Abstract
Alcohol misuse is associated with dysfunction of the amygdala-prefrontal cortical circuit. The amygdala and its cortical targets decrease activity during a variety of task challenges in individuals engaged in problem drinking. On the other hand, it is less clear how amygdala resting state functional connectivity (rsFC) may be altered in association with alcohol misuse and whether such changes are restricted to prefrontal cortical structures. Further, the influences of comorbid substance use and depression and potential sex differences have not been assessed in earlier work. Here, with fMRI data from a Nathan Kline Institute/Rockland sample of 83 non-dependent alcohol drinkers (26 men), we addressed changes in whole brain rsFC of the amygdala in association with problem drinking as indexed by an alcohol involvement score. Imaging data were processed with Statistical Parametric Mapping following standard routines and all results were examined at voxel p<0.001 uncorrected in combination with cluster p<0.05 corrected for false discovery rate. Alcohol misuse was correlated with decreased amygdala connectivity with the dorsal anterior cingulate cortex (dACC) irrespective of depression and other substance use. Changes in amygdala-dACC connectivity manifested in the latero-basal subdivision of the amygdala. Further, men as compared to women showed a significantly stronger relationship in decreased amygdala-dACC connectivity and problem drinking, although it should be noted that men also showed a trend toward higher alcohol involvement score than women. The findings add to a growing literature documenting disrupted amygdala-prefrontal cortical functions in relation to alcohol misuse.
Keywords: amygdala, alcohol, dACC, resting state functional connectivity
1. Introduction
The amygdala plays a critical role in processing emotional stimuli, showing vigorous response during emotional facial recognition (Morris et al., 1999; Ohman, 2005; Vuilleumier et al., 2001) and interacting with a large array of cortical and subcortical structures to support emotional experience (Stein et al., 2007). In particular, the amygdala-prefrontal cortical circuit has been widely studied for its role in emotional control (Hariri et al., 2000; Kim et al., 2011; Oscar-Berman and Marinkovic, 2007), especially in regulating responses to fear (Hariri et al., 2000) and resolving valence ambiguity (Neta et al., 2009). In decision making tasks involving surprising faces, the medial prefrontal cortex (PFC) increased and amygdala decreased activation when the surprise was seen as a benefit or gain, and vice versa when the surprise was interpreted negatively as a threat or loss (Kim et al., 2004). With amygdala’s innate response to negative valence, the medial PFC may help support a balance between top-down and bottom-up processes in emotion regulation (Kienast et al., 2013).
Alcohol is known to influence emotion, increasing positive and weakening negative affects (Sher and Grekin, 2007). Although less of a focus for studies of the etiology of alcohol use disorders, alcohol affects amygdala and prefrontal circuit activities. Alcohol enhances the activities of gamma aminobutyric acid (GABA) interneurons in the nucleus accumbens and amygdala, and dampens PFC functions in emotional and cognitive processing (George et al., 2012). Acute administration of alcohol in healthy adults resulted in decreased amygdala activation during exposure to threatening facial expressions (Gilman et al., 2008; Sripada et al., 2011). A common precipitator of drinking, stress triggers the release of corticotropin-releasing factor via the amygdala hypothalamus circuit, resulting in increased anxiety that can be alleviated by alcohol consumption (Koob and Volkow, 2016). Compared to non-drinking controls, alcoholic patients showed lower amygdala activation during a facial recognition task (Marinkovic et al., 2009). Children with a family history of alcoholism showed decreased amygdala activation when they identified negative facial emotions (Glahn et al., 2007). Social drinkers showed reduced amygdala activity to a risk-taking motor response (Yan and Li, 2009) and to fearful and angry faces after drinking (Sripada et al., 2011). Compared to healthy controls, patients with alcohol dependence showed decreased amygdala PFC functional connectivity during a Trier social stress task (Wade et al., 2017). Acute alcohol as compared to placebo administration decreased right amygdala PFC connectivity in heavy social drinkers viewing angry faces (Gorka et al., 2013). Weaker functional connectivity between the amygdala and superior frontal gyrus was reported in children with a family history of alcoholism viewing emotional stimuli (Cservenka et al., 2014) as well as in alcoholic patients performing an emotional face recognition task (O’Daly et al., 2012) and an inhibition task (Courtney et al., 2013). These studies suggest disrupted amygdala-prefrontal circuitry in association with alcohol use and misuse or a family history of alcoholism.
The amygdala interacts with the PFC to support emotional regulation. On the other hand, the relationship between the intrinsic amygdala-PFC connectivity and alcohol consumption or problem drinking has remained under-studied. Resting state functional connectivity (rsFC) has been widely used to examine functional organization and integrity of cerebral networks (Rosazza and Minati, 2011; van den Heuvel and Hulshoff Pol, 2010; Zhang et al., 2012; Zhang and Li, 2012). Previous work investigated the effects of alcohol on the rsFC seeded from the amygdala. For instance, Müller-Oehring et al. (2015) reported decreased rsFC with the frontal executive and subcortical reward networks in correlation with poor working memory performance in alcoholic patients as compared to healthy controls. In male adolescents and young adults, the amygdala-prefrontal including orbitofrontal connectivity was negatively associated with the severity of alcohol consumption (Peters et al., 2015) and predicted alcohol use in the same cohort of participants two years later (Peters et al., 2017). Together, these studies suggest that alcohol might disrupt resting state amygdala-prefrontal cortical connectivity.
In the current work, we used whole-brain analysis to examine the rsFC of the amygdala and its subdivisions as a function of problem drinking in adult non-dependent alcohol drinkers. In particular, as sex differences are widely implicated in the etiological processes and clinical manifestations of drug and alcohol addiction (Becker and Hu, 2008; Erol and Karpyak, 2015), we examined whether men and women differ in amygdala rsFC. As substance misuse is common in alcohol drinkers, an additional goal is to examine whether comorbid use of other substances may influence changes in amygdala rsFC. We posited decreased amygdala-prefrontal connectivity with increased severity of alcohol misuse and sex differences in the pattern of connectivity changes.
2. Methods
2.1 Data set
Resting-state fMRI data were obtained from the Nathan Kline Institute (NKI)/Rockland sample (Nooner et al., 2012) of the 1,000 Functional Connectomes project (http://www.nitrc.org/projects/fcon_1000/). This dataset has been used in previous research of brain circuitry in healthy adults and patients with medical and psychiatric conditions (Lee and Xue, 2017; Nakamura and Ikuta, 2017; Reid et al., 2016; Tremeau et al., 2014). Scans were collected using a multiband EPI sequence (Xu et al., 2012) with the following parameters: repetition time (TR)/echo time (TE) = 2500/30 ms, voxel size = 3.0 × 3.0 × 3.0 mm3, and 38 slices, covering the whole brain. Individual’s images were viewed one by one to ensure that the whole brain was covered.
Participants were assessed for drinking behavior with the NIDA Quick Screen V1.01 (11+). A total of 83 adult participants (18–89 years of age; 26 males; one five-minute scan per participant under identical imaging protocol) with a substance involvement (SI) score of alcohol use were included in the current cohort. Briefly, the screening asked about participants’ drinking behavior including the frequency, desire, amount of alcohol, as well as social disruptions that resulted from alcohol drinking, including whether they drink or not during their lifetime (yes/no), and drinking behavior in the past year and past 3 months. The total SI score of alcohol was summed from scores of each alcohol related question with a higher SI score indicating higher involvement in alcohol misuse (Appendix I). Of the 83 participants, 14 were diagnosed with drug dependence or abuse (1 cocaine dependence, 3 cannabis dependence, and 10 cannabis abuse), and 10 with a depressive disorder, according to the ICD-9.
2.2 Imaging data preprocessing
Brain imaging data were preprocessed using Statistical Parametric Mapping (SPM 8, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Standard image preprocessing was performed. Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were co-registered with the high resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (Ashburner and Friston, 1999; Friston et al., 1995). The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
Additional preprocessing was applied to reduce spurious blood-oxygenation-level dependent (BOLD) variances that were unlikely to reflect neuronal activity (Fair et al., 2007; Fox and Raichle, 2007; Fox et al., 2005; Rombouts et al., 2003). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, white matter, and whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Previous studies suggested that BOLD fluctuations below a frequency of 0.1Hz contribute to regionally specific BOLD correlations (Cordes et al., 2001). Thus, we applied a temporal band-pass filter (0.009 < f < 0.08 Hz) to the time course in order to obtain low-frequency fluctuations, as in previous studies (Fair et al., 2007; Fox and Raichle, 2007; Fox et al., 2005; Lowe et al., 1998).
2.3 Head motion
As extensively investigated by Van Dijk and his colleagues (2012), micro head motion (>0.1mm) is an important source of spurious correlations in resting state functional connectivity analysis. Therefore, we applied a “scrubbing” method proposed by Power and colleagues (Power et al., 2012) and successfully applied in previous studies (Power et al., 2012; Smyser et al., 2010; Tomasi and Volkow, 2014) to remove time points affected by head motions. Briefly, for every time point t, we computed the framewise displacement given by FD(t) = |Δdx(t)| + |Δdy(t)| + |Δdz(t)| + r|α(t)| + r|β(t)| + r|γ(t)|, where (dx, dy, dz)and (α, β, γ) are the translational and rotational movements, respectively, and r (= 50mm) is a constant that approximates the mean distance between center of MNI space and the cortex and transform rotations into displacements (Power et al., 2012). The second head movement metric was the root mean square variance (DVARS) of the differences in % BOLD intensity I(t) between consecutive time points across brain voxels, computed as follows: , where the brackets indicate the mean across brain voxels. Finally, to compute each subject’s correlation map, we removed every time point that exceeded the head motion limit FD(t)>0.5mm or DVARS(t)>0.5% (Power et al., 2012; Tomasi and Volkow, 2014). On average, 1% of the time points were removed across participants. We excluded individuals who moved > 1 voxel in any directions and those with greater than 10–20% of volumes affected by micromovements.
2.4 Seed regions: amygdala
The mask of amygdala was obtained from the SPM Anatomy Toolbox Version 2.2c (Eickhoff et al., 2006; Eickhoff et al., 2007; Eickhoff et al., 2005). It was created based on the cytoarchitectonic probabilistic map where four subdivisions were segregated, including the superficial, latero-basal, centro-medial, and amygdalostriatal transition area (Amunts et al., 2005) (Figure 1). In rsFC analyses, we focused on the whole amygdala, as well as the left and right amygdala separately, because of potential hemispheric functional differences (Baas et al., 2004), as seed regions. We also examined each of the four amygdala subdivisions and left and right subdivision in additional analyses to explore which subdivision accounts for the findings observed for the whole amygdala.
Figure 1.
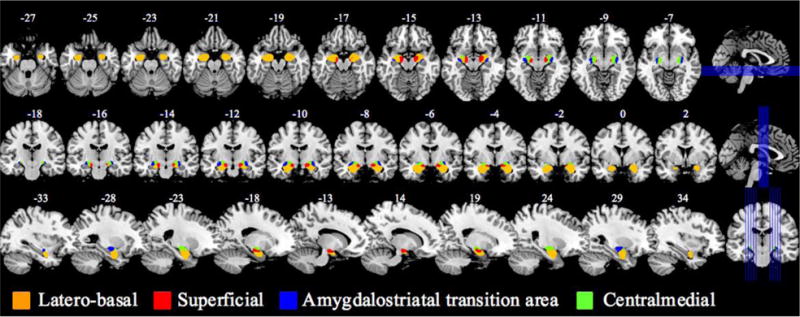
The amygdala and its subdivisions.
2.5 Seed region-based linear correlation and random effects analysis
The BOLD time courses were averaged spatially over each seed region. For individual participants, we computed the correlation coefficient between the averaged time course of each seed region and the time courses of all other brain voxels. To assess and compare the rsFC, we converted these image maps, which were not normally distributed, to z score maps by Fisher’s z transform (Berry and Mielke, 2000; Jenkins and Watts, 1968): . In the second level analysis, we correlated the Z map with the alcohol substance involvement (AlcSI) score with age as a covariate. In all analyses we evaluated the results at a voxelwise threshold of p<0.001 whole brain uncorrected in combination with cluster p<0.05, corrected for False Discovery Rate (FDR), following current reporting standards (Eklund et al., 2016; Poldrack et al., 2017).
3. Results
3.1 Age, sex, and alcohol substance involvement (AlcSI) score
The mean and standard deviation of age and alcohol SI score for the cohort were 49.39 ± 18.96 years and 4.77 ± 4.50. These two variables were not correlated (r = −0.2030, p = 0.0807). Age was used as a covariate in the regression analyses of rsFC. According to the NIH Resource Guide on NIDA Quick Screen V1.01 (https://www.drugabuse.gov/publications/resource-guide-screening-drug-use-in-general-medical-settings/nida-quick-screen), 58 participants were at the Lower Risk level, 25 at the Moderate Risk level, and none at the High Risk level. Men (5.69 ± 5.67) and women (3.68 ± 3.75) showed a trend difference in the AlcSI score (t = 1.9141, p = 0.0591). Individual item scores indicated that participants on average had more than four (women) or five (men) drinks approximately “Once” (0.96 ± 0.99 in men and women combined; 1.15 ± 0.98 in men; and 0.86 ± 1.00 in women) in the past year, and had been drinking almost “Monthly” (2.40 ± 1.83 in men and women combined; 2.88 ± 1.66 in men; and 2.18 ± 1.87 in women) in the past 3 months. These data suggested that participants are non-dependent social drinkers with varying degrees of problem drinking.
3.2 Resting state functional connectivity (rsFC) of the amygdala
At voxel p<0.001, uncorrected in combination with cluster p<0.05 corrected for false discovery rate (FDR), the results of one-sample t test on the rsFC of the whole amygdala and each of the subdivisions are shown in Figure 2. In general, the amygdala showed positive connectivity with subcortical areas including the basal ganglia and part of the thalamus, insula, inferior temporal cortex, hippocampus, ventromedial PFC, and a small region of the frontopolar cortex, and negative connectivity with the lateral frontal and posterior parietal areas as well as the occipital cortex (Roy et al., 2009).
Figure 2.
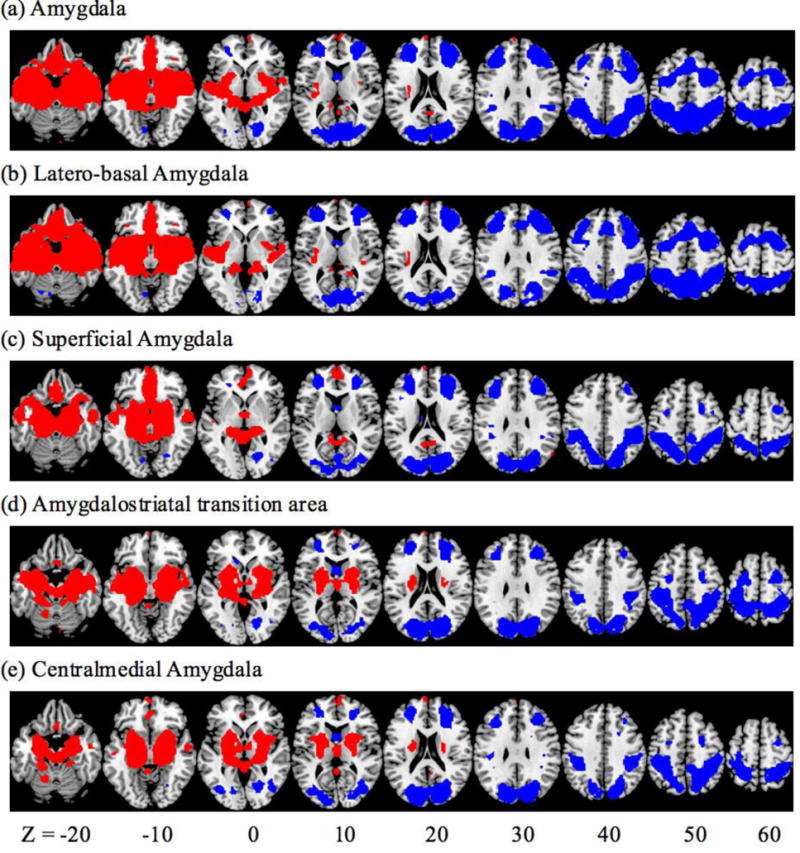
Whole brain resting state functional connectivity of the amygdala and each of its subdivisions. Voxel p<0.001, uncorrected, in combination with cluster p<0.05 corrected for FDR. Red: positive connectivity; Blue: negative connectivity.
3.3 rsFC and alcohol substance involvement (AlcSI) score
The results of regression against the AlcSI score showed a marginally significant negative correlation in the dorsal anterior cingulate cortex (dACC) (x = −6, y = 17, z = 34, k = 58, Z = 3.89, pFDR = .077). We repeated the analyses separately for the left and right amygdala. The results showed that the dACC connectivity was significantly and negatively correlated with AlcSI of the right (x = −6, y = 20, z = 25, k = 83, Z = 3.88, pFDR = .020) but not left amygdala. We further examined correlations of the AlcSI score and the rsFC of each subdivision of the amygdala. At the same threshold, dACC connectivity with the latero-basal amygdala was negatively correlated with AlcSI score (x = −6, y = 17, z = 34, k = 96, Z = 4.20, pFDR = .020). Again, this correlation was significant only for the right (x = −6, y = 23, z = 25, k = 95, Z = 3.99, pFDR = .024) but not left latero-basal amygdala (Figure 3a).
Figure 3.
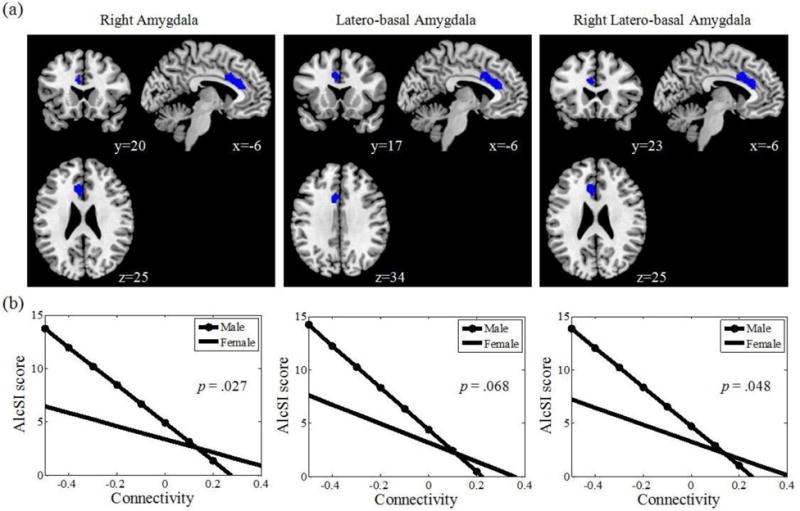
(a) Resting state amygdala connectivity with the dorsal anterior cingulate cortex (dACC) negatively correlated with alcohol substance involvement (AlcSI) score; (b) Gender differences in resting state amygdala connectivity with dACC.
To examine whether substance other than alcohol use or depressive disorder may influence the current results, we included an additional covariate coding for drug use or depressive disorder (1/0 for positive/negative diagnosis) in the regression. The results showed that the dACC was negatively correlated with AlcSI in connectivity with the amygdala (x = −9, y = 41, z = 22, Z = 3.98, pFDR = .014), right amygdala (x = −6, y = 41, z = 22, Z = 3.52, pFDR = .017), and latero-basal amygdala (x = −3, y = 35, z = 22, Z = 3.82, pFDR = .005). Thus, the rsFC findings remained even when individual variation in other drug use and depression was considered in data analyses.
In addition to controlling for age in the regressions between AlcSI and amygdala rsFC, we examined the age effects in the regression. The results revealed no clusters meeting voxel p<0.001 uncorrected and cluster p<0.05 FDR corrected. Further, no voxels in the dACC showed connectivity in relation to age even when the results were evaluated at voxel p<0.05 uncorrected.
3.4 Sex difference in the relationship between amygdala dACC connectivity and AlcSI score
We examined whether there is a sex difference in the association of amygdala dACC connectivity with the AlcSI. Whole-brain regression between the amygdala connectivity and AlcSI score did not result in significant finding in the dACC in men or women, probably due to a small sample size in each group. We then created masks of the dACC from the correlations between AlcSI and the rsFC of the right amygdala, latero-basal amygdala, and the right latero-basal amygdala, as described earlier. In small volume correction (SVC) on the regressions against AlcSI, male participants showed a significant negative correlation with dACC rsFC for the right amygdala and latero-basal amygdala whereas female participants did not show any significant correlations (Table 1; Figure 3b). Further, male and female participants differed significantly in the regression slope between AlcSI and the rsFC of the dACC with right amygdala (Z = 1.93, p = .027) and right latero-basal amygdala (Z = 1.66, p = .048), and marginally with bilateral latero-basal amygdala (Z = 1.93, p = .068) in slope test.
Table 1.
Amygdala connectivity with the dorsal anterior cingulate cortex in negative correlation with alcohol involvement score, shown separately for male and female participants.
| Seed Region | Cluster Size | Peak Voxel P value FDR | Voxel Z Value | MNI Coordinate | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Right amygdala | ||||||
| Male | 76 | 0.039 | 3.56 | −6 | 17 | 34 |
| Female | 39 | 0.392 | 2.55 | −12 | 32 | 22 |
| Latero-basal amygdala | ||||||
| Male | 82 | 0.040 | 3.43 | −6 | 17 | 34 |
| Female | 68 | 0.094 | 3.15 | −12 | 32 | 22 |
| Right latero-basal amygdala | ||||||
| Male | 82 | 0.063 | 3.20 | −9 | 29 | 16 |
| Female | 69 | 0.145 | 2.91 | −6 | 32 | 25 |
4. Discussion
Whole-brain regression revealed a negative correlation between right amygdala-dACC connectivity and alcohol involvement score, suggesting disrupted amygdala-prefrontal cortical connectivity in association with more severe alcohol consumption and problem drinking in adult drinkers. The findings remained significant after other drug use and depression were taken into account. This correlation appeared to be carried primarily by the latero-basal subdivision of the right amygdala. Further, this correlation appeared to be more significant in men than in women, suggesting a sex difference in the effects of alcohol misuse on amygdala-dACC connectivity.
4.1 Alcohol misuse and amygdala-dACC connectivity
Decreased rsFC between the amygdala and dACC in alcohol misuse is consistent with a large literature on the effects of alcohol on amygdala and ACC functions. Amygdala activation decreased in association with chronic (Hariri et al., 2000) and acute (Sripada et al., 2011) alcohol consumption in processing stimulus valence and identifying threats. The ACC also showed a consistent pattern of lower activations under the chronic effects of alcohol. For example, compared to healthy controls, alcohol addicted individuals showed decreased activation in the ACC when viewing faces with negative valence (Salloum et al., 2007) including threatening stimuli (Yang et al., 2013), and alcohol versus non-alcohol cues (Alba-Ferrara et al., 2016; Zakiniaeiz et al., 2017). ACC activation also inversely correlated with lifetime alcohol intake in alcoholic patients during emotional face recognition (Charlet et al., 2014). The ACC decreased activation in participants receiving acute alcohol administration, and greater decrease in activation was correlated with poorer performance in a working memory task (Gundersen et al., 2008). Alcohol drinkers showed decreased ACC activation as compared to non-drinkers during inhibitory control in a stop signal (Hu et al., 2015) and go/no-go task (Ahmadi et al., 2013; Claus et al., 2013), and during spatial working memory (Vollstadt-Klein et al., 2010). The current findings are thus in line with an extensive body of literature.
The current results are also consistent with previous research showing diminished amygdala PFC connectivity in individuals addicted to alcohol (Muller-Oehring et al., 2015; Wade et al., 2017) and in healthy adults under alcohol administration (Gorka et al., 2013). For instance, the amygdala-ACC functional connectivity was dampened in alcoholic patients with multiple detoxifications (O’Daly et al., 2012) and when they processed aversive emotional stimuli (Kienast et al., 2013). Disrupted resting state amygdala-ACC connectivity was also reported in patients with PTSD (Rabinak et al., 2011; Sripada et al., 2012), social anxiety disorder (Bishop et al., 2004; Hahn et al., 2011; Prater et al., 2013; Toazza et al., 2016), and bipolar disorder (Singh et al., 2015), likely reflecting a general stress-related etiological processes that implicate the amygdala (Koob and Volkow, 2016). The current results thus extend the literature on the links of alcohol misuse to amygdala-prefrontal circuit dysfunction.
Our results also suggest a potential laterality effect, with right amygdala – dACC connectivity more related to alcohol misuse. Previous research of the effects of alcohol use on the amygdala has produced mixed results as to laterality. For example, participants with acute alcohol administration showed reduced right amygdala (Gilman et al., 2008) or bilateral amygdala activation (Sripada et al., 2011) when viewing angry faces. Heavy social drinkers with acute alcohol administration showed decreased right amygdala-PFC connectivity when they viewed angry faces (Gorka et al., 2013), whereas abstinent individuals with alcohol dependence showed decreased bilateral amygdala-PFC connectivity (Wade et al., 2017). Further, structural brain imaging showed enlarged volumes in bilateral amygdala (Senatorov et al., 2015), decreased volumes in bilateral amygdala (Wrase et al., 2008), decreased volume in left but not right amygdala (Makris et al., 2008), suggesting the issue of laterality in the effects of alcohol misuse on amygdala structure and function remains to be investigated.
4.2 Alcohol misuse and latero-basal amygdala
Of all subdivisions, the finding of decreased dACC connectivity in association with alcohol involvement was significant only for the latero-basal amygdala. The amygdala can be divided into subregions based on distinct anatomical and functional organization (Kim et al., 2011). The latero-basal amygdala receives inputs from sensory cortices and projects to the frontal and temporal cortices (Ghashghaei and Barbas, 2002) and supports facial and bodily expressions associated with unpredictable aversive outcomes (Madarasz et al., 2016) and under threat (Bzdok et al., 2013). The latero-basal amygdala showed greater negative connectivity with the ACC than other amygdala subdivisions (Roy et al., 2009). In rats, acute alcohol administration enhances GABAergic transmission in the basolateral amygdala (Lindemeyer et al., 2014) and inhibits spontaneous neuronal activity of basolateral amygdala and its projection targets (Perra et al., 2008). The finding of the decreased latero-basal amygdala connectivity with the dACC is broadly consistent with this literature.
4.3 Sex differences in amygdala dACC connectivity in link with alcohol misuse
Men showed a stronger relationship than women in decreased amygdala-dACC connectivity in correlation with alcohol misuse. Previous studies have reported sex-related morphometric and functional difference in the amygdala, with men showing larger amygdala and involving the right amygdala more than women (Cahill, 2006 for a review; see Hamann, 2005; but see Marwha et al., 2017), especially during exposure to emotional stimuli (Andreano et al., 2014; Weisenbach et al., 2014). In addition, resting state connectivity seeded from the amygdala demonstrated a sex difference, with the right amygdala showing greater connectivity in men than in women (Kilpatrick et al., 2006) and the left amygdala showing stronger connectivity in women than men (Kogler et al., 2016). Granger causality analysis also revealed stronger right amygdala-prefrontal cortical connectivity in men than in women exposed to negative emotional stimuli (Lungu et al., 2015). Importantly, the right amygdala appeared to be more susceptible to aging and drug effects in men. In a longitudinal study amygdala activation decreased with age in male adolescents at risk of substance use while remaining largely unchanged in females when they viewed words with negative valence (Hardee et al., 2017). Reduced amygdala-prefrontal cortical connectivity was associated with increased alcohol intake in male but not female adolescents and young adults and the relationship appeared to be mediated by testosterone (Peters et al., 2015). The current findings add to this literature of sex differences in amygdala-prefrontal connectivity and alcohol misuse.
4.4 Limitations of the study and conclusions
Several limitations of the study need to be considered. First, the imaging data set did not include cognitive behavioral assessments; thus, the functional implications of disrupted amygdala dACC connectivity remained unclear. Second, there were more women than men in the cohort and men showed higher alcohol involvement index than women. It is possible that women with higher level of problem drinking were under-represented in the current cohort and the sex-related findings remain to be confirmed. Third, the age range in the current cohort was large. Although age was included as a covariate in the regressions, and separate analyses were performed to rule out age effects as a possible confound, these results need to be confirmed by studies with a larger sample size and of a narrower age range. Fourth, the amygdala subregions are small with the latero-basal nucleus being the largest. Thus, we cannot rule out the possibility that the findings obtained of latero-basal but not other nuclei simply reflect a volume-related signal-to-noise issue. Further, a number of models were tested and the issue of multiple comparisons was not considered. Fifth, although we used the term “alcohol misuse” and “problem drinking”, the current findings should be considered as specific to a non-dependent social drinking populations. Lastly, the alcohol assessment captured recent but not cumulative drinking, and it remains to be investigated whether or how history of alcohol use may have influenced amygdala connectivity.
In summary, we showed that problem drinking as indexed by the alcohol involvement score was associated with reduced amygdala-dACC resting state functional connectivity in non-dependent drinkers. The reduced connectivity appeared to be specific to the right amygdala and the latero-basal subdivision. In addition, increased alcohol involvement influenced amygdala-ACC connectivity more strongly in men than in women, suggesting a potential sex difference. These findings support previous reports of the effects of alcohol misuse on amygdala-prefrontal circuit and identify the latero-basal amygdala as a key subregion to mediate these influences.
Supplementary Material
Highlights.
Decreased amygdala-dACC connectivity was associated with increased alcohol misuse.
Interrupted connectivity was found in the right amygdala and latero-basal amygdala.
Men showed more severe alcohol-related disruption of connectivity than women.
Acknowledgments
This study was supported by NIH grants AA021449 and DA040032. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
SH and C-SL designed the experiments; SH, JI, and S. Zhang analyzed the data; all authors contributed to the literature review and writing of the manuscript. All authors approved of the final version of the manuscript before submission.
Conflicts of interest
None.
References
- Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, Austad CS, Raskin SA, Fallahi CR, Tennen H, Wood RM, Stevens MC. Influence of alcohol use on neural response to Go/No-Go task in college drinkers. Neuropsychopharmacology. 2013;38(11):2197–2208. doi: 10.1038/npp.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Ferrara L, Muller-Oehring EM, Sullivan EV, Pfefferbaum A, Schulte T. Brain responses to emotional salience and reward in alcohol use disorder. Brain Imaging Behav. 2016;10(1):136–146. doi: 10.1007/s11682-015-9374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210(5–6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Dickerson BC, Barrett LF. Sex differences in the persistence of the amygdala response to negative material. Soc Cogn Affect Neurosci. 2014;9(9):1388–1394. doi: 10.1093/scan/nst127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Rev. 2004;45(2):96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KJ, Mielke PW., Jr A Monte Carlo investigation of the Fisher Z transformation for normal and nonnormal distributions. Psychol Rep. 2000;87(3 Pt 2):1101–1114. doi: 10.2466/pr0.2000.87.3f.1101. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2013;34(12):3247–3266. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner CE, Konig F, Walaszek B, Schubert F, Muller CA, Gutwinski S, Seissinger A, Schmitz L, Walter H, Beck A, Gallinat J, Kiefer F, Heinz A. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol. 2014;19(3):439–451. doi: 10.1111/adb.12045. [DOI] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE. Behavioral control in alcohol use disorders: relationships with severity. J Stud Alcohol Drugs. 2013;74(1):141–151. doi: 10.15288/jsad.2013.74.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol. 2013;18(3):593–604. doi: 10.1111/adb.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol Clin Exp Res. 2014;38(7):1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015;156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith C, Polone J, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109(44):18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28(18):4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61(11):1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, King AC, Phan KL. Alcohol attenuates amygdala-frontal connectivity during processing social signals in heavy social drinkers: a preliminary pharmaco-fMRI study. Psychopharmacology (Berl) 2013;229(1):141–154. doi: 10.1007/s00213-013-3090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Gruner R, Specht K, Hugdahl K. The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimag J. 2008;2:65–72. doi: 10.2174/1874440000802010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11(4):288–293. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Hardee JE, Cope LM, Munier EC, Welsh RC, Zucker RA, Heitzeg MM. Sex differences in the development of emotion circuitry in adolescents at risk for substance abuse: A longitudinal fMRI study. Soc Cogn Affect Neurosci. 2017;12(6):965–975. doi: 10.1093/scan/nsx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Sinha R, Li CS. Conflict anticipation in alcohol dependence - A model-based fMRI study of stop signal task. Neuroimage Clin. 2015;8:39–50. doi: 10.1016/j.nicl.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral Analysis and Its Applications. Holden-Day; San Francisco: 1968. [Google Scholar]
- Kienast T, Schlagenhauf F, Rapp MA, Wrase J, Daig I, Buchholz HG, Smolka MN, Grunder G, Kumakura Y, Cumming P, Charlet K, Bartenstein P, Hariri AR, Heinz A. Dopamine-modulated aversive emotion processing fails in alcohol-dependent patients. Pharmacopsychiatry. 2013;46(4):130–136. doi: 10.1055/s-0032-1331747. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16(10):1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Muller VI, Seidel EM, Boubela R, Kalcher K, Moser E, Habel U, Gur RC, Eickhoff SB, Derntl B. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage. 2016;134:410–423. doi: 10.1016/j.neuroimage.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Xue SW. Linking graph features of anatomical architecture to regional brain activity: A multi-modal MRI study. Neurosci Lett. 2017;651:123–127. doi: 10.1016/j.neulet.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, Spigelman I. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res. 2014;39(6):1162–1170. doi: 10.1007/s11064-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lungu O, Potvin S, Tikasz A, Mendrek A. Sex differences in effective fronto-limbic connectivity during negative emotion processing. Psychoneuroendocrinology. 2015;62:180–188. doi: 10.1016/j.psyneuen.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Madarasz TJ, Diaz-Mataix L, Akhand O, Ycu EA, LeDoux JE, Johansen JP. Evaluation of ambiguous associations in the amygdala by learning the structure of the environment. Nat Neurosci. 2016;19(7):965–972. doi: 10.1038/nn.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33(11):1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwha D, Halari M, Eliot L. Meta-analysis reveals a lack of sexual dimorphism in human amygdala volume. Neuroimage. 2017;147:282–294. doi: 10.1016/j.neuroimage.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci U S A. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. The Resting Brain of Alcoholics. Cereb Cortex. 2015;25(11):4155–4168. doi: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Ikuta T. Caudate-Precuneus Functional Connectivity Is Associated with Obesity Preventive Eating Tendency. Brain Connect. 2017;7(3):211–217. doi: 10.1089/brain.2016.0424. [DOI] [PubMed] [Google Scholar]
- Neta M, Norris CJ, Whalen PJ. Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion. 2009;9(5):640–648. doi: 10.1037/a0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, Sikka S, Gutman D, Bangaru S, Schlachter RT, Kamiel SM, Anwar AR, Hinz CM, Kaplan MS, Rachlin AB, Adelsberg S, Cheung B, Khanuja R, Yan C, Craddock CC, Calhoun V, Courtney W, King M, Wood D, Cox CL, Kelly AM, Di Martino A, Petkova E, Reiss PT, Duan N, Thomsen D, Biswal B, Coffey B, Hoptman MJ, Javitt DC, Pomara N, Sidtis JJ, Koplewicz HS, Castellanos FX, Leventhal BL, Milham MP. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Front Neurosci. 2012;6:152. doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37(10):2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol Clin Exp Res. 2008;32(3):443–449. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Peters S, Jolles DJ, Van Duijvenvoorde AC, Crone EA, Peper JS. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Peters S, Peper JS, Van Duijvenvoorde ACK, Braams BR, Crone EA. Amygdala-orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Dev Sci. 2017;20(4) doi: 10.1111/desc.12448. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, Nichols TE, Poline JB, Vul E, Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18(2):115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30(3):234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AT, Lewis J, Bezgin G, Khundrakpam B, Eickhoff SB, McIntosh AR, Bellec P, Evans AC. A cross-modal, cross-species comparison of connectivity measures in the primate brain. Neuroimage. 2016;125:311–331. doi: 10.1016/j.neuroimage.2015.10.057. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a “no task condition”. Evidence for hippocampal connectivity using fMRI. NeuroImage. 2003;20(2):1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- Rosazza C, Minati L. Resting-state brain networks: literature review and clinical applications. Neurol Sci. 2011;32(5):773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D, Hommer DW. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31(9):1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Damadzic R, Mann CL, Schwandt ML, George DT, Hommer DW, Heilig M, Momenan R. Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain. 2015;138(Pt 1):69–79. doi: 10.1093/brain/awu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER. Alcohol and affect regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. pp. 560–580. [Google Scholar]
- Singh MK, Kelley RG, Chang KD, Gotlib IH. Intrinsic Amygdala Functional Connectivity in Youth With Bipolar I Disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(9):763–770. doi: 10.1016/j.jaac.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL. Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage. 2011;55(1):371–380. doi: 10.1016/j.neuroimage.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37(4):241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Toazza R, Franco AR, Buchweitz A, Molle RD, Rodrigues DM, Reis RS, Mucellini AB, Esper NB, Aguzzoli C, Silveira PP, Salum GA, Manfro GG. Amygdala-based intrinsic functional connectivity and anxiety disorders in adolescents and young adults. Psychiatry Res. 2016;257:11–16. doi: 10.1016/j.pscychresns.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb Cortex. 2014;24(4):935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F, Antonius D, Nolan K, Butler P, Javitt DC. Immediate affective motivation is not impaired in schizophrenia. Schizophr Res. 2014;159(1):157–163. doi: 10.1016/j.schres.2014.08.001. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Hermann D, Rabinstein J, Wichert S, Klein O, Ende G, Mann K. Increased activation of the ACC during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol Clin Exp Res. 2010;34(5):771–776. doi: 10.1111/j.1530-0277.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wade NE, Padula CB, Anthenelli RM, Nelson E, Eliassen J, Lisdahl KM. Blunted amygdala functional connectivity during a stress task in alcohol dependent individuals: A pilot study. Neurobiol Stress. 2017;7:74–79. doi: 10.1016/j.ynstr.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenbach SL, Rapport LJ, Briceno EM, Haase BD, Vederman AC, Bieliauskas LA, Welsh RC, Starkman MN, McInnis MG, Zubieta JK, Langenecker SA. Reduced emotion processing efficiency in healthy males relative to females. Soc Cogn Affect Neurosci. 2014;9(3):316–325. doi: 10.1093/scan/nss137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165(9):1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Xu J, Moeller S, Strupp J, Auerbach E, Chen L, Feinberg D, Ugurbil K, Yacoub E. Highly accelerated whole brain imaging using aligned-blipped-controlled aliasing multiband EPI. the 20th Annual Meeting of ISMRM; Melbourne, Australia. 2012. p. 2306. [Google Scholar]
- Yan P, Li CS. Decreased amygdala activation during risk taking in non-dependent habitual alcohol users: A preliminary fMRI study of the stop signal task. Am J Drug Alcohol Abuse. 2009;35(5):284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Devous MD, Briggs RW, Spence JS, Xiao H, Kreyling N, Adinoff B. Altered neural processing of threat in alcohol-dependent men. Alcohol Clin Exp Res. 2013;37(12):2029–2038. doi: 10.1111/acer.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, Constable RT. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clin. 2017;13:181–187. doi: 10.1016/j.nicl.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex. 2012;22(1):99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


