Abstract
Background
Asthma morbidity is high among low-income children living in rural U.S. regions, yet few interventions have been designed to reduce asthma burden among rural populations.
Objective
Examine the impact of a school-based asthma education program delivered via telemedicine among children living in an impoverished, rural region.
Methods
We conducted a cluster randomized trial with rural children, ages 7–14 years, comparing a school-based telemedicine asthma education intervention to usual care. The intervention provided comprehensive asthma education via telemedicine to participants and provided evidence-based treatment recommendations to primary care providers.
Results
Of the 393 enrolled children, median age was 9.6 years, 81% were African-American and 47% lived in households with <$14,999 annual income. At enrollment, 88% of children reported uncontrolled asthma symptoms. At the end of the intervention, there were no statistically significant differences in reported symptom free days (primary outcome) for either the intervention or usual care group. Participants in the intervention group reported significantly higher utilization of peak flow meters to monitor asthma and reported taking their asthma medications as prescribed more frequently when compared to the usual care group. There were no changes in other outcome measures including quality of life, self-efficacy, asthma knowledge, or lung function between groups.
Conclusion
Although there was some evidence of behavior change among intervention participants, these changes were inadequate to overcome the significant morbidity experienced by this highly symptomatic rural, impoverished population. Future interventions should be designed with a multifaceted approach that considers caregiver engagement, distance barriers and inadequate access to asthma providers in rural regions.
Keywords: Telemedicine, education, asthma, adolescents, rural, public schools
INTRODUCTION
Asthma, a major public health concern, is the leading chronic illness of childhood and a major cause of childhood morbidity.1–3 Although asthma is a significant cause of childhood disability in all regions of the US, large-scale interventions to reduce asthma burden have largely focused on children living in urban settings. Previous reports by our team and others 4–7 suggest that asthma prevalence is similar between rural and urban populations with many studies revealing significant morbidity among rural children. Despite these findings, comprehensive asthma initiatives addressing asthma morbidity in rural pediatric populations are lacking. Nationwide, data have consistently shown that children from minority and low-income families are at increased risk for poor asthma outcomes2 and are also less likely to receive adequate medical therapy for asthma.8, 9 These disparate findings are even more compelling when one considers high-risk populations living in rural regions have significant travel/transportation needs that present additional barriers to care. We and others have shown that low-income and minority children in Arkansas have high prevalence of asthma and increased asthma morbidity.7, 10–12 These data, consistent with national trends in asthma disparities, underscore the need for innovative programs targeting high-risk rural populations, particularly those living in medically underserved communities.
Telemedicine is defined as the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status. Telemedicine has the potential to improve health care access in rural and medically underserved regions and offers opportunities for advancing the care of pediatric asthma patients through clinical services, remote monitoring, and medical education.13–15 Technology-based healthcare solutions such as telemedicine are particularly appealing in regions such as the Arkansas Delta, one of the most medically underserved, impoverished US regions with significant disparities in health outcomes.16–18 Because our prior work revealed significant morbidity among school aged children in the region, we implemented a school-based telemedicine asthma education program with the aim of improving asthma symptoms, caregiver knowledge, parental and child self-efficacy, and other health outcomes.
METHODS
Study population
The study population included children with current asthma who were enrolled in public schools in Arkansas’ Delta region. Participating school districts had ≥51% minority enrollment and/or ≥51% of students were eligible for free/reduced lunch. Inclusion criteria were: 1) age 7–14 years, 2) current asthma per national guidelines,19 and 3) use of prescribed medications for asthma symptoms (rescue or controller) in the 6 months prior to enrollment. Exclusion criteria were: 1) significant respiratory disease other than asthma, 2) co-morbid conditions precluding participation in an education-based intervention, 3) inability to speak/understand English, 4) no telephone access (needed for follow-up surveys), and 5) exercise-induced asthma only. The study was approved by the University of Arkansas for Medical Sciences Institutional Review Board, and all participants gave written informed consent/assent.
Study procedures
We conducted a cluster randomized trial with 393 children and their caregivers. Prior to the enrollment period, 19 school districts in the Delta region of Arkansas were randomized to an intervention arm or usual care (UC) arm. Since rural individuals frequently travel to neighboring towns/counties to seek medical care, we employed randomization rules to districts located within a 50 mile radius of each other. These measures were employed to decrease the risk of experimental contamination by shared rural primary care providers (PCPs).
Enrolled children, parents/caregivers, and school nurses were assigned by randomized cluster (school district) to receive the intervention or UC. All components of the intervention were completed during one school year. The school-based telemedicine intervention included: 1) comprehensive asthma education via telemedicine for the child, his/her parent/caregiver(s) and school nurse, 2) prospective monitoring over a 6-month period for asthma symptoms and lung function, and 3) dissemination of asthma management prompts to the PCP. Children in the UC arm received medical care from their PCP as usual. UC participants completed follow-up surveys at the same intervals as the intervention group.
Asthma Education via Telemedicine
Each student participated in five 30–45 minute age-appropriate asthma education sessions via telemedicine (live interactive video). Each session was led remotely by a board certified allergist, respiratory therapist, or trained asthma educator at Arkansas Children’s Hospital in Little Rock, Arkansas. Sessions were conducted weekly or bi-weekly over a 5–9 week period depending on school needs/preferences. To ensure accuracy and consistency in delivery of the intervention, education was delivered by trained personnel utilizing a standardized script and structured format. Education included lung anatomy, common asthma symptoms, proper use of rescue and controller asthma medications, how to follow a personalized asthma action plan (AAP), asthma triggers, and proper use of asthma medication devices including inhalers, peak flow meters and spacer devices.
Telemedicine sessions were also conducted for intervention parents/caregivers and school nurses. Each 60–90 minute session was conducted at the school (local community center on weekend/nights). Caregivers participated in 2 sessions and school nurses participated in 1 session. Topics were the same as those conducted for students and included an interactive question/answer session. To accommodate working caregivers, sessions were offered weekdays, weekends, late evening and early morning. After ≥3 missed appointments, caregivers were asked to complete educational sessions via telephone with educational materials mailed to the caregiver prior to the session. In an effort to minimize transportation burden due to study participation, caregivers were provided transportation vouchers via a local medical transportation service to attend telemedicine sessions if needed. Further, to minimize barriers related to lack of phone service, we also provided caregivers with mobile minutes through their mobile service provider to complete interviews (both groups) and/or telephone education sessions (intervention group) if needed.
Prospective TeleMonitoring
Each intervention participant was assessed via remote telemonitoring at the school site. Telemonitoring sessions were completed prior to the first child education session and at 3 months. During the session, intervention participants described symptoms for the preceding 2 weeks and completed the PedsQL 3.0 survey.20 Lung function was also obtained using the Collins Eaglet System (Ferraris Respiratory, Louisville, CO) with values compared to age-matched normal data. Forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and forced expiratory flow (FEF) were measured and the best of 3 maneuvers was used for analysis.
Caregiver-reported asthma medications were assessed via structured interview at baseline, 3- and 6-months. In addition to reporting prescribed medications, caregivers reported if their child took medications as prescribed all of the time, most of the time, some of the time, little of the time or none of the time. Prescription refill data was used to calculate asthma medication ratio (AMR) prior to enrollment and during the study period. AMR is a measure defined by (units of controllers dispensed) / (units of controllers + rescue inhalers dispensed). In prior studies, AMR ≥ 0.5 has been associated with improved outcomes including asthma severity and control and healthcare utilization.21
PCP Prompts
The PCP of each intervention participant received a prompt with guidelines-based asthma management19 at baseline and 3 months. Caregivers and school nurses received copies of the prompt to reinforce recommendations. The prompt included: 1) a summary of education sessions, 2) blank AAP with completion instructions, 3) synopsis of caregiver-reported symptoms and medications and 4) treatment recommendations according to guidelines.19 For example, if a participant’s caregiver reported uncontrolled, recommendations for initiation or step-up of controller therapy were given.19 PCPs received a 7-question survey to confirm receipt of the prompt, verify accuracy of asthma severity and control assessment. We provided a self-addressed stamped envelope to return the survey and phone/fax/mailing contacts for the research team to answer any questions or to receive additional feedback.
Survey and Outcome Measures
Telephone interviews were conducted to examine caregiver-reported outcomes including the change in symptom free days (SFD) at 3 months (primary outcome) and 6 months. This outcome measure is consistent with the symptom monitoring suggested by the asthma guidelines and has been suggested as an appropriate surrogate marker for asthma control.22 Caregivers reported the number of days their child experienced no symptoms of asthma (defined as a 24 hour period with no coughing, wheezing, chest tightness, or shortness of breath, and no need for rescue medications) during the prior 2 weeks. Additional asthma outcomes included the Children’s Health Survey for Asthma (CHSA), a 48-item validated questionnaire divided into 5 scales: 1) physical health, 2) child activity, 3) family activity, 4) child emotional health, and 5) family emotional health. The survey distinguishes levels of disease severity and has internal consistency reliability (Cronbach’s α = 0.81–0.92).23, 24 For intervention participants, PedsQL 3.0 was utilized to measure health-related quality of life at baseline and 3- months. PedsQL 3.0 is a 22-question survey with reliable internal consistency (Cronbach’s α > 0.70) that has been validated in 9–17 year old African-American low-income populations.20, 23 For both groups, we assessed the mini-Pediatric Asthma Quality of Life Questionnaire (PAQLQ) via structured interview at baseline and at 6 months.25, 26 The mini-PAQLQ is a 13-item validated survey utilizing 3 domains: 1) symptoms, 2) activity limitations, and 3) emotional function. PAQLQ has strong internal consistency reliability (Cronbach’s α =0.88).
Caregiver asthma knowledge survey was administered at baseline and 6 months. This 20-item questionnaire measures asthma symptoms identification, appropriate use of controller vs. rescue medications, and knowledge of asthma triggers. This survey was based on NHLBI guidelines and showed high content validity against expert panel assessment in a previous study of asthma knowledge among rural caregivers.27 We also measured caregiver and child self-efficacy at baseline and 6 months. The caregiver self-efficacy questionnaire is an 18-item validated tool measuring caregiver self-efficacy in 3 subscales: 1) asthma attack prevention, 2) treatment efficacy, and 3) attack management. All subscales require caregivers to select one of five responses ranging from “not at all” (1 point) to “completely sure” (5 points). Cronbach’s α reliability = 0.77 for asthma attack prevention, 0.76 for treatment efficacy, and 0.82 for attack management.28 Child self-efficacy survey is a 9-item questionnaire designed to measure the child’s self-efficacy with regard to attack prevention and attack management. The child was required to select one of 4 responses ranging from “none of the time” (0 points) to “all of the time” (3 points). The child self-efficacy range is 0–27 points. The Cronbach’s α reliability = 0.75. 28
Statistical Methods
Sample size
The sample size for this study was calculated to detect a clinically meaningful difference in mean number of SFD between the two groups. Since the design of the study was a cluster randomized trial, the sample size to detect a desired treatment effect depends on the number of school districts (units of randomization) and also on the number of participants per school district.29 We estimated an overall sample size of 288 subjects with 9 school districts in each treatment arm and 16 participants per school district would yield at least 80% power to detect a 1 day difference in mean SFD between groups, assuming a standard deviation of 2.8. This calculation assumed an intra-cluster correlation of 0.01. After adjusting for a 20% attrition rate, we calculated an average of 20 participants in each school district with up to 10 school districts in each arm for a total of 180 participants in each arm or 360 participants overall.
Statistical analysis
All data were summarized using appropriate summary statistics such as median and quartiles for continuous variables and number and percent for categorical variables. We compared the two groups to ensure that they were appropriately balanced with respect to all baseline characteristics such as age, child gender, child race, child ethnicity, insurance (private/state-issued/self-pay), income groups, and maternal education. Continuous variables were compared between groups using a Wilcoxon rank sum test while categorical variables were compared using a Chi-squared test for association. We compared the number of SFD, and other outcomes at 3 and 6-months using linear or generalized linear mixed models after adjusting for baseline values as covariates. The number of SFD was compared between groups using a linear mixed model. Binomial distribution with a logit link function was used for binary outcomes while a negative binomial distribution with a log link was used to compare overdispersed count data. Due to the clustered nature of the data, all models comparing outcomes used random effects to account for clustering of individuals within school districts and the nesting of school districts within the intervention group. We performed analyses comparing outcomes in the UC group and intervention group vs. completed outcomes. We further did a sensitivity analysis by comparing outcomes in the UC group vs. intervention participants who completed protocol (per protocol group). Per protocol group was defined as participants who completed each of the five education sessions and their parents who completed both their education sessions. Since family income was significantly different between groups at baseline, we did a sensitivity analysis comparing primary and secondary outcomes after adjusting for family income. We further compared outcomes by stratifying participants based on smoking status (whether or not the participant lived in a home with a smoker). All tests conducted were two-sided and all P-values ≤ 0.05 were considered to be statistically significant. Statistical analysis was performed using SAS/STAT ® version 9.4 software (2002–2012 SAS institute Inc., Cary, North Carolina) and the R software (R Core Team, Vienna Austria).
RESULTS
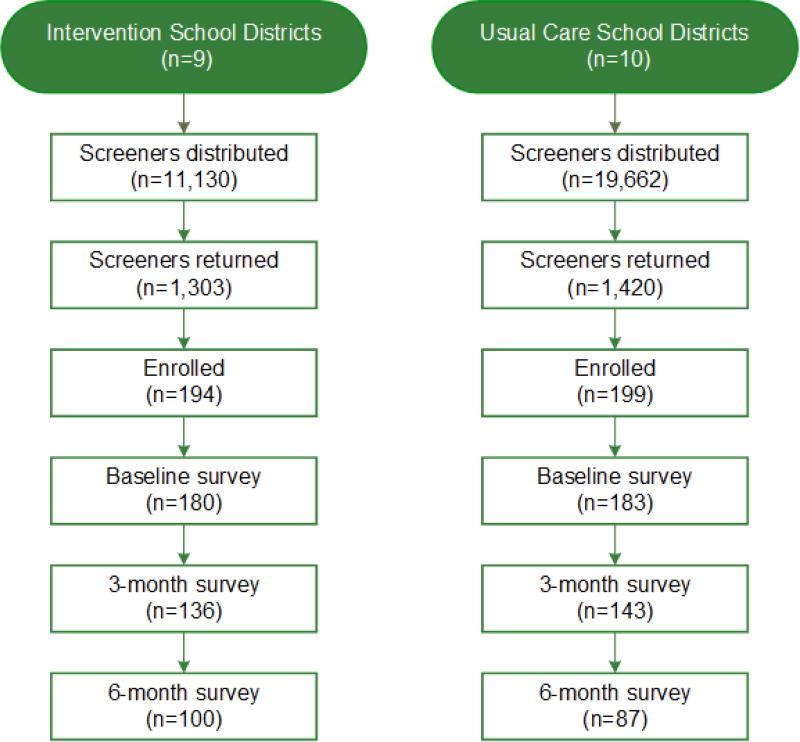
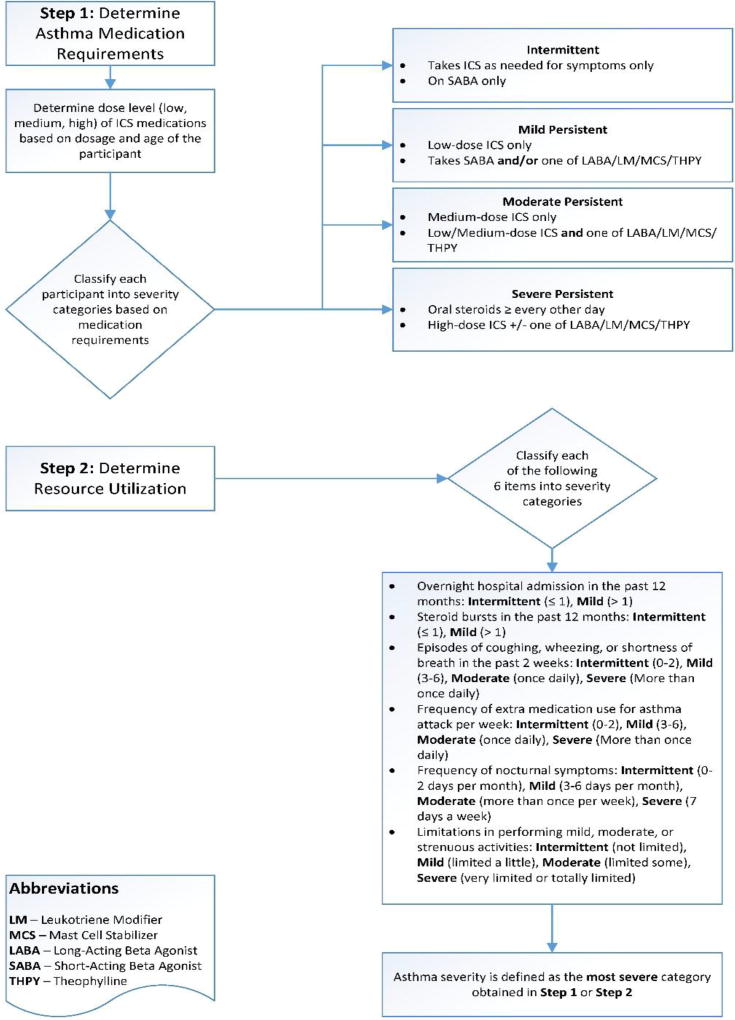
In total, 30,792 potentially eligible participants attending 19 rural Delta school districts were included in the screening process (9 intervention schools and 10 usual care schools) [Figure 1]. Through convenience sampling at each school, 194 child-caregiver dyads were enrolled in the intervention schools, and 199 were enrolled in the UC schools. After enrollment, 14 dyads in the intervention group and 16 in the UC group were dropped because they failed to complete the baseline interview. The demographics of the study population are depicted in Table 1 and included 180 dyads in the intervention group and 183 dyads in the UC group. The median child age was 9.6 years; 81% were African-American and 83% had state-issued or no insurance. Forty-seven percent were from households with an annual income ≤$14,999 and 35% of maternal caregivers had a high school education or less. More children in the intervention group were from families with household income <$14,999 (54%) compared to the UC group (39%) [p=0.029] otherwise there were no statistically significant differences between groups at baseline. Participants in both groups were highly symptomatic. Based symptoms, healthcare utilization, activity limits and prescribed controller medication requirements, 90% of participants were classified as having mild-severe persistent asthma (Table 2 and Figure 2).19 Further, only 22% reported well-controlled symptoms at baseline when guidelines-based criteria were applied.19 A median of 2.5 outpatient asthma sick visits were reported at baseline for the 363 enrolled participants, however, reported emergency healthcare utilization was low with no reported overnight hospitalizations and a median of 0 (range 0–2) emergency room visits in the previous 12 months.
Figure 1. Enrollment and Retention.
Children from 19 rural school districts were screened. Screeners were distributed at the beginning of each school year and at school events by the school nurse.
Table 1.
Demographics of enrolled child participants
| Demographics | N* | Usual Care (n=183) |
Intervention (n=180) |
Overall (n=363) |
|---|---|---|---|---|
| Age, Median (Q1, Q3), years | 359 | 9.6 (8.1, 11.4) | 9.6 (8.6, 11.6) | 9.6 (8.4, 11.6) |
| Child Gender, N (%) | 363 | |||
| Male | 107 (58%) | 98 (54%) | 205 (56%) | |
| Child Race, N (%) | 363 | |||
| African American | 152 (83%) | 143 (79%) | 295 (81%) | |
| White | 24 (13%) | 29 (16%) | 53 (15%) | |
| More than one race | 7 (4%) | 8 (4%) | 15 (4%) | |
| Ethnicity | 362 | |||
| Hispanic or Latino, N (%) | 8 (4%) | 3 (2%) | 11 (3%) | |
| Insurance, N (%) | 346 | |||
| Private | 35 (20%) | 24 (14%) | 59 (17%) | |
| State-issued | 136 (79%) | 144 (83%) | 280 (81%) | |
| Self-Pay | 2 (1%) | 5 (3%) | 7 (2%) | |
| Income, N (%) | 345 | |||
| ≤ $14,999 | 67 (39%) | 94 (54%) | 161 (47%) | |
| $15,000–$29,999 | 50 (29%) | 45 (26%) | 95 (28%) | |
| $30,000–$60,000 | 40 (23%) | 27 (16%) | 67 (19%) | |
| >$60,000 | 14 (8%) | 8 (5%) | 22 (6%) | |
| Maternal Education, N (%) | 360 | |||
| HS Graduate or less | 59 (32%) | 68 (38%) | 127 (35%) | |
| Some College or College Graduate | 105 (58%) | 100 (56%) | 205 (57%) | |
| Post-Graduate | 18 (10%) | 10 (6%) | 28 (8%) |
Data missing for some participants
Table 2.
Characteristics of the study population at enrollment. Findings based on parental report of symptoms, medication use and healthcare utilization.
| Symptoms and medication use | N | Usual Care (n=183) |
Intervention (n=180) |
Total (n=363) |
p |
|---|---|---|---|---|---|
| How many DAYS symptom free in past 2 weeks? | 363 | 8 (2, 12) | 7 (2, 12) | 8 (2, 12) | 0.843 |
| How many times admitted overnight in hospital in past 12 months? | 363 | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.865 |
| How many times seen in ED EVER? | 361 | 3 (1, 7) | 2 (0, 6) | 3 (0, 6) | 0.060 |
| How many times seen in ED past 12 months? | 363 | 0 (0, 2) | 0 (0, 1) | 0 (0, 2) | 0.415 |
| How many times seen in doctor’s office/clinic for sick visit past 12 months? | 362 | 2 (1, 4) | 3 (1, 5) | 2.5 (1, 5) | 0.088 |
| How many courses of systemic steroids (by mouth or shot) past 12 months? | 363 | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.991 |
| Asthma severity | 360 | 0.185 | |||
| Intermittent asthma | 22 (12%) | 14 (8%) | 36 (10%) | ||
| Mild persistent asthma | 38 (21%) | 31 (17%) | 69 (19%) | ||
| Moderate-severe persistent asthma | 120 (67%) | 135 (75%) | 255 (71%) | ||
| Asthma control | 360 | 0.842 | |||
| Well controlled | 40 (22%) | 39 (22%) | 79 (22%) | ||
| Not well controlled | 40 (22%) | 36 (20%) | 76 (21%) | ||
| Very poorly controlled | 100 (56%) | 105 (58%) | 205 (57%) | ||
| Prescribed controller* | 363 | 80 (44%) | 86 (48%) | 166 (46%) | 0.44 |
Controller medications include inhaled corticosteroids +/− long-acting beta agonist, leukotriene modifiers, mast cell stabilizers, theophylline or systemic steroids.
Figure 2.
Asthma severity algorithm. Step 1 determined asthma severity based on reported prescribed controller medication requirements. Because <50% of participants reported being prescribed a controller asthma medication, Step 2 determined asthma severity based on reported symptoms, rescue medication use, activity limits, and healthcare utilization. Asthma severity was defined as the most severe category in either Step 1 or Step 2.
Of the enrolled intervention participants, 88% of children completed all 5 education sessions and 61% of caregivers completed all parent education sessions (8% completed via telephone, n=14). Forty-nine (27%) of caregivers didn’t complete either parent session. Follow-up survey completion rates for the intervention group was 75% at 3 months and 55% at 6 months. For the UC group, follow-up rates were 78% and 48% at 3 and 6 months, respectively. PCP prompts with recommendations to initiate or step up therapy due to poorly controlled asthma were sent for 141/180 intervention participants. We received feedback from only 1 PCP.
The primary outcome measure, SFD in the prior 2 weeks, improved for both groups with no statistically significant difference between groups (Table 3). UC participants reported on average 7.4 SFDs/2 weeks at baseline and 8.4 at the end of the intervention. Intervention participants reported on average 7.2 SFDs at baseline and 8.0 at the end of the intervention (p=0.51). After adjusting for baseline SFDs, there was no significant difference in SFD between groups at 3 months, even when the analysis included only the per protocol population. According to national guidelines standards, participants in both groups remained uncontrolled at the end of the intervention period as indicated by <10 SFDs/2 weeks. The family activity domain of the CHSA improved for the UC group but not the intervention group (p = 0.02). There were no changes in other CHSA domain scores for either group.
Table 3.
Asthma Outcomes at baseline, 3 months (end of the intervention) and 6 months.
| Baseline
|
3 months
|
6 months
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | N | Usual Care (n=183) |
Intervention (n=180) |
N | Usual Care (n=143) |
Intervention (n=136) |
p1 | N | Usual Care (n=87) |
Intervention (n=100) |
p2 |
| Symptom free days | 363 | 7.4 (5.3) | 7.2 (5.3) | 279 | 8.4 (5.3) | 8.0 (5.6) | 0.51 | 186 | 9.4 (5.1) | 8.8 (5.1) | 0.55 |
| Asthma knowledge | 361 | 15.4 (2.8) | 15.2 (2.3) | -- | -- | -- | -- | 184 | 15.3 (2.5) | 15.3 (2.3) | 0.62 |
| CHSA physical health | 361 | 78.1 (15.9) | 74.3 (16.7) | 277 | 79.7 (16.6) | 81.6 (15.8) | 0.16 | 185 | 83.6 (14.5) | 77.5 (17.3) | 0.19 |
| CHSA activity child | 356 | 83.7 (16.9) | 80.9 (17.9) | 275 | 85.5 (15.7) | 85.2 (17.5) | 0.61 | 179 | 86.6 (14.6) | 83.8 (18.5) | 0.83 |
| CHSA activity family | 362 | 91.5 (12.8) | 89.8 (11.5) | -- | -- | -- | -- | 186 | 94.6 (8.7) | 89.2 (14.4) | 0.02 |
| CHSA emotion health child | 363 | 76.2 (25) | 73.9 (25.9) | 279 | 80.1 (23.6) | 80.1 (23.8) | 0.46 | 186 | 81.7 (23.5) | 74.4 (25.2) | 0.31 |
| CHSA emotion health family | 362 | 77.3 (13.2) | 77.3 (13) | -- | -- | -- | -- | 185 | 81.9 (11.3) | 78.2 (12) | 0.12 |
| Peak flow meter use (yes) | 363 | 60 (33%) | 45 (25%) | 278 | 51 (36%) | 104 (76%) | <0.01 | 187 | 39 (45%) | 79 (79%) | <0.01 |
| Takes medication as prescribed all of the time or almost all of the time | 363 | 133 (73%) | 124 (69%) | 279 | 107 (75%) | 97 (71%) | 0.77 | 187 | 55 (63%) | 78 (78%) | 0.03 |
| Child self-efficacy, mean (SD) | 309 | 50.3 (9.7) | 49 (10.4) | -- | -- | -- | -- | 145 | 51.8 (9.1) | 52.1 (11.0) | 0.66 |
| Caregiver self-efficacy (SD) | 357 | 46 (5.4) | 46 (5.4) | -- | -- | -- | -- | 186 | 46.9 (5.4) | 47.2 (5) | 0.38 |
| Asthma control | 360 | 273 | 184 | 0.37 | |||||||
| Well controlled | 40 (22%) | 39 (22%) | 28 (20%) | 31 (23%) | 0.33 | 15 (18%) | 19 (19%) | ||||
| Not well controlled | 40 (22%) | 36 (20%) | 33 (24%) | 28 (21%) | 25 (30%) | 22 (22%) | |||||
| Very poorly controlled | 100 (56%) | 105 (58%) | 77 (56%) | 76 (56%) | 44 (52%) | 59 (59%) | |||||
After the intervention, more intervention caregivers reported that their child used peak flow meters to monitor asthma compared to the UC group (45% UC group vs 79% intervention group p < 0.0001). More intervention caregivers reported that their child took asthma medications as prescribed “most of the time” or “all of the time” as compared to the UC group (78% vs 63%, p = 0.03). At the end of the study period, intervention caregivers also reported higher rates of being prescribed a controller asthma medications compared to baseline and compared to the control group but the change was not statistically significant (Table 4). Further, there was no change in AMR for either group with both groups reporting an AMR of 0.5 before and after the intervention. There were no significant differences in AMR between groups when participants categorized as intermittent asthmatics at baseline were excluded from the analysis.
Table 4.
Caregiver report of any prescribed controller medication at 3-months and 6-months after adjusting for baseline.
| Prescribed Controller** | All (n=363)* |
Usual Care (n=183) |
Intervention (n=180) |
p |
|---|---|---|---|---|
| Baseline | 46% | 44% | 48% | - |
| 3 months | 48% | 45% | 52% | 0.41 |
| 6 months | 49% | 44% | 53% | 0.77 |
Controller medications include inhaled corticosteroids +/− long-acting beta agonist, leukotriene modifiers, mast cell stabilizers, theophylline or systemic steroids
Data missing for some participants
PedsQL 3.0 scores showed a trend in improvement for intervention participants at the 3- month assessment compared to baseline; however, this improvement did not reach statistical significance (p= 0.06). There was no change from baseline in mini-PAQLQ scores at 6 months for either group. There was no change from baseline in caregiver or child self-efficacy, lung function, or caregiver asthma knowledge in either the intervention or UC group. Seventy children (30 usual care and 40 intervention) were exposed to a smoker in the home. There were no significant differences in results after analyzing participants with and without exposure to environmental tobacco smoke in the home (data not shown).
DISCUSSION
Asthma in the rural setting is an important public health issue and previous studies have confirmed significant asthma morbidity in these areas.10, 30, 31 Rural asthma is confounded by a shortage of medical providers and inadequate access to subspecialty providers. Because our prior studies in rural Arkansas found high asthma prevalence and morbidity, we examined the impact of a comprehensive school-based telemedicine asthma education program on asthma outcomes among this rural cohort. At the completion of the intervention, we found no significant differences between groups in SFD, our primary outcome. While there was some evidence of behavior change among intervention participants as evidenced by an increase in peak flow monitoring and improvement in caregivers’ report of taking medications as prescribed, we did not find evidence that these behaviors related to improved asthma outcomes. Our school-based telemedicine asthma education program was not sufficient to overcome the significant morbidity experienced by the population. Importantly, both groups were very symptomatic at baseline and remained symptomatic with continued evidence of uncontrolled asthma at the end of the study period.
Several factors likely played a role in study outcomes. Enrolled participants reported significant baseline morbidity with ≤ 8 SFD in the 2 weeks prior to enrollment. In addition to calculation of SFD, we conducted a structured interview to query controller and rescue medication use, healthcare utilization, frequency of daytime symptoms, and nocturnal awakenings, and the vast majority of participants (88%) fit guidelines19 criteria for uncontrolled and/or persistent asthma.19 Although it is possible that our survey measure overestimated rates of uncontrolled asthma, this high level of poorly controlled asthma is consistent with our previous reports among children in the Delta region.10, 30 Despite reports of significant morbidity, fewer than 50% were prescribed a controller (Table 4) and these findings suggests a major gap in implementation of guidelines-based asthma care for this rural population. Consistent with our previous reports,10, 11, 30 findings of high morbidity and low rates of controller medication use suggest that a more targeted approach that includes provider education and interventions to ensure implementation of guidelines-based care are needed to improve outcomes. An intervention to engage the PCP in the educational or decision-making components, a mechanism to ensure that controller medications were prescribed or adjusted as a part of the intervention, along with asthma education delivered via telemedicine would have likely been more effective. Simply providing a treatment prompt to PCPs with medication recommendations was proven to be ineffective. Although prompts were sent to all participants’ PCPs, we did not require compliance with our recommendations for continued enrollment in the study. Although more intervention participants were prescribed a controller at the end of the follow up period and intervention participants reported better compliance with prescribed medications, these changes were insufficient to improve asthma outcomes. PCPs may have been reluctant to change or initiate asthma medications based on our recommendations without a formal referral to a subspecialty clinical setting. Further, families may not have been able to connect with PCPs due to difficulty accessing care within the 3-month timeframe when the primary outcome was measured. Although we asked for feedback from enrolled participants’ PCPs, we only received correspondence from one provider, and better provider engagement may have resulted in more robust controller medication prescription rates for participants. Further, the AMR did not improve for intervention participants thus indicating that our intervention did not change prescription possession rates for participants.
Other limiting factors were likely related to the relatively low participation of caregivers in the educational sessions and survey response rates. The majority of child participants (88%) completed all 5 education sessions. However, only 61% of caregivers completed both educational sessions, despite sessions being offered on multiple attempts, with free transportation and weekend/night opportunities. Although caregivers reported high asthma burden in their children; it is possible that participation in an asthma education program was not engaging, or families had other priorities (i.e. work) or barriers that made it difficult to attend sessions. Further, survey completion rates were not ideal with ≤78% survey completion at 3 months and ≤55% at 6 months despite caregivers receiving free mobile minutes to complete telephone interviews when needed. These retention rates coupled with 27% of caregivers not completing either of the caregiver education sessions likely impacted measured outcomes and may have contributed to our inability to find differences between the groups at the end of the intervention. The poor attendance and dropout rates of caregivers may also reflect an inherent characteristic of this population suggesting a failure by these caregivers to adequately monitor and intervene in their child's disease process. These findings also suggest to this study team that more emphasis on caregiver engagement and innovative teaching strategies need to be employed to address these issues in the future.
As evidenced by the high symptom burden described in the current report, our previous investigations,10, 30 and as reported by others,6, 32–34 asthma is a significant public health concern for rural, low-income populations. Interventions to address the significant gap in implementation of guidelines-based asthma care are needed. Potential co-morbid conditions that may have impacted outcomes and precluded adoption of an education-based intervention such as psychological stress,35, 36 maternal depression,37 and caregiver literacy38 should be explored in future interventions. Interventions specifically aiming to ensure access to appropriate, effective medical therapy, improve medication adherence, and the ability to define and remediate specific barriers to care should also be explored. School-based interventions that include supervised administration of controller asthma medications39, 40 and school-based clinical programs such as the Breathmobile Program have successfully improved asthma outcomes and reduced annual costs associated with asthma morbidity in other high risk population.41, 42 The success of these school-based programs coupled with the high child participant retention rates noted in the current study suggest that a school-based model, with improved provider and caregiver engagement, may be ideal for this rural region given the lower population densities that would make implementation of case management intervention difficult. Other interventions such as subspecialty referral could potentially be tailored for rural populations, but limited access to subspecialty providers or other community resources and efforts to overcome these barriers have to be carefully considered. Using technology such as telemedicine or mobile clinics to reach these traditionally underserved populations hold promise, but a multifaceted approach such as the recently created SAMPRO (School-based Asthma Management Program)43 appears to be necessary to overcome the many barriers to care.
Acknowledgments
Funding provided by: The National Institutes of Health (R01HL102388), Arkansas Children’s Research Institute and Arkansas Biosciences Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution of each Author:
Perry: Concept and design of study, interpretation of data, manuscript preparation and edits
Halterman: Concept and design of study, interpretation of data, critical revision of manuscript
Brown: Design of study, data collection, data generation, and critical revision of manuscript
Randle and Hunter: Data acquisition and manuscript preparation
Luo and Rettiganti: Design of study, analysis and interpretation of the data; manuscript preparation and critical revision of manuscript
ClinicalTrials.gov Identifier: NCT01167855
References
- 1.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 3.Akinbami LJ, Sullivan SD, Campbell JD, et al. Asthma outcomes: healthcare utilization and costs. J.Allergy Clin.Immunol. 2012;129:S49–S64. doi: 10.1016/j.jaci.2011.12.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buescher PA, Jones-Vessey K. Using Medicaid data to estimate state- and county-level prevalence of asthma among low-income children. Matern Child Health J. 1999;3:211–6. doi: 10.1023/a:1022377405914. [DOI] [PubMed] [Google Scholar]
- 5.Chrischilles E, Ahrens R, Kuehl A, et al. Asthma prevalence and morbidity among rural Iowa schoolchildren. J Allergy Clin Immunol. 2004;113:66–71. doi: 10.1016/j.jaci.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Ownby DR. Asthma in rural America. Ann Allergy Asthma Immunol. 2005;95:S17–22. doi: 10.1016/s1081-1206(10)61005-8. [DOI] [PubMed] [Google Scholar]
- 7.Pesek RD, Vargas PA, Halterman JS, Jones SM, McCracken A, Perry TT. A comparison of asthma prevalence and morbidity between rural and urban schoolchildren in Arkansas. Ann.Allergy Asthma Immunol. 2010;104:125–31. doi: 10.1016/j.anai.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in the United States. Ambul Pediatr. 2002;2:382–7. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein JA, Lozano P, Farber HJ, Miroshnik I, Lieu TA. Underuse of controller medications among Medicaid-insured children with asthma. Arch Pediatr Adolesc Med. 2002;156:562–7. doi: 10.1001/archpedi.156.6.562. [DOI] [PubMed] [Google Scholar]
- 10.Perry TT, Jones SM, McCracken A, Vargas PA. Under-diagnosed and uncontrolled asthma: Findings in rural schoolchildren from the delta region. Annals. 2008;101:375–81. doi: 10.1016/S1081-1206(10)60313-4. [DOI] [PubMed] [Google Scholar]
- 11.Perry TT, Vargas PA, Brown RH, Watkins DR, McCracken A, Jones SM. Asthma Morbidity in High Risk Rural Children in the Delta Region of Arkansas. J Allergy Clin Immunol. 2008;121:S231. [Google Scholar]
- 12.Vargas PA, Bushmiaer M, Goel R, Jones CA, Feild CR, Tilford JM. Symptom profile and asthma control in school-aged children. Ambul Pediatr. 2005 doi: 10.1016/S1081-1206(10)61340-3. [DOI] [PubMed] [Google Scholar]
- 13.Brown W, Odenthal D. The uses of telemedicine to improve asthma control. J Allergy Clin Immunol Pract. 2015;3:300–1. doi: 10.1016/j.jaip.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Kew KM, Cates CJ. Remote versus face-to-face check-ups for asthma. Cochrane Database Syst Rev. 2016;4:CD011715. doi: 10.1002/14651858.CD011715.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson R. Telemedicine and Telehealth: The Potential to Improve Rural Access to Care. Am J Nurs. 2017;117:17–8. doi: 10.1097/01.NAJ.0000520244.60138.1c. [DOI] [PubMed] [Google Scholar]
- 16.Gennuso KP, Jovaag A, Catlin BB, Rodock M, Park H. Assessment of Factors Contributing to Health Outcomes in the Eight States of the Mississippi Delta Region. Prev Chronic Dis. 2016;13:E33. doi: 10.5888/pcd13.150440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felix H, Stewart MK. Health status in the Mississippi River Delta region. South Med J. 2005;98:149–54. doi: 10.1097/01.SMJ.0000145304.68009.02. [DOI] [PubMed] [Google Scholar]
- 18.Goldhagen J, Remo R, Bryant T, 3rd, et al. The health status of southern children: a neglected regional disparity. Pediatrics. 2005;116:e746–53. doi: 10.1542/peds.2005-0366. [DOI] [PubMed] [Google Scholar]
- 19.National asthma education and prevention program expert panel report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart Lung and Blood Institute. 2007:NIH Publication No.07-4051. 2012 [Google Scholar]
- 20.Greenley RN, Josie KL, Drotar D. Self-reported quality of life among inner-city youth with asthma: an empirical examination of the PedsQL 3.0 Asthma Module. Ann Allergy Asthma Immunol. 2008;100:106–11. doi: 10.1016/S1081-1206(10)60418-8. [DOI] [PubMed] [Google Scholar]
- 21.Schatz M, Zeiger RS, Vollmer WM, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006;130:43–50. doi: 10.1378/chest.130.1.43. [DOI] [PubMed] [Google Scholar]
- 22.Szefler SJ. Challenges in assessing outcomes for pediatric asthma. J Allergy Clin Immunol. 2001;107:S456–64. doi: 10.1067/mai.2001.114947. [DOI] [PubMed] [Google Scholar]
- 23.Josie KL, Greenley RN, Drotar D. Health-related quality-of-life measures for children with asthma: reliability and validity of the Children's Health Survey for Asthma and the Pediatric Quality of Life Inventory 3.0 Asthma Module. Ann.Allergy Asthma Immunol. 2007;98:218–24. doi: 10.1016/S1081-1206(10)60710-7. [DOI] [PubMed] [Google Scholar]
- 24.Asmussen L, Olson LM, Grant EN, Fagan J, Weiss KB. Reliability and validity of the Children's Health Survey for Asthma. Pediatrics. 1999;104:e71. doi: 10.1542/peds.104.6.e71. [DOI] [PubMed] [Google Scholar]
- 25.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual.Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 26.Wing A, Upton J, Svensson K, Weller P, Fletcher M, Walker S. The standardized and mini versions of the PAQLQ are valid, reliable, and responsive measurement tools. J Clin Epidemiol. 2012;65:643–50. doi: 10.1016/j.jclinepi.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Butz A, Pham L, Lewis L, et al. Rural children with asthma: impact of a parent and child asthma education program. J.Asthma. 2005;42:813–21. doi: 10.1080/02770900500369850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bursch B, Schwankovsky L, Gilbert J, Zeiger R. Construction and validation of four childhood asthma self-management scales: parent barriers, child and parent self-efficacy, and parent belief in treatment efficacy. J.Asthma. 1999;36:115–28. doi: 10.3109/02770909909065155. [DOI] [PubMed] [Google Scholar]
- 29.Donner A, Birkett N, Buck C. Randomization by cluster. Sample size requirements and analysis. Am J Epidemiol. 1981;114:906–14. doi: 10.1093/oxfordjournals.aje.a113261. [DOI] [PubMed] [Google Scholar]
- 30.Perry TT, Rettiganti M, Brown RH, Nick TG, Jones SM. Uncontrolled asthma and factors related to morbidity in an impoverished, rural environment. Ann.Allergy Asthma Immunol. 2012;108:254–9. doi: 10.1016/j.anai.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Valet RS, Perry TT, Hartert TV. Rural health disparities in asthma care and outcomes. J Allergy Clin Immunol. 2009;123:1220–5. doi: 10.1016/j.jaci.2008.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estrada RD, Ownby DR. Rural Asthma: Current Understanding of Prevalence, Patterns, and Interventions for Children and Adolescents. Curr Allergy Asthma Rep. 2017;17:37. doi: 10.1007/s11882-017-0704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ownby DR, Tingen MS, Havstad S, Waller JL, Johnson CC, Joseph CL. Comparison of asthma prevalence among African American teenage youth attending public high schools in rural Georgia and urban Detroit. J Allergy Clin Immunol. 2015;136:595–600. doi: 10.1016/j.jaci.2015.02.007. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner city asthma epidemic. J Allergy Clin Immunol. 2015;135:655–62. doi: 10.1016/j.jaci.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shalowitz MU, Berry CA, Quinn KA, Wolf RL. The relationship of life stressors and maternal depression to pediatric asthma morbidity in a subspecialty practice. Ambul Pediatr. 2001;1:185–93. doi: 10.1367/1539-4409(2001)001<0185:trolsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Yonas MA, Lange NE, Celedon JC. Psychosocial stress and asthma morbidity. Curr Opin Allergy Clin Immunol. 2012;12:202–10. doi: 10.1097/ACI.0b013e32835090c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medsker BH, Brew BK, Forno E, et al. Maternal depressive symptoms, maternal asthma, and asthma in school-aged children. Ann Allergy Asthma Immunol. 2017;118:55–60. doi: 10.1016/j.anai.2016.10.026. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosas-Salazar C, Apter AJ, Canino G, Celedon JC. Health literacy and asthma. J Allergy Clin Immunol. 2012;129:935–42. doi: 10.1016/j.jaci.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the School-Based Asthma Therapy trial. Arch.Pediatr.Adolesc.Med. 2011;165:262–8. doi: 10.1001/archpediatrics.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halterman JS, Tajon R, Tremblay P, et al. Development of School-Based Asthma Management Programs in Rochester, NY. Acad Pediatr. 2017 doi: 10.1016/j.acap.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morphew T, Scott L, Li M, et al. Mobile health care operations and return on investment in predominantly underserved children with asthma: the breathmobile program. Popul Health Manag. 2013;16:261–9. doi: 10.1089/pop.2012.0060. [DOI] [PubMed] [Google Scholar]
- 42.Bollinger ME, Morphew T, Mullins CD. The Breathmobile program: a good investment for underserved children with asthma. Ann Allergy Asthma Immunol. 2010;105:274–81. doi: 10.1016/j.anai.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Lemanske RF, Jr, Kakumanu S, Shanovich K, et al. Creation and implementation of SAMPRO: A school-based asthma management program. J Allergy Clin Immunol. 2016;138:711–23. doi: 10.1016/j.jaci.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]