Abstract
A subset of patients with non-alcoholic fatty liver disease develop an inflammatory condition, termed nonalcoholic steatohepatitis (NASH). NASH is characterised by hepatocellular injury, innate immune cell-mediated inflammation and progressive liver fibrosis. The mechanisms whereby hepatic inflammation occurs in NASH remain incompletely understood, but appear to be linked to the proinflammatory microenvironment created by toxic lipid-induced hepatocyte injury, termed lipotoxicity. In this review, we discuss the signalling pathways induced by sublethal hepatocyte lipid overload that contribute to the pathogenesis of NASH. Furthermore, we will review the role of proinflammatory, proangiogenic and profibrotic hepatocyte-derived extracellular vesicles as disease biomarkers and pathogenic mediators during lipotoxicity. We also review the potential therapeutic strategies to block the feed-forward loop between sublethal hepatocyte injury and liver inflammation.
INTRODUCTION
Hepatic steatosis unassociated with alcohol use is present in up to 25% of the world population and is often referred to as non-alcoholic fatty liver disease (NAFLD).1 The etiopathogenesis is complex and has been ascribed to several non-mutually exclusive conditions including obesity and a sedentary lifestyle, the composition of nutrient intake (eg, fructose in corn syrup), insulin resistance with or without overt diabetes, alterations of the microbiome termed dysbiosis and genetic predispositions.2 Primary and secondary changes in the bile acid pool have also been implicated in NAFLD pathogenesis.3 For the majority of patients, isolated hepatic steatosis is non-pathogenic and has been referred to as simple steatosis,4 although patients with isolated hepatic steatosis may be at risk for neoplastic and cardiovascular diseases.5 In contrast, a subset of patients (up to 30%) develop hepatocellular injury, hepatic inflammation and liver fibrosis1; this constellation of pathological findings is termed non-alcoholic steatohepatitis (NASH).2 This group is clinically relevant due to the risks for end-stage liver disease and its sequela.6 It is unclear why some patients with hepatic steatosis develop NASH and/or why most patients with steatosis do not. Several postulates have been developed to explain this observation. These concepts are based on: (1) variations in the etiopathogenesis of hepatic steatosis with some pathogenic mechanisms being more aggressive than others resulting in a broad phenotypic spectrum; (2) the advent of a secondary process occurring in the context of pre-existing hepatic steatosis; (3) or perhaps simple steatosis and NASH are distinctly two different pathogenic diseases which are conflated due to lack of information.
Accumulation of lipid intermediates in hepatocytes causes hepatocellular lipotoxicity, leading to cellular stress, dysfunction and eventually cell death. Lipotoxicity-induced hepatocyte cell death appears to be mainly mediated by the apoptotic machinery activated by death receptors and endoplasmic reticulum (ER) stress,7 and potentiated by enhanced fatty acid uptake due to upregulation of fatty acid transport proteins.8 In this review of current advances in basic science regarding the pathogenesis of NASH, we explore the concept that toxic lipids initiate signalling processes converging on common pathways to incite monocyte recruitment into the liver with the differentiation and polarisation of these monocytes into inflammatory macrophages. The toxic lipid mediators such as free fatty acids, ceramides, free cholesterol, diacyl-glycerol and phospholipids have been previously reviewed elsewhere9 and will not be discussed in detail. Rather we will review how the signalling processes occurring during hepatocyte lipotoxic stress initiate macrophage-associated inflammation. We will explore the concept of sublethal hepatocellular lipotoxic injury which can be defined as lipid-induced hepatocyte stress and dysfunction which is of an insufficient magnitude to cause cell death, but is sufficient to trigger aberrant, proinflammatory signalling cascades. The relationship between immune cell hepatic infiltration and further liver injury as a feed-forward loop will be discussed. This information is topical and timely because of recent advances in the field and also because this information can be exploited to aid in the diagnosis and therapy of NASH.
SUBLETHAL LIPOTOXIC HEPAT OCYTE INJURY
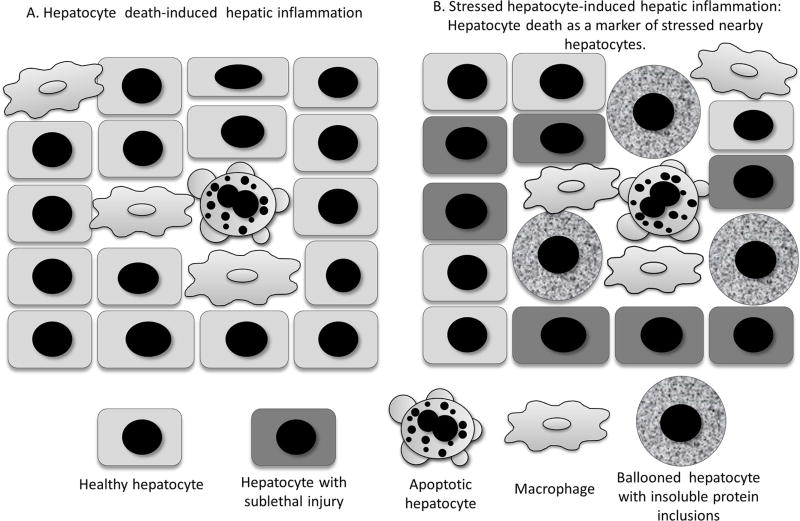
Sublethal lipid-induced injury in hepatocytes can readily be studied in vitro; however, its identification in vivo is hindered by lack of appropriate detection assays. Due to relatively easy detection of cell death in liver tissue, it is plausible that dead cells may simply represent a marker for large populations of neighbouring cells with sublethal injury.10 This hypothesis assumes that cell death signalling cascades are insufficient to induce cell death in the majority of cells but only in a small minority. This minor cell population would undergo apoptosis, which by itself may not promote high-level proinflammatory activity in contrast to the larger stressed cell subpopulation (figure 1). Indeed, cells with sublethal injury have been demonstrated to initiate an inflammatory response, for example by the release of extracellular vesicles (EVs).11–13 To better understand the sublethal stress signalling in vivo, new techniques and methods that would identify cells with activated stress signalling in the absence of cell death are needed. Such assays would include the identification of proinflammatory or cell injury mediating protein complexes by proximity ligation assays,14 as an example.
Figure 1.
Two concepts of cell death role in NASH-associated inflammation. (A) Apoptotic hepatocytes can directly initiate inflammation via apoptotic bodies engulfed by macrophages. (B) Apoptotic hepatocytes serve as a marker for widespread proapoptotic/stress signalling occurring in the majority of nearby stressed hepatocytes. These stressed cells with sublethal injury may promote inflammation, for example, via release of proinflammatory extracellular vesicles. (Modified from Hirsova et al10). NASH, non-alcoholic steatohepatitis.
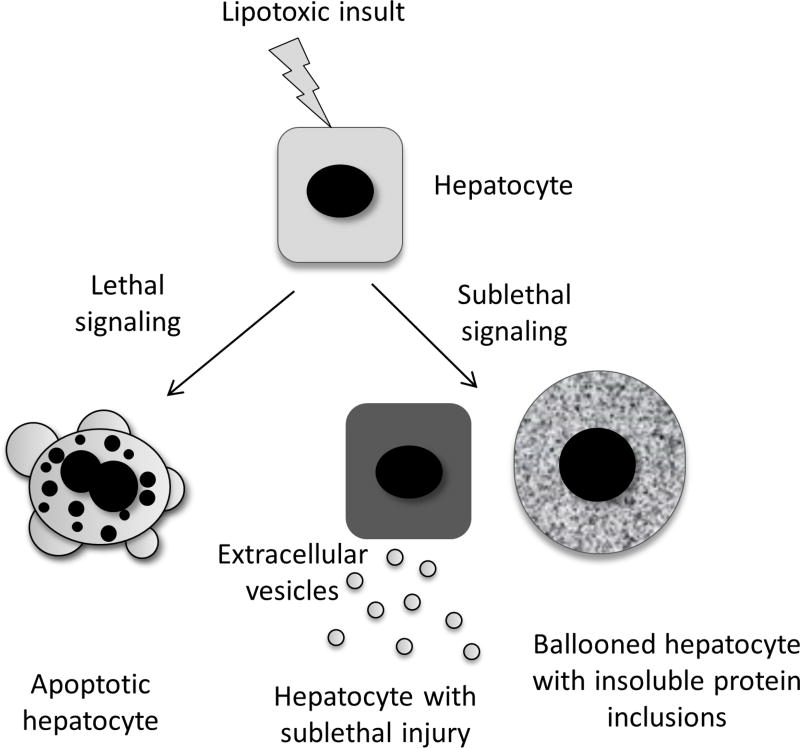
We will review two forms of sublethal hepatocyte injury, where recent advances have elucidated novel signalling mechanisms between injured hepatocytes and other cell types in the liver. One is the concept of the undead hepatocyte (which includes ballooned hepatocytes and hepatocytes with Mallory-Denk bodies’ inclusions) and the other is the release of EVs induced by sublethal, proapoptotic signalling in hepatocytes (figure 2).
Figure 2.
Lipotoxic lethal and sublethal injury in hepatocytes. Toxic lipid-induced lethal signalling in hepatocytes causes apoptotic cell death (lipoapoptosis). Sublethal proapoptotic signalling induced by lipotoxicity results in release of extracellular vesicles. Sublethal stress also occurs in ‘undead’ hepatocytes which include ballooned hepatocytes with Mallory-Denk bodies’ inclusions.
Ballooned hepatocyte: undead cells
Hepatocellular ballooning is a prominent feature of lipotoxic liver injury. The presence and magnitude of hepatocellular ballooning are used for histological grading and staging of NAFLD and NASH diagnosis.15 The term ballooned hepatocytes is used for hepatocytes with a special form of cellular degeneration characterised by cellular swelling, a central nucleus and reticulated cytoplasm, disorganised cellular polarity, loss of keratin 8 and 18 and accumulation of ubiquitinated proteins.16 However, relatively little are known about these cells and their isolation (eg, by laser capture microdissection) coupled with single cell RNA sequencing studies are needed.
Ballooned hepatocytes are a hallmark of NASH and they have been implicated in the disease pathogenesis. Ballooned hepatocytes generate sonic hedgehog (Shh), a ligand of the developmental hedgehog signalling pathway.17 The Hedgehog pathway is a complex and tightly regulated signal transduction pathway that consists of 4 main components: the ligand Hedgehog, the inhibitory receptor Patched, the signal transducer Smoothened and the effector transcription factors of the Gli family. In healthy adult liver, the Hedgehog pathway is dormant, but it activates in response to liver injury. The Hedgehog pathway is critical for liver repair and regeneration but if persistently activated, it induces liver fibrosis.18
Furthermore, ballooned hepatocytes exist in a state of initiated cell death that cannot be executed (‘undead’) and secrete various factors, including Shh, to promote tissue repair and healing.19 Models of undead ballooned hepatocytes using lipotoxic treatment in hepatocytes lacking caspase 9, a protease critical for the execution of apoptosis, are characterised by activation of the stress kinase c-Jun N-terminal kinase, which lead to an upregulation of Shh in the absence of cell death. In these cells, lipotoxicity-induced hepatocyte-derived Shh functioned as an autocrine survival factor.20 These observations imply that inhibition of hedgehog signalling may prevent the development of ballooned hepatocytes, a hypothesis yet to be tested.
ER stress has also been shown to promote expression and secretion of Shh by hepatocytes.17 Shh may then promote fibrogenesis by activating hepatic stellate cell (HSC), a major target cell of hepatic Shh signalling. Hepatocytes are also an important hedgehog-responsive cell type during lipotoxicity. First, hepatocytes express different components of the hedgehog signalling cascade, including smoothened.21 Second, hedgehog signalling in hepatocytes can regulate osteopontin expression and secretion via a Gli1-dependent mechanism.18 Pharmacological inhibition of the hedgehog pathway using smoothened inhibitors prevents liver injury, inflammation and fibrosis in a mouse model of NASH.21 Likewise, liver-specific deletion of smoothened attenuates liver inflammation in high fat diet-fed mice.22 Interestingly, in patients with NASH enrolled in the PIVENS trial, improvement in liver injury correlated with a decreased number of Shh-positive hepatocytes. On the other hand, a reduction of Shh-positive hepatocytes was not associated with improvement of fibrosis in these patients.23 Hence, further studies are needed to determine the role of hepatocyte hedgehog signalling in liver injury during NASH.
Hepatocytes with insoluble protein inclusions
The formation of insoluble protein inclusions in hepatocytes referred to as Mallory-Denk bodies is a histological feature of NASH and closely linked to sublethal hepatocyte lipotoxic injury.24 These inclusions consist of ubiquitinated proteins such as keratins and the ubiquitin- binding autophagy receptor p6224,25 and accumulate secondary to attenuated autophagic flux. P62 accumulation can promote liver fibrosis.26 In hepatocytes under toxic lipid treatment, reduced ER membrane fluidity inhibits Sarco-ER calcium pump, which results in elevated cytosolic calcium and impaired autophagosome-lysosome fusion. Calcium channel blockers can restore the autophagosomes-lysosomes fusion, autophagic removal of protein inclusions and fat droplets in vivo and reduce liver inflammation.25 Hence, insoluble protein inclusion accumulation in hepatocytes under lipotoxic stress appears to be linked to liver inflammation and fibrosis, though studies are needed to further delineate the signalling pathways involved in their accumulation and identify potential therapeutic targets. Big data analysis could also be used to determine whether calcium channel blockers administration reduces NASH or obesity-associated liver morbidity and mortality; such approach was recently employed to show a relationship between beta-blockers and Parkinson disease.27
LIPOTOXIC STRESS AND EXTRAC ELLULAR VESICLE (EV) RELEASE
EVs as pathogenic mediators in NASH
Cells release diverse types of membrane-bound EVs into the extracellular milieu. These can be further classified into three main subgroups based on their cellular biogenesis: exosomes, microvesicles and apoptotic bodies.28 Exosomes (~50–100 nm diameter) originate from the multivesicular body (MVB); MVBs are well-characterised endosomal precursors of the lysosomal degradation pathway. MVBs can also fuse with the plasma membrane. In this case, their intraluminal vesicle contents are released into the extracellular space, thus becoming ‘exosomes’.29 Microvesicles (~50–1000 nm diameter) bud directly from the plasma membrane. Apoptotic bodies (more than 500 nm in diameter) represent cell fragments generated during apoptosis. In addition, certain cancer cells shed large (~1–10 µm diameter) vesicles from the plasma membrane; these vesicles are termed ‘large oncosomes’.30 Apoptotic bodies and oncosomes are not the focus of this review, where we focus on EVs released by stressed hepatocytes under sublethal insults. In this review, we use the term ‘extracellular vesicle’ to refer to both exosomes and microvesicles.
EVs mediate intercellular communication and regulate the function of target cells in NASH (figure 3). EVs are efficient messengers, with superior stability and bioavailability of their signature cargoes.31 EVs transmit from their cells of origin selected cargoes such as surface receptors, proteins (membrane, cytosolic and nuclear), RNAs (including mRNAs and non-coding RNAs) and lipids.28 EVs deliver their cargoes to the target cells through interaction with surface receptors, internalisation or fusion.32 The EVs role in health and disease has been recognised; EVs are constantly released under physiological conditions into different body fluids,33 while various insults may further increase the number of released vesicles and may modify their contents.28 Hence, circulating EV number is significantly increased in mouse models34 and patients with NASH.13
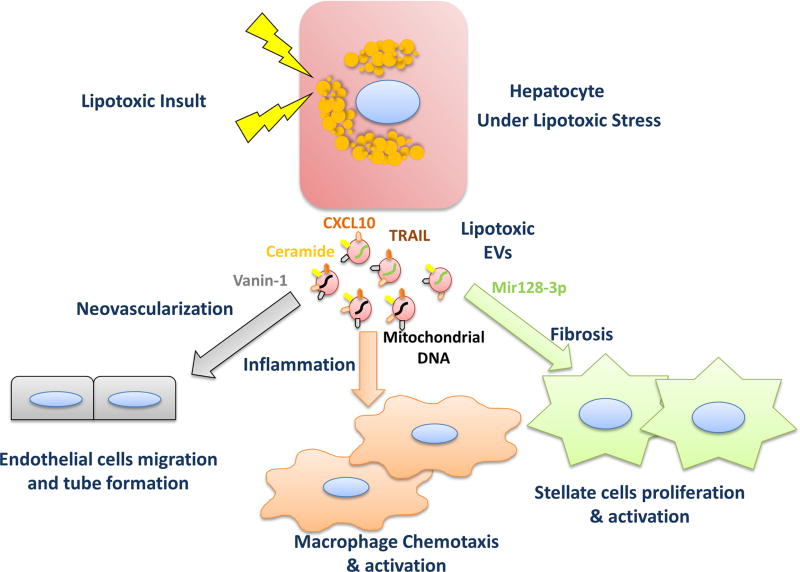
Figure 3.
Signalling events mediated by lipotoxic hepatocyte-derived EVs. Hepatocytes under lipotoxic conditions release increased amount of extracellular vesicles of distinct cargo. Recent in vitro and in vivo studies have described important roles of lipotoxic EVs in NASH pathogenesis through intercellular communication. CXCL10 and ceramide-enriched EVs mediate monocyte/macrophage hepatic trafficking and infiltration. Mitochondrial DNA and TRAIL-enriched EVs promote macrophage activation. VNN1-enriched EVs mediate endothelial cell migration and neovascularisation. miR-128–3 p-laden EVs induce HSC proliferation and activation. (Modified from Hirsova et al9). CXCL10, C-X-C motif chemokine ligand 10; EV, extracellular vesicle; HSC, hepatic stellate cells; NASH, non-alcoholic steatohepatitis; TRAIL, tumour necrosis factor-like apoptosis-inducing ligand; VNN1, vanin-1.
EVs have been implicated as mediators of toxic lipid-induced intercellular signalling in several recent studies.11–13,35 Adoptive transfer of EVs isolated from the serum of high fat diet-fed mice into chow diet-fed mice results in immature myeloid cells activation and homing to the liver, increased levels of hepatic proinflammatory markers and serum aminotransferases.35 Furthermore, hepatocytes from different species treated with toxic lipids such as palmitate and its active metabolite lysophosphatidylcholine (LPC), release an increased number of EVs.11,12,34 EVs derived from hepatocyte under lipotoxic stress are heterogeneous regarding their biogenesis, selected cargo, release and intended target cells. For example, LPC-induced EV release is mediated by the stress kinase mixed lineage kinase 3 (MLK3).12 Furthermore, MLK3 regulates the chemotactic cargo of the EVs. Genetic or pharmacological inhibition of MLK3 results in a reduced cellular induction36 and abundance of the potent C-X-C motif chemokine ligand 10 (CXCL10) in vesicles derived from LPC-treated hepatocytes.12 Likewise, MLK3−/− mice fed a NASH-inducing diet have reduced CXCL10 levels in their plasma EVs. This, in turn, is associated with hepatoprotection against injury and inflammation.37 Furthermore, the release of EVs by LPC-treated hepatocytes was dependent on tumour necrosis factor-like apoptosis-inducing ligand (TRAIL) receptor 2 (TRAIL-R2) signalling cascade, involving TRAIL-R2, caspase 8 and caspase 3.11 This process engages the proapoptotic machinery and the effector caspase 3 that subsequently induce proteolytic activation of Rho-associated kinase1 (ROCK1). ROCK1 in turn mediates the formation and shedding of microvesicles from the plasma membrane. Hence, EV release is reduced in the presence of fasudil, a ROCK1 inhibitor.11 These EVs are biologically active, as they induce macrophage chemotaxis in a CXCL10-dependent manner12 and macrophage activation by a TRAIL-dependent mechanism.11 Consistent with these in vitro data, fasudil decreased the number of circulating EVs in an experimental murine NASH model, resulting in reduced liver injury and inflammation.11 Furthermore, lipotoxic hepatocyte-derived EVs mediate neovascularisation, an important pathological feature in NASH that correlates with fibrosis severity. These EVs were enriched with the surface protein vanin-1 (VNN1), which mediates EV internalisation by endothelial cells and subsequent endothelial cells migration and tube formation in vitro and angiogenesis in a NASH mouse model.34 Treating methionine and choline-deficient (MCD) diet-fed C57BL/6 mice with siRNA against VNN1 protected mice from the pathological angiogenesis associated with NASH.34
Emerging data also highlight the role of lipid cargo on EVs in NASH pathogenesis. A recent report demonstrated that palmitate-induced EV release is mediated by the unfolded protein response sensor, inositol-requiring protein 1α. These EVs are enriched in C16:0 ceramide and promote macrophage chemotaxis via ceramide-derived sphingosine-1-phosphate signalling pathway.13 Interestingly, pharmacological inhibition of sphingosine-1-phosphate ameliorates NASH in an experimental mouse model.38
Furthermore, recent data suggest that miRNAs within EVs are key mediators of NASH-associated fibrosis. EVs released by lipotoxic hepatocytes are enriched with miR-128–3 p and efficiently internalised by HSCs.39 miR-128–3 p regulates several proteins involved in liver fibrosis and HSCs activation. miR-128–3 p level in circulating EVs was markedly associated with the extent of fibrosis in different NASH mouse models. HSCs treated with miR-128–3 p-depleted EVs upregulate the quiescent marker peroxisome proliferator-activated receptor (PPAR)-γ and downregulate profibrogenic markers. Likewise, miR-128–3 p depleted EVs attenuated HSC proliferation and migration.39
Mitochondrial DNA is a newly recognised EV cargo that plays a role in NASH pathogenesis. Increased levels of oxidised mitochondrial DNA within EVs were detected in the serum of mice and patients with NASH.40 These EVs drive toll-like receptor (TLR) 9 activation and enhance the sterile inflammatory response associated with NASH.40 Although, further studies are needed to elucidate the mechanism of mitochondrial DNA packaging into lipotoxic EVs. There are probably other mediators of macrophage chemotaxis and activation within the lipotoxic EVs, like danger-associated molecular patterns (DAMPs) proteins which are known to activate inflammatory responses in mammalians.41 We have identified by mass spectrometry several DAMPS on EVs derived from lipotoxic hepatocytes (box 1). The role of DAMPs proteins-enriched EVs in peripheral blood monocyte trafficking to the liver and macrophage activation and their regulatory relationship merits further studies.
Box 1. Damage-associated molecular patterns (DAMPs) identified on lipotoxic hepatocyte-derived extracellular vesicles (EVs) by mass spectrometry.
DAMPs
-
►
High-mobility group box 1
-
►
Serum Amyloids
-
►
S100s
-
►
Hepatoma-derived growth factor
-
►
Heat shock proteins
-
►
Galectins
-
►
Nucleolin
-
►
Annexins
-
►
Histones
Primary mouse hepatocytes were treated with 20 µM
lysophosphatidylcholine for 4 hours; EVs were isolated from the supernatant by ultracentrifugation. Proteomic analysis on vesicles lysate was achieved by mass spectrometry as described in detail.12
EVs as biomarkers in NASH
There is a critical unmet need for the development of non-invasive biomarkers to diagnose, risk stratify and monitor patients with NAFLD. EVs as disease mediators can also function as disease biomarkers, with the evolving concept that serum EVs may serve as a ‘liquid biopsy’ and abrogate the risk and inconvenience of the traditional liver biopsy.28 Circulating EVs can derive from diverse cell types. Using immune cell-derived EVs as disease signature, patients with chronic hepatitis C could be differentiated from patients with NASH.42 EVs derived from invariant natural killer T (NKT) cells and CD14+ macrophages/monocytes were prevalent in the circulation of patients with NASH. The level of these EVs correlated with the levels of alanine aminotransferase and the histological severity of NASH.42 Although circulating EVs mirror tissue injury, current techniques provide a diverse pool of EVs that might differentially represent various body tissues/cells, confounding their use as biomarkers without isolation of tissue specific EVs.
Recently identified hepatocyte-specific EV markers include CYP2E111,12 and asialoglycoprotein receptor 1.32 These markers are still in the preclinical phase, but have the potential to specifically track and examine circulating EVs of hepatocyte origin. Emerging sophisticated technologies have the potential to study circulating hepatocyte-derived EV in the peripheral blood. These new techniques include nanoscale flow cytometry43 and integrated nanotechnique-based strategies for biomarkers discovery, using nanoplasmon-enhanced scattering assay.44 The assay uses the binding of antibody-conjugated gold nanospheres and nanorods to EVs captured by EV-specific antibodies on a sensor chip to produce a local plasmon effect that enhances detection of a specific subset of EVs.44
EV cargoes that could be potential biomarkers in NASH are summarised in table 1. Despite the potential of EVs use as biomarkers, a number of challenges remain. Isolation of EVs and their quantitative and functional analysis is challenging due to the requirement of prolonged differential ultracentrifugation and sophisticated instrumentation. Furthermore, different EV subpopulations isolated from different fractions obtained by ultracentrifugation may have different biological functions.45 Hence, the concept of EVs as biomarkers in NASH warrants careful validation in clinical studies.
Table 1.
Potential EV-associated biomarkers of NAFLD
| Type of study | EV source | EV cargo | Reference |
|---|---|---|---|
| Preclinical | Plasma/serum | miR-122 and miR-192 | 32 |
| Plasma | Ceramide and S1P | 13 | |
| Plasma | CXCL10 | 12 | |
| Plasma | VNN1 | 34 | |
| Clinical | Plasma | Ceramide and S1P | 13 |
| Plasma/serum | CD14 | 42 |
CD, cluster of differentiation; CXCL10, C-X-C motif chemokine ligand 10; EV, extracellular vesicle; NAFLD, non-alcoholic fatty liver disease; S1P, sphingosine-1-phosphate; VNN1, vanin-1.
HEPATOCYTE LIPOTOXICITY AND LIVER INFLAMMATION
Besides hepatocyte injury and death, inflammation is another histological hallmark of NASH. The inflammation during NASH is described as sterile inflammation, as the inflammatory response occurs in the absence of pathogens or external antigens.46 This sterile inflammation may be a consequence of lipid-induced hepatocyte stress, damage and cell death. Indeed, cell death can trigger an inflammatory response by innate immune cells.7 On the other hand, a sustained inflammatory response may contribute to hepatocellular injury and death, creating a feed-forward loop between tissue injury and inflammation. Inflammation in NASH is most strikingly associated with activation of the innate immune system,47 although cells of the adaptive immune system are also involved in the inflammatory response.48 We will briefly discuss how immune cells can recognise hepatocyte injury and death and describe major immune cell types implicated in NASH-associated inflammation. We will also highlight how sublethal hepatocyte injury can contribute to this inflammation.
Damage-associated molecular patterns (DAMPs) and their receptors
Hepatocyte lipotoxicity can cause cellular stress and eventual cell death that can trigger the release of danger signals in the form of intracellular molecules termed DAMPs. DAMPs may then activate a sterile inflammatory response in immune cells in order to restore tissue homeostasis. However, if the proinflammatory stimulus persists, the inflammatory response becomes exacerbated and can lead to chronic inflammation, tissue remodelling and fibrosis. To date, a wide spectrum of DAMPs has been identified. High-mobility group box 1 (HMGB1), nuclear and mitochondrial DNA, purine nucleotides (ATP, UTP), uric acid and interleukin (IL)-33 represent some of the DAMPs implicated in liver diseases.46 DAMPs are thought to be released from the cell as soluble molecules. In addition, recent reports have demonstrated that DAMPs, such as HMGB1 and heat-shock proteins, are also packaged and released in EVs; hence, cell death is not requisite for their cellular release.49
DAMPs are recognised by so-called pattern recognition receptors (PRRs), which were initially studied as receptors for bacterial products (pathogen-associated molecular patterns). PRRs comprise a variety of receptors expressed both on the cell surface and intracellularly. PRRs and their ligands are summarised in table 2. In NAFLD, the family of TLRs is the best characterised PRRs. Cell surface TLR4 is expressed by Kupffer cells, hepatocytes, liver sinusoidal endothelial cells and HSCs and can be activated by multiple ligands including lipopolysaccharide, HMGB1, heat shock proteins and free fatty acids.46 In vitro, treatment of macrophages with free fatty acids activates TLR4 signalling and macrophage proinflammatory polarisation.50 In isolated hepatocytes, free fatty acids stimulate secretion of HMGB1, which in turn activates hepatocyte TLR4 signalling in an autocrine manner, leading to hepatocyte NF-κB activation and cytokine expression.51 TLR4 deletion in hepatocytes prevented obesity-induced insulin resistance and systemic inflammation, during high-fat diet feeding.52 Furthermore, whole-body deletion of TLR4 attenuated the development of NASH in MCD-diet fed mice.53 Likewise TLR9 activation in a choline-deficient, amino acid-defined (CDAA) diet murine NASH model enhanced hepatic inflammation and fibrosis.54 Taken together, these studies suggest that inhibition of TLR4 and/or TLR9 activation appears to be a promising therapeutic strategy in NASH.
Table 2.
An overview of select PRRs and their ligands implicated in NASH
| Receptor | DAMP | Notes | Ref. |
|---|---|---|---|
| TLR2 | HMGB1 HSPs | TLR2 promotes liver injury, inflammation and fibrosis in CDAA diet-fed mice. | 86 |
| TLR4 | HMGB1 HSPs | MCD diet-fed TLR4 knockout mice display attenuated liver injury and inflammation. | 53 |
| TLR7/TLR8 | Single-stranded RNA | TLR7/8 role in NASH unknown. | |
| TLR9 | Mitochondrial DNA Histones | CDAA diet-fed TLR9 knockout mice display attenuated liver injury, inflammation and fibrosis. Hepatocyte-derived mitochondrial DNA in EVs promotes NASH via TLR9 activation. | 40 54 |
CDAA, choline-deficient, amino acid-defined; DAMP, damage-associated molecular pattern; EVs, extracellular vesicles.; HMGB1, high-mobility group box 1; HSPs, heat shock proteins; MCD, methionine-choline deficient; NASH, non-alcoholic steatohepatitis; PRR, pattern recognition receptor; TLR, toll-like receptor.
Immune cell types implicated in NASH
A variety of immune cells reside in healthy liver. Liver resident macrophages, referred to as Kupffer cells, are the most abundant immune cells in the liver. The healthy liver also contains natural killer (NK) cells, NKT cells and dendritic cells.47 There are several interspecies differences in the liver-resident immune cell populations; for example, the hepatic NKT cells population in the mouse is much higher than in humans, while human liver contains more NK cells than mouse liver.47 Hepatic inflammation during NASH is characterised by a striking accumulation of recruited monocytes/monocyte-derived macrophages and increased numbers of neutrophils and NK cells.55 Hepatic macrophage accumulation correlates with the severity of histological activity in human NASH. Since the hepatocyte injury has mainly been linked to an activation of the innate immune system, we will briefly discuss the involvement of resident macrophages, recruited monocyte-derived macrophages and neutrophils in NASH-associated inflammation. The role of the adaptive immune system during NASH has been recently reviewed elsewhere.47,56
Kupffer cells
Kupffer cells (liver resident macrophages) originate from progenitor cells derived from the yolk sac, reside in the sinusoidal space and maintain themselves by self-renewal.47,57 They represent sentinel cells that constantly remove pathogen or pathogen-derived products coming to the liver via portal blood.58 They also participate in antigen presentation and disposal of dead cells by phagocytosis. Kupffer cells are negative for CX3CR1, owing to their non-monocytic origin, they specifically express Clec4f,59 but their expression of other surface markers overlaps with monocytes.57 A recent report suggests that CD11c+ resident macrophages play a key role in the progression of simple steatosis to NASH in a murine model.60
In NASH, Kupffer cells appear to have a critical role in the development of inflammation and fibrosis by initiating the recruitment of other immune cells into the liver. Kupffer cells polarised towards a proinflammatory phenotype secrete inflammatory cytokines, such as tumour necrosis factor and chemokines, including C-C motif chemokine ligand (CCL) 2 and IL-8, thereby recruiting monocytes and neutrophils to the liver. 57 However, these and other chemokines, such as CXCL10, can be also secreted by stressed and dying hepatocytes in a soluble or EV-associated form.12,61 The contribution of each hepatic cell type to the pool of liver-derived chemokines is not currently known. It is also not entirely clear how Kupffer cells become activated during NASH. Likely multiple factors are involved, including hepatocyte-derived DAMPs and EVs, as discussed earlier in this review. Activated macrophages were demonstrated to express death receptor ligands,62 which may further exacerbate hepatocyte injury in a feed-forward loop.7 Nevertheless, experimental depletion of Kupffer cells attenuates hepatic inflammation in mice fed the MCD or CDAA diet.53,63,64
Monocyte-derived macrophages
During hepatic lipotoxic injury, circulating blood monocytes infiltrate the liver and give rise to monocyte-derived macrophages. Similar to resident macrophages, monocyte-derived macrophages play a vital role in perpetuating inflammation and tissue remodelling, but they may also promote the resolution of these processes.57 In NASH, monocytes infiltrate the liver via mechanisms largely dependent on chemokine receptors expressed by monocytes, such as C-C chemokine receptor (CCR) 2 and CXCR3.63,65 Liver-derived CCR2 ligands, such as CCL2, attract CCR2+Ly6Chigh monocytes which may later differentiate into CCR2lowCX3CR1+Ly6Clow monocyte-derived macrophages.47 Therefore, in experimental NASH models, CCR2−/− mice were protected against inflammation.63 Similarly, inflammation is significantly attenuated in CXCR3−/− mice and mice lacking CXCR3 ligands (eg, CXCL10) during MCD diet or obesity-induced NASH.65,66
Monocyte-derived macrophages, as well as Kupffer cells, change their phenotype according to the local microenvironment and contextual cues, which contribute to their substantial disease subtypes. Often macrophages have been classified as proinflammatory (M1) and wound-healing or reparative (anti-inflammatory, M2) macrophages based on markers they express. However, it is now clear that there is a wide continuous spectrum of macrophage activation states that cannot be simply described as M1 or M2 polarisation. A recent proposal suggests that a set of standards encompassing three principals should be used to describe macrophage activation status.67 These three principals relate to the source of macrophages, the definition of the activation stimulus and a consensus collection of markers.
Neutrophils
Hepatic neutrophil infiltration is another feature of NASH. Although the magnitude of their accumulation appears to be lower than in alcoholic steatohepatitis,68 they are likely important players in NASH progression. Mice lacking key neutrophilic enzymes, such as myeloperoxidase or elastase, displayed attenuated hepatic inflammation or improved insulin resistance in murine models of NAFLD.69,70 Recently, neutrophil extracellular trap formation has been implicated in liver sterile injury.71 However, whether this phenomenon occurs in the lipotoxic liver is not known. Future studies are needed to delineate the role of neutrophil extracellular trap formation in NASH pathogenesis.
CLINICAL IMPLICATIONS AND THERAPEUTIC STRATEGIES
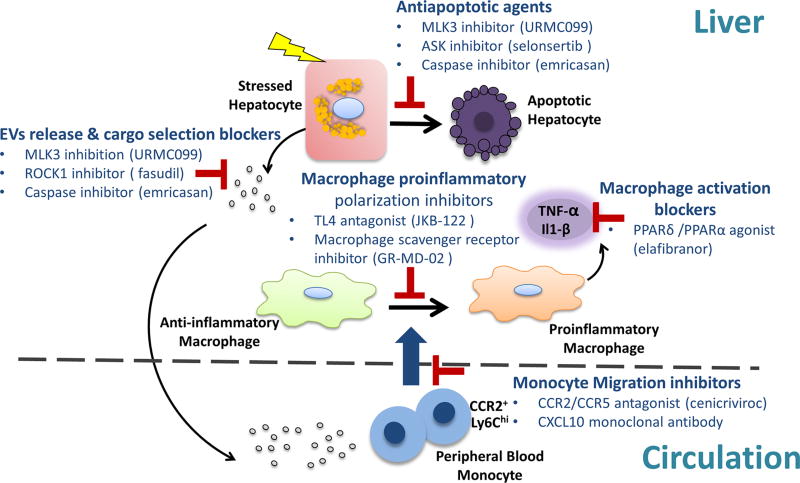
A plethora of therapeutic clinical trials are ongoing in NASH and have been extensively reviewed.72 Herein, we review only studies relevant to sublethal hepatocyte injury and monocyte-associated inflammation. Potential therapeutic agents directed to prevent sublethal hepatocyte injury-induced proinflammatory cascade in NASH are under different phases of development (figure 4). These therapeutic agents can be classified into two main categories. The first category targets hepatocyte-derived lipotoxic EVs release11 and chemotactic cargo selection.12 The second category modulates the inflammatory response by either blocking monocyte-derived macrophage recruitment to the liver, activation or proinflammatory polarisation.73 Relevant preclinical therapeutic agents in NASH are summarised in table 3.
Figure 4.
Therapeutic agents that target hepatocyte injury and the sterile inflammatory response in NASH. Current therapeutic strategies are outlined and include antiapoptotic agents, inhibitors of vesicle release and pathogenic cargoes sorting, inhibitors of macrophage chemotaxis, proinflammatory polarisation and activation. ASK, apoptosis signal-regulating kinase; CCR, C-C chemokine receptor; EVs, extracellular vesicles; MLK3, mixed lineage kinase 3; NASH, non-alcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptor; ROCK1, Rho-associated, coiled-coil containing protein kinase 1.
Table 3.
Preclinical therapeutic agents in NASH
| Drug | Mechanism of action | Results (drug vs placebo) | Reference/registered study |
|---|---|---|---|
| Selonsertib | ASK inhibitor | Improved fibrosis by ≥1 stage in drug combination | 75 |
| Obeticholic acid | FXR agonist | Improved NAS score by >2 points (45% vs 21%) | 87 |
| Emricasan (IDN-6556) | Pan-caspase inhibitor | Not yet released | 76, NCT02686762 |
| Elafibranor (GFT505) | PPARδ/PPARα agonist | NASH reversal (19% vs 12%) | 85 |
| Cenicriviroc | Dual CCR2/CCR5 antagonist | Improved fibrosis by ≥1 stage (20% vs 10%) | 78 |
| GR-MD-02 | Macrophage scavenger receptor inhibitor | Not yet released | NCT02421094 |
| JKB-122 | TLR4 receptor antagonist | Not yet released | NCT02442687 |
ASK, apoptosis signal-regulating kinase; CCR, C-C chemokine receptor; FXR, farsenoid X receptor; NAS, non-alcoholic fatty lived disease activity score; NASH, non-alcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptor; TLR, toll-like receptor.
Inhibition of proapoptotic signalling may serve as a therapeutic strategy for NASH,74 as it reduces the release of proinflammatory extracellular vesicles. Few antiapoptotic agents are currently in clinical trials including the selective apoptosis signal-regulating kinase 1 inhibitor selonsertib75 and the pancaspase inhibitor emricasan (IDN-6556). Selonsertib has shown improvement in fibrosis by ≥1 stage without worsening of NASH,75 while emricasan has shown improvement in ALT in preliminary reported data.76 The effect of these agents on EV release as potential biomarker for efficiency merits attention.
Blocking hepatic macrophage infiltration is achieved by either inhibiting the chemokines or their receptors. For example, cenicriviroc, a dual CCR2/CCR5 antagonist improved hepatic inflammation and fibrosis in a murine model of NASH.77 A phase IIb trial of cenicriviroc in patients with NASH patients with fibrosis78 showed improvement of fibrosis without worsening of steatohepatitis. Furthermore, the pharmacological MLK3 inhibitor URMC099 reduced circulating CXCL10 and attenuated murine NASH.79 Likewise, CXCL10 monoclonal antibody improved NASH in MCD-fed mice.66 CXCL10 hepatic expression12 and serum levels were significantly elevated in patients with NASH and correlated with the lobular inflammation,66 suggesting that CXCL10 may serve as a biomarker of disease activity. Since the CXCL10 monoclonal antibody has shown efficacy in patients with inflammatory bowel disease,80 it could potentially be repurposed for the treatment of human NASH.
Kupffer cells express galectin-3, the main scavenger receptor involved in the hepatic uptake of advanced lipid oxidation end products (ALE). Receptor-mediated endocytosis of ALE contributes to macrophage activation, progressive inflammation and fibrosis in NASH.81 In a NASH mouse model, treatment with the complex carbohydrate drug that binds galectin-3 (GR-MD-02) ameliorated NASH.82 In subjects with biopsy-proven NASH with advanced fibrosis, GR-MD-02 was safe and well tolerated,83 and a phase II clinical trial (NCT02421094) was recently completed. Furthermore, blocking macrophage activation can be achieved by employing a TLR4 antagonist like JKB-122, which is currently in early phase clinical trial (NCT02442687). Taken together, these studies support blocking macrophage activation as a therapeutic strategy to abrogate the hepatic inflammation in NASH.
Shifting macrophages from the proinflammatory to the anti-inflammatory phenotype is a potential therapeutic strategy. Anti-inflammatory macrophages rely on fatty acid oxidation. This alternative pathway is maintained by the fatty acid sensor PPARδ. Thus, mice lacking PPARδ develop more severe NASH compared with the wild type mice in an experimental model.84 Likewise, PPARα stimulation in hepatocytes facilitates oxidation of lipids and decreases hepatic steatosis.48 A phase IIb study of elafibranor, an agonist of PPARδ and PPARα, versus placebo in patients with NASH was recently published.85 Elafibranor resolved NASH without fibrosis worsening in 19% of patients on 120 mg/day for 1 year versus 12% of patients on placebo. Hence, PPAR agonists are potential therapeutic target in NASH.
Taken together, these studies suggest that potential future therapeutic directions in human NASH will arise with more in depth understanding of the mechanisms of sublethal hepatocyte injury and the role of EVs as pathogenic mediators and biomarkers in NASH.
CONCLUSION AND REFLECTION
Lethal lipotoxic injury is a histological hallmark of NASH and may contribute to disease pathogenesis. However, emerging concepts suggest that sublethal signalling by the release of EVs actually may be more important in triggering and maintaining a proinflammatory microenvironment in the liver. NASH-associated inflammation is largely macrophage related; the recruited monocyte-derived macrophages in response to hepatocyte injury, in turn, contribute to a vicious circle of liver injury, which is associated with an impaired resolution resulting in progressive hepatic fibrosis and end-stage liver disease. Interruption of these pathways, for example by inhibiting EV generation or release, may be therapeutic in NASH. This concept is an attractive therapeutic strategy, where therapy could be coupled with biomarkers in specific EVs subpopulations. Coupling therapy with a biomarker would help select and stratify patients for specific targeted therapies, permitting a precision medicine approach to NASH. These concepts suggest a myriad of potential pharmacological approaches for treating a heterogeneous disease that constitute a public health problem with no approved therapies.
Key messages.
-
►
Excess toxic lipids induce sublethal hepatocyte injury in non-alcoholic steatohepatitis (NASH).
-
►
Sublethal lipotoxic injury engages the proapoptotic machinery and induces hepatocyte stress and dysfunction, but is of an insufficient magnitude to execute cell death.
-
►
Sublethal hepatocyte injury creates a proinflammatory microenvironment in the liver and triggers aberrant, proinflammatory cascades.
-
►
Sublethal hepatocyte injury induces the release of proinflammatory extracellular vesicles; these vesicles mediate peripheral blood monocytes-derived macrophages hepatic infiltration and activation.
-
►
Current therapeutic strategies are directed to block the vicious circle created by sublethal hepatocyte injury and the sterile inflammatory response in NASH.
Acknowledgments
Funding This work was supported by NIH grants DK41876 (to GJG) and DK111397 (to SHI), North American Society of Pediatric Gastroenterology Hepatology and Nutrition Young Investigator Award/Nestle Nutrition Award and Gilead Sciences (to SHI), MH CZ–DRO (UHHK, 00179906) and the Mayo Clinic.
Footnotes
Correction notice This article has been corrected since it published Online First. The author’s corresponding address and the third affiliation has been corrected.
Contributors All authors have contributed equally in the outline of the review, research and writing of the manuscript. PH and SHI prepared the figures.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:1769–77. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arab JP, Karpen SJ, Dawson PA, et al. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–62. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13:2062–70. doi: 10.1016/j.cgh.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Bonci E, Chiesa C, Versacci P, et al. Association of nonalcoholic fatty liver disease with subclinical cardiovascular changes: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:1–11. doi: 10.1155/2015/213737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 7.Hirsova P, Gores GJ. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2015;1:17–27. doi: 10.1016/j.jcmgh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechmann LP, Gieseler RK, Sowa JP, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010;30:850–9. doi: 10.1111/j.1478-3231.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirsova P, Ibrahim SH, Gores GJ, et al. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–70. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsova P, Ibrahim SH, Malhi H, et al. Hepatocyte lethal and nonlethal lipotoxic injury. In: Ding WX, Yin XM, editors. Cellular injury in liver diseases. 1. Springer International Publishing; 2017. [Google Scholar]
- 11.Hirsova P, Ibrahim SH, Krishnan A, et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150:956–67. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim SH, Hirsova P, Tomita K, et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–44. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakazu E, Mauer AS, Yin M, et al. Hepatocytes release ceramide-enriched proinflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res. 2016;57:233–45. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsova P, Weng P, Salim W, et al. TRAIL deletion prevents liver, but not adipose tissue, inflammation during murine diet-induced obesity. Hepatol Commun. 2017;1:648–62. doi: 10.1002/hep4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Hirsova P, Gores GJ. Ballooned hepatocytes, undead cells, sonic hedgehog, and vitamin E: therapeutic implications for nonalcoholic steatohepatitis. Hepatology. 2015;61:15–17. doi: 10.1002/hep.27279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangwala F, Guy CD, Lu J, et al. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–10. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado MV, Diehl AM. Hedgehog signalling in liver pathophysiology. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakisaka K, Cazanave SC, Werneburg NW, et al. A hedgehog survival pathway in ’undead’ lipotoxic hepatocytes. J Hepatol. 2012;57:844–51. doi: 10.1016/j.jhep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsova P, Ibrahim SH, Bronk SF, et al. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013;8:e70599. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon H, Song K, Han C, et al. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease. Hepatology. 2016;63 doi: 10.1002/hep.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy CD, Suzuki A, Zdanowicz M, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–21. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stumptner C, Fuchsbichler A, Heid H, et al. Mallory body-a disease-associated type of sequestosome. Hepatology. 2002;35:1053–62. doi: 10.1053/jhep.2002.32674. [DOI] [PubMed] [Google Scholar]
- 25.Park HW, Park H, Semple IA, et al. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun. 2014;5:4834. doi: 10.1038/ncomms5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umemura A, He F, Taniguchi K, et al. p62, Upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell. 2016;29:935–48. doi: 10.1016/j.ccell.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal S, Bjørnevik K, Im DS, et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science. 2017;357:891–8. doi: 10.1126/science.aaf3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsova P, Ibrahim SH, Verma VK, et al. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology. 2016;64:2219–33. doi: 10.1002/hep.28814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyenne V, Apaydin A, Rodriguez D, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211:27–37. doi: 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–88. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Povero D, Eguchi A, Li H, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Povero D, Eguchi A, Niesman IR, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng ZB, Liu Y, Liu C, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412–20. doi: 10.1002/hep.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomita K, Kabashima A, Freeman BL, et al. Mixed lineage kinase 3 mediates the induction of CXCL10 by a STAT1-dependent mechanism during hepatocyte lipotoxicity. J Cell Biochem. 2017;118:3249–59. doi: 10.1002/jcb.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charlton M, Krishnan A, Viker K, et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–G834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauer AS, Hirsova P, Maiers JL, et al. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2017;312:G300–G313. doi: 10.1152/ajpgi.00222.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Povero D, Panera N, Eguchi A, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell Mol Gastroenterol Hepatol. 2015;1:646–63. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Martinez I, Santoro N, Chen Y, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–64. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–45. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornek M, Lynch M, Mehta SH, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–58. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brett SI, Lucien F, Guo C, et al. Immunoaffinity based methods are superior to kits for purification of prostate derived extracellular vesicles from plasma samples. Prostate. 2017;77:1335–43. doi: 10.1002/pros.23393. [DOI] [PubMed] [Google Scholar]
- 44.Liang K, Liu F, Fan J, et al. Nanoplasmonic quantification of tumor-derived extracellular vesicles in plasma microsamples for diagnosis and treatment monitoring. Nat Biomed Eng. 2017;1:0021. doi: 10.1038/s41551-016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tkach M, Kowal J, Zucchetti AE, et al. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. Embo J. 2017;36:3012–28. doi: 10.15252/embj.201696003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–72. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Heymann F, Tacke F. Immunology in the liver-from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 48.Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64. doi: 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buzas EI, György B, Nagy G, et al. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–64. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 50.Huang S, Rutkowsky JM, Snodgrass RG, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–13. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Chen L, Hu L, et al. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620–30. doi: 10.1002/hep.24552. [DOI] [PubMed] [Google Scholar]
- 52.Jia L, Vianna CR, Fukuda M, et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–9. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–34. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahraman A, Schlattjan M, Kocabayoglu P, et al. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology. 2010;51:92–102. doi: 10.1002/hep.23253. [DOI] [PubMed] [Google Scholar]
- 56.Sutti S, Jindal A, Bruzzì S, et al. Is there a role for adaptive immunity in nonalcoholic steatohepatitis? World J Hepatol. 2015;7:1725–9. doi: 10.4254/wjh.v7.i13.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–21. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 58.Dixon LJ, Barnes M, Tang H, et al. Kupffer cells in the liver. Compr Physiol. 2013;3:785–97. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott CL, Zheng F, De Baetselier P, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itoh M, Suganami T, Kato H, et al. CD11c+ resident macrophages drive hepatocyte death-triggered liver fibrosis in a murine model of nonalcoholic steatohepatitis. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cullen SP, Henry CM, Kearney CJ, et al. Fas/CD95-induced chemokines can serve as "find-me" signals for apoptotic cells. Mol Cell. 2013;49:1034–48. doi: 10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 62.Canbay A, Feldstein AE, Higuchi H, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–98. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 63.Miura K, Yang L, van Rooijen N, et al. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tosello-Trampont AC, Landes SG, Nguyen V, et al. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287:40161–72. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomita K, Freeman BL, Bronk SF, et al. CXCL10-mediates macrophage, but not other innate immune cells-associated inflammation in murine nonalcoholic steatohepatitis. Sci Rep. 2016;6:28786. doi: 10.1038/srep28786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Shen J, Man K, et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J Hepatol. 2014;61:1365–75. doi: 10.1016/j.jhep.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greuter T, Malhi H, Gores GJ, et al. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: exploiting similarities and differences in pathogenesis. JCI Insight. 2017;2 doi: 10.1172/jci.insight.95354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rensen SS, Bieghs V, Xanthoulea S, et al. Neutrophil-derived myeloperoxidase aggravates non-alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLoS One. 2012;7:e52411. doi: 10.1371/journal.pone.0052411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Talukdar S, Oh DY, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–87. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 72.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–90. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]
- 73.Lanthier N. Targeting Kupffer cells in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why and how? World J Hepatol. 2015;7:2184–8. doi: 10.4254/wjh.v7.i19.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barreyro FJ, Holod S, Finocchietto PV, et al. The pan-caspase inhibitor emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35:953–66. doi: 10.1111/liv.12570. [DOI] [PubMed] [Google Scholar]
- 75.Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2017 doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shiffman M, Freilich B, Vuppalanchi R, et al. LP37: a placebo-controlled, multicenter, double-blind, randomised trial of emricasan in subjects with non-alcoholic fatty liver disease (NAFLD) and raised transaminases. J Hepatol. 2015;62:S282–S82. [Google Scholar]
- 77.Lefebvre E, Hashiguchi T, Jenkins H, et al. Anti-fibrotic and anti-inflammatory activity of the dual CCR2 and CCR5 antagonist cenicriviroc in a mouse model of NASH. Hepatology. 2013;58:221–2. [Google Scholar]
- 78.Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2017 doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomita K, Kohli R, MacLaurin BL, et al. Mixed-lineage kinase 3 pharmacological inhibition attenuates murine nonalcoholic steatohepatitis. JCI Insight. 2017;2 doi: 10.1172/jci.insight.94488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayer L, Sandborn WJ, Stepanov Y, et al. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut. 2014;63:442–50. doi: 10.1136/gutjnl-2012-303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–11. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 82.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrison SA, Marri SR, Chalasani N, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther. 2016;44(11–12):1183–98. doi: 10.1111/apt.13816. [DOI] [PubMed] [Google Scholar]
- 84.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:2084–84. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 86.Miura K, Yang L, van Rooijen N, et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577–89. doi: 10.1002/hep.26081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]