.1179/027249303235002161. [DOI] [PubMed] [Google Scholar]
- Parachure RS, Kulkarni VV, Darak TS, Mhaskar R, Miladinovic B, Emmanuel PJ1. Growth patterns of HIV infected indian children in response to ART: A clinical based cohort study. Indian J Pediatr. 2015;82:519–524. doi: 10.1007/s12098-014-1659-1. [DOI] [PubMed] [Google Scholar]
- Paramesparan Y, Garvey LJ, Ashby J, Foster CJ, Fidler S, Winston A1. High rates of asymptomatic neurocognitive impairment in vertically acquired HIV-1-infected adolescents surviving to adulthood. J Acquir Immune Defic Syndr. 2010;55:134–136. doi: 10.1097/QAI.0b013e3181d90e8c. [DOI] [PubMed] [Google Scholar]
- Penazzato M, Bendaud V, Nelson L, Stover J, Mahy M1. Estimating future trends in paediatric HIV. AIDS. 2014;28:S455–S251. doi: 10.1097/QAD.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Francis K, Sconza R, Horn A, S Peckham C, Tookey PA, Thorne C1. UK mother-to-child HIV transmission rates continue to decline: 2012–2014. Clin Infect Dis. 2017;64:527–528. doi: 10.1093/cid/ciw791. [DOI] [PubMed] [Google Scholar]
- Piazza FV, Segabinazi E, Centenaro 1LA, do Nascimento PS, Achaval M, Marcuzzo S. Enriched environment induces beneficial effects on memory deficits and microglial activation in the hippocampus of type 1 diabetic rats. Metab Brain Dis. 2014;29:93–104. doi: 10.1007/s11011-013-9467-2. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J1. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WC, Ibrahim WG, Kim SJ, Denaro F, Casas R, Lee DE, Maric D, Hammoud DA1. Characterization of neuropathology in the HIV-1 transgenic rat at different ages. J Neuroimmunol. 2016a;292:116–125. doi: 10.1016/j.jneuroim.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WC, Casas R, Papadakis GZ, Muthusamy S, Lee DE, Ibrahim WG, Nair A, Koziol D, Maric D, Hammoud DA1. Neurobehavioral abnormalities in the HIV-1 transgenic rat do not correspond to neuronal hypometabolism on 18F-FDG-PET. PLoS One. 2016b;11:e0152265. doi: 10.1371/journal.pone.0152265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lefebvre C, George O, Kawamura T, Morales M, Koob GF, Califano A, Masliah E, Sanna PP1. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol Neurodegener. 2014;9:26. doi: 10.1186/1750-1326-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reliquet V, Winer N, Chereau N, Launay E, Lamberet A, Andre-Garnier E, Raffi F, Brunet C1. The spectrum of HIV mother-to-child transmission risk. Journal of the International AIDS Society. 2014;17:19703. doi: 10.7448/IAS.17.4.19703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter SN, Palu G1. Inhibitors of HIV-1 Tat-mediated transactivation. Curr Med Chem. 2006;13:1305–1315. doi: 10.2174/092986706776872989. [DOI] [PubMed] [Google Scholar]
- Rigardetto R, Vigliano P, Boffi P, Marotta C, Raino E, Arfelli P, Bonassi E, Gandione M, Vigna Taglianti M, Tovo PA, Russo R1. Evolution of HIV-1 encephalopathy in children. Panminerva medica. 1999;41:221–226. [PubMed] [Google Scholar]
- Riordan A, Bugembe T1. Update on antiretroviral therapy. Archives of disease in childhood. 2009;94:70–74. doi: 10.1136/adc.2007.128744. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM1. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Shiau S, Strehlau R, Technau KG, Patel F, Arpadi SM, Coovadia A, Abrams EJ, Kuhn L1. Early age at start of antiretroviral therapy associated with better virologic control after initial suppression in HIV-infected infants. AIDS. 2017;31:355–364. doi: 10.1097/QAD.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Wilkins M1. Perinatally acquired HIV infection: Long-term neuropsychological consequences and challenges ahead. Chil Neuropsychol. 2015;21:234–268. doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- Sodroski J, Patarca R, Rosen C, Wong-Staal F, Haseltine W1. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985;229:74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Sohn AH, Hazra R1. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents. Journal of the International AIDS Society. 2013;16:18555. doi: 10.7448/IAS.16.1.18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksomboon N, Poolsup N, Ket-Aim S1. Systematic review of the efficacy of antiretroviral therapies for reducing the risk of mother-to-child transmission of HIV infection. J Clin Pharm Ther. 2007;32:293–311. doi: 10.1111/j.1365-2710.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Trudeau D, Arnold B, Wang J, Gerrow K, Summerfeldt K, Holmes A, Zamani A, Brocardo PS, Brown CE1. VEGF can protect against blood brain barrier dysfunction, dendritic spine loss and spatial memory impairment in an experimental model of diabetes. Neurobiol Dis. 2015;78:1–11. doi: 10.1016/j.nbd.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO1. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA1. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22:973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- Townsend CL, Byrne L, Cortina-Borja M, Thorne C, de Ruiter A, Lyall H, Taylor GP, Peckham CS, Tookey PA1. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS. 2014;28:1049–1057. doi: 10.1097/QAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- UNAIDS;1. Global HIV Statistics. Fact Sheet. 2017 Available at http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
- Vallat AV, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith TW, Gabuzda D1. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- van der Plas A, Scherpbier H, Kuijpers T, Pajkrt D1. The effect of different intervention programs on treatment adherence of HIV-infected children, a retrospective study. AIDS care. 2013;25:738–743. doi: 10.1080/09540121.2012.748864. [DOI] [PubMed] [Google Scholar]
- Van Rie A, Harrington PR, Dow A, Robertson K1. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: A global perspective. Eur J Paediatr Neurol. 2007;11:1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Van Rie A, Mupuala A, Dow A1. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–128. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie A, Dow A, Mupuala A, Stewart P1. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2009;52:636–642. doi: 10.1097/QAI.0b013e3181b32646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL1. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Vijayan T, Benin AL, Wagner K, Romano S, Andiman WA1. We never thought this would happen: transitioning care of adolescents with perinatally acquired HIV infection from pediatrics to internal medicine. AIDS care. 2009;21:1222–1229. doi: 10.1080/09540120902730054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmink J, Siegfried NL, van der Merwe L, Brocklehurst P1. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2007:CD003510. doi: 10.1002/14651858.CD003510.pub2. [DOI] [PubMed] [Google Scholar]
- Walker SY, Pierre RB, Christie CD, Chang SM1. Neurocognitive function in HIV-positive children in a developing country. Int J Infect Dis. 2013;17:e862–867. doi: 10.1016/j.ijid.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS1. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang L, Fang L, Wang A, Jin X, Wang F, Wang X, Qiao Y, Sullivan SG, Rutherford S, Zhang L1. Timely antiretroviral prophylaxis during pregnancy effectively reduces HIV mother-to-child transmission in eight counties in China: a prospective study during 2004–2011. Sci Rep. 2016;6:34526. doi: 10.1038/srep34526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb KM, Mactutus CF, Booze RM1. The ART of HIV therapies: dopaminergic deficits and future treatments for HIV pediatric encephalopathy. Expert Rev Anti Infect Ther. 2009;7:193–203. doi: 10.1586/14787210.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb KM, Aksenov MY, Mactutus CF, Booze RM1. Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2010;16:168–173. doi: 10.3109/13550281003690177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ1. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whitehead N, Potterton J, Coovadia A1. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. 2014;26:497–504. doi: 10.1080/09540121.2013.841828. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Masliah E, Achim CL1. Measurement of CNS HIV burden and its association with neurologic damage. Adv Neuroimmunol. 1994;4:319–325. doi: 10.1016/s0960-5428(06)80272-x. [DOI] [PubMed] [Google Scholar]
- Willen EJ1. Neurocognitive outcomes in pediatric HIV. Ment Retard Dev Disabil Res Rev. 2006;12:223–228. doi: 10.1002/mrdd.20112. [DOI] [PubMed] [Google Scholar]
- Winer BJ1. Statistical principles in experimental design. McGraw-Hill; New York: 1971. [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I1. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwas M, Trouche S, Richetin K, Escude T, Halley H, Gerardy-Schahn R, Verret L, Rampon C1. Environmental enrichment rescues memory in mice deficient for the polysialytransferase ST8SiaIV. Brain Struct Funct. 2015;221:1591–1605. doi: 10.1007/s00429-015-0991-1. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chen X, Lin Y, Tan T, Yang Z, Dayao C, Liu L, Jiang R, Zhang J1. Impairment of synaptic plasticity in hippocampus is exacerbated by methylprednisolone in a rat model of traumatic brain injury. Brain Res. 2011;1382:165–172. doi: 10.1016/j.brainres.2011.01.065. [DOI] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM1. Recombinant human immunodeficiency virus-1 transactivator of transcription1–86 allosterically modulates dopamine transporter activity. Synapse. 2011;65:1251–1254. doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchini S, Pittaluga A, Brocca-Cofano E, Summa M, Fabris M, De Michele R, Bonaccorsi A, Busatto G, Barbanti-Brodano G, Altavilla G, Verlengia G, Cifelli P, Corallini A, Caputo A, Simonato M1. Increased excitability in tat-transgenic mice: role of tat in HIV-related neurological disorders. Neurobiol Dis. 2013;55:110–119. doi: 10.1016/j.nbd.2013.02.004. [DOI] [PubMed] [Google Scholar]