Abstract
Background
While the notion that smokers reliably show higher reactivity to cigarette-related versus neutral cues is both theoretically and empirically supported, it is unclear why never-smokers also show enhanced brain responses to cigarette-related cues.
Methods
Using a repetitive picture viewing paradigm, in which responses evoked by affective cues are more resistant to habituation, we assessed the effects of stimulus repetition on event-related potentials (ERPs) evoked by pleasant, unpleasant, cigarette-related, and neutral images in 34 smokers (SMO) and 34 never-smokers (NEV). We examined the early posterior negativity (EPN) and the late positive potential (LPP), two ERP components which are sensitive to a picture's motivational qualities.
Results
Before stimulus repetition, pleasant, unpleasant, and cigarette-related cues produced greater EPN and LPP amplitudes than neutral cues in all subjects. During stimulus repetition, both components were similarly modulated by emotional arousal, such that pleasant, unpleasant, and cigarette-related cues evoked greater EPN and LPP amplitude, relative to neutral. Smoking status did not modulate these effects. While there were no group differences in self-reported stimulus ratings of valence for pleasant, unpleasant, or neutral stimuli, NEV rated cigarette-related cues as unpleasant. We observed a moderate, negative correlation between LPP amplitude and self-reported valence ratings of cigarette-related cues among NEV.
Conclusions
These data suggest that cigarette-related cues capture attentional resources of both SMO and NEV, but for different reasons. For SMO, cigarette-related cues have acquired motivational significance through repeated associations with nicotine delivery, whereas for NEV, cigarette-related cues are perceived as unpleasant.
Keywords: Event-related potentials, repetition, addiction, smoking
1. Introduction
Neurobiological models of drug dependence propose that cues preceding drug delivery can acquire motivational properties through associative learning processes (Koob and Volkow, 2010; Robbins and Everitt, 1996; Robinson and Berridge, 1993). In fact, smokers report that the presence of cigarette-related cues (e.g., ashtrays, other people smoking) is sufficient to induce cravings and spur compulsive smoking (Shiffman et al., 2007; Stewart, 2008). Neurophysiological measures support the idea that for smokers, cigarette-related cues are motivationally relevant cues that attract attention. Cigarette-related cues increase the amplitude of both the early posterior negativity (EPN) and the late positive potential (LPP), two components of the event-related potential (ERP) reflecting both the engagement of attentional resources by emotional stimuli and the activation of cortico-limbic appetitive and defensive systems (Littel et al., 2012; Versace et al., 2011; Codispoti et al., 2016). However, recent studies have reported that never-smokers also show enhanced brain responses to cigarette-related cues relative to neutral (e.g., Deweese et al. 2016; Littel et al. 2012; McDonough and Warren 2001; Minnix et al. 2013; Robinson et al. 2015; Oliver et al. 2016). Since never-smokers have not experienced the effects of nicotine, researchers hypothesized that reactivity to cigarette-related cues for never-smokers might be driven by an overall more negative perception of smoking (i.e., a top-down driven process), rather than by the motivational relevance of the cigarette-related stimuli (Yiend, 2010; Robinson et al., 2015). In fact, both cognitive (top-down) and affective (bottom-up) processes can have similar effects on brain responses evoked by natural scenes and increase the amplitude of the late positive potential (LPP) over centro-parietal sensors (Ferrari et al., 2008). Codispoti and colleagues (2006) employed a repetitive picture viewing paradigm, in which a small set of images (e.g., 1 pleasant, 1 unpleasant, 1 neutral) is repeated many times, to disentangle the effects that cognitive and affective processes exert on ERPs. An advantage of this paradigm is that repeated presentation reduces stimulus novelty (Öhman,1992; Siddle and Spinks, 1992; Kahneman, 1973), which may allow for motivational effects to emerge more clearly. Several studies consistently showed that even after massive repetition, pleasant and unpleasant cues continue to elicit larger EPNs and LPPs compared to neutral ones, a result suggesting that, also when the images are no longer novel, affectively engaging pictures to continue to activate mental representations with strong associations to motivational circuits (Bradley et al., 2006; Codispoti et al., 2007; Ferrari et al., 2011; Ferrari et al., 2017; Mastria et al., 2017).
In the present study, we used this same paradigm to investigate the extent to which top-down and bottom-up processes influence reactivity to cigarette-related cues in smokers and never-smokers. We examined the effects of stimulus repetition on the amplitude of the EPN and LPP components, and whether smoking status modulated any observed differences among a group of 34 smokers and 34 never-smokers. For never-smokers, in particular, this paradigm allows us to assess whether cigarette-related cues continue to elicit enhanced ERP amplitude (relative to neutral) similar to that of other motivationally salient cues (bottom-up), or whether these cues habituate as they lose novelty and salience as a function of stimulus repetition (top-down). Existing cue-reactivity studies assessing reactivity to cigarette-related cues among smokers and never-smokers were not designed to test whether enhanced responses to cigarette-related cues were due to the motivational significance of the stimulus or to stimulus novelty (e.g., Littel et al. 2012; Minnix et al. 2013; Deweese et al. 2016). Thus, the repetition paradigm will allow us to explore the cognitive processes underlying the amplitude and modulation of the EPN and LPP by emotional arousal during picture viewing (Codispoti et al., 2007).
We expected pleasant, unpleasant, and cigarette-related stimuli to produce larger EPNs and LPPs relative to neutral in both smokers and never-smokers when presented for the first time. Following stimulus repetition, we expected emotional pictures (pleasant, unpleasant) to produce larger EPNs and LPPs relative to neutral in both smokers and never-smokers. We hypothesized that only smokers would maintain enhanced brain responses to cigarette-related cues, relative to neutral. This finding would indicate that cigarette-related cues are motivationally salient stimuli only for smokers, as attenuation of ERP amplitude to cigarette-related cues across blocks in the repetition phase for never-smokers could reflect changes in resource allocation as the pictures become less novel with repetition. In the reinstated novel phase, we expected full recovery of all habituated responses, except for the EPN and LPP evoked by cigarette-related cues in never-smokers, as these cues should have limited motivational significance for this group.
2. Material and Methods
2.1 Participants
We recruited 86 participants from the Houston metropolitan area using radio and newspaper advertisements. Participants were eligible for the study if they were: between 18 and 65 years of age, were fluent in English, had access to a working telephone, were neither pregnant nor breastfeeding, were not currently enrolled in a formal smoking-cessation activity, did not report any history of psychiatric disorders, substance abuse disorder, history of seizures or seizure disorder, head injuries with a loss of consciousness, uncorrected visual or auditory impairments or use of any non-cigarette tobacco products (e.g., pipe tobacco, cigars, snuff, chewing tobacco, hookah), or be unwilling to change hairstyle (e.g., braids, pony tails, or dread locks) or remove a wig to accommodate application of the EEG net. Smoking participants had to report a baseline expired carbon monoxide (CO) at least six-parts per million (ppm) and reported smoking 5 or more cigarettes per day for the last six-months. To be eligible for the never-smoker group, participants must have smoked less than 100 cigarettes in their lifetime (Bondy et al., 2009) and produce a baseline expired CO less than 4 ppm. All participants received monetary compensation for their time and parking/travel, totaling $60.
2.2 Procedures
The procedures were approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. All participants were initially screened in a 30-minute telephone interview to establish initial eligibility for the study. Eligible participants were then invited to attend an in-person visit, where a trained member of the staff explained the study procedures to the potential participants and collected written, informed consent. Biochemical verification of smoking status was assessed by measuring expired carbon monoxide levels, and participants completed questionnaires regarding demographics, medical, mood, and smoking history. After questionnaire completion, participants then completed the EEG recording session (see Stimuli and experimental paradigm).
2.3 Self-report Measures
For smokers, nicotine dependence was measured using the Fagerström Test for Nicotine Dependence (FTND), a 6-item questionnaire that assesses various components of smoking behavior such as daily intake and time to the first cigarette after waking (Heatherton et al., 1991). In all participants, affect was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item self-report measure developed to assess depressive symptoms in community (non-clinical) populations (Ross and Mirowsky, 1984), as well as the Positive and Negative Affect Scale (PANAS; Watson et al., 1988), comprised of two 10-item mood scales, Positive Affect (PA) and Negative Affect (NA), rated on a scale of 1-5. One participant chose not to respond to self-report measures; thus, data from 33 smokers and 34 never-smokers were included in the final demographic and questionnaire analysis.
2.4 Stimuli and Experimental Paradigm
Stimuli were presented with a PC using E-Prime software (version 2.0.8.74; PST Inc., Pittsburgh, PA) on a 42-inch high-definition plasma screen approximately 2.25 meters from the participant's eyes. From this distance, the stimuli subtended a visual angle of 21 degrees. Picture stimuli consisted of 48 pleasant (PLE; 24 erotica and 24 romance), 48 cigarette-related (CIG; people smoking), 48 neutral (NEU; neutral people), and 48 unpleasant (UNP; 24 mutilation and 24 attack) images selected in part from the International Affective Picture System (Lang et al., 2005), the International Smoking Image Series (Gilbert and Rabinovich, 1999), the Emotional Picture Set (Wessa et al., 2010) and other images previously used in our laboratory (Deweese et al., 2016; Minnix et al., 2013; Robinson et al., 2015). Because the LPP varies with emotional arousal (Schupp et al., 2004b), only high-arousing PLE (e.g., erotica; ERO) and UNP (e.g., mutilations; MUT) images were used in the repetition phase.
The repetitive picture-viewing paradigm was modified from Codispoti et al., 2006, and consisted of three phases: an initial novel phase (block 1), a repetition phase (blocks 2, 3, 4) and a reinstated novel phase (block 5; see Figure 1). In all blocks, stimuli were presented for 2 seconds, followed by a variable (2-3 second) inter-trial interval during which a white fixation cross appeared on a black screen. In block 1, the initial novel phase, 96 unique images (24 PLE, 24 CIG, 24 NEU, and 24 UNP) were presented with no stimulus repetition. In blocks 2, 3, and 4, the repetition phase, the last image from each stimulus category presented in block 1 was repeated a total of 24 times, for a total of 96 trials per block. Thus, the same 4 stimuli (1 ERO, 1 CIG, 1 NEU, and 1 MUT) were repeated 24 times each in blocks 2, 3, and 4. In block 5, the reinstated novel phase, a previously unseen set of 96 images (24 PLE, 24 CIG, 24 NEU, and 24 UNP) was presented with no stimulus repetition. Stimulus presentation was pseudo-randomized such that no more than two images of the same category were presented consecutively. Images repeated in the repetition phase were counterbalanced across participants.
Figure 1.
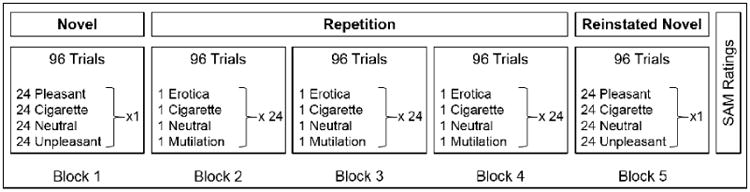
Schematic diagram depicting the sequence of events in the study.
Following the experimental session, participants were asked to rate a subset of the images presented during the experiment using a computerized version of the Self-Assessment Manikin (SAM; Lang, 1980) on the dimensions of affective valence and arousal. The total duration of the EEG session, including SAM ratings, was approximately 50 minutes.
2.5 ERP Recording
During the picture presentation, we recorded EEG using a high-input impedance (200 MΩ) 129-channel Geodesic Sensor System (Geodesic EEG System 200; Electrical Geodesics, Inc., Eugene, OR) and referenced to Cz. We used a sampling rate of 250 Hz, and data were filtered online by using 0.1 Hz high-pass and 100 Hz low-pass filters. Scalp impedances were kept below 70 KΩ, as suggested by the manufacturer.
2.6 ERP Scoring
Following the EEG recording session, a 60-Hz low-pass filter was applied off-line. Data were visually inspected, and channels contaminated by artifacts for more than 50% of the recording were interpolated with use of spherical splines. On average, approximately 3.4% of the channels met this criterion and were interpolated. Eye blinks were corrected using a spatial filtering method implemented in BESA version 5.1.8.10 (MEGIS Software GmbH, Gräfelfing, Germany), and the EEG data were then transformed to the average reference. The Brain Vision Analyzer (version 2.0.2; Brain Products GmbH, Munich, Germany) software program was used to extract single epochs from the continuous EEG signal. The data were segmented into 1100 ms segments, beginning 100 ms before the onset of the image, and baseline was defined as the 100 ms interval preceding picture onset. Using the segmented data, artifacts affecting sensors within specific trials were identified by the following criteria: EEG amplitude above 100 or below −100 μV; absolute voltage difference between any two data points within the segment larger than 100 μV; voltage difference between two contiguous data points above 25 μV and less than 0.5 μV variation for more than 100 ms. A segment was excluded from the subsequent averages if more than 10% of the sensors within the segment were contaminated by artifacts. The average number of trials for the EPN and LPP components by phase and condition are presented in Table 2. There were no significant differences among the number of trials retained per condition during the novel (EPN: p=.4; LPP: p=.3) or repetition (EPN: p=.1; LPP: p=.08) phases.
Table 2.
Number of trials per condition for the EPN and LPP components during the Novel and Repetition phases, for each picture type.
| Novel | Repetition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| PLE | CIG | NEU | UNP | PLE | CIG | NEU | UNP | ||
|
|
|||||||||
| EPN | Average | 19.38 | 19.66 | 19.35 | 19.27 | 18.67 | 19.08 | 18.71 | 19.15 |
| Percent | 80.80% | 81.90% | 80.60% | 80.30% | 77.80% | 79.50% | 78% | 79.80% | |
| Maximum | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | |
| Minimum | 7 | 7 | 9 | 5 | 6 | 6 | 8 | 7 | |
| SD | 4.08 | 3.88 | 3.72 | 4.11 | 4.03 | 3.83 | 3.76 | 3.81 | |
| LPP | Average | 19.19 | 19.53 | 19.16 | 19.09 | 18.57 | 19.01 | 18.62 | 19.12 |
| Percent | 80% | 81.40% | 79.80% | 79.50% | 77.40% | 79.60% | 77.60% | 79.70% | |
| Maximum | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | |
| Minimum | 7 | 7 | 9 | 5 | 6 | 6 | 8 | 7 | |
| SD | 4.06 | 3.86 | 3.68 | 4.03 | 4.01 | 3.81 | 3.73 | 3.84 | |
|
|
|||||||||
Due to poor recording quality, largely attributed to excessive movement, eighteen participants were excluded from further analysis. Laboratory data from 34 smoking (SMO) and 34 never-smoking (NEV) participants were included in the final EEG analyses.
2.7 Statistical Analysis
To identify time regions and sensors (within time regions) to include in our ERP analyses, we used a functional localizer approach, which accounts for between-subject differences in latency and scalp distribution (Luck and Gaspelin, 2017). First, we calculated the mean global field power (GFP, the sum of the squared potential differences of all 129 sensors (Lehmann and Skrandies, 1980), for each time point for each picture type for each participant, separately for each of the 5 blocks. Then, analyzed variance (ANOVA) to test for the main effect of Picture Type (four levels: pleasant, cigarette-related, neutral, and unpleasant) on each time point, separately by block. If the F value observed at a given time point was significant at the p<.05 level (F[3, 201] = 2.65) in each of the 5 blocks, that time point was included in a temporal region of interest (ROI; Figure 2A). Within each temporal ROI, separately by block, we computed the mean voltage at each electrode for the four picture conditions. We used these values to conduct an ANOVA to test for the main effect of Picture Type at each sensor, separately by block. If the F value observed for a given electrode was significant at the p<.05 level in each of the 5 blocks, that electrode was included in the spatial ROI (Figure 2B).
Figure 2.
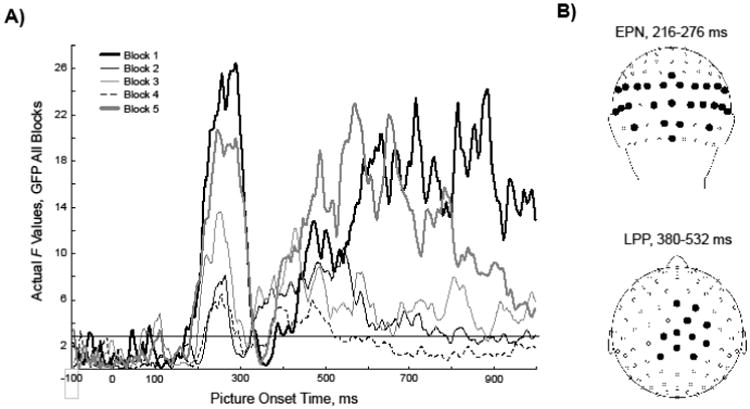
A) F values for the main effect of Picture Type on the global field power at each time point, plotted separately for each block. The dotted line indicates the F value corresponding to p<.05 (F=2.65). B) Filled circles indicate electrodes included in the EPN (top) and LPP (lower) spatial regions of interest.
This approach identified two segments of time in which cortical activity was differentially modulated by picture type: a segment extending from 216 to 276 ms post-picture onset and another from 380 to 532 ms post-picture onset. The 216-276 ms time window, which we refer to as the early posterior negativity (EPN) component of the ERP, included 26 electrodes in three spatially distinct clusters: (left) 50, 51, 52, 57, 58, 59, 60, 64, (center), 70, 71, 72, 74, 75, 76, 77, 81, 82, 83, 84, 85 and (right) 91, 92, 95, 96, 97, 100, and 101 (Figure 2B). Left, right, and center EPN clusters did not differ statistically; thus, results are presented as an average of all sensors. The 380-532 ms time window, which we refer to as the late positive potential (LPP), included 11 centro-parietal electrodes: 6, 31, 54, 55, 79, 80, 87, 105, 106, 112, and 129 (Figure 2B).
As a first step, we sought to confirm that the presentation of emotionally arousing (including cigarette) cues produced larger EPN and LPP amplitudes, relative to neutral, in the initial novel block. Then, to test whether smoking status modulated ERPs to picture type as a function of stimulus repetition, we examined the significance of the interaction Group (NEV, SMO) by Picture Type (PLE, CIG, NEU, UNP) by Block (2, 3, 4). Finally, to determine whether smoking status affected recovery of habituated responses following stimulus repetition, we tested the significance of the interaction group (NEV, SMO), by Picture Type (ERO, CIG, NEU, UNP) by Block (4, 5). The above statistical tests were computed separately for the LPP and EPN components, and where appropriate, significant effects were further evaluated using Bonferroni error corrected pairwise comparisons (Luck and Gaspelin, 2017).
3. Results
3.1 Participant Characteristics
Participant characteristics for smokers and never-smokers are presented in Table 1. Smokers did not differ from never-smokers in racial composition (p=.8), but included significantly more males (p<.0003) and were, on average, 6.6 years older (p<.04) than the never-smoking sample. After controlling for age and gender, smokers had significantly lower PANAS Positive Affect scores (p<.003) and significantly higher CES-D scores (p<.001), indicating that smokers were reporting less positive affect and more depressive symptoms, relative to never-smokers. There were no group differences in PANAS Negative Affect scores (p=.7).
Table 1.
Demographic and smoking characteristics.
| Never-smokers n=34 | Smokers n=34 | |
|---|---|---|
| Gender | %(N) | %(N) |
|
|
||
| Male | 47(16) | 87(28)* |
| Race | ||
| African American, non-Hispanic | 32.4(11) | 39.4(13) |
| White, non-hispanic | 52.9(18) | 48.5(16) |
| Other | 14.7(5) | 9.1(3) |
| Mean (SE) | Mean (SE) | |
|
|
||
| Age | 40.44(2.22)* | 47.03(2.29) |
| Cigarettes/day | -- | 19.13(12.35) |
| FTNDa | -- | 4.66(2.15) |
| PANAS Positive Affectb | 37.93(1.09) | 32.75(1.12)* |
| PANAS Negative Affectb | 14.94(0.83) | 15.38(0.86) |
| CES-Dc | 5.5(0.66) | 8.625 (0.68)* |
p<.05
Fagerström test for nicotine dependence
Positive and Negative Affect Scale
Center for Epidemiologic Studies Depression Scale
3.2 EPN Component (216 – 276 ms)
3.2.1 Novel phase
When shown for the first time, PLE, UNP, and CIG stimuli evoked significantly greater negativity than NEU (all ps<.0001, ; Figure 3A). PLE was significantly more negative than both CIG and UNP cues (all ps<.0001, ; CIG and MUT did not differ (p>.3).
Figure 3.
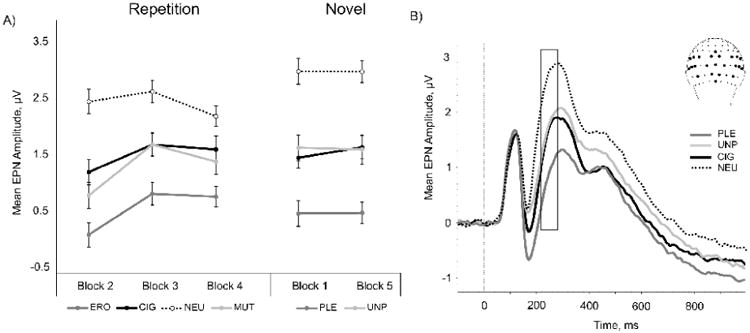
A) Mean amplitude for the early posterior negativity (216-276 ms) component evoked by picture stimuli during the repetition and novel phases, averaged across all subjects. Bars denote standard error of the mean. Note: High-arousing erotica and mutilations were repeated in the repetition phase; high- and low-arousing pleasant (erotica and romance) and unpleasant (mutilations and attack) cues were presented in the novel phase. B) Event-related potential waveforms of mean channel regions of interest (inset) are averaged across all participants and blocks, and are plotted by condition. Box denotes time region of the EPN (216-276 ms). Note different scales.
Repetition phase
For the EPN component, we found a significant interaction of Picture Type and Block (F[6, 396] = 4.45, p <.0001, ), where for each repetition block, ERO evoked significantly greater negativity than CIG (all ps<.001, ) and MUT (all ps<.006, ), and all emotional stimuli (including cigarettes) evoked significantly greater negativity than NEU (all ps<.003, ). MUT and CIG were not significantly different within any repetition blocks (all ps>.1); see Figure 3A). Although attention to ERO, MUT, and CIG cues decreased as a function of stimulus repetition (block 2 vs. block 4, F[1, 67] = 11.325, p <.001, ), these cues remained significantly more negative than NEU (all ps<.03).
3.2.2 Reinstated novel phase
When a novel set of images was presented following the repetition phase, PLE, UNP, and CIG evoked significantly greater negativity than NEU (all ps<.0001, ; Figure 3A). PLE was significantly more negative than both CIG and UNP cues (all ps<.0001, ); CIG and UNP did not differ (p>.8).
We did not observe any effects of Group on the EPN during either the novel or repetition phases, suggesting that the EPN was not modulated by smoking status. Grand averaged ERP waveforms are averaged across all blocks and participants and are plotted by the condition in Figure 3B.
3.3 LPP Component (380 – 532 ms)
3.3.1 Novel phase
Similar to the EPN, when presented for the first time, LPP amplitude evoked by PLE, UNP, and CIG was significantly greater than NEU (all ps<.0001, ; Figure 4A). PLE and UNP evoked significantly greater LPP amplitude relative to CIG (all ps<.0001, ). PLE and UNP did not differ (p>.3, )
Figure 4.
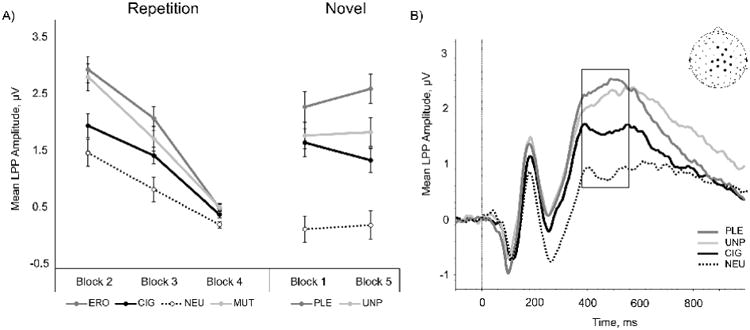
A) Mean late positive potential (380-532 ms) amplitude evoked by picture stimuli during the repetition and novel phases, averaged across all subjects. Bars denote standard error of the mean. Note: High-arousing erotica and mutilations were repeated in the repetition phase; high- and low-arousing pleasant (erotica and romance) and unpleasant (mutilations and attack) cues were presented in the novel phase. B) Event-related potential waveforms of mean channel regions of interest (inset) are averaged across all participants and blocks, and are plotted by condition. Box denotes time region of the LPP (380-532 ms). Note different scales.
3.3.2 Repetition phase
We observed an interaction of Picture Type and Block for the LPP component, F(6, 396) = 5.88, p <.0001, . Pairwise comparisons indicated that in all blocks of the repetition phase, ERO, MUT, and CIG evoked greater LPP amplitude relative to NEU (all ps<.04, ; Figure 4A), and ERO and MUT images evoked significantly greater LPP amplitude than CIG (all ps<.0001, ). Follow-up tests indicated a significant decrease in LPP amplitude across the repetition phase (block 2 vs block 4, F[1, 66] = 215.3, p <.0001, ), replicating previous studies (Codispoti et al., 2006, 2007; Mastria et al., 2017). In other words, there was a significant decrease in observed LPP amplitude for all picture types (ERO, MUT, CIG, NEU), such that the amplitude for a given condition in block 3 was less than the amplitude observed in block 2 (all ps < .006), and the amplitude observed in block 4 was less than the amplitude observed in block 3 (all ps < .001). Further, the introduction of novel stimuli following the repetition phase elicited a significant reinstatement of cortical positivity (block 4 vs. block 5, F[1,67] = 65.15, p <.0001, ), suggesting that the observed decrease in amplitude was due to stimulus repetition rather than study fatigue.
3.3.3 Reinstated Novel Phase
When a novel set of images was presented following the repetition phase, LPP amplitude evoked by emotional stimuli (including cigarettes) was significantly greater than NEU (all ps<.0001, ; Figure 4A). PLE and UNP evoked significantly greater LPP amplitude relative to CIG images (all ps<.0001, ). PLE evoked significantly greater amplitude than UNP (p<.02, ).
We did not observe any effects of Group on the LPP during either the novel or repetition phases, suggesting that smoking status did not modulate the LPP Grand averaged ERP waveforms are averaged across all blocks and participants and are plotted by the condition in Figure 4B.
3.4 Potential Covariates of the ERP Response
To determine whether any of the baseline demographic variables functioned as a covariate of the ERP response, we ran a Group × Picture Type × Block analysis, separately for each ERP component, and included the following potential moderators as covariates in the model: gender, race, and age. We found no main effects for any of the baseline measures on either of the ERP components, indicating that none of the baseline measures covaried with ERP response during the EPN or LPP time window. Likewise, the presence of the covariates did not alter significant main effects or interactions within the EPN and LPP time windows.
3.5 SAM Ratings
A significant interaction between smoking status and picture type emerged when we analyzed ratings of emotional valence, F(3, 198) = 6.88, p <.0001, . There were no group differences in self-reported valence ratings of pleasant and unpleasant cues, which were rated by all participants as more pleasant (p<.0001) and more unpleasant (p<.0001) than neutral cues. However, unlike smokers, never-smokers rated cigarette cues as significantly more unpleasant than neutral (p <.0001, ). Groups did not differ on ratings of arousal (p=0.5); all subjects rated pleasant and unpleasant stimuli as significantly more arousing than neutral (all ps<.0001), and arousal ratings for cigarette cues did not differ from neutral (p=.2).
To determine whether never-smokers' rating of cigarette-related cues was related to attentional biases toward cigarette-related stimuli, we ran a correlation analysis with SAM ratings and grand-averaged ERP amplitude as variables. We ran these analyses across all blocks, separately for each group (smokers, never-smokers) and ERP component (EPN, LPP). There was a moderate, negative correlation between LPP amplitude and self-reported valence ratings of cigarette-related cues among never-smokers, ρ(30) = -.32, p = .04. In other words, a more unpleasant rating of cigarette-related cues was related to higher LPP amplitude. No other correlations reached statistical significance.
4. Discussion
In the present study, we used a repetitive picture viewing paradigm to assess the extent to which cognitive (top-down) and affective (bottom-up) processes influence neurophysiological responses to cigarette-related cues in smokers and never-smokers. Overall, our findings are in line with a large body of research demonstrating that emotional cues are processed in a mandatory fashion, and continue to engage cortico-limbic appetitive and defensive systems even after massive repetition, as suggested by the affective modulation of the LPP (Codispoti et al., 2006; Codispoti et al., 2016; Ferrari et al., 2017; Mastria et al., 2017). Contrary to our predictions, our results indicate that cigarette-related cues continue to evoke larger ERPs than neutral images in both smokers and never-smokers, even when the stimuli have been repeated 96 times and are no longer novel.
While a lack of group differences may be surprising, our findings are in line with those from other ERP (Bloom et al., 2013; Deweese et al., 2016; Engelmann et al., 2016; Littel et al., 2012; Robinson et al., 2015) and reaction time (Oliver and Drobes, 2012) studies showing a cognitive, attentional bias toward cigarette-related cues in both smokers and non-smokers.
We think that it is unlikely that a purely perceptual explanation would account for our findings. Using a second-order conditioning paradigm, we recently showed that both smokers and non-smokers respond to perceptually identical cues when they precede both pleasant and cigarette-related images (Deweese et al., 2016). However, because pleasant and unpleasant stimuli evoke comparable ERP responses (Bradley et al., 2012; Keil et al., 2002; Schupp et al., 2004a; Wiens and Syrjänen, 2013; Leite et al., 2012), we are unable to discriminate whether cigarette-related cues are being processed as pleasant or unpleasant cues. Our self-report ratings, however, support the notion that, unlike smokers, never-smokers find cigarette-related cues unpleasant (Engelmann et al., 2011; Robinson et al., 2016; Robinson et al., 2015). These findings are also supported by studies that measured peripheral physiology and showed that in never-smokers, cigarette-related cues elicited responses similar to those evoked by unpleasant stimuli (Dempsey et al., 2007; Gantiva et al., 2016; Geier et al., 2000). The moderate, negative correlation between LPP amplitude and self-reported valence ratings of cigarette-related cues further supports the conclusion that never-smokers maintain enhanced ERP responses to cigarette-related cues because they perceive them as unpleasant.
Although we did not find evidence of a correlation between EPN amplitude and self-reported valence ratings of cigarette-related cues, these results were also not surprising. The EPN is largely associated with amplitude modulation of primarily pleasant stimuli (De Cesarei and Codispoti, 2006; Schupp et al., 2004a; Keil et al., 2002). Because smokers' valence ratings of cigarette-related cues did not differ from neutral, and never-smokers rated cigarette-related cues as more unpleasant than neutral, we would not anticipate a correlation among these measures.
Our findings may also be interpreted in the light of results from studies showing that the imminent possibility of smoking increases craving intensity (Carter and Tiffany, 2001; Wilson and Sayette, 2015) and brain responses (Wilson et al., 2005; Jasinska et al., 2014) evoked by cigarette-related cues. In fact, a recent study investigating the effects of nicotine exposure and dose expectancy on the EPN and LPP reported that the anticipated effects of nicotine improve attention similar to receiving nicotine (Robinson et al., 2016). In our study, however, smokers were aware that smoking was not possible during or immediately following the EEG recording session, which may have affected the salience of cigarette-related cues.
In a recent study designed to examine the impact of an explicit categorization task on the emotional processing of repeated pictures, Mastria and colleagues (2017) found that while affective modulation of the LPP was unaffected by the categorization task, stimulus-specific effects emerged as a function of task-relevance. Task-relevant images evoked LPPs on the level of non-target emotional cues, whereas the same images evoked LPP amplitude comparable to neutral ones when presented in the passive viewing condition. Through years of repeated use, smoking cues have acquired strong motivational properties powerful enough to capture attention, activate affective states, and guide behavior (Robinson and Berridge, 1993). However, even following massive repetition, we observed an increase in ERP amplitude to cigarette-related cues in never-smokers who have not experienced the associative learning processes associated with the effects of nicotine. Thus, future studies might capitalize on how cognitive mechanisms, such as task relevance (implicit and explicit), and external cues such as nicotine availability, might influence reactivity to cigarette-related cues in smokers and never-smokers.
Taken together, we believe these data make an important contribution to the literature by demonstrating that cigarette-related cues are relevant to smokers and never-smokers. Even following massive repetition, never-smokers produced higher EPNs and LPPs to cigarette-related cues relative to neutral, despite never having been exposed to nicotine. In combination with the self-report data, we conclude that smoking cues capture attentional resources of smokers and never-smokers, but for different reasons. While never-smokers perceive cigarette-related cues as unpleasant stimuli, smokers preferentially allocate attention to cigarette-related cues because these cues have been imbued with incentive salience through years of repeated associations with the delivery of nicotine.
Highlights.
We measured event-related potentials in smokers and never-smokers.
Stimuli were repeated up to 96 times.
Emotional stimuli evoked greater EPN and LPP amplitude than neutral.
These effects were not modulated by smoking status.
Unlike smokers, never-smokers rated cigarette-related cues as unpleasant.
Acknowledgments
The authors thank Hannah L. Stewart for help with data collection and Kimberly Claiborne for study coordination. This research has been presented as posters at the annual meetings of the Society for Psychophysiological Research (2016) and Society for Research on Nicotine and Tobacco (2017).
Role of Funding Sources: Menton M. Deweese and the research reported in this publication were supported by the National Cancer Institute of the National Institutes of Health under award number R25CA057730 and by MD Anderson's Cancer Center Support Grant under award number CA016672 funded by the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors: MC and FV conceptualized. MMD and FV designed the experiment. MMD collected the data. MMD and FV performed the data analyses. All authors contributed to the interpretation of the results. MMD drafted the manuscript. FV, MC, JDR, and PMC reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bloom EL, Potts GF, Evans DE, Drobes DJ. Cue reactivity in smokers: An event-related potential study. Int J Psychophysiol. 2013;90:258–264. doi: 10.1016/j.ijpsycho.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob Control. 2009;18:317–323. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Keil A, Lang PJ. Orienting and emotional perception: Facilitation, attenuation, and interference. Front Psychol. 2012;3:493. doi: 10.3389/fpsyg.2012.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9:183–190. doi: 10.1037/1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: Autonomic and cortical correlates. Brain Res. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: Distinguishing early and late processes in affective picture perception. J Cognitive Neurosci. 2007;19:577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Codispoti M, De Cesarei A, Biondi S, Ferrari V. The fate of unattended stimuli and emotional habituation. Behavioral interference and cortical changes. Cogn Affect Behav Neurosci. 2016;16:1063–1073. doi: 10.3758/s13415-016-0453-0. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. When does size not matter? Effects of stimulus size on affective modulation. Psychophysiology. 2006;43:207–215. doi: 10.1111/j.1469-8986.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Dempsey JP, Cohen LM, Hobson VL, Randall PK. Appetitive nature of drug cues re-confirmed with physiological measures and the potential role of stage of change. Psychopharmacology (Berl) 2007;194:253–260. doi: 10.1007/s00213-007-0839-3. [DOI] [PubMed] [Google Scholar]
- Deweese MM, Robinson JD, Cinciripini PM, Versace F. Conditioned cortical reactivity to cues predicting cigarette-related or pleasant images. Int J Psychophysiol. 2016;101:59–68. doi: 10.1016/j.ijpsycho.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Gewirtz JC, Cuthbert BN. Emotional reactivity to emotional and smoking cues during smoking abstinence: Potentiated startle and P300 suppression. Psychophysiology. 2011;48:1656–1668. doi: 10.1111/j.1469-8986.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Gewirtz JC, Cinciripini PM. Individual differences in brain responses to cigarette-related cues and pleasant stimuli in young smokers. Drug Alcohol Depend. 2016;163:229–235. doi: 10.1016/j.drugalcdep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ. Repetitive exposure. Brain and reflex measures of emotion and attention. Psychophysiology. 2011;48:515–522. doi: 10.1111/j.1469-8986.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari V, Codispoti M, Bradley MM. Repetition and ERPs during emotional scene processing. A selective review. Int J Psychophysiol. 2017;111:170–177. doi: 10.1016/j.ijpsycho.2016.07.496. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Codispoti M, Cardinale R, Bradley MM. Directed and motivated attention during processing of natural scenes. J Cogn Neurosci. 2008;20:1753–1761. doi: 10.1162/jocn.2008.20121. [DOI] [PubMed] [Google Scholar]
- Gantiva C, Ballén Y, Casas M, Camacho K. Respuestas psicofisiológicas ante estímulos asociados al tabaco. Diferencias entre fumadores y no fumadores. Av Psicol Latinoam. 2016;34:557. doi: 10.12804/apl34.3.2016.09. [DOI] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. The international smoking image series (with neutral counterparts) Carbondale, IL: Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. In Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Englewood Cliffs N.J: Prentice-Hall (Prentice-Hall series in experimental psychology); 1973. [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1111/1469-8986.3950641. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: Computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in mental health care delivery systems. Norwood, NJ: Ablex; 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report no A-6. Gainesville, FL: University of Florida; 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Galdo-Alvarez S, Alves J, Sampaio A, Gonçalves OF. Affective picture modulation. Valence, arousal, attention allocation and motivational significance. Int J Psychophysiol. 2012;83:375–381. doi: 10.1016/j.ijpsycho.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Littel M, Euser AS, Munafo MR, Franken IH. Electrophysiological indices of biased cognitive processing of substance-related cues: A meta-analysis. Neurosci Biobehav Rev. 2012;36:1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gaspelin N. How to get statistically significant effects in any ERP experiment (and why you shouldn't) Psychophysiology. 2017;54:146–157. doi: 10.1111/psyp.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastria S, Ferrari V, Codispoti M. Emotional picture perception. Repetition effects in free-viewing and during an explicit categorization task. Front Psychol. 2017;8:1001. doi: 10.3389/fpsyg.2017.01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough BE, Warren CA. Effects of 12-h tobacco deprivation on event-related potentials elicited by visual smoking cues. Psychopharmacology (Berl) 2001;154:282–291. doi: 10.1007/s002130000647. [DOI] [PubMed] [Google Scholar]
- Minnix JA, Versace F, Robinson JD, Lam CY, Engelmann JM, Cui Y, Brown VL, Cinciripini PM. The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: A content comparison. Int J Psychophysiol. 2013;89:18–25. doi: 10.1016/j.ijpsycho.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A. Orienting and attention: Preferred preattentive processing of potentially phobic stimuli. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1992. pp. 263–295. [Google Scholar]
- Oliver JA, Drobes DJ. Visual search and attentional bias for smoking cues: The role of familiarity. Exp Clin Psychopharmacol. 2012;20:489–496. doi: 10.1037/a0029519. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Jentink KG, Drobes DJ, Evans DE. Smokers exhibit biased neural processing of smoking and affective images. Health Psychol. 2016;35:866–869. doi: 10.1037/hea0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Versace F, Engelmann JM, Cui Y, Gilbert DG, Waters AJ, Gritz ER, Cinciripini PM. Attentional bias to smoking and other motivationally relevant cues is affected by nicotine exposure and dose expectancy. J Psychopharmacol. 2016;30:627–640. doi: 10.1177/0269881116642879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Versace F, Engelmann JM, Cui Y, Slapin A, Oum R, Cinciripini PM. The motivational salience of cigarette-related stimuli among former never and current smokers. Exp Clin Psychopharmacol. 2015;23:37–48. doi: 10.1037/a0038467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Components of depressed mood in married men and women. The Center for Epidemiologic Studies' Depression Scale. Am J Epidemiol. 1984;119:997–1004. doi: 10.1093/oxfordjournals.aje.a113819. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cogn Emot. 2004a;18:593–611. [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology. 2004b;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:59–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle DAT, Spinks JA. Orienting, habituation, and the allocation of processing resources. In: Campbel BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1992. pp. 263–295. [Google Scholar]
- Stewart J. Review. Psychological and neural mechanisms of relapse. Philos Trans R Soc Lond B Biol Sci. 2008;363:3147–3158. doi: 10.1098/rstb.2008.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addict Biol. 2011;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wessa M, Kanske P, Neumeister P, Bode K, Heissler J, Schönfelder S. EmoPics: Subjektive und psychophysiologische Evaluation neuen Bildmaterials für die klinisch-bio-psychologische Forschung. Zeitschrift für Klinische Psychologie und Psychotherapie. 2010;39(Suppl. 1/11) [Google Scholar]
- Wiens S, Syrjänen E. Directed attention reduces processing of emotional distracters irrespective of valence and arousal level. Biol Psychol. 2013;94:44–54. doi: 10.1016/j.biopsycho.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine Tob Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA. Neuroimaging craving. Urge intensity matters. Addiction. 2015;110:95–203. doi: 10.1111/add.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention. A review of attentional processing of emotional information. Cogn Emot. 2010;24:3–47. doi: 10.1080/02699930903205698. [DOI] [Google Scholar]


