Abstract
Background
Individuals with alcohol use disorder (AUD) do not always respond to currently available treatments, and evaluation of new candidate pharmacotherapies is indicated. N-acetylcysteine (NAC), an over-the-counter supplement, has shown promise in treating a variety of substance use disorders, but little research has evaluated its merits as a treatment for AUD. This secondary analysis from the National Drug Abuse Treatment Clinical Trials Network examined the effects of NAC versus placebo on alcohol use among participants with cannabis use disorder (CUD) enrolled in a 12-week, multi-site cannabis cessation trial.
Methods
Participants (N=302, ages 18–50) were randomized to double-blind NAC (1200mg, twice daily) or placebo. Neither alcohol use nor desire for alcohol cessation were requirements for participation. Participants that returned for at least one treatment visit and had recorded alcohol use data (i.e., total drinks per week, drinking days per week, and binge drinking days per week) were included in the analysis (n=277).
Results
Compared to the placebo group, participants in the NAC group had increased odds of between-visit alcohol abstinence [OR=1.37; 95% CI=1.06–1.78; p=0.019], fewer drinks per week [RR=0.67; 95% CI=0.48–0.99; p=0.045], and fewer drinking days per week [RR=0.69; 95% CI=0.51–0.92; p=0.014]. Changes in concurrent cannabis use amounts were not correlated to any of the alcohol use variables.
Discussion
These findings indicate that NAC may be effective at reducing consumption of alcohol by ~30% among treatment-seeking adults with CUD, suggesting a need for further trials focused on the effects of NAC on alcohol consumption among individuals seeking treatment for AUD.
Keywords: alcohol, cannabis, marijuana, N-acetylcysteine, medication, treatment
1. INTRODUCTION
N-Acetylcysteine (NAC) is an over-the-counter antioxidant with potential promise as a treatment option for substance use disorders. NAC targets glutamate transporters affected by substance use (McClure et al., 2014; Roberts-Wolfe and Kalivas, 2015), which have been shown to play a role in craving and drug seeking (Kalivas, 2009; Kalivas and Volkow, 2011). Previous trials have demonstrated the potential of NAC in treating substance use disorders, including tobacco (Froeliger et al., 2015; Knackstedt et al., 2009; Van Schooten et al., 2002), cannabis (Gray et al., 2012), and cocaine (LaRowe et al., 2007). NAC may also reduce compulsive behaviors such as pathological gambling (Grant et al., 2007), trichotillomania (Grant et al., 2009), and skin-picking (Grant et al., 2016). Part of the appeal of NAC is its safety and tolerability. NAC has a long history of clinical use as a treatment for acetaminophen overdose, has been FDA approved for adult and pediatric medical use since 1963, and has an established record of being safe and well tolerated (Grandjean et. al., 2000; Gray et. al., 2010; Rhodes and Braakhuis, 2017).
To our knowledge, there are no published, large-scale clinical trials examining NAC as a treatment option for adults with alcohol use disorder (AUD), but animal, adolescent, and pilot adult trials have been promising. In a preclinical trial with NAC, alcohol-consuming rats showed NAC-treated rats reduced their consumption of alcohol by up to 70% compared to rats treated with saline (p < 0.0001)(Quintanilla et al., 2016). Reduced alcohol consumption persisted for up to four days, suggesting enduring effects of NAC on glutamate transmission. A subsequent preclinical trial studying the effects of NAC on alcohol self-administration in rats showed an 81% decrease in alcohol consumption for the NAC-treated group compared to placebo, as well as reduced rates of reacquisition in rats that had been abstinent from alcohol for 17 days (Lebourgeois et al., 2017).
A pilot clinical trial examined the efficacy of NAC for reduction of alcohol and drug craving and posttraumatic stress among Veterans (N=35) with comorbid substance use disorder and trauma (Back et al., 2016). Though an AUD diagnosis was not required for inclusion, 82% of the sample met criteria for an AUD. NAC significantly reduced amount and frequency of alcohol and drug craving relative to the placebo group. However, possibly due to low overall substance use and required initial abstinence prior to treatment start, no group differences in substance use post-treatment were observed. This study suggests that reductions in alcohol use observed in animal models may translate to humans. However, larger studies are needed to determine the effect of NAC on alcohol consumption specifically.
An earlier secondary analysis (Squeglia et al., 2016) examined alcohol use data from a NAC treatment trial for cannabis use disorder (CUD) among adolescents ages 15 to 21 (Gray et al., 2012). In the parent trial, youth randomized to receive NAC had more than double the odds of negative urine cannabinoid tests during treatment compared to the placebo group (Gray et al., 2012). In the secondary analyses examining alcohol use within the parent trial, there was a significant relationship between lowering levels of cannabis use and alcohol use in the NAC-treated group, but not in the placebo group. This was encouraging, as it suggested NAC was able to reduce both alcohol and cannabis use in the treatment group. No “substitution effect” was found, wherein decreased use of one substance correlates with increased use of another (Chaloupka and Laixuthai, 1997; Copersino et al., 2006; Schaub et al., 2010).
The goal of this secondary analysis was to examine the effect of NAC on alcohol use to further gauge the potential of NAC to treat AUD based on promising preclinical (Lebourgeois et al., 2017; Quintanilla et al., 2016) and clinical (Back et al., 2016) findings. The parent study was a twelve-week trial that focused on changes in cannabis use in adults seeking treatment for CUD when treated with NAC compared to placebo (Gray et al., 2017). Unlike the adolescent trial (Gray et al., 2012), the adult study did not find NAC to be effective in reducing cannabis use (Gray et al., 2017). The current study evaluated: (1) the effect of NAC versus placebo on alcohol use over a twelve-week CUD treatment trial and (2) the role of cannabis use (reductions and/or abstinence) on subsequent alcohol use. This is the first exploratory analysis from a randomized treatment trial examining the effects of NAC specifically on adult alcohol use and provides a unique opportunity to explore alcohol use during NAC-assisted CUD treatment.
2. METHODS
2.1 Participants
The parent study participants were 302 adults ages 18–50 who were seeking treatment for cannabis dependence. Participants were recruited from a multisite clinical trial sponsored by the National Drug Abuse Treatment Clinical Trials Network (NIDA CTN) using community/media advertisements (Clinicaltrials.gov: NCT01675661) (Gray et al., 2017). Inclusion criteria included: a positive urine cannabinoid test at screening, Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) diagnosis of cannabis dependence, interest in treatment for cannabis dependence, and, if female, agreement to use birth control. Exclusion criteria included: DSM-IV-TR substance dependence other than cannabis or tobacco, a urine drug test positive for non-cannabinoid substances, synthetic cannabinoid use in the past 30-days, current use of NAC, allergy to NAC, current treatment for substance use, asthma, pregnant or breastfeeding (if female), and any uncontrolled medical or psychiatric illness. The average age of participants was 30 (SD=9), and the sample was 72% male, 58% White, 28% Black or African American (see Table 1). On average, participants were using cannabis almost daily. As this was a cannabis cessation trial, participants were not required to be alcohol users or interested in alcohol cessation to qualify and were excluded from study participation if they met criteria for DSM-IV alcohol dependence. Of the 302 participants, 277 had at least one study visit available for analysis; 207 reported alcohol use in the past 30-days. On average, participants were drinking alcohol once per week and binge drinking less than once per month.
Table 1.
Demographic and use characteristics
| Demographic Characteristics | All Participants (n=277) | NAC (n=142) | Placebo (n=135) |
|---|---|---|---|
| Gender, male, % (n) | 72.2 (200) | 77.5 (110) | 66.7 (90) |
| Age (years) at Randomization, mean (SD) | 30.3 (9.0) | 29.8 (8.8) | 30.8 (9.2) |
| Race | |||
| Caucasian | 57.8 (160) | 54.9 (78) | 60.7 (82) |
| African American | 28.2 (78) | 28.2 (40) | 28.2 (38) |
| Other | 14.1 (39) | 16.9 (24) | 10.6 (15) |
| Baseline Use Characteristics | |||
|
|
|||
| Age at First Alcohol Use* | 16.7 (2.9) | 16.7 (3.0) | 16.7 (2.9) |
| Lifetime history of Alcohol Use % (n) | 98.6 (273) | 100.0 (142) | 97.0 (131) |
| Any Alcohol Use 30 Days Prior to Study % (n) | 74.7 (207) | 75.4 (107) | 74.1 (100) |
| Lifetime Alcohol Abuse % (n) | 22.7 (63) | 22.5 (32) | 22.9 (31) |
| Full Sample: # of Drinks 30 Days Prior to Study | 17.8 (28.4) | 17.6 (29.5) | 18.1 (27.3) |
| Full Sample: # of Drinking Days 30 Days Prior to Study | 4.7 (6.2) | 4.2 (5.8) | 5.1 (6.6) |
| Full Sample: # of Binge Drinking Days 30 Days Prior to Study | 0.3 (1.4) | 0.1 (0.6) | 0.5 (1.9) |
| Baseline Drinkers: # of Drinks 30 Days Prior to Study† | 23.9 (30.6) | 23.3 (32.0) | 24.5 (29.2) |
| Baseline Drinkers: # of Drinking Days 30 Days Prior to Study† | 6.2 (6.4) | 5.6 (6.1) | 6.9 (6.8) |
| Baseline Drinkers: # of Binge Drinking Days 30 Days Prior to Study† | 0.4 (1.6) | 0.2 (0.7) | 0.6 (2.2) |
| Cannabis Use Days 30 Days Prior to Study | 26.0 (6.2) | 26.2 (5.8) | 25.9 (6.6) |
| Cannabis Use Grams 30 Days Prior to Study+ | 69.2 (97.9) | 65.5 (83.3) | 73.0 (111.3) |
| Smokes Cigarettes % (n) | 37.6 (104) | 39.4 (56) | 35.6 (48) |
Note: There were no significant baseline differences between groups with respect to demographics or substance use characteristics.
273 of 277 participants reported any prior lifetime alcohol use.
N=275 (2 participants did not have cannabis gram data)
Baseline drinkers noted as participants reporting any drinks in the past 30 days at study randomization (n=207).
2.2 Procedures and Measures
Detailed procedures and main outcomes from the primary clinical trial have been previously published (Gray et al., 2017; McClure et al., 2014). Participants received abstinence-based contingency management for cannabis use and were randomized to receive either NAC (1200 mg two times per day) or matched placebo for a 12-week duration. No psychosocial treatment targeted alcohol use and no specific instruction to reduce alcohol use was provided. Participants self-reported their substance use and provided urine samples for quantitative cannabinoid testing at an initial screening visit, a pre-treatment visit, weekly study visits, and at a one-month follow-up visit.
2.3 Measures
2.3.1 Substance Use
Quantity and frequency of past 30-day alcohol and cannabis use were assessed at the initial screening visit via the Timeline Follow-Back (TLFB; (Sobell and Sobell, 1992). For alcohol use, participants reported total standard drinks (based on NIAAA guidelines (http://rethinkingdrinking.niaaa.nih.gov/tools/Calculators/drink-size-calculator.aspx) consumed each day. For cannabis, participants reported whether they had used cannabis (yes/no) and the number of joints, blunts, pipes, bowls, vaporizers, spliffs, edibles, or other administration methods used. Using dried motherwort as a proxy for cannabis, participants were asked to weigh on a scale the amount of cannabis they typically used for each administration method (e.g., joints, blunts) in the previous 30-days. This is consistent with a scale-based method used by Mariani and colleagues to estimate grams of cannabis use, with the exception that oregano was used as their proxy substance (Mariani et al., 2011). At weekly and follow-up visits, participants reported daily cannabis use in between visits and the number of joints/blunts/etc. used on days which cannabis use was endorsed. Weekly grams of cannabis used were computed by multiplying the number of joints by the typical grams per joint, number of blunts by typical grams per blunt, and so forth for each method endorsed, and summing the total across methods.
2.3.2 Psychopathology
The Mini International Neuropsychiatric Interview 6.0 (MINI) ascertained current or lifetime history of the major DSM-IV and ICD-10 psychiatric disorders (Sheehan et al., 1998; Sheehan et al., 2010). None of the participants met criteria for alcohol dependence.
3. Outcomes
Total standard drinks consumed, drinking days, and binge drinking days (4 or more drinks for women and 5 or more drinks for men) were calculated at each weekly study visit as the primary alcohol use outcomes. When missing visit data occurred between attended visits, the TLFB summary alcohol use data for the next attended visit were calculated back to the last previously attended visit (3.6% of study visits data). This allowed for the collection of continuous TLFB data even in the presence of missing visit data. To account for the possible variable time frame of data collection between attended visits, all statistical models adjusted for the number of days since the last attended visit. Out of the 302 participants included in this analysis, there were 277 with at least one study visit available for analysis. The average number of attended study visits in this cohort was 10 (SD=3; Range 1– 12) and 61% (170) attended all 12 treatment visits [Placebo 63% (n=85/135) vs. NAC 60% (n=85/142); χ21=0.3, p=0.596].
3.1 Statistical Analysis
Standard descriptive statistics were used to quantify demographic, clinical, and substance use characteristics for the cohort as well as between study randomization groups. A Wilcoxon rank sum test statistic assessed differences among continuous variables at screening while differences in categorical variables were assessed using a Pearson Chi-square test statistic. The effect of NAC versus placebo on secondary abstinence from alcohol use was analyzed over the twelve-week treatment period.
Alcohol use is a two-part correlated process that includes both abstinence and reductions in drinking. Drinking behavior is reported as both the presence (any) and intensity (amount) of drinking at each attended study visit. The reported weekly drinking intensity data contained a preponderance of zeros (standard drinks, drinking days, binge drinking days) across the duration of the study. Thus, these zeros were assumed to be from a mixture of two distinct processes: 1) abstinence from alcohol since the last study visit in the presence of past or current use (sampling zeros) and 2) abstinence from alcohol since the last study visit in a non-alcohol user (structural zeros). Since the zeros likely came from more than one source, mixed effects zero-inflated negative binomial models (ZINB) were chosen as appropriate to estimate both treatment efficacy of NAC and the effect of concurrent cannabis use on alcohol use across all post-baseline visit data. The zero-inflated models extend the Negative Binomial models with the inclusion of a logistic regression component that distinguishes between sampling and structural zeros. The parameter estimates from the Negative Binomial portion of the model assessed the increased or decreased effects of the treatment on the reported weekly number of standard drinks, drinking days, and binge drinking days (He et al., 2014; Lambert, 1992).
In the presented models, the primary model predictors were: randomized treatment assignment (NAC vs. Placebo), baseline levels of drinking intensity (average weekly drinking days, binge drinking days, or total drinks over the 30-days prior to study entry, dependent on the model outcome), age at study entry, week of study visit (Study time), and the number of days since last treatment visit contact. Additional predictors were chosen as those that were associated with each of the alcohol use outcomes, possible effect modifiers, or confounders of the treatment effect (race, sex, concurrent cannabis use amounts, etc.).
Interactions between covariates and the randomized treatment assignment were investigated and noted when significant. These expanded models were used to investigate the effect of cannabis use patterns on drinking behavior during the study. Weekly cannabis use amounts (grams) were independently included in the regression models to assess whether cannabis use amounts were associated with alcohol use patterns. Treatment interactions with model covariates were independently added to the adjusted models. Of the 277 participants included in the primary study/outcome analysis, 275 (99.3%) had cannabis use data at the gram level and were included in the analysis assessment of the association between co-occurring use of cannabis and alcohol; 2 participants had missing gram amount information, one participant attended only 1 treatment visit, and another completed the treatment protocol, and both were randomized to NAC study treatment. Although not specifically powered to detect interactions of interest at p<0.05, those that reached a p<0.15 level were further stratified by treatment assignment to investigate possible treatment effect modification. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
4. RESULTS
4.1 Study Characteristics and Baseline Associations
Of the 302 participants randomized in the study, 277 (92%) returned for at least one study treatment visit and had recorded alcohol use data (even if abstinent from alcohol) and were included in the longitudinal analysis. Of those 277, 142 were randomized to receive NAC and 135 to receive placebo; 2,786 of the possible 3,324 (84%) weekly study visits had alcohol use and abstinence data available (NAC: 1,409 vs. placebo: 1,377), and each attended visit was, on average, 7.0 (SD=1.3) days apart (max=13 days). Overall, participants attended a mean of 10.0 of the 12 possible weekly visits (SD=3.4; Median=12.0) with the NAC group attending a mean of 9.9 (SD=3.5; Median=12.0) study visits and the placebo group attending a mean of 10.2 (SD=3.2; Median=12.0; group difference z=0.64, p=0.525) study visits. Demographic, psychiatric, and use characteristics between study groups are presented in Table 1. Out of the 277 participants with study data available, 273 (99%) reported lifetime alcohol use and 207 (75%) reported at least one drink during the 30-days prior to study entry; 107 (75%) in the NAC group and 100 (74%) in the placebo group (χ21= 0.06; p=0.807). Similar to the overall study cohort, there was a slightly greater proportion of male participants in the group randomized to NAC as compared to placebo (χ21= 4.0; p=0.045). There were no differences between study groups with respect to age, race, or other substance use characteristics.
4.2 Efficacy of N-Acetylcysteine on Drinking Outcomes: Main Effects
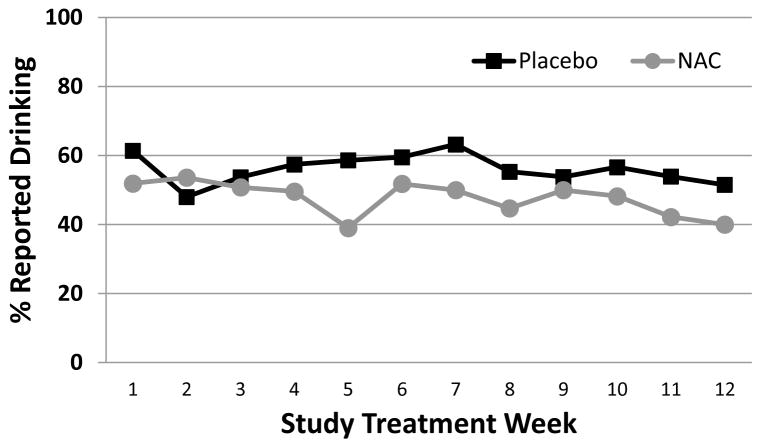
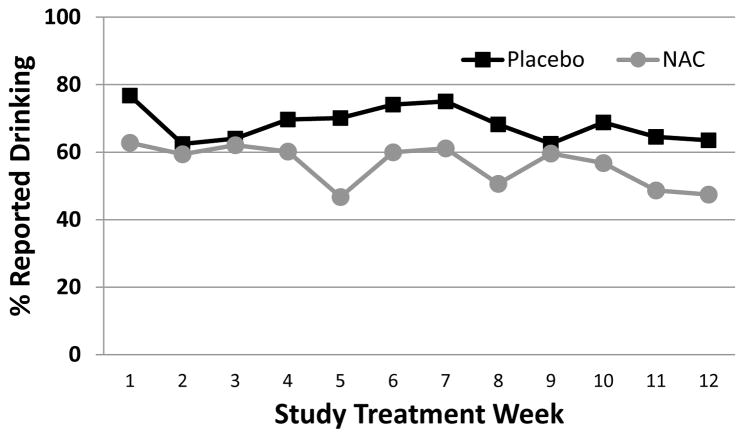
Percentages of abstinence from drinking/binge drinking as well as mean total standard drinks, mean drinking days, and binge drinking days between each of the study visits in those that failed to achieve abstinence from drinking/binge drinking between visits are listed in Table 2. During the study, participants randomized to receive treatment with NAC had increased odds of weekly alcohol abstinence relative to placebo [any drinks: Adjusted OR=1.37 (95% CI=1.06–1.78), p=0.019; Figure 1] and fewer weekly drinks [total Weekly Drinks: Adjusted RR=0.67 (95% CI=0.48–0.99), p=0.045]. Similarly, participants randomized to receive treatment with NAC reported fewer drinking days between each visit as compared to those who received placebo [drinking days: Adjusted RR=0.69 (95% CI=0.51–0.92), p=0.014]. Although there was a treatment group difference with the expected total number of drinks and drinking days reported between visits, NAC did not affect the rate of binge drinking days [number of binge drinking days: RR=0.77 (0.53–1.13), p=0.186]. Additional analysis of the subgroup that reported drinking during the 30-days prior to study treatment (207/277) was consistent with results from the full cohort (See Figure 2).
Table 2.
Zero-Inflated Negative Binomial (ZINB) Outcomes for alcohol use taken at study treatment visits (n=277 participants).
| Outcome | Placebo
|
NAC
|
Odds RatioƗ (95% CI) | Rate Ratio╪ (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| % Abstinent | Nonzero M | SD | % Abstinent | Nonzero M | SD | |||
| Total Weekly Drinks | 43.9% | 8.7 | 9.0 | 52.2% | 8.2 | 9.2 | 1.37 (1.06–1.78) * | 0.67 (0.48–0.99) * |
| Weekly Drinking Days | 43.9% | 2.4 | 1.7 | 52.2% | 2.3 | 1.7 | 1.37 (1.06–1.78) * | 0.69 (0.51–0.92) * |
| Weekly Binge Drinking Days | 78.2% | 1.6 | 1.0 | 80.7% | 1.5 | 0.9 | 1.25 (0.82–1.90) | 0.77 (0.53–1.13) |
The odds ratio (OR) represents the odds of reported abstinence from drinking outcomes at treatment study visits in the NAC group as compared to Placebo and is derived from the adjusted logit portion of the ZINB model.
The rate ratio (RR) represents the decreased risk of drinking outcome rates between study treatment visits in the NAC group as compared to Placebo and is derived from the negative binomial portion of the ZINB model.
p<0.05
OR and RR results are shown adjusted for visit number, baseline drinking, days since last visit, and age. Means and percentages are calculated from reported TLFB data are represented as unadjusted raw values taken during study treatment visits.
Figure 1.
Percentage of participants reporting alcohol use during treatment among all participants (n=277). Data are shown as raw weekly proportions of those that report no drinking at each visit since the last visit.
Figure 2.
Percentage of participants reporting alcohol use during treatment among participants who endorsed alcohol use in the 30 days before treatment (n=207). Data are shown as raw weekly proportions of those that report no drinking at each visit since the last visit.
Models were additionally investigated to test the modifying effect of age, sex, and race on treatment efficacy. There were no significant differences in the number of standard weekly drinks, drinking days, or binge drinking days reported across age, race, or gender (p>0.15).
4.3 Other Predictors of Drinking Behavior
Greater baseline drinking [Total Drinks: RR=4.46 (2.76–7.23), p<0.001; Drinking Days: RR=6.33 (4.35–9.21), p<0.001; Binge Drinking Days: RR=4.10 (2.45–6.87), p<0.001] and increased number of days since last visit contact [Total Drinks: RR=1.13 (1.10–1.17), p<0.001; Drinking Days: RR=1.15 (1.12–1.18), p<0.001; Binge Drinking Days: RR=1.14 (1.09–1.20), p<0.001] were the greatest predictors of increased drinking behavior during the treatment portion of the study. Younger age at study entry was also associated with increased drinking during the treatment portion of the study [Total Drinks: RR=0.95 (0.93–0.97), p=0.001; Drinking Days: RR=0.98 (0.96–1.00), p=0.017; Binge Drinking Days: RR=0.92 (0.90–0.94), p<0.001].
4.4 The Relationship between Cannabis Use and Drinking Outcomes: Interactive Effects
It was also hypothesized that cannabis use patterns would be associated with drinking behavior. Concurrent cannabis use (grams per week) was added to the count portion of the model to assess if the recent use was associated with greater concurrent self-reported alcohol consumption. Concurrent cannabis use amounts were not associated with weekly reported total drinks [RR=1.03 (0.99–1.08), p=0.132], drinking days [RR=1.00 (0.96–1.05), p=0.870], or binge drinking days between visits [RR=1.01 (0.91–1.11), p=0.833].
5. DISCUSSION
This secondary analysis examined alcohol use in a sample of cannabis-dependent adults (ages 18–50) enrolled in a medication-assisted cannabis cessation trial (Gray et al., 2017). Despite prior promising findings in adolescents (Gray et al., 2012), NAC was not found to be efficacious in reducing cannabis use in an adult sample (Gray et al., 2017). However, secondary analyses suggest participants who were randomized to NAC versus placebo significantly reduced their alcohol use during treatment, even after controlling for baseline level of drinking. The increase in the odds of complete abstinence from alcohol was 37% in the NAC group. Not only did those treated with NAC have increased odds of abstinence but also an attenuated risk of drinking when they were at risk of non-abstinence. Although the benefits were moderate, the risk of an increased number of standard drinks was 33% less and drinking days was 31% less in the NAC group as compared to placebo, when adjusting for covariates. NAC did not affect the number of binge drinking days; however, participants were binge drinking, on average, less than one time per month, so a significant decrease may be hard to detect in this sample. Age, sex, and race did not affect findings.
There are notable differences between the current results and alcohol reduction/abstinence results of the NAC adolescent trial. In previous secondary analyses from the original NAC adolescent trial (Gray et al., 2012), alcohol reductions were associated with cannabis reductions in the NAC group (Squeglia et al., 2016). The current findings are more direct, in that, regardless of cannabis use, adults randomized to the NAC group showed reduced alcohol use when compared to the placebo group. It is possible that adolescents and adults with CUD differ in alcohol-cannabis co-use patterns, and perhaps alcohol and cannabis use are distinct and maintained by different variables and contexts among adults. In both the adolescent and adult trials, no evidence of compensatory alcohol use was found during cannabis cessation treatment, which is consistent with previous tobacco findings from the same parent trial (McClure et al., 2014). In fact, in both studies, NAC-treated participants reduced their alcohol use. Taken together, it appears that NAC may be a promising candidate medication for AUD and possibly co-occurring alcohol and cannabis use.
These findings are considered in the context of promising preclinical NAC alcohol findings. Two recent studies found that rats who were treated with NAC reduced self-administered alcohol intake between 70% (Quintanilla et al., 2016) and 81% (Lebourgeois et al., 2017) compared to placebo-treated rats. Additional preclinical data suggests NAC may also be useful for alcohol withdrawal (Schneider et al., 2015), and abstinence before initiating NAC may further improve alcohol-related outcomes (Lebourgeois et al., 2017). The only published human studies specifically examining alcohol use reduction have been in the context of cannabis cessation trials. A pilot trial with a general substance use disorder sample (with a high rate of AUD) also suggested that NAC may be a promising treatment for AUD through craving reduction (Back et al., 2016). NAC did not reduce alcohol use relative to placebo in the pilot trial; however, 7-day abstinence was required prior to initiation of treatment. In the current study, no specific instruction about alcohol reduction was provided, yet those who received NAC reduced their use. Fully-powered clinical trials focused on youth and adults with AUD are warranted.
Limitations to the current findings exist. First, participants were recruited specifically for their cannabis use, and none met criteria for alcohol dependence. Participants were drinking, on average, once per week, with less than one binge drinking episode in the past month. Significant, phase-like neurochemical changes occur with the progression of AUD (Koob and Volkow, 2016), so it is necessary to assess whether NAC is effective at more problematic levels of alcohol use. Existing preclinical research suggests that NAC may be more effective at later stages of addiction after chronic drug exposure, as this is when extracellular increases in glutamate are often observed (Koob and Volkow, 2016; Spencer and Kalivas, 2017). However, there are multiple proposed mechanisms via which NAC may reduce substance use (Spencer and Kalivas, 2017) and the current data with lighter drinkers may suggest that NAC’s effect on drug seeking can occur earlier in the addiction process. More research is needed to establish when in the progression of AUD NAC may be most effective. Second, alcohol was assessed via self-report. Though there were no incentives to underreport alcohol use, reports were still subject to retrospective recall biases. Future studies should obtain biomarkers of alcohol consumption. Third, medication adherence for the overall trial was low at 55% (Gray et al., 2017); however, that suggests current findings may underestimate the effect of NAC on alcohol use.
This study, in the context of recent preclinical and clinical findings, suggests NAC may be a promising candidate pharmacotherapy for alcohol use. Further studies are needed in individuals with AUD.
Highlights.
N-acetylcysteine (NAC) is a promising target medication for treating addiction.
Cannabis-users in the NAC group reduced their alcohol use by ~30% compared to placebo.
More research is warranted to understand the effect of NAC on alcohol use disorder.
Acknowledgments
Role of Funding Source
The authors wish to acknowledge the funding sources for this study. Funding was provided by NIAAA (K23 AA025399) and NIDA (UG1 DA013727, R01 DA042114, U01 DA031779, K01DA036739). Additional funding was provided by the South Carolina Clinical and Translational Institute at the Medical University of South Carolina (UL1TR000062). These funding sources had no further role in study design, the collection, analysis, or interpretation of the data, writing of the report, or in the decision to submit the paper for publication.
Footnotes
Contributors
LS, RT, EM, and KG developed the study concept and design. NB performed the data analyses. LS, NB, and RT drafted the initial manuscript. GB performed the literature review and assisted with writing the Introduction. All authors provided critical manuscript review. All authors approved the final version of the manuscript for submission.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, Gray KM, Hamner MB, DeSantis SM, Malcolm R, Kalivas PW. A double-blind, randomized, controlled pilot trial of N-acetylcysteine in Veterans with posttraumatic stress disorder and substance use disorders. J Clin Psychiatry. 2016;77:e1439–e1446. doi: 10.4088/JCP.15m10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloupka FJ, Laixuthai A. Do youths substitute alcohol and marijuana? Some econometric evidence. East Econ J. 1997;23:253–276. [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Quitting among non-treatment-seeking marijuana users: Reasons and changes in other substance use. Am J Addict. 2006;15:297–302. doi: 10.1080/10550490600754341. [DOI] [PubMed] [Google Scholar]
- Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI study. Drug Alcohol Depend. 2015;156:234–242. doi: 10.1016/j.drugalcdep.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean EM, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: A meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Redden SA, Leppink EW, Odlaug BL, Kim SW. N-Acetylcysteine in the treatment of excoriation disorder: A randomized clinical trial. JAMA Psychiatry. 2016;73:490–496. doi: 10.1001/jamapsychiatry.2016.0060. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: A pilot study. Biol Psychiatry. 2007;62:652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: A double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae-Clark AL, Carroll KM, Potter JS, Wiest K, Mooney LJ, Hasson A, Walsh SL, Lofwall MR, Babalonis S, Lindbald RW, Sparenborg S, Wahle A, King JS, Baker NL, Tomko RL, Haynes LF, Vandrey RG, Levin FR. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249–257. doi: 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Carpenter MJ, Larowe SD. N-acetylcysteine (NAC) in young marijuana users: An open-label pilot study. Am J Addict. 2010;19:187–189. doi: 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Tang W, Wang W, Crits-Christoph P. Structural zeroes and zero-inflated models. Shanghai Arch Psychiatry. 2014;26:236–242. doi: 10.3969/j.issn.1002-0829.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. Zero-inflated poisson regression, with an application to defects in manufacturing. Technometrics. 1992;34:1–14. [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- Lebourgeois S, González-Marín MC, Jeanblanc J, Naassila M, Vilpoux C. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol. 2017 doi: 10.1111/adb.12521. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Brooks D, Haney M, Levin FR. Quantification and comparison of marijuana smoking practices: Blunts, joints, and pipes. Drug Alcohol Depend. 2011;113:249–251. doi: 10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. Am J Drug Alcohol Abuse. 2014;40:285–291. doi: 10.3109/00952990.2013.878718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs. 2014;28:95–106. doi: 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Sonne SC, Winhusen T, Carroll KM, Ghitza UE, McRae-Clark AL, Matthews AG, Sharma G, Van Veldhuisen P, Vandrey RG, Levin FR, Weiss RD, Lindblad R, Allen C, Mooney LJ, Haynes L, Brigham GS, Sparenborg S, Hasson AL, Gray KM. Achieving cannabis cessation -- evaluating N-acetylcysteine treatment (ACCENT): Design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials Commun. 2014;39:211–223. doi: 10.1016/j.cct.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Rivera-Meza M, Berríos-Cárcamo P, Salinas-Luypaert C, Herrera-Marschitz M, Israel Y. Beyond the “First Hit”: Marked inhibition by N-acetyl cysteine of chronic ethanol intake but not of early ethanol intake. Parallel effects on ethanol-induced saccharin motivation. Alcohol Clin Exp Res. 2016;40:1044–1051. doi: 10.1111/acer.13031. [DOI] [PubMed] [Google Scholar]
- Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: A systematic review and meta-analysis. Sports Med. 2017;47:1619–1636. doi: 10.1007/s40279-017-0677-3. [DOI] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14:745–756. doi: 10.2174/1871527314666150529144655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M, Gmel G, Annaheim B, Mueller M, Schwappach D. Leisure time activities that predict initiation, progression and reduction of cannabis use: A prospective, population-based panel survey. Drug Alcohol Rev. 2010;29:378–384. doi: 10.1111/j.1465-3362.2009.00156.x. [DOI] [PubMed] [Google Scholar]
- Schneider RJ, Santos CF, Clarimundo V, Dalmaz C, Elisabetsky E, Gomez R. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol. 2015;49:259–263. doi: 10.1016/j.alcohol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:S22–S33. [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, Milo KM, Stock SL, Wilkinson B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Spencer S, Kalivas PW. Glutamate transport: A new bench to bedside mechanism for treating drug abuse. Int J Neuropsychopharmacol. 2017;20:797–812. doi: 10.1093/ijnp/pyx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Baker NL, McClure EA, Tomko RL, Adisetiyo V, Gray KM. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172–177. doi: 10.1016/j.addbeh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schooten FJ, Besaratinia A, De Flora S, D’Agostini F, Izzotti A, Camoirano A, Balm AJ, Dallinga JW, Bast A, Haenen GR, Van’t Veer L, Baas P, Sakai H, Van Zandwijk N. Effects of oral administration of N-acetyl-L-cysteine: A multi-biomarker study in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:167–175. [PubMed] [Google Scholar]