Abstract
Background
The Fagerström Test for Nicotine Dependence (FTND), a derivation of the Fagerström Tolerance Questionnaire, was first published in 1991. The FTND remains one of the most widely used measures of nicotine dependence for studying genetic and epidemiological risk factors and the likelihood of smoking cessation. However, it is unclear whether secular trends in patterns of smoking alter the psychometric properties of the FTND and its interpretation.
Methods
We examined measurement invariance in the lifetime and current FTND scores across birth cohorts using participants drawn from six study samples (N=13,775).
Results
We found significant (p<0.05) measurement non-invariance in means and factor loadings of most FTND items by birth cohort, but effect sizes, ranging from r2=0.0001 to r2=0.0035, indicated that less than 0.5% of the model variance was explained by the measurement non-invariance for each factor loading. To assess its impact, we regressed the lifetime FTND latent variable on well-established factors associated with nicotine dependence (quitting smoking and the nicotinic acetylcholine receptor gene [CHRNA5] variant rs16969968, separately), and we observed that the regression coefficients were unchanged between models with and without adjustment for measurement non-invariance.
Conclusions
These findings suggest that possible FTND non-invariance that occurs across study samples of various birth years has a negligible impact on study results.
Keywords: measurement invariance, birth year, nicotine dependence, Fagerström Test for Nicotine Dependence, tobacco smoking
1. Introduction
Nicotine dependence studies are increasingly combining samples of participants to increase statistical power and make comparisons across groups of diverse age, race/ethnicity, and sex (Belsky et al., 2013; Bierut et al., 2007; Fagerstrom and Furberg, 2008; John et al., 2003). In studies that compare an underlying latent trait, like nicotine dependence, it is assumed that the instrument is measuring the trait on a consistent scale (i.e., it is invariant, measuring the trait similarly across groups) (Widaman and Reise, 1997). Measurement non-invariance is a type of measurement error that can bias study results toward or away from the null hypothesis, thereby leading to incorrect results in statistical comparisons and increasing the chances of both Type 1 and Type 2 errors. A non-invariant measure of nicotine dependence might incorrectly suggest that groups differ in their dependence levels (Schroeder and Moolchan, 2007) or the relation between dependence and other key variables (e.g., estimating the association between nicotine dependence and cessation, in which cessation is correlated with group membership like age (Johnson et al., 2008)). Measurement invariance may also obscure true associations, making them appear non-significant. Moreover, if a non-invariant measure is used as an inclusion criterion across groups, it might allow recruitment of groups that unintentionally differ on trait dependence because the same score may be differently related across groups to the underlying latent dependence (Robinson et al., 2006).
The Fagerström Test for Nicotine Dependence (FTND; also called the Fagerström Test for Cigarette Dependence (Fagerstrom, 2012)) is perhaps the most widely used measure for studying genetic and epidemiological risk factors of nicotine dependence and likelihood of smoking cessation (Haddock et al., 1999; Heatherton et al., 1991). It focuses on core dependence criteria, including heavy use/tolerance and withdrawal (Baker et al., 2012), and remains an especially strong predictor of smoking cessation (Fagerstrom et al., 2012; Fidler et al., 2011). Its use across diverse studies with varying participant characteristics makes measurement invariance a vital psychometric issue to support the accuracy of analytic findings across a variety of studies.
Secular trends in smoking might produce measurement non-invariance in longitudinal studies and studies that incorporate cross-sectional data collected at different times across multiple samples if the salience of dependence symptoms were affected across different birth cohorts. Smoking prevalence was relatively low in the U.S. before 1939 but increased up until the 1960s, when almost half of adults smoked. The 1964 Surgeon General’s report (U.S. Surgeon General’s Advisory Committee on Smoking and Health) marked another turning point, and smoking prevalence has fallen since (Figure S1)1 (U.S. Department of Health Human Services, 2014). A concomitant evolution in the social stigma and legal context of smoking have also affected smoking behaviors that are key indicators of dependence in the FTND (e.g., more difficulty refraining from smoking, fewer cigarettes per day [CPD] because smoking is forbidden in many public places), potentially making them more salient indicators of nicotine dependence.
Our study assessed measurement invariance in FTND by birth cohort and quantified the magnitude of significant effects. This address whether FTND scores have the same meaning when collected in different individuals studied at different times and whether results of studies using FTND across multiple birth cohorts are likely to be biased by this measurement error.
2. Methods
2.1 Study Samples
We used five study samples that collected FTND data from 1989 to 2013: African American Nicotine Dependence (AAND), Collaborative Genetic Study of Nicotine Dependence (COGEND), Center for Oral Health Research in Appalachia (COHRA1), Chronic Obstructive Pulmonary Disease Gene (COPDGene®), and University of Wisconsin Transdisciplinary Tobacco Use Research Center (UW-TTURC). See Supplementary Material and Table S11 for detailed sample descriptions. All protocols received Institutional Review Board approval at their respective sites. All study participants provided informed consent.
2.2 Measures
2.2.1 Exposure
Birth cohort was categorized into three groups (Figure S1)1 (U.S. Department of Health Human Services, 2014): (1) those born before 1945, a period of low but increasing cigarette consumption; (2) those born 1945–1975, the period of highest per capita cigarette consumption that peaked around the 1964 report (U.S. Surgeon General’s Advisory Committee on Smoking and Health); and (3) those born after 1975, when cigarette consumption steadily declined.
2.2.2 Outcomes
The FTND is a six-item questionnaire with scores ranging from 0 (no dependence) to 10 (highest dependence level). Our study focused on FTND scores based on habits among current smokers (current FTND) and compared them with results from when they reported smoking the most (lifetime FTND).
To evaluate the impact of any measurement non-invariance, we examined the relationship of lifetime FTND on quitting smoking. Quitting smoking was defined among lifetime smokers as either a self-reported status of “quit” or a frequency of 0 cigarettes smoked in the past month (depending on which measure was available).
Finally, we conducted analyses to evaluate the impact of any measurement non-invariance on rs16969968, the functional coding single nucleotide polymorphism (SNP) in the nicotinic acetylcholine receptor gene CHRNA5, that is robustly associated with nicotine dependence (Hancock et al., In-press). Rs16969968 was either genotyped or imputed with high quality (IMPUTE2 “info” quality metric = 0.99–1) in each of the five study samples and additively coded (ranging from 0 to 2 for the number of G alleles carried) for analysis, as previously described (Hancock et al., 2017a).
2.3 Statistical Analyses
2.3.1 Measurement Invariance
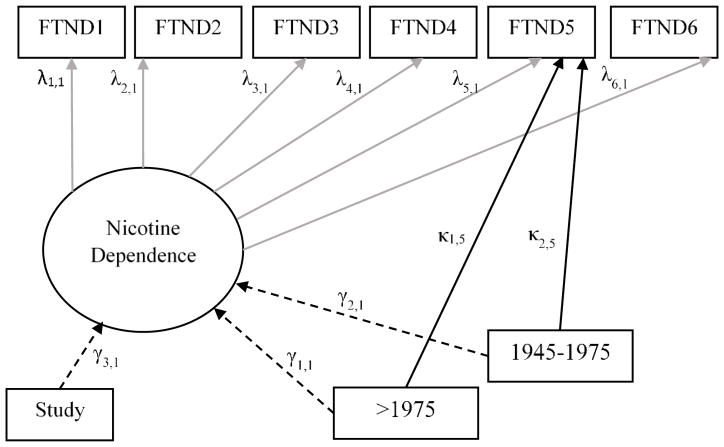
We tested item-level measurement invariance for birth cohort by using multiple-indicator, multiple-cause (MIMIC) models with a weighted least squares parameter estimates with standard errors and a mean- and variance-adjusted chi-square test statistic that used a full weight matrix (an estimator appropriate for use with categorical data) (Johnson et al., 2008; Kline, 2010). MIMIC models are structural equation models that can examine group-specific effects on item responses relative to a reference group, without mediation through a latent variable (i.e., nicotine dependence). Figure 1 provides an example of a MIMIC model testing an item-level difference in FTND item 5 (smoke more frequently during the first hours after waking than during the rest of the day) by birth cohort, with those born <1945 as the reference. Each FTND item was tested this way. Modeling the direct paths from each birth cohort, adjusting for nicotine dependence level, results in estimates of response differences attributable to measurement non-invariance. Models including direct paths from each birth cohort to each FTND item were compared to nested models, where direct paths were fixed to zero using Mplus difftest to determine the statistical significance of non-invariance (Muthén and Muthén, 1998–2015). Separate models were run with different reference groups to examine all pairwise differences (e.g., birth cohorts 2 and 3 vs. 1; birth cohorts 1 and 3 vs. 2). Results for other reference groups are presented in the Supplementary Material. For significant item-level invariance (direct-path parameter estimates with p<0.05 in the final model), eta-squared (r2) effect sizes were calculated (Ferguson, 1966).
FIGURE 1.
Example MIMIC Model Testing for Item-level Differences in FTND Item 5 (Smoke More Frequently During the First Hours After Waking than During the Rest of the Day) by Birth Cohort Using <1945 as the Reference Group.
Note: Study sample is dummy coded into indicator variables but has been simplified in this figure for illustrative purposes.
All MIMIC and regression models controlled for study sample and final models included an adjustment for measurement invariance. Statistical testing was two-tailed with α=0.05. Betas are reported as standardized parameter estimates. Analyses were conducted using Mplus version 7 (Muthén and Muthén, 1998–2015).
We present results of models that included a control variable for study sample because of different study enrollment criteria. There was overlap between birth cohort and study (e.g., some studies had younger or older participants), and to test whether controlling for study removed variance related to birth cohort, we conducted sensitivity analyses uncontrolled for study. Results (not shown) were substantively the same. Additionally, the cohort exposure group was changed from birth year to birth year plus 10 to mimic age of first tobacco exposure. Results (not shown) were consistent with the birth cohort findings.
2.3.2 Regression Analyses
Two regression models were tested to assess the impact of item-level invariance. First, quitting smoking was regressed on the FTND latent variable in baseline models that did not account for item invariance and compared to adjusted models that accounted for model invariance (Figure S2)1. Second, a similar procedure was conducted to evaluate the impact of measurement non-invariance on the association between FTND and Rs16969968. The regression coefficients were compared between baseline and adjusted models. All regression analyses, included sex (male or female), race (European American or African American), and study sample (AAND, COGEND, COHRA1, COPDGene, or UW-TTURC) as covariates. All statistical testing was two-tailed with p<.05 used to declare statistical significance.
3. RESULTS
3.1 Descriptive Statistics
Current FTND analyses included 9,865 participants (Table S1)1. AAND, COGEND, and COHRA1 had the lowest proportions of participants in the oldest birth cohorts; COPDGene had the oldest participants, and UW-TTURC had a more normally shaped distribution for the year of birth.
Current FTND scores differed significantly by birth cohort. FTND was highest among those born 1945–1975 (mean=5.1; Table 1), followed by those born <1945 (mean=4.7), and lowest among those born >1975 (mean=4.2). Moreover, birth cohort differed significantly for each FTND item (X2 p<0.05). Response patterns for FTND items varied greatly. For example, 27.1% of those born <1945 reported having their first cigarette within 5 minutes of waking, compared with 38.8% of those born 1945–1975, and 39.3% of those born >1975.
TABLE 1.
CURRENT FAGERSTRÖM TEST FOR NICOTINE DEPENDENCE (FTND) ITEM DISTRIBUTION BY BIRTH COHORT
| <1945 | 1945–1975 | >1975 | |
|---|---|---|---|
| N=806 | N=7794 | N=1265 | |
| Mean FTND (s.d.) a,b,c | 4.7 (2.2) | 5.1 (2.2) | 4.2 (2.1) |
| Time to first cigarette (FTND1) a,b | |||
| 60+ minutes | 16.0 | 9.0 | 11.8 |
| 31–60 minutes | 15.9 | 14.6 | 16.3 |
| 5–30 minutes | 41.0 | 37.6 | 32.6 |
| <5 minutes | 27.1 | 38.8 | 39.3 |
| Difficult to refrain (FTND2) a,b | |||
| No | 80.6 | 62.6 | 65.3 |
| Yes | 19.4 | 37.4 | 34.7 |
| Cigarette most hate to give up (FTND3) c | |||
| Any other | 36.8 | 35.1 | 39.4 |
| First one of the morning | 63.2 | 64.9 | 60.6 |
| Cigarettes smoked per day (FTND4) a,b,c | |||
| <=10 | 12.9 | 22.1 | 56.0 |
| 11–20 | 52.6 | 49.8 | 35.5 |
| 21–30 | 18.4 | 17.8 | 7.3 |
| 30+ | 16.1 | 10.3 | 1.2 |
| Smoke more in the morning (FTND5) a,c | |||
| No | 57.6 | 51.7 | 59.6 |
| Yes | 42.4 | 48.3 | 40.4 |
| Smoke while sick (FTND6) a,b,c | |||
| No | 75.2 | 55.0 | 61.6 |
| Yes | 24.8 | 45.0 | 38.4 |
Difference between first birth cohort and second birth cohort are significant at p<0.05
Difference between first birth cohort and third birth cohort are significant at p<0.05
Difference between second birth cohort and third birth cohort are significant at p<0.05
3.1.1 Measurement Invariance
Table 2 presents the results of baseline and fitted MIMIC models testing for birth cohort measurement invariance for current FTND models using birth <1945 as the reference. This model demonstrated poor fit (X2= 4364.34 (39), p<.001) with comparative fit index (CFI)=0.478 and root mean square error of approximation (RMSEA)=0.108 and a worsening fit with adjustment for FTND measurement non-invariance (X2=4213.75 (20), p<.001, CFI=0.495, and RMSEA=0.123) (Hu and Bentler, 1999). Results evaluating measurement invariance for current FTND using 1945–1975 as a reference group were largely similar to analyses using <1945 as the reference, including a low level of measurement non-invariance that accounted for little of the model variance and did not greatly affect the modelling results when invariance was adjusted for (Table S2)1.
TABLE 2.
Baseline and Final MIMIC Model Estimates of Current Fagerström Test for Nicotine Dependence (FTND): Testing Measurement Invariance Across Birth Cohort Using <1945 as the Reference Group
| Parameter | β, Baseline Model | β, Final Model | Effect Size r2 |
|---|---|---|---|
| Standardized loadings | |||
| FTND1 | 0.680*** | 0.680*** | |
| FTND2 | 0.574*** | 0.560*** | |
| FTND3 | 0.415*** | 0.419*** | |
| FTND4 | 0.454*** | 0.466*** | |
| FTND5 | 0.463*** | 0.466*** | |
| FTND6 | 0.598*** | 0.583*** | |
| Nicotine dependence on cohort | |||
| Cohort: <1945 | Reference | Reference | |
| Cohort: 1945–1975 | 0.171*** | 0.178*** | |
| Cohort: >1975 | 0.034 | 0.039 | |
| Direct effects | |||
| FTND1 Cohort: < 1945 |
Reference | ||
| Cohort: 1945–1975 | n.s. | ||
| Cohort: >1975 | n.s. | ||
| FTND2 Cohort: <1945 |
Reference | ||
| Cohort: 1945–1975 | 0.100*** | 0.0015 | |
| Cohort: >1975 | 0.126*** | 0.0024 | |
| FTND3 Cohort: <1945 |
Reference | ||
| Cohort: 1945–1975 | −0.041* | 0.0003 | |
| Cohort: >1975 | −0.061** | 0.0007 | |
| FTND4 Cohort: <1945 |
Reference | ||
| Cohort: 1945–1975 | −0.118*** | 0.0022 | |
| Cohort: >1975 | −0.150*** | 0.0035 | |
| FTND5 Cohort: <1945 |
Reference | ||
| Cohort: 1945–1975 | −0.046* | 0.0004 | |
| Cohort: >1975 | −0.061** | 0.0007 | |
| FTND6 Cohort: <1945 |
Reference | ||
| Cohort: 1945–1975 | 0.103*** | 0.0016 | |
| Cohort: >1975 | 0.111*** | 0.0019 | |
| Model fit summary | |||
| Chi-Square (d.f.), p- value | 4364.34 (39), p<.001 | 4213.75 (29), p<.001 | |
| CFI | 0.478 | 0.495 | |
| RMSEA | 0.108 | 0.123 |
Notes: FTND1: Time to first cigarette; FTND2: Difficult to refrain in places; FTND3 Cigarette most hate to give up; FTND4: Cigarettes Per Day; FTND5: Smoke more in the morning; FTND6: Smoke while sick. n.s., not significant. The baseline model is unadjusted for measurement invariance. The final model adjusts for significant measurement invariance. All models also controlled for study sample.
p<0.05,
p<0.01,
p<0.001.
Mean latent variable differences in current dependence were examined for both birth cohort reference groups. Mean dependence among those born <1945 was significantly different than those born 1945–1975; this difference remained largely unchanged after accounting for measurement non-invariance (unadjusted β=0.171, p<.001, adjusted β=0.178, p<.001). Birth cohorts 1945–1975 and <1945 did not differ significantly in mean dependence in both unadjusted and adjusted models (unadjusted β=0.034, p=0.118, adjusted β=0.039, p=0.149).
Evaluating item-level non-invariance in the model using birth cohort <1945 as the reference, 10 parameters show statistically significant measurement non-invariance. Time to first cigarette in the morning was the only current FTND item that did not show measurement non-invariance. The effect sizes for these direct paths ranged from r2=0.0003 to r2=0.0035 indicating that, despite statistical significance, little variance (<0.5%) of the model was explained by measurement non-invariance. Fewer significant parameters were noted for the model using birth cohort 1945–1975 as the reference group, and they were of lower magnitude (Table S2)1. A similar pattern of results was observed for lifetime FTND.
3.1.2 Regression Analyses
Chi-square tests for differences in quitting smoking by birth cohort indicated that those in the oldest cohort were the most likely to have quit (Table S3)1. For example, among participants with lifetime FTND, 49.2 % of those born before 1945 had quit, compared with 22.4% of those born between 1945 and 1975 and 15.5% of those born after 1975. To determine whether these differences were due to measurement non-invariance rather than other causes, regression models were created with and without accounting for non-invariance (Table 3). In baseline models for the birth cohort (using birth cohort 1945–1975), higher lifetime FTND scores were associated with less quitting, as expected (odds ratio =0.48, 95% confidence interval [CI] =0.40–0.44, p<.001). After adjusting for measurement non-invariance, the odds ratio and 95% CI estimates did not change.
Table 3.
Regression Models for Quitting Cigarette Smoking and rs16969968 Regressed on Lifetime FTND and Birth Cohort Before and After Adjusting for Measurement Non-Invariance.
| Baseline1 Model | Invariance Adjusted Model | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Model | Parameter | β (S.E.) | OR | 95% CI | β (S.E.) | OR | 95% CI |
|
| |||||||
| Quit Smoking (Logistic Model) | FTND | −0.74 (0.01)*** | 0.48 | 0.40–0.44 | −0.74 (0.01)*** | 0.48 | 0.40–0.44 |
| Cohort <1945 | −0.30 (0.05)*** | 0.74 | 0.34–0.56 | −0.31 (0.05)*** | 0.73 | 0.34–0.56 | |
| Cohort >1975 | −0.42 (0.05)*** | 0.67 | 0.23–0.40 | −0.42 (0.05)*** | 0.67 | 0.23–0.40 | |
| Chi-Square (d.f.) | 1002.87 (48)*** | N/A | N/A | 978.89 (44)*** | N/A | N/A | |
| CFI | 0.960 | N/A | N/A | 0.961 | N/A | N/A | |
| RMSEA | 0.062 | N/A | N/A | 0.064 | N/A | N/A | |
|
| |||||||
| Additive rs16969968 genotype (Linear Model) | FTND | −0.09 (0.02)*** | N/A | −0.16–0.07 | −0.09 (0.02)*** | N/A | −0.16–0.07 |
| Cohort <1945 | 0.01 (0.04) | N/A | −0.07–0.11 | 0.01 (0.04) | N/A | −0.20–0.26 | |
| Cohort >1975 | 0.02 (0.04) | N/A | −0.11–0.07 | 0.02 (0.04) | N/A | −0.30–0.19 | |
| Chi-Square (d.f.) | 871.045 (48)*** | N/A | N/A | 849.23 (44)*** | N/A | N/A | |
| CFI | 0.060 | N/A | N/A | 0.062 | N/A | N/A | |
| RMSEA | 0.954 | N/A | N/A | 0.955 | N/A | N/A | |
N/A, not applicable. All models controlled for study sample, sex, and race. Birth cohort 1945–1975 was used as reference.
p<0.05,
p<0.01,
p<0.001.
The baseline model is unadjusted for measurement invariance.
Similar findings were observed when examining the association between rs16969968 and lifetime FTND. Consistent with prior studies (Hancock et al., In-press), carrying the rs16969968-G protective allele was associated with a lower lifetime FTND score (β= −0.09 (0.02), 95% CI= −0.16–−0.07, p<.001). Moreover, as expected given the low level of measurement non-invariance detected in MIMIC modes, the association between rs16969968 and FTND did not change when controlling for the measurement non-invariance.
4. Discussion
Measurement invariance is important when attempting to compare groups on underlying latent characteristics like nicotine dependence. Measurement non-invariance can lead to incorrect conclusions about the differences across groups, obscuring differences that exist or creating differences due to measurement non-invariance rather than actual differences, as well as add heterogeneity to analyses that combine cohorts from different eras (e.g., meta-analytic genome-wide association studies). For the first time, we assessed FTND measurement invariance by birth cohort using cross-sectional studies that collectively comprise individuals born from 1926 through 1998. The pattern of responses to current FTND items has changed over time, including higher CPD and lower tendency to smoke when sick in the oldest respondents (birth cohort <1945). Thus, verifying measurement invariance across different birth cohorts is important for instilling confidence in study results that combine or compare FTND across different age groups.
Our findings revealed some statistically significant non-invariance for FTND items, including CPD, which is often collected and analyzed on its own. However, the effect sizes of the item-level non-invariance accounted for <0.5% of the model variance, indicating that while it is advisable to control for year of birth in analyses using FTND, failure to do so should not greatly alter analytic results, which was confirmed through analyses examining the impact of adjusting for the measurement non-invariance in regression models. In models evaluating the association between FTND and quitting smoking, the odds ratios, and 95% CI estimates were unchanged when controlling for the measurement non-invariance. Moreover, even in genetic analyses where the effects of SNPs often account for small portions of the variance in nicotine dependence (altogether <15%; Hartz, et al., 2017), the measurement non-invariance is likely to have little demonstrable effect, as evidenced by β and 95% CI estimates of the FTND and rs16969968 association being unchanged when controlling for the measurement non-invariance. Therefore, although smoking patterns have changed over time, these changes have not affected the ability of the FTND to assess nicotine dependence.
This study has limitations. First, some study samples had small coverage over some birth cohorts (e.g., AAND had no one born <1945). Due to differences in recruitment criteria, we controlled for study sample in all models, but the overlap in sample and coverage may have led to over controlling for invariance with the addition of study sample in the model. However, no single study sample provided all data for a birth cohort, and sensitivity analyses indicated similar results when controlling versus not controlling for the study sample. A related concern involved the different eligibility criteria for the included studies, but controlling for sample should have helped to control this variability. Notably, the overall model fit indices revealed poor fit in baseline and final MIMIC models. While this suggests substantial variation in the ability of the model to reproduce the overall data, there was no notable difference in fit between models that did and did not adjust for measurement non-invariance. This suggests that model fit was driven by variation in the data (e.g., current FTND had poorer model fit than lifetime FTND, studies differed in their inclusion criteria) and not by measurement non-invariance. Finally, variation associated with birth cohort could be due to the participants’ age at assessment. However, this concern is reduced by broad variation in the median birth year, even among studies with similar recruitment periods (Table S1)1. Post hoc analyses evaluating model fit while controlling for study sample, sex, and race further support that the variability across studies may be a primary driver of poor fit, as model fit improved modestly (RMSEA = 0.090 vs. 0.108 and CFI= 0.595 vs. 0.478 for the unadjusted vs. covariate-adjusted models). Moreover, model fit was good for regression models that included latent variable modeling of FTND and covariates.
These findings build upon prior analyses across important demographic groups. Schroeder and Moolchan (2007) found some measurement non-invariance in FTND across European and African ancestry participants, which we confirmed but further found the FTND to have only minor measurement non-invariance by sex and race (Johnson et al., 2008). Altogether, the prior and current findings increase confidence for researchers concluding comparisons made across birth cohorts using the FTND, as manifest scores have similar relations with an underlying dependence dimension regardless of birth cohort. Likewise, meta-analyses incorporating results from different birth cohorts are unlikely biased by non-invariance–induced heterogeneity. The FTND, therefore, provides a robust basis for comparing nicotine dependence across populations and informing public health research.
Supplementary Material
Highlights.
The six-item Fagerström Test for Nicotine Dependence (FTND) is widely used.
Secular trends in smoking may have altered psychometric properties of the FTND.
We found item-level invariance by birth year, but effect sizes were very small.
The utility of the FTND is reinforced, and adjusting for birth year is ideal.
Not adjusting for birth year should have negligible impact on study results.
Acknowledgments
Role of Funding Source
This work was supported by the National Institutes of Health (NIH), National Institute on Drug Abuse (NIDA) grant numbers R01 DA035825, R01 DA036583, and R01 DA042090. Funding support for the collection of the African American Nicotine Dependence (AAND) dataset and its analyses were supported by NIH/NIDA grant number R01 DA025888. Funding support for collection of the Collaborative Genetic Study of Nicotine Dependence (COGEND) dataset and its analyses were supported by NIH, National Cancer Institute (NCI) grant number P01 CA089392. Funding support for the Center for Oral Health Research in Appalachia (COHRA1) study cohort was provided by NIH, National Institute of Dental and Craniofacial Research (NIDCR) grant numbers U01-DE018903 and R01-DE014899. Data and samples were provided by the Center for Oral Health Research in Appalachia (COHRA), a collaboration of the University of Pittsburgh and West Virginia University funded by NIDCR R01-DE014899. The COPDGene® project was supported by award numbers R01 HL089897 and R01 HL089856 from the National Heart, Lung, and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of NHLBI or NIH. The COPDGene® project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprising AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline. Funding support for the Study of Addiction: Genetics and Environment (SAGE) was provided through NIH GEI grant number U01 HG004422. Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center grant number U01 HG004446. Support for collection of datasets and samples used for this manuscript was provided through COGA grant number U10 AA008401 and FSCD grant number R01 DA013423. SAGE data were obtained via dbGaP accession number phs000092.v1.p1. The University of Wisconsin–Transdisciplinary Tobacco Use Research Center (UW-TTURC) sample was accessed via dbGaP as part of “Genetic Architecture of Smoking and Smoking Cession” (accession number phs000404.v1.p1). Funding support for collection of the UW-TTURC dataset and samples was provided by P50 DA019706 and P50 CA084724.
The authors acknowledge investigators of the COPDGene® project core units: Administrative (James Crapo [Principal Investigator] and Edwin Silverman [Principal Investigator]), Barry Make, and Elizabeth Regan); Genetic Analysis (Terri Beaty, Nan Laird, Christoph Lange, Michael Cho, Stephanie Santorico, Dawn DeMeo, Nadia Hansel, Craig Hersh, Peter Castaldi, Merry-Lynn McDonald, Emily Wan, Megan Hardin, Jacqueline Hetmanski, Margaret Parker, Marilyn Foreman, Brian Hobbs, Robert Busch, Adel El-Bouiez, Megan Hardin, Dandi Qiao, Elizabeth Regan, Eitan Halper-Stromberg, Ferdouse Begum, Sungho Won); Imaging (David Lynch, Harvey Coxson, MeiLan Han, Eric Hoffman, Stephen Humphries Francine Jacobson, Philip Judy, Ella Kazerooni, John Newell, Jr., Elizabeth Regan, James Ross, Raul San Jose Estepar, Berend Stoel, Juerg Tschirren, Eva van Rikxoort, Bram van Ginneken, George Washko, Carla Wilson, Mustafa Al Qaisi, Teresa Gray, Alex Kluiber, Tanya Mann, Jered Sieren, Douglas Stinson, Joyce Schroeder, Edwin Van Beek); Pulmonary Function Testing Quality Assurance (Robert Jensen); Data Coordinating Center and Biostatistics (Douglas Everett, Anna Faino, Matt Strand, Carla Wilson); and Epidemiology (Jennifer Black-Shinn, Gregory Kinney, Katherine Pratte). The authors also acknowledge the clinical center investigators: Jeffrey Curtis, Carlos Martinez, Perry G. Pernicano, Nicola Hanania, Philip Alapat, Venkata Bandi, Mustafa Atik, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Amit Parulekar, Arun Nachiappan, Dawn DeMeo, Craig Hersh, George Washko, Francine Jacobson, R. Graham Barr, Byron Thomashow, John Austin, Belinda D’Souza, Gregory D.N. Pearson, Anna Rozenshtein, Neil MacIntyre, Jr., Lacey Washington, H. Page McAdams, Charlene McEvoy, Joseph Tashjian, Robert Wise, Nadia Hansel, Robert Brown, Karen Horton, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Janos Porszasz, Hans Fischer, Matthew Budoff, Dan Cannon, Harry Rossiter, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Marilyn Foreman, Gloria Westney, Eugene Berkowitz, Russell Bowler, David Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Robert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditti Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, Gloria Vega-Sanchez, Mark Dransfield, William Bailey, J. Michael Wells, Surya Bhatt, Hrudaya Nath, Joe Ramsdell, Paul Friedman, Xavier Soler, Andrew Yen, Alejandro Cornellas, John Newell, Jr., Brad Thompson, MeiLan Han, Ella Kazerooni, Fernando Martinez, Joanne Billings, Tadashi Allen, Frank Sciurba, Divay Chandra, Joel Weissfeld, Carl Fuhrman, Jessica Bon, Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, and Mario Ruiz.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
CG, EOJ, and DBH designed the study. EOJ, NLS, SML, TBB, DWM, MLM, JEH, and LJB provided the study sample data. CG performed the statistical analyses. CG prepared the manuscript with contributions by DBH. All authors contributed to the interpretation of the study’s findings as well as reviewed and approved of the manuscript before submission.
Conflict of Interest
LJB and the spouse of NLS are listed as inventors on Issued U.S. Patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. The other authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addict. 2012;107:263–275. doi: 10.1111/j.1360-0443.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrington H, Houts R, Meier M, Sugden K, Williams B, Poulton R, Caspi A. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry. 2013;70:534–542. doi: 10.1001/jamapsychiatry.2013.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K, Furberg H. A comparison of the Fagerstrom Test for Nicotine Dependence and smoking prevalence across countries. Addict. 2008;103:841–845. doi: 10.1111/j.1360-0443.2008.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: A pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14:1467–1473. doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- Ferguson GA. Statistical Analysis in Psychology and Education. McGraw-Hill; New York, N.Y: 1966. p. 244. [Google Scholar]
- Fidler JA, Shahab L, West R. Strength of urges to smoke as a measure of severity of cigarette dependence: comparison with the Fagerstrom Test for Nicotine Dependence and its components. Addict. 2011;106:631–638. doi: 10.1111/j.1360-0443.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- Haddock CK, Lando H, Klesges RC, Talcott GW, Renaud EA. A study of the psychometric and predictive properties of the Fagerstrom Test for nicotine dependence in a population of young smokers. Nicotine Tob Res. 1999;1:59–66. doi: 10.1080/14622299050011161. [DOI] [PubMed] [Google Scholar]
- Hancock DB, Markunas CA, Bierut LJ, Johnson EO. Human genetics of addiction: New insights and future directions. Curr Psychiatry Rep. doi: 10.1007/s11920-018-0873-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Guo Y, Reginsson GW, Gaddis NC, Lutz SM, Sherva R, Loukola A, Minica C, Han Y, Markunas CA, Young KA, Gu F, McNeil DW, Qaiser B, Glasheen C, Olson S, Landi MT, Madden P, Farrer LA, Vink J, Saccone NL, Neale MC, Kranzler HR, McKay J, Hung J, Amos CI, Marazita ML, Boomsma DI, Baker TB, Gelernter J, Kaprio J, Caporaso NE, Thorgeirsson TE, Hokanson JE, Bierut LJ, Stefansson K, Johnson EO. Multi-ancestry genome-wide association study identifies novel DNMT3B SNP that contributes to nicotine dependence and influences RNA expression in cerebellum. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.193. In press. https://doi.org/10.1038/mp.2017.193. [DOI] [PMC free article] [PubMed]
- Hartz SM, Horton AC, Hancock DB, Baker TB, Caporaso NE, Chen LS, Hokanson JE, Lutz SM, Marazita ML, McNeil DW, Pato CN, Pato MT, Johnson EO, Bierut LJ. Genetic correlation between smoking behaviors and schizophrenia. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.02.022. In Press. https://doi.org/10.1016/j.schres.2017.02.022. [DOI] [PMC free article] [PubMed]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- John U, Meyer C, Hapke U, Rumpf HJ, Schumann A, Adam C, Alte D, Ludemann J. The Fagerstrom test for nicotine dependence in two adult population samples-potential influence of lifetime amount of tobacco smoked on the degree of dependence. Drug Alcohol Depend. 2003;71:1–6. doi: 10.1016/s0376-8716(03)00038-3. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Morgan-Lopez AA, Breslau N, Hatsukami DK, Bierut LJ. Test of measurement invariance of the FTND across demographic groups: assessment, effect size, and prediction of cessation. Drug Alcohol Depend. 2008;93:260–270. doi: 10.1016/j.drugalcdep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. The Guilford Press; New York: 2010. [Google Scholar]
- Muthén L, Muthén B. Mplus User’s Guide. 7. Muthén & Muthén; Los Angeles, C.A: 1998–2015. [Accessed February 2, 2018]. https://www.statmodel.com/download/usersguide/MplusUserGuideVer_7.pdf. [Google Scholar]
- Robinson ML, Schroeder JR, Moolchan ET. Adolescent smokers screened for a nicotine replacement treatment trial: correlates of eligibility and enrollment. Nicotine Tob Res. 2006;8:447–454. doi: 10.1080/14622200600670413. [DOI] [PubMed] [Google Scholar]
- Schroeder JR, Moolchan ET. Ethnic differences among adolescents seeking smoking cessation treatment: a structural analysis of responses on the Fagerstrom Test for Nicotine Dependence. Nicotine Tob Res. 2007;9:137–145. doi: 10.1080/14622200601078400. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health Human Services. The health consequences of smoking 50 years of progress. [Accessed February 2, 2018];A report of the Surgeon General. 2014 https://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html.
- U.S. Surgeon General’s Advisory Committee on Smoking Health. Smoking and health: Report of the Advisory Committee to the Surgeon General of the public health service. [Accessed February 2, 2018];US Public Health Service. 1964 https://profiles.nlm.nih.gov/ps/access/nnbbmq.pdf.
- Widaman KF, Reise SP. Exploring the measurement invariance of psychological instruments: Applications in the substance use domain. In: Bryant KJ, Eindle M, West SG, editors. The science of prevention: Methodological advances from alcohol and substance abuse research. 1997. pp. 281–324. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.