Abstract
We examined the effect of irradiance on the synthesis of β-carotene and its isomers by Dunaliella salina. Growth irradiance had a marked effect both on growth of the alga (which was suppressed at both low and high irradiances) and on the accumulation of β-carotene. The accumulation of β-carotene but not α-carotene was closely linked to an increase in irradiance. Growth at low irradiances (20–50 μmol m−2 s−1) promoted a high ratio of 9-cis to all-trans β-carotene (>2:1), while exposure to high irradiances (200–1,250 μmol m−2 s−1) resulted in a large reduction in this ratio (to <0.45:1). A similar pattern was seen for the geometric isomers of α-carotene, with exposure to low irradiance favoring the accumulation of the 9-cis form. The carotenoid biosynthesis inhibitors 4-chloro-5(methylamino)-2-(α-α-α-trifluoro-m-tolyl)-3-(sH)-pyridazinone and 2-(4-chlorophenylthio)triethylamine caused the accumulation of the precursors phytoene and lycopene, respectively, in D. salina. High-performance liquid chromatography and infrared analysis showed that phytoene adopted the 15-cis and all-trans forms (as in higher plants), and that lycopene primarily adopted the all-trans form. This indicates that isomerization of β-carotene takes place during or after cyclization.
The ability of some species of microalgae to accumulate carotenoids such as β-carotene (β,β-carotene) and astaxanthin (3,3′-dihydroxy-β,β-carotene) is well known. Algae such as Dunaliella salina and Trentopholia sp. can accumulate high levels of β-carotene under growth-limiting conditions (typically nutrient deprivation and/or exposure to high irradiances). The β-carotene accumulated within globules in the inter-thylakoid spaces of the chloroplast of D. salina is composed mainly of two isomers, namely 9-cis and all-trans (Ben-Amotz et al., 1988, 1989; Jimenez and Pick, 1994). Ben-Amotz et al. (1988) reported that the level of the 9-cis isomer of β-carotene accumulated in D. salina was proportional to the integral light intensity to which the alga was exposed during a division cycle. Photocontrol of accumulation of 9-cis β-carotene has also been reported for the ripening of fruit such as apricot, peach, pepper, etc. (Ben-Amotz et al., 1988).
There is, however, considerable debate concerning the regulation of the biosynthesis of β-carotene and its isomers in D. salina. There are conflicting data from separate studies (e.g. Ben-Amotz et al., 1988; Jimenez and Pick, 1994) as to whether the synthesis of 9-cis β-carotene is promoted at high or low irradiance. This may be strain dependent, and Jimenez and Pick (1994) suggested major differences in the regulation of carotene synthesis between D. salina and Dunaliella bardawil (which are reported be different strains of a single species, namely D. salina; Borowitzka and Borowitzka, 1988). Unlike D. bardawil, 9-cis β-carotene biosynthesis in D. salina was reported to be greater at low but not high irradiances (Jimenez and Pick, 1994). When cells of D. salina are transferred to high irradiances, high levels of β-carotene are accumulated. Such light-activation of β-carotene synthesis could occur as the result of the photo-activation of carotenogenic enzymes already present in the cell, or more probably, to the de novo synthesis of these enzymes. The light-activated synthesis of β-carotene can be halted by the use of transcriptional inhibitors or inhibitors of protein synthesis, suggesting that the activation of specific genes and protein synthesis are necessary for the biosynthesis of β-carotene (Lers et al., 1990). In conditions of high irradiance, Lers and colleagues (1990) found that the expression of certain genes in D. salina was suppressed but others were induced. Indeed, differences in this response to high irradiances was observed between D. salina and D. bardawil.
The analysis of the geometrical isomers of carotenoids is fraught with difficulties (Pfander et al., 1994), and many of the readily available chromatographic systems do not allow complete separation of the cis- and trans- isomers (Craft, 1992; Orset and Young, 1999). The normal-phase chromatographic system based on a Ca(OH)2 stationary phase developed by Koyama and colleagues (Tsukida et al., 1982; Tsukida, 1992) does provide a comprehensive separation of a range of geometrical isomers of β-carotene (and other carotenoids). An advantage of this system is that the original identification of carotene isomers was based on NMR analysis of each component. Using this chromatographic system, a wide range of cis forms (both mono-cis and di-cis isomers) in addition to 9-cis β-carotene has been identified in D. salina (S.C. Orset and A.J. Young, unpublished data). Together these geometrical isomers were present at levels >20% of total β-carotene, with the 9-cis form being the major isomer present. This contrasts with the majority of previous studies on D. salina in which only the presence of all-trans and 9-cis isomers of β-carotene has been reported, possibly due to poor chromatographic separation.
In the present study, the effect of irradiance on the biosynthesis of cis/trans isomers of β-carotene, especially the 9-cis form, by D. salina has been examined. The conditions leading to a high ratio of 9-cis to all-trans β-carotene have been identified.
MATERIALS AND METHODS
Dunaliella salina (Teorodesco) CCAP 19/30 was cultivated in De Walne medium adjusted to 2.5 m NaCl (Orset and Young, 1999). Cells of D. salina were maintained at 25°C under constant illumination (provided by cool-white fluorescent lights) at 20 μmol m−2 s−1.
For all light treatments, the density of the starting cultures was kept low (approximately 0.3 × 106 cell mL−1) in order to observe possible changes of the isomeric composition of β-carotene during the division period of the cells and to minimize self-shading. The cultures were incubated at 29°C to 31°C and illuminated at seven different irradiances (20, 50, 260, 500, 700, 960, and 1,250 μmol m−2 s−1) for up to 42 d.
Inhibitors
Stock solutions of 4-chloro-5(methylamino)-2-(α-α-α-trifluoro-m-tolyl)-3-(sH)-pyridazinone (norflurazon) (1 mmol L−1) and 2-(4-chlorophenylthio)triethylamine (CPTA) (0.05 mol L−1) were prepared by first dissolving each inhibitor in a small volume of ethanol (<2% of final volume) and then adjusting to the required dilution with distilled water. Aliquots of the inhibitors were added to 100 mL of culture medium to obtain final concentrations of 10 and 0.1 μmol L−1 for norflurazon and CPTA, respectively.
Pigment Extraction
An aliquot (5 mL) of algal culture was diluted with 2.5 mL of distilled water and centrifuged at 2,500g for 7 min (Mistral 1000 centrifuge, Sanyo MSE, Itasca, IL). The pellet was then resuspended in 2.5 mL of ethanol by vortex mixing, 5 mL of diethyl ether was added, and the mixture was vortex mixed until a white precipitate appeared. The extract was filtered through absorbent cotton wool, and the centrifuge tube was rinsed with diethyl ether until no pigment remained. The sample was dried by evaporation of the solvent in the dark under a gentle flow of O2-free N2.
Before analysis of the carotenes by HPLC to avoid contamination of the stationary phase, all carotenoid extracts were purified on a 3-cm alumina column (aluminum oxide 90, activity I, Merck, Rahway, NJ) to remove chlorophylls and xanthophylls (Britton and Young, 1993). Alumina was stored at 80°C to guarantee total dryness. The column was equilibrated using redistilled petroleum ether (40°C–60°C) dried on a molecular sieve (3 Å, Fluka, Milwaukee, WI). Redistilled diethyl ether was used to elute the carotenes. Samples were dried under O2-free N2 and analyzed by HPLC as soon after preparation as possible: prolonged sample storage was avoided. Using appropriate mixtures of standards, this procedure was shown not to alter the isomeric composition of β-carotene.
Pigment Analysis
Total carotenoid and chlorophyll levels were determined by UV/Vis spectroscopy (Cecil CE5501 split-beam spectrophotometer) of samples resuspended in 100% (v/v) acetone using the equations of Lichtenthaler (1987). The concentrations of β-carotene and α-carotene were determined using the appropriate extinction coefficients (Britton, 1995).
The chromatographic system used for the analysis of the geometrical isomers of β-carotene was based on a system originally developed by Koyama and colleagues (Tsukida et al., 1982; Koyama et al., 1988a; Tsukida, 1992). This system has the advantage that the individual isomers have been previously identified by NMR (Tsukida et al., 1982; Koyama et al., 1988a). A stationary phase of Ca(OH)2 (300 × 4 mm, packed at 300 kg cm−2, Nakalai Chemical, Kyoto) was used because it affords the most complete and reproducible separation of the isomers of both α-carotene and β-carotene (O'Neil and Schwartz, 1992; Orset and Young, 1999). Separation of 12 different isomers of algal β-carotene was achieved (see Fig. 1; Table I). An isocratic mobile phase of hexane/acetone (99.5:0.5, v/v) with a flow rate of 1 mL min−1 was used. The column was fitted with a water jacket (Alltech, Deerfield, IL) linked to a low-temperature thermostat (RM6B, Lauda-Königshofe, Germany). All chromatography was performed at 24°C. The sample was dissolved in a small volume of hexane for injection. Solvents were thoroughly dried and stored on a molecular sieve (3 Å, Fluka). A comparison of retention time and on-line absorption spectra (maxima and Q-ratio) was made using authentic standards of β-carotene isomers (Table I).
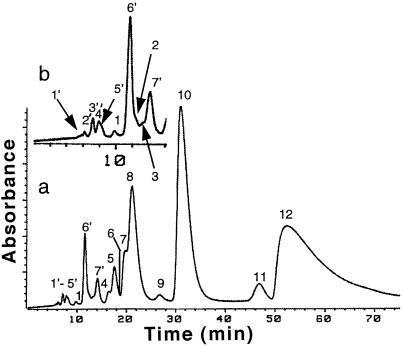
Figure 1.
HPLC separation of isomers of α- and β-carotene extracted from orange cells of D. salina (detection at 450 nm). Peaks 1 through 12 (see chromatogram a) are isomers of β-carotene: peaks 1 through 3, not identified; peak 4, 15-cis; peak 5, 13,13′-di-cis; peak 6, 9–13-di-cis; peak 7, 13-cis; peak 8, 9,15-cis; peak 9, 9,13′-di-cis; peak 10, all-trans; peak 11, 9,9′-di-cis; peak 12, 9-cis. Peaks 1′ through 7′ (see enlargement, chromatogram b, for clarity) are isomers of α-carotene: peaks 1′, 3′, and 4′, not identified; peak 2′, 15-cis; peak 6′, 13-cis; peak 7′, 9-cis. See text and Table I for details.
Table I.
Wavelength of the absorption spectrum maxima and Q-ratio (ɛ at λmax/ɛ at cis peak) of geometrical isomers of β-carotene or α-carotene in hexane:acetone (99.5:0.5, v/v), se ± 2 nm
| Peak | Isomer | cis Peak | Maximum (II) | Maximum (III) | Q-Ratio Found | Q-Ratio Reporteda |
|---|---|---|---|---|---|---|
| λ (nm) | ||||||
| β-Carotene | ||||||
| 1 | – | 442 | 472 | 1.4 | ||
| 2 | – | – | 434 | 458 | 1.3 | |
| 3 | – | 328 | 436 | 464 | 4.1 | |
| 4 | 15-cis | 336 | 446 | 474 | 1.9 | 1.9 |
| 5 | 13,13′-cis | 336 | 439 | 469 | 3.7 | |
| 6 | 9,13-cis | 431 | 457 | 6.1 | ||
| 7 | 13-cis | 337 | 442 | 468 | 2.4 | 2.8 |
| 8 | 9,15-cis | 337 | 438 | 464 | 5.2 | 5.0 |
| 9 | 9,13′-cis | 432 | 459 | 5.5 | ||
| 10 | All-trans | 450 | 477 | 16.5 | 17.4 | |
| 11 | 9,9′-cis | 440 | 467 | 13.5 | ||
| 12 | 9-cis | 341 | 445 | 473 | 10.7 | 11.5 |
| α-Carotene | ||||||
| 1′ | – | 430 | 456 | |||
| 2′ | 15-cis | 330 | 440 | 468 | 1.8 | |
| 3′ | – | 330 | 436 | 464 | 2.4 | |
| 4′ | – | 328 | 432 | 460 | 4.2 | |
| 5′ | 13-cis | 330 | 436 | 464 | 2.8 | |
| 7′ | All-trans | 444 | 472 | 14.5 | ||
| 8′ | 9-cis | 330 | 438 | 466 | 11.6 | |
HPLC analysis was performed using a diode-array detector (model 1040, Hewlett-Packard, Palo Alto, CA) linked to a computer workstation. Solvents were delivered using a tertiary pump (CM4000, LDC Analytical, Riviera Beach, FL). Samples were injected onto the column via an injector (model 7125, Rheodyne, Rohnert Park, CA) using complete loop filling (20 μL). The on-line absorption spectrum and peak area were recorded for each component at its absorption maximum. All solvents were of HPLC grade (Merck) and were filtered and degassed prior to use.
The analysis of the geometric isomers of lycopene was based on the methods of Hengartner et al. (1992) and Schierle et al. (1996). Separation of the isomers was performed on a Nucleosil 5SIL column (25 × 0.46 cm; Macherey-Nagel GmbH & Co, Dueren, Germany) operating with an isocratic solvent system of hexane/hexane:n-ethyldiisopropylamine (99.85:0.15, v/v) at a flow rate of 0.5 mL min−1. Samples were dissolved and injected (20 μL) in 1% (v/v) acetone/hexane.
The analysis of the geometric isomers of phytoene was performed on a reversed-phase column (25 × 0.46 cm; 5-μm particles; model 201TP54, Vydac, Hesperia, CA). A mobile phase of methanol:acetonitrile (90:10, v/v) was delivered at a flow rate of 0.7 mL min−1. Samples (20 μL) were injected in 20% (v/v) diethyl ether-methanol.
Authentic carotenoid standards (all-trans and 9-, 13-, and 15-cis-isomers) together with a standard thermally isomerized mixture of cis isomers were provided by F. Hoffmann-La Roche, Basel and the laboratory of Prof. Y. Koyama (Kwansei Gakuin University, Nishinomiya, Japan). These were used alone or in combination to confirm that the chromatography protocols used throughout this study did not alter the isomeric composition of the carotenoids studied.
Infrared Spectroscopy
Infrared analysis of purified carotenoid isomers was performed on an FT-IR spectrophotometer (model 1600, Perkin-Elmer/Applied Biosystems, Foster City, CA) operating at a resolution of 4 cm−1. Carotenoids were carefully mixed with oven-dried KBr and discs formed on a hydraulic press at a pressure of 10 tons.
RESULTS AND DISCUSSION
Growth and Pigment Biosynthesis
In the first treatment, the algae were grown for up to 42 d in a constant environmental chamber at 26°C with continuous illumination at 20 μmol m−2 s−1. Under these conditions, β-carotene accumulated in the cells during the stationary phase at a rate three times greater than that observed during log-phase growth (Table II). At this low level of irradiance, levels of β-carotene remained low at all times (compare with Table III). The most apparent difference between cells in the log and stationary phases of growth was seen for the isomeric composition of both β-carotene and α-carotene (see below).
Table II.
Culture characteristics of D. salina in the logarithmic and stationary growth phases
| Characteristic | Culture Stage
|
|
|---|---|---|
| Log | Stationary | |
| Chlorophyll (pg cell−1) | 7.34 ± 1.37 | 8.73 ± 0.65 |
| Carotenoid (mg L−1) | 2.16 ± 0.43 | 5.73 ± 0.31 |
| Carotenoid (pg cell−1) | 2.74 ± 0.44 | 7.22 ± 0.32 |
| Carotenoid accumulation (pg cell−1 d−1) | 0.13 | 0.34 |
| Percent cis (other than 9-cis)a | 22.80 | 25.10 |
| Percent all-transa | 66.12 | 24.10 |
| Percent 9-cisa | 11.10 | 50.82 |
Algae were grown under an irradiance of 20 μmol m−2 s−1 (n = 3 ± se).
Data for isomeric composition of β-carotene is the mean of two separate analyses. Values are expressed as a percentage of total β-carotene.
Table III.
Culture characteristics of D. salina grown under irradiances ranging from 50 to 1,250 μmol m−2 s−1 after 8 d of treatment (n = 3 ± se)
| Irradiance | Cell Density | Growth Constant | Carotenoid | Chlorophyll | ||
|---|---|---|---|---|---|---|
| μmol m−2 s−1 | × 104 mL−1 | K | mg L−1 | pg cell−1 | pg cell−1 d−1 | pg cell−1 |
| 50 | 33 ± 2.87 | 0.14 | 2.12 ± 0.38 | 6.38 ± 1.11 | 0.80 | 17.38 ± 0.74 |
| 260 | 56 ± 4.49 | 0.29 | 7.28 ± 1.26 | 13.04 ± 1.27 | 1.63 | 5.53 ± 0.40 |
| 500 | 57 ± 8.30 | 0.30 | 10.06 ± 1.15 | 18.09 ± 0.03 | 2.26 | 5.35 ± 0.03 |
| 700 | 55 ± 5.69 | 0.29 | 10.31 ± 1.05 | 18.64 ± 0.01 | 2.33 | 4.16 ± 0.13 |
| 960 | 46 ± 2.19 | 0.24 | 9.34 ± 1.28 | 21.22 ± 4.66 | 2.65 | 3.82 ± 0.97 |
| 1,250 | 33 ± 6.04 | 0.14 | 10.35 ± 0.89 | 36.55 ± 4.20 | 4.57 | 5.10 ± 0.68 |
The effect of cultivating D. salina at higher irradiances (in the range 50–1,250 μmol m−2 s−1) affected both the rate of growth and the rate of accumulation of carotenoid (Table III). Slower growth rates were observed at very high irradiances (≥960 μmol m−2 s−1) but also when the cultures were grown under a low irradiance level (≤50 μmol m−2 s−1). Although at these “extreme” irradiances (≤50 and ≥960 μmol m−2 s−1) light acted as a growth-limiting factor, between 260 and 700 μmol m−2 s−1 the growth constant (k) was not significantly different between treatments (Table III). A stimulating effect of growth irradiance on carotenoid accumulation was clearly observed. The carotenoid content per cell was higher at higher irradiances, but the carotenoid content per unit volume of culture was not greatly different for any given level of irradiance >260 μmol m−2 s−1 due to the growth-limiting effects of these irradiances (see above).
At irradiances between 50 to 260 μmol m−2 s−1, a large decrease in the chlorophyll content of each algal cell was observed. Further increases in irradiance (i.e. >260 μmol m−2 s−1) did not further alter the chlorophyll content. A decrease of chlorophyll concentration in D. salina cells exposed to high irradiances has been extensively reported (Loeblich, 1982; Ben-Amotz, 1987; Gomez-Pinchetti et al., 1992). When the irradiance level exceeds that which can be used by the photosynthetic apparatus, photobleaching of chlorophylls occurs (Carpentier, 1996; Horton et al., 1996). Globular β-carotene is rapidly accumulated to protect the photosynthetic system, possibly by acting simply as a sunscreen (Ben-Amotz et al., 1988, 1989; Jimenez and Pick, 1993, 1994; Zamir, 1995) and thus increasing the photoinhibitory threshold of the system (Gomez-Pinchetti et al., 1992).
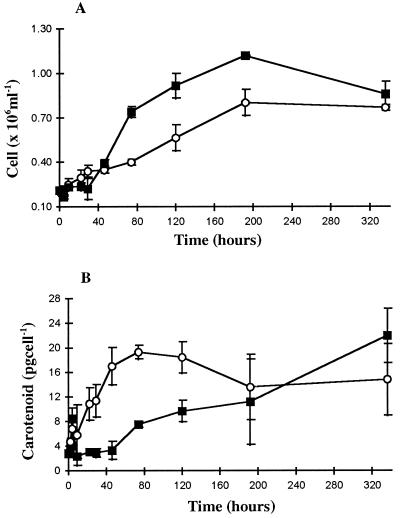
The effects of transfer of the alga from low (20 μmol m−2 s−1) to higher (200 and 1,250 μmol m−2 s−1) irradiances was studied in more detail. After transfer of the algal culture to higher irradiances, some cell death was recorded (Fig. 2). A similar observation was previously reported by Lers et al. (1990) when green cells of D. salina were transferred to a higher irradiance. A rapid accumulation of β-carotene was observed during the first 4 h of exposure (Fig. 2); however, this was followed (approximately 9 h after transfer) by a transient decrease in the β-carotene content per cell. At this stage, cell growth was still not apparent and cells only started dividing after approximately 29 h. While the growth rate was greater at 200 μmol m−2 s−1 than at 1,250 μmol m−2 s−1, carotenogenesis was much slower. Again, a two-phase response to higher irradiances was reported by Lers et al. (1990). The first phase occurred in the first few hours after the transfer of cells to high irradiance and involved carotenoid accumulation without cell division. This was followed by a period of 3 to 4 d in which no further carotenoid biosynthesis took place. The biosynthesis of β-carotene was only regained after this. In this study, carotenoid biosynthesis was halted and β-carotene levels actually decreased for the first few hours after transfer. This was a much shorter period than that recorded by Lers et al. (1990), but the overall response was similar. After 120 h at 1,250 μmol m−2 s−1, no further carotenoid synthesis was observed, but pigment concentration per cell still increased in the cells transferred to 200 μmol m−2 s−1. Finally, after 192 h, no difference was seen in the carotenoid content per cell between the cells exposed to 1,250 and 200 μmol m−2 s−1 (Fig. 2).
Figure 2.
The effect of irradiance on the culture characteristics of D. salina after transfer of green cells from 20 μmol m−2 s−1 to 1,250 (○) and 200 μmol m−2 s−1 (▪). A, Cell growth (cell × 106 ml−1); B, carotene content per cell (pg cell−1); n = 3 ± se.
Isomeric Composition of β-Carotene
The analysis of the geometrical isomers of carotenoids such as β-carotene requires that stringent conditions are used throughout the extraction, isolation, and analytical procedures. In this study all experimental procedures relating to the extraction, isolation, and analysis of carotenoids were performed in near-complete darkness at a low temperature (4°C). All procedures were fully validated using appropriate standards to ensure that the isomeric composition of β-carotene was not altered in any way by any methods employed.
The total carotenoid content of green cells in the exponential stage of growth at 20 μmol m−2 s−1 was in the range of 2 to 5 pg cell−1, with a total chlorophyll content of approximately 7 pg cell−1 (Table II). Reversed-phase HPLC analysis revealed the presence of a number of xanthophylls (neoxanthin, violaxanthin, and lutein), chlorophylls a and b, β-carotene, and traces of α-carotene (data not shown). This pigment composition is typical for the photosynthetic tissues of higher plants and the majority of green algae (Young, 1993). No carotenoid-rich globules were evident in these cells when studied by light microscopy, nor could globules be isolated from these green cells. The β-carotene isolated from these cells was therefore assumed to be fully associated with the photosynthetic apparatus (similar data have been reported for D. salina by Lers et al., 1990). Using normal-phase HPLC (with Ca[OH]2 as the stationary phase; see Fig. 1), the isomeric composition of the (thylakoidal) β-carotene was determined to be 75% all-trans and 25% cis isomers (of which 9-cis was <15% total β-carotene). The level of α-carotene was very low at <1% of total carotene. Similar values (approximately 80% all-trans) for the thylakoidal β-carotene was reported by Jimenez and Pick (1994) for both D. salina and D. bardawil.
After prolonged cultivation at 20 μmol m−2 s−1, the cells entered the stationary growth phase ( approximately 35–40 d); large changes in the isomeric composition of β-carotene were observed as this carotene was accumulated within globules (Table II). A range of isomers of both β-carotene and α-carotene (see below; Orset and Young, 1999), including some di-cis isomers, were accumulated within the algal cell, usually only at small levels (Table I, Fig. 1). The two main forms of β-carotene accumulated were all-trans and 9-cis, with the remaining isomers of β-carotene collectively termed “other-cis.” The ratio of 9-cis/all-trans β-carotene increased from approximately 0.2:1 in the logarithmic phase to >2.1:1 in the stationary phase. The relative levels of the other cis-isomers of β-carotene were unaltered. Overall, total carotenoid levels doubled compared with cells in the logarithmic phase of growth, even at this very low level of irradiance.
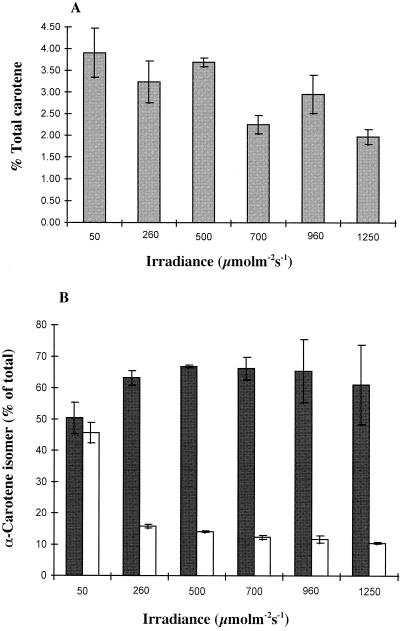
The effect of irradiance on the isomeric composition of β-carotene in D. salina was further studied over the range of 50 to 1,250 μmol m−2 s−1 for 8 d (Table III; Fig. 3). The relative levels of the all-trans form increased with increasing irradiance, with a parallel decrease in the levels of the 9-cis isomer. Again, levels of the other (minor) mono- and di-cis isomers remained relatively unchanged. An inverse response to increases in irradiance was observed by Ben-Amotz et al. (1988) for D. salina, which accumulated high levels of the 9-cis isomer when exposed to high irradiances. Jimenez and Pick (1994) reported that the increase in levels of 9-cis β-carotene in D. bardawil was not linked to changes in the isomeric composition within the globules, but was the result of a significant decrease in the levels of the pigment associated with the thylakoid membranes (mainly in all-trans form) coupled with significant accumulation within the globules (with a typical composition of 40% all-trans and 60% 9-cis β-carotene).
Figure 3.
The effect of irradiance on the isomeric composition of β-carotene in D. salina after 8 d of treatment (see text for full details); n = 3 ± se. Gray bars, cis (other than 9-cis); black bars, all-trans; white bars, 9-cis.
Conversely, in D. salina, the decrease in the level of the 9-cis isomer at high irradiances was assigned to a decrease in the level of the 9-cis isomer within the globules (Jimenez and Pick, 1994). In the present study the magnitude of the change in the level of β-carotene in the algal cells would preclude any significant effect due to thylakoidal β-carotene alone. Recently, Bialek-Bylka et al. (1995, 1996) reported that β-carotene in the photosystem I (PSI) and II (PSII) reaction centers of higher plants was 15-cis and not all-trans as previously reported. However, despite the use of very stringent conditions throughout this study for the extraction and analysis of green cells of D. salina (lacking globular β-carotene), the level of 15-cis β-carotene was consistently very low (<1% total β-carotene), and the all-trans form predominated.
The stability of the isomeric composition of β-carotene was verified by a second analysis 14 d after transfer. Although little change to the isomeric composition was observed in the cultures grown at irradiances ≥ 260 μmol m−2 s−1, at 50 μmol m−2 s−1, large changes in the ratio of 9-cis/all-trans β-carotene were observed. Eight days after transfer from 20 to 50 μmol m−2 s−1, the ratio was 0.95:1, increasing to 1.56:1 after 14 d of cultivation at 50 μmol m−2 s−1. This compares to a ratio for 9-cis/all-trans β-carotene of 0.42:1 achieved after 14 d of growth at 1,250 μmol m−2 s−1. Overall, however, the decrease observed in the levels of the 9-cis isomer was not directly proportional to the increase in irradiance over the range of light levels studied. This observation would be consistent with the model of regulation of the isomeric composition of β-carotene established above. During the first 8 d after transfer, the rate of carotenoid accumulation was very low (0.8 pg cell−1 d−1) and the potential contribution made by thylakoidal β-carotene (as estimated by following changes in the chlorophyll content) was deemed to be relatively unchanged (see Table II). However, between d 8 and 14 (when large changes to the ratio of 9-cis/all-trans occurred), a doubling of the carotenoid content per cell was observed, and it is the change in the composition of this globular β-carotene that results in an altered ratio for 9-cis and all-trans β-carotene.
The evolution of isomeric composition of β-carotene in the D. salina following the transfer of green cells (in the logarithmic phase of growth) from 20 μmol m−2 s−1 to 200 and 1,250 μmol m−2 s−1 is shown in Figure 4. A short and rapid period of accumulation of β-carotene was observed (Fig. 2) coupled with rapid changes in the isomeric composition of β-carotene. Thus, after only a few hours of exposure to higher irradiances, the accumulation of 9-cis β-carotene could be clearly observed in the algal cells. The relative levels of all-trans β-carotene decreased during this initial phase (which lasted approximately 10 h) so that the ratio of 9-cis/all-trans β-carotene increased from 0.24:1 in cells grown at 20 μmol m−2 s−1 to 0.72:1 and 0.53:1 at irradiances of 200 and 1,250 μmol m−2 s−1, respectively.
Figure 4.
Changes of the isomeric composition of β-carotene in D. salina after transfer of green cells (grown at 20 μmol m−2 s−1) to irradiances of 200 μmol m−2 s−1 (A) and 1,250 μmol m−2 s−1 (B), n = 2. ▪, All-trans; ●, cis (other than 9-cis); ▵, 9-cis.
At 1,250 μmol m−2 s−1, the relative levels of both the 9-cis and other-cis isomers of β-carotene increased in parallel (by approximately 10%), while at 200 μmol m−2 s−1, the relative increase in the levels of the 9-cis isomer (+20%) was double that observed for the other-cis isomers (Fig. 4). Little change to the isomeric composition of β-carotene was observed after this first phase (approximately 10 h) following transfer, and even when the cells entered the stationary phase (after approximately 190 h). The rapid change to the isomeric composition of β-carotene was coupled to the rapid synthesis of this carotenoid immediately following transfer of cells to a higher irradiance (Figs. 2 and 4) and again reflects the changes that occur upon the accumulation of globular rather than changes in thylakoidal β-carotene in D. salina.
Isomeric Composition of α-Carotene
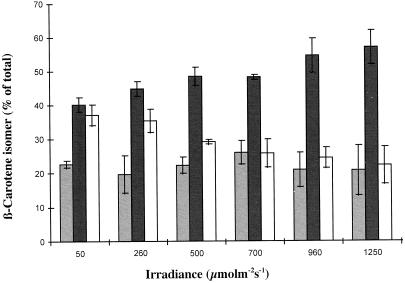
In green, rapidly dividing cells of D. salina, α-carotene accounts for a relatively minor proportion of the pigment pool, typically accounting for approximately 1% to 2% of total carotene (see above). As with β-carotene, light had a stimulatory effect on the synthesis of α-carotene, and a 3-fold increase in levels was observed at 1,250 μmol m−2 s−1 compared with 50 μmol m−2 s−1 (Table III). The ratio of α-carotene/β-carotene decreased when the alga was cultivated at higher irradiances; α-carotene accounted for up to approximately 4% of total carotene at 50 μmol m−2 s−1, falling to approximately 2% at 1,250 μmol m−2 s−1 (Fig. 5). This “shade-like” response appears to be characteristic of α-carotene biosynthesis in the photosynthetic tissues of higher plants (Cunningham and Gantt, 1998). As with β-carotene, the synthesis of 9-cis α-carotene (as the main cis-isomer of this carotenoid) was favored at low irradiances (Fig. 6). At 50 μmol m−2 s−1, the ratio of 9-cis/all-trans α -carotene was approximately 0.9:1, falling to 0.25 to 0.15:1 at higher irradiances. Although irradiance plays a role in determining both the level and composition of α-carotene, the main stimulatory factor for the synthesis of this carotenoid in D. salina has been identified as exposure to low growth temperatures (Orset and Young, 1999).
Figure 5.
The effect of irradiance on the level of α-carotene (total cis + trans) calculated as a percentage of total carotene (i.e. α-carotene + β-carotene; n = 3 ± se) (A) and the level of 9-cis and all-trans α-carotene (calculated as a percentage of total α-carotene; n = 3 ± se) (B). Black bars, All-trans; white bars, 9-cis.
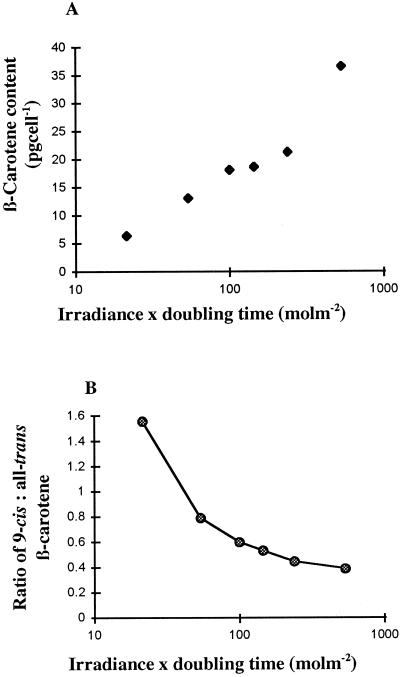
Figure 6.
A, Dependence of β-carotene content in D. salina on the integral irradiance per division cycle. B, Dependence of the ratio of 9-cis to all-trans β-carotene in D. salina on the integral irradiance per division cycle. Irradiance ranged from 50 to 1,250 μmol m−2 s−1.
Effect of Inhibitors of Carotenoid Biosynthesis
An important question concerning carotenogenesis in D. salina is when does the introduction of the 9-cis bond occur in the synthesis of β-carotene? Both Ben-Amotz et al. (1988) and Ebenezer and Pattenden (1993) have suggested that isomerization takes places early in the biosynthetic pathway during phytoene synthesis or during the desaturation of phytoene to phytofluene, respectively. We used a two-phase cultivation system to induce rapid synthesis of β-carotene in D. salina. Cells were initially cultivated at 20 μmol m−2 s−1 and transferred to higher irradiances (350 and 650 μmol m−2 s−1) for a period of 48 h to induce rapid β-carotene accumulation (including accumulation of the 9-cis form, which is absent from green, actively dividing cells; see above). During this exposure to altered growth irradiance, inhibitors were used to selectively block carotenoid biosynthesis and the isomeric composition of the resulting accumulated precursors determined. In the control cells (transferred to higher irradiances in the absence of the inhibitors CPTA and norflurazon), as expected, precursors of carotenoid biosynthesis were completely absent from cells of D. salina (see Britton et al., 1989).
In the presence of CPTA (an inhibitor of cyclization; 10 μmol L−1), lycopene and not β-carotene accumulated in the treated cells. Subsequent isolation of the total carotene fraction (including lycopene) from these cells and analysis by normal-phase HPLC allowed the separation of the geometric isomers of lycopene to be performed (Hengartner et al., 1992; Schierle et al., 1996). All-trans lycopene (λmax 442, 470, 500 nm in the HPLC eluting solvent) was identified as the main component of CPTA-treated D. salina (approximately 80% of the total) by co-chromatography and comparison of spectral characteristics with an authentic standard (F. Hoffmann-La Roche) and with a natural extract from tomato (Lycopersicum esculentum, which has the all-trans form as its main isomer; Schierle et al., 1996). Five (unidentified) cis-isomers of lycopene were separated from CPTA-treated D. salina. Collectively, these isomers accounted for only a fraction of total lycopene accumulated by the algal cell, with no particular isomer accounting for >5% (w/w) total lycopene. Thus, although the untreated algal cells did accumulate 9-cis β-carotene (at a ratio to the all-trans form of 0.73:1), no selective accumulation of any cis-isomers of lycopene in the CPTA-treated cells was observed. Traces of neurosporene (λmax = 440 nm in the HPLC eluting solvent) were also observed in CPTA-treated D. salina, indicating that cyclization of neurosporene to β-carotene (via γ-carotene) may also be inhibited by CPTA.
CPTA does not block cyclization completely in plants or algae (Britton et al., 1989) and some “leakage” to final product formation is therefore frequently seen in most species examined to date. Thus, the cells treated with this inhibitor do accumulate small amounts of β-carotene in addition to lycopene. It is important to note that the ratio of 9-cis/trans β-carotene in the CPTA-treated cells was lower than the control (untreated) cells maintained at the same irradiance (i.e. 0.73:1 in the control compared with 0.45:1 in CPTA-treated cells grown at 350 μmol m−2 s−1). This suggests that the synthesis of 9-cis β-carotene was preferentially blocked by CPTA. In addition, the lack of accumulation of any cis isomers of lycopene would further indicate that the isomerization of β-carotene in D. salina takes place after cyclization and not before.
In the presence of the phytoene desaturase inhibitor norflurazon (0.1 μmol L−1) both phytoene (see below) and mono-hydroxyphytoene (tentative identification based on retention time and spectral characteristics) were accumulated in D. salina. The carotene fraction was isolated by elution through an alumina grade 1 column and the isomeric composition determined by reversed-phase HPLC (see “Materials and Methods”). Two peaks were resolved, both with a λmax of 287 nm, with one component having a distinctive shoulder and higher A300 (characteristic of the all-trans form) and the other possessing a higher absorbance at another shoulder located at 278 nm. Co-chromatography and comparison of spectral characteristics with all-trans and 15-cis phytoene isolated from norflurazon-treated leaves of barley (Mayer et al., 1989) grown at high and low irradiances, respectively, demonstrated that norflurazon-treated cells of D. salina accumulated these two particular isomers of phytoene. In barley, the ratio of 15-cis to all-trans phytoene was very dependent upon the irradiance at which the plants were grown. Thus, the higher the irradiance the higher the ratio of all-trans to 15-cis phytoene accumulated in the bleached tissues (S.C. Orset and A.J. Young, unpublished data).
Further analysis of the two isomers of phytoene isolated from the norflurazon-treated cultures of D. salina by infrared spectroscopy compared with authentic isomer standards of β-carotene (provided by F. Hoffmann-La Roche) and phytoene isomers isolated by HPLC from norflurazon-treated seedlings of barley was also performed. One gave a spectrum identical to that of all-trans phytoene with a specific absorption at 960 cm−1 that is characteristic of all-trans phytoene (Ebenezer and Pattenden, 1993). In the other, the presence of an absorption band at 766 cm−1 (characteristic of the 15-cis configuration in carotenoids, e.g. β-carotene; Koyama et al., 1988b) was observed but it also revealed the absence of the absorption at 960 cm−1.
These data demonstrate that phytoene in D. salina primarily adopts the 15-cis and not the 9-cis configuration, as reported by Ben-Amotz et al. (1988). It should be noted that their identification of 9-cis phytoene was based on “an analogy with the 9-cis β-carotene and on the typical absorption spectra and mass spectra of phytoene” and that no firm chemical evidence was provided to establish its identity. To date, only the all-trans and 15-cis forms of phytoene have been detected in tissues (plant, algal, and microbial) that have been treated with inhibitors that block phytoene desaturase (G. Britton, personal communication). Ebenezer and Pattenden (1993) also found that 15-cis was the main form of phytoene accumulated and could not detect the 9-cis form in norflurazon-treated cells. They further suggested that the 9-cis configuration was introduced into the carotenoid molecule during the desaturation of phytoene into phytofluene. In the present study, the yield of phytofluene in norflurazon-treated cells was very small and, as a result, its configuration could not be determined. Nevertheless, the absence of any significant levels of 9-cis (or indeed any other cis isomers) of lycopene in the CPTA-treated cells (see above) indicates that the 9-cis isomer is not formed during the desaturation reactions but, rather, is formed during or after cyclization. Alternatively, the possibility of light-mediated trans ↔ cis isomerization of β-carotene taking place in situ in the globules of D. salina cannot be ruled out.
CONCLUSIONS
In D. salina the effect of irradiance on the biosynthesis of β-carotene is very marked. This relationship can be seen both for the level of β-carotene accumulated (the higher the irradiance at which the alga is cultivated, the higher the rate of β-carotene accumulation per cell; Fig. 6A) and also for the biosynthesis of the two main geometric isomers of β-carotene (the lower the irradiance, the higher the ratio of 9-cis/all-trans β-carotene; Fig. 6B). While the levels of the other mono- and di-cis isomers of β-carotene in the alga were unchanged whatever the level of irradiance, the data indicate a clear and distinctive relationship between irradiance and the levels of two main forms of this particular carotenoid, namely all-trans and 9-cis β-carotene. In contrast to the synthesis of β-carotene, the accumulation of α-carotene did not appear to be regulated by the level of irradiance. Nevertheless, as for β-carotene, the higher the irradiance, the lower the ratio of 9-cis/all-trans α-carotene in the cells.
Footnotes
This work was supported by the European Commission AIR Programme (grant no. AIR2–CT94–1283).
LITERATURE CITED
- Ben-Amotz A. Effect of irradiance and nutrient deficiency on the chemical composition of Dunaliella bardawil Ben-Amotz and Avron (Volvocales, Chlorophyta) J Plant Physiol. 1987;131:479–487. [Google Scholar]
- Ben-Amotz A, Lers A, Avron M. Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol. 1988;86:1286–1291. doi: 10.1104/pp.86.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A, Shaish A, Avron M. Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol. 1989;91:1040–1043. doi: 10.1104/pp.91.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek-Bylka GE, Hiyama T, Yumoto K, Koyama Y. 15-cis-Beta-carotene found in the reaction center of spinach photosystem I. Photosynth Res. 1996;49:245–250. doi: 10.1007/BF00034785. [DOI] [PubMed] [Google Scholar]
- Bialek-Bylka GE, Tomo T, Satoh K, Koyama Y. 15-cis-Beta-carotene found in the reaction center of spinach photosystem II. FEBS Lett. 1995;363:137–140. doi: 10.1016/0014-5793(95)00298-n. [DOI] [PubMed] [Google Scholar]
- Borowitzka MA, Borowitzka LJ. Dunaliella. In: Borowitzka LJ, Borowitzka MA, editors. Micro-Algal Biotechnology. Cambridge, UK: Cambridge University Press; 1988. pp. 27–58. [Google Scholar]
- Britton G. UV/Visible spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander HP, editors. Carotenoids 1B (Spectroscopy). Basel: Birkhäuser; 1995. pp. 13–62. [Google Scholar]
- Britton G, Barry P, Young AJ. Carotenoids and chlorophylls: herbicidal inhibition of pigment biosynthesis. In: Dodge AD, editor. Herbicides and Plant Metabolism. Cambridge, UK: Cambridge University Press; 1989. pp. 51–72. [Google Scholar]
- Britton G, Young AJ. Methods for the isolation and analysis of carotenoids. In: Young AJ, Britton G, editors. Carotenoids in Photosynthesis. London: Chapman & Hall; 1993. pp. 409–459. [Google Scholar]
- Carpentier R. Influence of high light intensity on photosynthesis. In: Pessarakli M, editor. Handbook of Photosynthesis. New York: Marcel Dekker; 1996. pp. 443–450. [Google Scholar]
- Craft NE. Carotenoid reversed-phase high-performance liquid chromatography methods: reference compendium. Methods Enzymol. 1992;213:185–205. doi: 10.1016/0076-6879(92)13121-d. [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- Ebenezer WJ, Pattenden G. cis-Stereoisomers of beta-carotene and its congeners in the alga Dunaliella bardawil, and their biogenetic interrelationships. J Chem Soc Perkin Trans. 1993;I:1869–1873. [Google Scholar]
- Gomez-Pinchetti JL, Ramazanov Z, Fontes A, Garcia-Reina G. Photosynthetic characteristics of Dunaliella salina (Chlorophyceae, Dunaliellales) in relation to β-carotene content. J Appl Phycol. 1992;4:11–15. [Google Scholar]
- Hengartner U, Bernhard K, Meyer K, Englert G, Glinz E. Synthesis, isolation, and NMR-spectroscopic characterization of fourteen (Z)-isomers of lycopene and some acetylenic didehydro- and tetradehydrolycopenes. Helv Chim Acta. 1992;75:1848–1865. [Google Scholar]
- Horton P, Ruban AV, Walters R. Regulation of light harvesting in plants. Annu Rev Plant Physiol Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Pick U. Differential reactivity of β-carotene isomers from Dunaliella bardawil toward oxygen radicals. Plant Physiol. 1993;101:385–390. doi: 10.1104/pp.101.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez C, Pick U. Differential stereoisomer compositions of β,β-carotene in thylakoids and in pigment globules in Dunaliella. J Plant Physiol. 1994;143:257–263. [Google Scholar]
- Koyama Y, Hosomi M, Miyata A, Hashimoto H, Reames SA, Nagayama K, Katto-Jippo T, Shimamura T. Supplemetary and revised assigment of the peaks of the 7,9-, 9,9′-, 13,13′-, 9,13′-di-cis and 9,9′,13-tri-cis isomers of β-carotene in high performance liquid chromatography using a column of calcium hydroxide. J Chromatogr. 1988a;439:417–422. [Google Scholar]
- Koyama Y, Takatsuka I, Nakata M, Tasumi M. Raman and infrared spectra of the all-trans, 9-cis, 13-cis and 15-cis isomers of β-carotene: key bands distinguishing stretched or terminal-bent configurations from central-bent configurations. J Raman Spec. 1988b;9:37–49. [Google Scholar]
- Lers A, Biener Y, Zamir A. Photoinduction of massive β-carotene accumulation by the alga Dunaliella bardawil. Plant Physiol. 1990;93:389–395. doi: 10.1104/pp.93.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Loeblich LA. Photosynthesis and pigments influenced by light intensity and salinity in the halophile Dunaliella salina (Chlorophyta) J Mar Biol Ass UK. 1982;62:493–508. [Google Scholar]
- Mayer MP, Bartlett DL, Beyer P, Kleining H. The in vitro mode of action of bleaching herbicides on the desaturation of 15-cis phytoene and cis-ζ-carotene in isolated daffodil chromoplasts. Pestic Biochem Physiol. 1989;34:111–117. [Google Scholar]
- O'Neil CA, Schwartz SJ. Chromatographic analysis of cis/trans carotenoid isomers. J Chromatogr Biomed Appl. 1992;624:235–252. doi: 10.1016/0021-9673(92)85682-j. [DOI] [PubMed] [Google Scholar]
- Orset S, Young AJ. Low temperature-induced synthesis of α-carotene in the microalga Dunaliella salina. J Phycol. 1999;35:520–527. [Google Scholar]
- Pfander H, Riesen R, Niggli U. HPLC and SFC of carotenoids: scope and limitations. Pure Appl Chem. 1994;66:947–954. [Google Scholar]
- Schierle J, Bretzel W, Faccin N, Hess D, Steiner K, Wurz C, Schuep W. (E/Z)-Isomers of lycopene in processed food and human blood plasma. Abstract, XIth International Symposium on Carotenoids, Leiden, The Netherlands, August 18–23; 1996. [Google Scholar]
- Tsukida K. Separation of isomers of cis-β-carotenes. Methods Enzymol. 1992;213:291–298. [Google Scholar]
- Tsukida K, Saiki K, Takii T, Koyama Y. Separation and determination of cis/trans β-carotene by high performance liquid chromatography. J Chromatogr. 1982;245:359–364. [Google Scholar]
- Young AJ. Occurrence and distribution of carotenoids in photosynthetic systems. In: Young AJ, Britton G, editors. Carotenoids in Photosynthesis. London: Chapman & Hall; 1993. pp. 17–31. [Google Scholar]
- Zamir A. Plant defences against excessive light studied in the microalga Dunaliella. Endeavour. 1995;19:152–156. [Google Scholar]