1. Introduction
Over 70 years ago, Renee Spitz described the phenomenon of anaclitic depression in institutionalized children, in which children deprived of social contact would eventually appear emotionally withdrawn and demonstrate impaired physical and intellectual development (Spitz and Wolf, 1946). Since then, an impressive amount of research has investigated the health consequences of early life adversity. Consistent with the biological vulnerability of the developing brain, early life stress (ELS; defined as childhood maltreatment, abuse or neglect) increases the risk for later psychopathology. ELS is associated with illnesses across the diagnostic spectrum, including major depressive disorder (MDD) (Heim and Binder, 2012), anxiety (Phillips et al., 2005), substance use (Andersen and Teicher, 2008), personality disorders (Ball and Links, 2009), attention-deficit/hyperactivity disorder (ADHD) (Harold et al., 2013), and psychotic disorders (Varese et al., 2012). The particular psychopathological trajectory might depend on the type of ELS (Carr et al., 2013) and the developmental window in which the stress was experienced (Andersen et al., 2008), as well as genetic and epigenetic risk and protective factors (Caspi et al., 2003; Palma-Gudiel and Fañanás, 2016). In this review, we argue that ELS, instead of being considered as having a direct association with specific diagnoses, is better conceptualized as altering core dimensional aspects of affective and cognitive function related to psychiatric illness.
That reward processing would be affected by exposure to ELS has a conceptual basis in learning and attachment theories, as well as in empirical data on stress neurobiology. Because learning about reward cues and contingencies occurs early on in the context of the parent-child relationship, when that relationship is abusive, inconsistent, or absent, learning how various cues and behaviors lead to reward is impaired (Pollak, 2015). For example, when a child grows up in an abusive home, they may become hypervigilant to cues predicting danger at the expense of attending to cues predicting reward. This is supported by evidence that those with a history of abuse demonstrate impairments in adjusting reward-seeking behavior based on changing environmental information (Guyer et al., 2006; Pechtel and Pizzagalli, 2013)
From a neurobiological perspective, perhaps one of the most well-known effects of ELS is alteration of the hypothalamic-pituitary-adrenal (HPA) axis (for reviews, see McCrory et al., 2010; Strüber et al., 2014; Tyrka et al., 2013). The HPA axis modulates the endocrine response to stress, regulating the release of glucocorticoids such as cortisol via interactions between corticotropin releasing factor (CRF), adrenocorticotropic hormone (ACTH) and receptors for glucocorticoids that provide negative feedback. CRF, besides inducing the release of ACTH, also acts within the brain to enhance sympathetic arousal (Dunn and Swiergiel, 2008; Valentino et al., 1983). Early life stress has been found to both exaggerate and attenuate the activity of the HPA axis in response to stress, both phenomena which have been linked to psychopathology (Carpenter et al., 2009; Heim C et al., 2000; Tyrka et al., 2008). Such alterations are relevant to reward processing, as receptors for CRF and glucocorticoids are located throughout the reward circuitry of the brain, with their activation or blockade affecting reward-sensitive neurotransmission and behaviors (Bryce and Floresco, 2016; Craenenbroeck et al., 2005; Walsh et al., 2014). This overlap between stress neurobiology and reward neurobiology suggests that any change in stress neurobiology is likely to influence reward.
Reward processing encompasses the response to rewarding stimuli, the ability to learn from reward, the anticipation of future rewards, and engagement in goal-directed behavior towards rewards (Berridge and Robinson, 2003; Rizvi et al., 2016). Deficits in reward processing are found in MDD (Admon and Pizzagalli, 2015), substance use disorders (Garfield et al., 2014; Tobler et al., 2016), posttraumatic stress disorder (PTSD) (Kalebasi et al., 2015), schizophrenia (McCarthy et al., 2016), and ADHD (van Hulst et al., 2016). Even in individuals who do not meet full diagnostic criteria, alterations in reward processing predict later diagnoses of MDD (Nelson et al., 2016), substance use disorder (Castellanos-Ryan et al., 2011; Garfield et al., 2014), and conduct disorder (Rubia et al., 2009). Accordingly, identifying at-risk individuals and tailoring interventions to target the reward processing system could represent an effective strategy to prevent later psychopathology (McCrory and Viding, 2015). While this remains to be tested, there are data showing that cognitive remediation, including working memory training and mindfulness, can enhance reward processing in non-clinical populations with high trait social anhedonia (Li et al., 2016) and in prescription opioid abusers (Garland, 2016).
The goal of this review is to summarize the research to date on ELS and reward processing, with the intent of providing clinicians and clinical scientists interested in ELS an introduction to this emerging field of inquiry and encouraging additional investigation. By integrating results from both the human and animal literatures, we aim to provide a perspective that synthesizes studies benefiting from the control and specificity of animal models with the more complex human work. For example, animal studies in ELS can utilize a specific strain of species (e.g., Sprague Dawley rats, rhesus monkeys) that are bred from a limited number of parents, and are thus genetically similar. Furthermore, test animals are housed in uniform conditions, with precise control over the type, timing, and dosage of ELS. Variability and co-variation of these factors represent a major challenge in human studies. However, animal models cannot replicate the complexity of human ELS, and the significance of findings for human conditions is not always clear. Consideration of these two approaches together should provide perspective on the limitations of each approach and new insight into the effects of ELS on reward processing. We start with a brief overview of reward processing relevant to the ELS literature, introducing the neurobiological and conceptual basis of reward processing as well as methods employed in its measurement. Next, we review findings in humans and animals, followed by a synthesis of these two domains. Finally, we describe the implications of reward processing dysfunction, as a risk factor and target for intervention.
2. Methods
A systematic review was completed by searching PubMed to identify studies conducted in humans and animals evaluating the effects of ELS on reward processing. Keywords relevant to ELS and reward processing were chosen, based on keywords and terms used in prior research. For human studies, we used the following search terms: (“early life stress” OR “childhood maltreatment” OR “adverse childhood experiences” OR “childhood adversity” OR “childhood trauma” OR “emotional abuse” OR “emotional neglect” OR “ sexual abuse” OR “physical abuse” OR “physical neglect”) AND (“reward” OR “striatum” OR “reinforcement”). To identify animal studies, a similar strategy was used, except with keywords specific to common ELS paradigms: (“maternal separation” OR “social isolation” OR “early life stress”) AND (“reward” OR “reinforcement” OR “sucrose preference”). In addition, we cross-checked these search results with relevant studies cited by other authors.
We included studies published prior to December 9, 2017 in which humans or animals had experienced or been subjected to some form of ELS and that assessed some aspect of reward processing via measurements of behavior. In the case of humans, we only included studies in which functional magnetic resonance imaging (fMRI) was utilized in conjunction with a reward processing task. Studies that did not meet these criteria, such as animal studies that did not include a measurement of reward behavior or human studies that only utilized fMRI without a reward task, were excluded. Initial literature searches, especially within the animal literature, revealed that many of the studies evaluated reward processing in the context of drugs of abuse and risk for substance use disorders. Given that the topic of ELS and addiction is reviewed extensively elsewhere (Andersen and Teicher, 2009; Burke and Miczek, 2014; Delavari et al., 2016; Enoch, 2012), we restricted our review to studies that dealt with non-drug reward (food, social interaction, money, positive feedback). Data were extracted by no fewer than two separate authors to ensure accuracy and limit bias (Tables 1, 2).
Table 1.
Studies of Reward Processing in Animals.
| Maternal Separation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year |
Species/ Strain |
Sex | Timing of Exposure |
Nature of Exposure |
Methods/Group Design |
Inferred Reward Construct |
Method to Assess Reward |
Object | PND Test |
Main Findings | Effect on Reward |
| Amiri et al., 2016 | NMRI mice | M | PND 2-14 | MS | MS: separated from their mothers for 180 min/day. | Reward responsiveness | SPT | 1% sucrose solution | PND 50 | Reduced consumption of sucrose compared to control rats. | Decreased reward responsiveness. |
| Bai et al., 2014 | Sprague Dawley rats | M+F |
MS: PND 1-13 CUPS: 10 weeks |
MS, CUPS, MS+CUPS |
MS: separated from their mothers for 6 hrs/day for 2 wks. CUPS: exposed to one of various stressors once a day for 3 wks beginning at PND 70. DUAL STRESS: MS + CUPS. CONTROL: standard husbandry care. |
Reward responsiveness | SPT | 1% sucrose solution | 13 wks | Reduced consumption of sucrose in male MS and CUPS, but not MS + CUPS rats compared to controls. Female MS+CUPS rats consumed less than control rats. |
Decreased reward responsiveness in males with only one exposure and in females with only dual exposure. |
| Bolton et al., 2017 | Sprague Dawley rats | M | PND 2-9 | Limited bedding |
Limited bedding: Pups and dam reduced nesting material PND 2-9. Standard bedding on PND 10. Controls: Normal bedding. |
Reward responsiveness, approach motivation | SPT, social interaction | 1.5% sucrose solution, social play with conspecific | PND 56 | Decreased sucrose preference and duration of social play in rats exposed to limited bedding. | Decreased reward responsiveness. |
| Campbell et al., 2017 | Wistar rats | M | PND 2-14 | MS |
MS: separated from mother for 3 hrs/day. Control: undisturbed. |
Approach motivation | Sucrose self-administration (PR) | 10% sucrose solution | PND 70-75 | MS rats similar self-administration on FR, decreased self-administration on PR. | Decreased approach motivation. |
| Ferreira et al., 2013 | Wistar rats | M | PND 1-10 | MS |
MS: pups removed from home cages and placed in separate cages for 3 hrs/day. NH: not handled until weaning. H: pups were removed from home cages and placed in separate cages for 10 min/day. |
Reward responsiveness | SPT | 1% sucrose solution | Not specified | MS rats consumed more sucrose in the second hr of exposure. | Increased reward responsiveness. |
| Hill et al., 2014 | Wistar rats | M+F | PND 2-14 | MS |
MS: pups separated from dam 3 hrs/day. CONTROL: pups exposed to 10-15s of separation/day. At 8 wks, all rats randomly assigned to receive 2 wks of cort or vehicle. |
Reward responsiveness | SPT | 1% sucrose solution | Wk 14 | No difference with MS in male or female. Decreased preference in MS females receiving cort. | Decreased reward responsiveness with “two hit” of MS + cort in females but not males. |
| Hui et al., 2011 | Sprague Dawley rats | M+F | PND 2-14 | MS with or without EE post-weaning |
MS: litters separated from dams for 180 min/day. Non-MS: stayed with dams. Post weaning on PND 21, half the pups housed in standard conditions and half the pups housed in EE. |
Reward responsiveness | SPT | 1% sucrose solution | PND 61-75 | Reduced sucrose preference in MS rats. EE rescued effect of MS sucrose preference. |
Decreased reward responsiveness. |
| Klug and Van de Bluse., 2012 | Wistar rats | M+F | PND 2-14 | MS |
MS: pups separated from dam 3 hrs/day. CONTROL: exposed to 10-15s of separation. At 8 wks, all rats received 2 wks of cannabinoid receptor agonist or vehicle. |
Reward responsiveness | SPT | 1% sucrose solution | Wk 12 | No difference with just MS in either sex. Decreased preference in MS males receiving cort. | Decreased reward responsiveness with “two hit” of MS + cannabinoid in males but not females. |
| Kosten et al., 2006 | Sprague Dawley rats | F | PND 2-9 | MS |
MS: pups isolated individually for 1 hr/day. CONTROL: NH. |
Approach motivation | Food self-administration (PR) | Food pellets | ~PND 90 (adult rats≥ 90) | MS females demonstrated increased lever pressing for food on escalating PR schedule. | Increased approach motivation/willingness to work. |
| Kundakovic et al. 2013 | Balb/cJ (Balb/c) mice and C57BL/6J (B6) mice | M+F | PND 1-14 | MS + Maternal Unpredictable stress |
MS: pups isolated together for 2 hours daily. Maternal Stress: Dams exposed to either 20 min restraint stress or 2 min forced swim during separation. Controls: left undisturbed. |
Reward responsiveness, approach motivation |
SPT | 1% sucrose | PND 36-39 (SPT) | -MS C57BL/6J males and females decreased sucrose preference vs controls. -MS Balb/c males decreased sucrose preference vs controls. -MS Balb/c females increased sucrose preference vs controls. |
Decreased reward responsiveness in C57BL/6J M+F and Balb/c M. Increased reward responsiveness in Balb/c females. |
| Lomanowska et al., 2006 | Sprague Dawley rats | M | PND 5-17 | Artificial Rearing |
Artificial Rearing: feeding though gastric cannulae. Maternal Rearing: reared by foster dam. |
Reward responsiveness | SPT | Table sugar | PND 42-63 | Increased sucrose preference in artificially reared rats. | Increased reward responsiveness. |
| Maniam &; Morris, 2010 | Sprague Dawley rats | M+F | PND 2-14 | MS |
NH: litters not handled MS15: litters separated from dams for 15 min/day. MS180: litters separated from dams for 180 min/day. |
Reward responsiveness | SPT | 2.5% sucrose solution | PND 34 | Male MS180 rats decreased sucrose preference compared to NH and MS15 rats. No differences in females. | Decreased reward responsiveness in males only. |
| Matthews et al., 1996a | Lister-hooded rats | M+F | PND 5-20 | MS |
MS: removed from home cages for 6-hr periods on 10 random days. CONTROL: litters removed from home cages and placed into identical wire baskets for 5 min/separation day. |
Approach motivation, learning (valuation) | Conditioned locomotor activity to food, contrast effects on sucrose preference | Food pellets, sucrose solutions | 10 wks, 12 wks (experimentally naïve) or 28 wks | MS females failed to develop conditioned locomotor response. No effect on sucrose preference. Controls greater downward shift in lick frequency during negative contrast. Controls increased lick frequency more than MS during positive contrast. |
Decreased approach motivation and decreased learning. |
| Matthews et al., 1996b | Lister- hooded rats | M+F | PND 5-20 | MS |
MS: 6-hr separations on 10 random days. CONTROL: separated for 2 min/separation day. |
Approach motivation | Conditioned locomotor response to food, effects of amphetamine, sulpiride, SCH-23390, and clonidine | Food pellets | 10 wks | MS rats attenuated and delayed conditioned locomotor response; blunted response to enhancing effect of amphetamine and enhanced sensitivity to depressant effects of D2 blockade. | Decreased approach motivation. Pharmacological manipulations suggestive of decreased DA tone. |
| Matthews and Robbins, 2003 | Lister-hooded rats | F | PND 5-20 | MS |
MS: 6-hr separations performed on 10 random days. CONTROL: separated for 2 min/separation day. |
Approach motivation, “hedonic capacity” | Intracranial self-stimulation threshold | Electrical current to lateral hypothalamus | >20 wks | No difference in baseline threshold for stimulation. MS rats more sensitive to D2 antagonist. |
No baseline differences. |
| Michaels and Holtzman, 2006 | Long-Evans hooded rats | M | PND 3-14 | MS |
MS: 15 1-hr or 3-hr separations. CONTROL: NH. |
Reward responsiveness | SPT | 10% sucrose solution vs deionized water | ~ 3 months | MS rats drank more fluid total, but no differences in sucrose preference. | No differences. |
| Mrdalj et al., 2016 | Wistar rats | M | PND 2-14 | MS with or without CMS |
LMS: offspring separated from dams 180 min/day. BMS: offspring separated from dams 10 min/day. NH CONTROL: no separation. At PND 90, half of each group was exposed to CMS for 4 wks. |
Reward responsiveness | SPT | 1% sucrose solution | ~PND 90, 120 | No effect of early life condition on sucrose preference. Decreased preference with CMS. | Reduced reward responsiveness in rats with CMS. |
| Øines, Murison, Mrdalj, Grønli, &; Milde, 2012 | Wistar rats | M | PND 2-14 | MS with or without SIS |
LMS: offspring separated from dams for 180 min/day. BMS: offspring separated from dams for 10 min/day. NH CONTROL: dams and offspring left completely undisturbed. From PND 72-75 until PND 85-88, rats from each group exposed to SIS or received standard animal care. SIS consisted of pairing each rat with a new rat daily for 14 days. |
Reward responsiveness | SPT | 1% sucrose solution | PND 64-68, 86-90 | No difference in preference for LMS offspring compared to NH and BMS offspring. No effect of SIS observed on sucrose preference, but overall liquid consumption less for LMS rats. | No differences: LMS rats exhibited decreased liquid consumption overall. |
| Paul et al., 2000 | Rhesus-Macaque monkeys | M+F | 3 days-7 months | MS |
MS: separated from mothers at 3 days old to be hand/nursery reared. Pair housed at 7 months. CONTROL: reared normally. |
Reward responsiveness | Sucrose and quinine consumption | Sucrose (2.74-175.0 mM) and quinine-HCl (0.1-5.0 mM) solutions | 4-5 years | MS monkeys consumed less sucrose containing solutions and more quinine containing solution. | Unclear; authors speculate there may be reduced gustatory sensitivity overall. |
| Pryce et al., 2004 | Marmoset monkeys | M+F | PND 2-28 | MS |
MS: infant brought to remote isolation chamber for 30-120 min/day. CONTROL: carrying parent restrained briefly and released. Monkeys’ performance on VD/VR/VRR task was assessed. |
Reinforcement learning, approach motivation, reward responsiveness | CANTAB (stimulus response with reversal learning, PR), free consumption | Banana flavored milk | 9 months | MS monkeys made more errors during reversal learning, decreased responding on PR. Similar consumption of freely available reward. | Decreased learning and approach motivation, similar reward responsiveness. |
| Rüedi-Bettschen et al., 2005 | Wistar rats | M | PND 1-14 | MS |
MS – pup isolated for 4 hrs/day. MSxLightxWarm. MSxLightxCold. MSxDarkxWarm. MSxDarkxCold. NH: NHxDark. NHxLight. Light (lights on 0700-1900) vs dark phase (lights off 0700-1900). Cold (21° C) vs warm (32-33° C). |
Approach motivation, reward responsiveness | PR responding, free consumption | 7% sucrose solution | 6 months | MS during dark cycle (warm or cold) resulted in decreased responding and reinforcement on PR. No difference in free consumption. | Decreased approach motivation and no change in reward responsiveness. |
| Sadeghi, Peeri, &; Hosseini, 2016 | Albino Wistar rats | M | PND 2-14 | MS |
CONTROL: no maternal separation. MS: litters separated from mothers for 180 min/day. MS + fluoxetine: 5 mL administered intraperitoneally from PND 28 to 60. MS + voluntary wheel running: free access to running wheel 24 hrs/day from PND 28 to 60. MS + mandatory treadmill: rats treated to exercise for increasing interval of time over 4 wk period. |
Reward responsiveness | SPT | 1% sucrose solution | PND 60 | Reduced sucrose consumption in MS rats compared to control rats. | Decreased reward responsiveness. |
| Sasagawa et al., 2017 | C57BL/6N mice | M+F | PND 1-14 | MS+SI |
MS+SI: Each pup separated from dam and placed in separate container for 3 hours daily. Control: Left undisturbed until weaning. All mice rehoused at weaning in groups of 3-4. |
Reward responsiveness, approach motivation | Free consumption + CPP |
Milk Chocolate | 3-4 Months | Similar free consumption of chocolate in MS+SI mice. Reduced CPP for chocolate in MS+SI females. | Decreased approach motivation in females but not males. |
| Shalev and Kafkafi., 2002 | Long-Evans rats | M | PND 3-14 | MS |
EH: pups separated from dams for 15 min/day at room temperature. MS: pups handled similarly to EH pups, but separated from dams for 180 min/day. NH: pups were undisturbed. |
Reward responsiveness, reinforcement learning, approach motivation | SPT, concentration response test, progressive reinforcement test |
1%, 3% and 0.5% sucrose solutions | 10 wks, 3 months, 11 wks | No differences for SPT, concentration response test or progressive reinforcement test. MS rats increased locomotor response compared to NH rats. | No changes. |
| Shu et al., 2015 | Sprague Dawley rats | M | PND 2-14 | MS with or without CMS |
MS: litters separated 3 hrs/day from mothers. CONTROL: no separation. After reaching adulthood, half of pups in control group and half of pups in MS group randomly assigned to CMS from PND 91 to 112. |
Reward responsiveness | SPT | 1% sucrose solution | PND 91, 112 | Decreased sucrose consumption in rats with MS compared to control rats without MS. Sucrose consumption of rats with CMS alone was lower than sucrose consumption of rats with MS. | Decreased reward responsiveness. |
| Uchida et al., 2010 | Sprague Dawley rats | M+F | PND 2-14 | MS with or without repeated restrained stress |
AFR: animals handled twice a wk during regular cage changes. MS15: pups separated from dams 15 min/day. MS180: pups separated from dams 180 min/day. At 8 wks old, adult rats subjected to 2 hrs/day of restraint stress for 14 days (held in wire mesh restrainers with head and feet secured). |
Reward responsiveness | SPT | 1% sucrose solution | 10 wks | No effect of MS alone on sucrose preference. MS180 rats given repeated restraint stress had decreased sucrose preference compared with non-restrained controls and MS 180 rats. | Decreased reward responsiveness with combination of MS and restraint. |
| Vazquez et al., 2005 | Long-Evans rats | M | PND1-14 | MS |
MS: pups separated from dam for 3 hrs/day. CONTROL: no handling other than cage cleaning. |
Approach motivation, reward responsiveness | SPT, morphine CPP, morphine consumption | 0.025% sucrose solution, 2mg/kg morphine for CPP, 25mg/L morphine solution for consumption | 2.5-3 months | Slightly increased sucrose preference in MS rats. Increased CPP and morphine consumption for morphine in MS rats. | Increased approach motivation and reward responsiveness for morphine; increased reward responsiveness for sucrose. |
| Zhang et al., 2005 | Sprague Dawley rats | M | PND 2-9 | MS |
MS: pups isolated individually for 1 hr/day. CONTROL: NH. Responding for food under FR15 and PR5 and PR escalation schedules was tested. |
Approach motivation | Food self-administration (FR, PR) | Food pellets | ~PND 70-90 | Lower responding rates at FR15 for food for MS rats than NH rats, but similar performance at PR for food. | Decreased approach motivation. |
| Social Isolation | |||||||||||
| Author, year | Strain | Sex | Timing of Exposure | Nature of Exposure | Methods/Group Design | Inferred Construct | Method to Assess Reward | Object | PND Test | Main Findings | Effect on Reward |
| Amitai et al., 2014 | Long- Evans rats | M | PND 24 | SI |
SI: single-housed Socials: housed in groups of 3. |
Reward learning | Reversal learning | Strawberry milkshake | 18 and 52 wks post-weaning | SI rats required more sessions in order to reach criterion performance when reward contingencies were reversed. | Decreased reward learning. |
| Brenes &; Fornaguera, 2008 | Sprague Dawley rats | M | PND 30- 114 | SI |
SI: housed individually under standard conditions. GH: housed under standard conditions in groups of 3. EE: housed in special cages with plastic objects, PVC tubes, food dispensers and water bottles. |
Reward responsiveness | SPT | 32% sucrose solution | PND 65-66, 93-94, 108-109 | SI rats showed greater preference for sucrose at PND 66 and consumed significantly more sucrose at PND 94 than GH and EE groups. | Increased reward responsiveness. |
| Brenes &; Fornaguera, 2009 | Sprague Dawley rats | M | PND 28- 94 | SI |
SI: housed singly in cages. GH: housed in groups of 3. |
Reward responsiveness | SPT | 32% sucrose solution | PND 90-91, 94-95 | SI rats had significantly increased sucrose consumption at PND 91 compared with GH rats. | Increase reward responsiveness. |
| Colonnello, Iacobucci, Anderson, &; Panksepp, 2011 | Octodon Degus | M+F | PND 25 | SI |
SI: housed in isolation for 4 wks; handled daily to be moved to treatment cages alone 1 hr/day. Partial SI: housed in isolation for 4 wks; allowed 1 hour of socialization with 2 same-sex sibs/day. Social Housing Group: housed with sex- matched sib; allowed 1 hour of socialization with sex-matched partial SI sib/day. |
Reward responsiveness | SPT | 2% sucrose solution | PND 25, 39 and 53-56 | Greater preference for sucrose at wk 4 in SI group than the partial SI and social groups. | Increased reward responsiveness. |
| Cuenya et al., 2015 | Wistar rats | M | PND 21-36 | SI |
SI: housed alone. CONTROL: housed in groups of 5-6. From PND 36-60, isolated subjects were regrouped. |
Reinforcement learning, Approach motivation | Sucrose consumption following shifts in concentration | 32% and 4% sucrose solution | PND 90 | SI enhanced consumption compared to controls following increase positive contrast (4%-32%). | Increased approach motivation. |
| Hall et al., 1997 | Lister-hooded rats | M | PND 21 onward | SI |
SI: housed individually. CONTROL: housed 4 rats/cage . |
Reward responsiveness, approach motivation | SPT in fed and deprived states, positive and negative contrast | 0.7%, 2.1%, 7.0%, 21.0%, 34.0% sucrose solutions under non-deprived conditions, 0.7%, 7.0% and 34.0% sucrose solutions under deprived conditions and for tests of positive and negative contrast | 8 wks | No difference between groups in consumption of sucrose in fed or deprived conditions. SI rats consumed more sucrose under conditions of positive and negative contrast and were more sensitive to positive contrast/less sensitive to negative contrast. |
Increased approach motivation due to isolation. |
| Hall et al., 1998a | Fawn- hooded and Wistar rats | M | PND 21 | SI |
SI: housed singly for 8 wks. CONTROL: housed 2 animals per cage for 8 wks. |
Reward responsiveness | Voluntary consumption of sucrose and saccharine | 0.7%, 2.1%, 7.0%, 21.0% and 34% sucrose solutions, 0.01%, 0.04%, 0.16%, and 0.64% saccharin solutions | ~PND 77 | SI rats had increased sucrose consumption at all concentrations. Isolated rats increased saccharin consumption at high concentrations. |
Increased reward responsiveness. |
| Hong et al., 2012 | Sprague Dawley rats | M+F | PND 30-49 | SI |
SI: housed one per cage. CONTROL: housed in groups of 2-3. Forced swim test at P49-50. All rats group-housed P50-P70. 2nd FST P70-P71. Rats isolated around ~P90 for habituation to sucrose. |
Reward responsiveness | SPT following 30 min of restraint | 1% sucrose solution | ~PND 100 | Females but not males increased sucrose intake during SPT. | Increased reward responsiveness in female rats isolated in adolescence. |
| Jones, Marsden, &; Robbins, 1990 | Lister- hooded rats | F | PND 21 | SI |
SI: housed individually for the duration of the experiment. GH: housed 6 rats/cage for the duration of the experiment. |
Approach motivation | Exp 1: Locomotor activity conditioned to food presentation Exp 2: Operant responding to a conditioned reinforcer (stimulus previously associated with sucrose) |
Standard rat chow, 10% sucrose solution | ~14 wks | SI resulted in increased conditioned locomotor activity and increased responding for conditioned reinforcer. | Increased approach motivation. |
| Li et al., 2007 | Sprague Dawley rats | M | PND 21 | SI |
SI: housed individually. Control: housed 3 per cage. |
Reward learning | Reversal learning in alternating T-maze | Food pellets | 8 wks post-weaning | No differences in initial acquisition of task. SI rats required more trials to acquire reversal learning. | Decreased reward learning. |
| McCool and Chappell, 2009 | Long-Evans rats | M | Starting between PND 28-32 | SI |
SI: housed individually. GH: housed 4 animals/cage. Wks 1, 2 &; 4: self-administration of either 10% ethanol or 3% sucrose. Wks 3&; 5: extinction trials. |
Reward responsiveness, approach motivation | Sucrose self-administration (FR) | 3% sucrose solution | ~PND 70 | No difference in response rate for sucrose between SI and GH animals. | No differences. |
| Morgan and Einon, 1975 | Lister-hooded rats | F | PND 25-120 | SI |
SI: housed in plastic mesh cages. CONTROL: housed in groups of 4. |
Reinforcement learning, Approach motivation | Two-lever DRL in which rat must alternate levers for food reward, waiting 30s in between responding | 45 mg Noyes sucrose pellet | PND 120 | SI rats show both increased perseverative (pressing same lever before 30s interval is up) and anticipatory (pressing alternate lever before 30s interval is up) responses compared to controls. | Increased approach motivation. |
| Pisu et al., 2011 | Sprague Dawley CD rats | M | ~ PND 25 | SI |
SI: housed individually in smaller cages for 30 days. GH:6-8 rats/cage for 30 days. |
Reward responsiveness | SPT | 32% sucrose solution | PND 55-60 | SI rats exhibited reduced intake and preference for sucrose compared to group-housed animals. | Decreased reward responsiveness. |
| Schrijver and Würbel, 2001 | Lister-hooded rats | M | PND 21 | SI |
SI: housed individually. Control: housed 3 per cage. |
Reward learning | Reversal Learning and Set Shifting Spatial and Non-Spatial Radial Arm Maze | Food pellets | PND 80 | No difference on acquisition or reversal learning within Spatial or Non-Spatial task. SI rats required more trials to acquire shift between spatial and non-spatial cues. | Decreased reward learning. |
| Van den Berg et al., 1999 | Wistar rats | M | PND 22-35 | SI |
SI: individually housed at 4 wks. GH: pair housed in cages. |
Approach motivation | CPP for social interaction, anticipation | Social interaction, 5% sucrose solution | ~Wks 8-11 | Isolation resulted in decreased CPP for social interaction and decreased anticipatory locomotion for sucrose. | Decreased approach motivation for social and food rewards. |
| Van den Berg, Van Ree, &; Spruijt, 2000 | Wistar rats | M | PND 21 | SI |
SI: housed in isolation with no social contact. PARTIAL SI: housed in isolation with 30 min of social contact/day. GH: housed 5 rats per cage. |
Reward responsiveness | Sucrose consumption | 5% sucrose solution | PND 22-35 | Increased sucrose consumption in SI rats compared to partial SI and GH. Partial SI rats had increased consumption compared to non-isolated rats. | Increased reward responsiveness. |
| Other ELS Paradigms | |||||||||||
| Author, year | Strain | Sex | Timing of Exposure | Nature of Exposure | Methods/Group Design | Inferred Construct | Method to Assess Reward | Object | PND Test | Main Findings | Effect on Reward |
| Bourke and Neigh., 2011 | Wistar rats | M+F | PND 37-49 | Combination SD and restraint |
Stress group: 6 total exposures to social defeat, 6 total exposures to 60-min restraint over 12 days. Adolescent CONTROL: pair-housed rats of each sex. Adult CONTROL: individually housed. |
Reward responsiveness | Sucrose consumption | 0.8% sucrose solution | Adoles-cent: PND 48-55 Adult: PND 96-103 | Adolescent stress resulted in decreased sucrose consumption in adolescence and adulthood in female but not male rats. | Decreased reward responsiveness in females but not males. |
| Novick et al., 2013 | Sprague Dawley rats | M | PND 35-40 | SD |
SD: put in cage of larger, aggressive adult rat 1x/day for 5 days. CONTROL: adolescent rats removed from home cages and put into novel cages for duration of SD task each day. |
Approach motivation | Sweetened condensed milk (SCM) consumption &; CPP | SCM | ~PND 60 | No difference in CPP or total consumption. | No difference. |
| Pohl et al., 2007 | Long-Evans rats | M+F | PND 23-51 | CMS, SSS |
CMS: exposed to variable stressors everyday for 4 weeks. SSS: exposed to 45 min restraint during water immersion or 5 min of 3x0.6 shocks, at random intervals twice a wk throughout the stress period. CONTROL: handled twice per wk. |
Reward responsiveness | Sucrose consumption | 0%, 7%, and 10% sucrose solutions | 45 wks | Females but not males in SSS and CMS stress groups consumed significantly less 10% sucrose solution. | Decreased reward responsiveness in females following stress. |
| Toth et al., 2008 | Sprague Dawley rats | M | PND 30 | CMS |
CMS: exposed to variable stressors. everyday for 4 weeks CONTROL: ordinary care. |
Reward responsiveness | SPT | 0.2% sucrose solution | ~PND 60 (directly after CMS) | No difference in sucrose preference between CMS and control animals. | No difference. |
Abbreviations: AFR, animal facility rearing; BMS, brief maternal separation; CMS, chronic mild stress; cort, corticosterone; CPP, conditioned place preference; CUPS, chronic unpredictable stress; EE, enriched environment; EH, early handling; FR, Fixed Ratio; GH, group housed; H, neonatal handling; LMS, long maternal separation; MS, maternal separation; NH, non-handled; ; PND, post-natal day; PR, Progressive Ratio; SD, social defeat; SI, Social Isolation; SIS, Social Instability Stress; SPT, sucrose preference test; SSS, severe sporadic stress
Table 2.
Studies using fMRI and Reward Tasks.
| Study Characteristics | Early Stress Exposure Characteristics | Reward Task Characteristics | Results in ELS Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Neural response findings
|
Other findings | ||||||||||||||
| Author, year |
Study N (N exposed) |
Gender | Mean Age |
Diagnosis or symptoms |
Nature of exposure |
Timing of exposure |
Method to assess exposure |
Reward phase/type |
Task | Object | |||||
| Striatum* | mPFC | Amygdala | Other Areas and Striatal Subregions |
||||||||||||
| Baranger, 2016 | 665 (-) | Both | 19.6 | 7.8% met DSM-IV criteria for Axis I disorder, including alcohol use disorder | Abuse and neglect | <17 years | CTQ | Delivery | Reward-guessing task | Monetary | ↑ ventral striatal reactivity to reward-related stimuli, NS after controlling for confounders | PER1 rs3027172 genotype interacted with ELS to predict both problematic drinking. | |||
| Birn, 2017 | 42 (23) | Both | 20.5 | NR | “Severe negative life events and circum-stances” | < 11 years | YLSI | Anticipation and delivery | MID | Monetary | ↓posterior cingulate, middle temporal gyrus, lingual gyrus, right middle frontal gyrus and cerebellum in anticipation, but not delivery of reward vs no-reward | On separate gambling task, high childhood stress correlated with decreased latency to place bets, the choice of low probability targets, and failure to adjust behavior to feedback. | |||
| Boecker, 2014 | 162 (-) | Both | 24.4 | Lifetime ADHD symptoms | Early family adversity (low educational level, overcrowd-ing, parental psychiatric disorder, etc.) | 3 months | Parent interview | Anticipation and delivery | MID | Monetary or verbal feedback | ↓ during anticipation ↑during delivery of verbal, but not monetary, reward |
↓ putamen, pallidum, left thalamus, left insula, left ACC and right anterior hippocampus during anticipation; ↑ bilateral insula, right pallidum and bilateral putamen during delivery of verbal, but not monetary, reward |
↑ reaction time. No effect on number of win trials. ↓ contingent negative variation on EEG. |
||
| Boecker-Schlier, 2016 | 168 (-) | Both | 24.5 | None meeting DSM criteria | Childhood family adversity (low educational level, overcrowd-ing, parental psychiatric disorder, etc.) |
<11 years | Parent interview | Anticipation and delivery | MID | Monetary and verbal | ↓left ventral striatum during anticipation, ↑right ventral striatum during delivery in COMT Met homozygotes |
↓ACC and left medial frontal gyrus during anticipation, ↑ACC during delivery in COMT Met homozygotes |
↓EEG contingent negative variation signal during anticipation. No significant effect of adversity on reaction time. | ||
| Casement, 2014 | 120 (-) | Female | 16 | 2.5% major depression | Social stressors (low parental warmth, peer victimiza-tion) | 11–12 years | PCRS and PES | Anticipation | Reward-guessing task | Monetary | ↑ for low parental warm | ↑ for low parental warm ↓ for higher peer victimization |
↑ for low parental warm | = OFC | Stress-related neural response to potential rewards correlated with depressive symptoms. |
| Casement, 2015 | 157 (-) | Male | 20 | NR | Cumulative stressful life events | 15–18 years | LEQ and IPSI | Anticipation and delivery | Reward-guessing task | Monetary | ↓ for anticipation and delivery | ↑ cumulative stressful life events associated with more problematic alcohol use. mPFC response mediated association between stressful life events and later symptoms of alcohol dependence. | |||
| Dennison, 2016 | 59 (21) | Both | 17 | 14% of maltreated sample met criteria for MDD | Abuse and neglect | <17 years | CTQ and CECA | Response to positive and neutral stimuli | Viewing social images | Socio-affective cues | ↑ | ↑left NAcc, and left putamen for positive relative to neutral stimuli | Higher levels of reward response in left putamen associated with decreased depression in maltreated youth. | ||
| Dillon, 2009 | 44 (13) | Both | 24.6 | 77% of ELS group met DSM-IV criteria for an Axis I disorder at some time | Abuse | ≤13 years | AAI, TSS, CTS and protective services records | Anticipation | MID | Monetary | ↓ left globus pallidus | ↑ symptoms of anhedonia and depression. Rated reward cues less positively. ↑ reaction time. ↑ putamen volumes. |
|||
| Goff, 2013 | 69 (38) | Both | 9.9 | 33% in ELS group with clinically significant externalizing/internalizing symptoms | Previously institutional-ized | < 2 years | - | Viewing happy faces |
Emotional faces task | Socio-affective cues | ↓ | ↓ NAcc | ↑ depression. Atypical NAcc development. Ventral striatum activation to happy faces negatively correlated with depression scores in ELS individuals. |
||
| Hanson, 2015 | 106 (-) | Both | 13.8 | Free of all psychopath-ology, except for anxiety disorders, at baseline | Emotional neglect | < 15 years | CTQ | Delivery | Reward-guessing task | Monetary | ↓ to positive feedback = to negative feedback |
↓ in this reward-related ventral striatum activity associated with ↑ symptomatology and partially mediated association between emotional neglect and subsequent depression. | |||
| Hanson, 2016 | 72 (-) | Male | 26.3 | High risk for long-term antisocial behavior | Parental loss, medical problems, other major life stressors | <Grade 12 | Life Changes measure | Delivery | Card-guessing task | Monetary | ↓ response to reward ↓ response to positive feedback = for negative feedback |
Effect significant for stress during early, but not later, childhood. No association between ventral striatum activity and adult internalizing or externalizing symptoms. | |||
| Holz, 2017 | 171(-) | Both | 25 | Conduct Disorder | Childhood family adversity (low educational level, overcrowd-ing, parental psychiatric disorder, etc.) | <11 years | Parent interview | Anticipation | MID | Monetary and verbal | ↓ventral striatum and dorsal striatum during anticipation | Conduct disorder partially mediated effects of adversity on brain activity. Negative relationship between reward dependence and adversity. | |||
| Mehta, 2010 | 23 (12) | Both | 16 | “Quasi-autism,” hyper-activity, cognitive impairment, disinhibited attachment | Romanian adoptees – previously institutional-ized | < 2 years | - | Anticipation | MID | Monetary | ↓ | = | ↓ Caudate nucleus = Insula and thalamus |
Adoptees did not recruit striatum during reward anticipation despite comparable performance accuracy and latency. Accuracy on MID task did not differ. |
|
| Morgan, 2014 | 120 (-) | Male | 20 | 13% MDD or dysthymia, 14% history of substance dependence, 8% history of ASPD | Low maternal warmth and maternal depression | < 2 years | Observation during mother–child interactions and SCID with mothers | Anticipation and delivery | Reward-guessing task | Monetary | ↑ for anticipation | ↑ for anticipation | |||
| Takiguchi, 2015 | 36 (16) | Both | 12.6 | RAD | Abuse and neglect | < 12 years | CATS | Gambling task | Monetary | ↓ | ↓NAcc and caudate | Earlier age of stress better predictor of decreased activity. Negative correlations between bilateral striatal activity and avoidant attachment. ↓ reaction time. |
|||
Notes: AAI, Adult Attachment Interview; ACC, anterior cingulate cortex; ADHD, attention-deficit/hyperactivity disorder; ASPD, antisocial personality disorder; CATS, Child Abuse and Trauma Scale; CS, conditioned stimulus; COMT, catechol-o-methyltransferase; CTQ, Childhood Trauma Questionnaire; CTS, Conflict Tactics Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; ELS, early life stress; fMRI, functional magnetic resonance imaging; IPSI, Interpersonal Problem Situations Inventory for Urban Adolescents; LEQ, Life Event Questionnaire for Adolescents; MDD, major depressive disorder; met, methionine; MID, monetary incentive delay; mPFC, medial prefrontal cortex; NAcc, nucleus accumbens; NR, not reported; NS, not significant; PCRS, Parent-Child Rating Scale; PES, Peer Experiences Scale; RAD, reactive attachment disorder; SCID, Structured Clinical Interview for DSM; TSS, Traumatic Stress Schedule; YLSI, Youth Life Stress Interview.
, Neural response findings included under striatum whenever subregions or whole striatum was activated
↑, higher relative to non-exposed subjects
=, same as non-exposed subjects
↓, lower relative to non-exposed subjects
3. Overview of Reward Processing
Reward processing broadly encompasses the biological and behavioral functions that serve to facilitate the acquisition of rewarding stimuli (Berridge and Robinson, 2003; Rizvi et al., 2016). The three broad components of reward processing are drive towards a reward, hedonic experience/liking, and reward learning (Berridge and Robinson, 2003). There have been various attempts to parse out these processes with even greater specificity. The NIMH Research Domain Criteria (RDoC; https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml) provides a framework for components of reward processing under its “Positive Valence Systems.” This consists of several main constructs which map onto the above-noted components: approach motivation (similar to the drive component), initial responsiveness to reward (hedonic experience), and reward learning. Within approach motivation, RDoC has defined additional subconstructs, including reward valuation, willingness to work, expectancy, and preference-based decision making. Although the RDoC framework is relatively new, we have applied this terminology wherever possible for the purposes of this review (see Figure 1). It should be noted that parsing of various reward processes using the RDoC framework is limited by the precision of the paradigm used, with several paradigms engaging more than one process.
Figure 1.
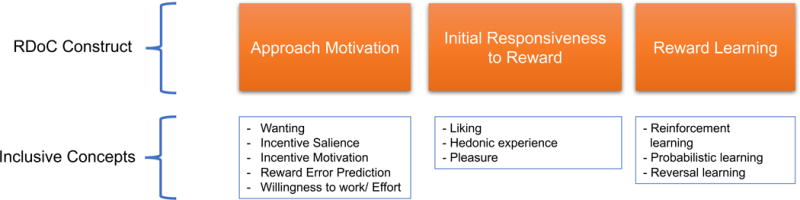
Three of the major NIMH Research Domain Criteria constructs relevant to reward processing. Inclusive concepts are subconstructs as well as phrases/concepts used in the reward processing literature that map onto each construct.
The importance of parsing out reward processes can be illustrated by the fact that in certain conditions, one reward function may be impaired while the others remain intact. For example, individuals with MDD have similar hedonic responses to sweet solutions compared to controls (Berlin et al., 1998), but are less likely to engage in tasks requiring higher amounts of effort (Treadway et al., 2012).
Evidence for these separate aspects of reward processing comes from research in animals on the neurobiological basis of reward and motivated behavior. In particular, attempts to understand the role of dopamine in the brain have demonstrated a differentiation between motivational and hedonic components of reward. Antagonism of dopamine receptors or dopamine depletion in the ventral striatum (specifically the nucleus accumbens) reduce the amount of effort an animal will expend to obtain a reward, but often leave intact reward consumption and positive hedonic responses (e.g., affective facial expressions) (Berridge and Robinson, 1998; Salamone and Correa, 2002). In addition, dopamine from the nucleus accumbens is important for the ability of rewarding cues to gain incentive salience and elicit instrumental behavior (Peciña et al., 2003; Wyvell and Berridge, 2000). Thus, dopamine in the nucleus accumbens appears to be necessary for the approach motivation aspects of reward processing, but is less involved in the hedonic experience/initial responsiveness to reward. Responsiveness to reward also involves the nucleus accumbens (in addition to other regions, such as the ventral pallidum), but opioids and endocannabinoids are the major neurochemical mediators of reward responsiveness (Mahler et al., 2007; Peciña and Berridge, 2005).
Dopamine signaling to the basal ganglia, including the nucleus accumbens, also plays a role in what is known as the reward prediction error, a subconstruct of approach motivation. The reward prediction error, in which changes in dopamine firing encode differences between an expected vs. actual reward outcome, allows for dynamic learning from experience and updates in reward expectations (Gradin et al., 2011; Schultz, 2016).
While a complete discussion of all the neuroanatomical structures and molecules involved in reward processing is beyond the scope of this review, regions of the prefrontal cortex (PFC) and amygdala are additional key brain areas involved in reward circuitry. Within the PFC, the orbital frontal cortex encodes and maintains reward value, while the anterior cingulate cortex plays a role in monitoring and determining effort required to obtain rewards (for review see (Rushworth et al., 2011)). Reward value and deviations of expected and actual outcomes are encoded via the amygdala through its projections to the PFC (Lichtenberg et al., 2017; Wassum and Izquierdo, 2015). Taken together, connections between midbrain dopamine cells, the basal ganglia (including structures within ventral striatum, such as the nucleus accumbens) and the PFC, with input from other structures, including the amygdala, thus form a reward circuit. It is in this circuit that information about reward cues, reward value, and required effort is processed, communicated, and then integrated to guide decision-making, facilitate motivation and readiness for action, and eventually result in the experience of pleasure (Rizvi et al., 2016) (Figure 2).
Figure 2.
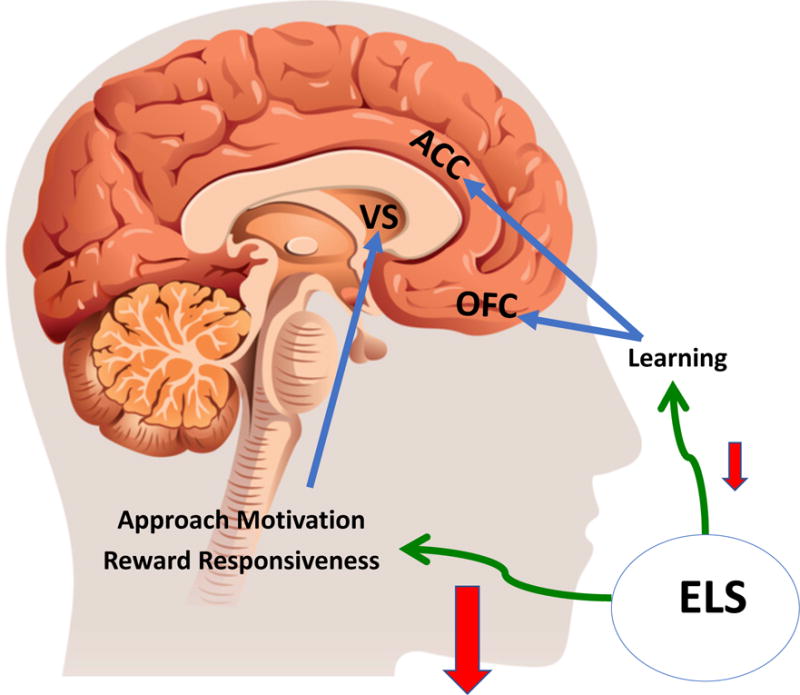
A simplified overview of brain regions involved in reward processing and the influence of early life stress. Areas of the ventral striatum (VS) are involved in approach motivation and reward responsiveness via activity of dopamine and endogenous opioids, respectively. The anterior cingulate cortex (ACC) and orbital frontal cortex (OFC) are linked to reward learning. Available evidence suggests overall decrease in approach motivation and reward responsiveness functions by ELS. While ELS can also impair reward learning, there is less available evidence for this, as indicated by the size of the red arrow.
Normative development of the brain regions involved in reward processing demonstrates structural and functional changes from childhood to adulthood, with maturation of PFC regions typically delayed compared to that of subcortical regions (Galvan et al., 2006; Mills et al., 2014; Somerville et al., 2011). For example, animal models show protracted pruning of dopamine receptors in the PFC compared to the striatum and accumbens (Andersen et al., 2000; Tarazi et al., 1999). Given that brain regions undergoing the most extensive maturation are thought to be the most vulnerable to insult (Schneider et al., 1999), we might expect differing effects of ELS based on developmental timing. Thus, stressors occurring earlier in life might be more likely to affect subcortical reward functions (approach motivation and reward responsiveness), with those later in adolescence more likely affecting learning, valuation, and effort prediction.
4. ELS and Reward Processing: Preclinical Data
Early animal studies on developmental adversity, such as those by Harry Harlow (Harlow, 1958) and Seymour Levine (Levine, 1957), emerged in the 1950s, inspired both by early psychoanalytic theories and by the deprivations of civilian populations during World War II (Suomi et al., 2008). Observations of institutionalized children highlighted the importance of both maternal care and peer relationships (Spitz and Wolf, 1946). Accordingly, it is not surprising that the most common manipulations in animals to model ELS consist of maternal and peer separation. While the majority of available data on ELS and reward processing is from animals subjected to either neonatal maternal separation or juvenile peer isolation, we also highlight results from other paradigms, such as social defeat stress, social instability, and chronic variable stress.
4.1 Methods Used to Measure Reward Processing in Animals
Because most components of reward processing are ultimately subjective phenomena (motivation, hedonic experience), their occurrence in animals can only be inferred by the measurement of behavior. While there are multiple well-validated tests of reward behavior in animals that allow some differentiation between various reward processing components, close scrutiny often reveals that more than one component is likely involved.
4.1.1 Responsiveness to reward/Liking
Observation of orofacial movements is probably the only measure of liking independent of any learning or motivational process (Berridge and Robinson, 1998), but this is rarely studied. Reward consumption as a proxy for liking is frequently evaluated using the sucrose preference test (Rizvi et al., 2016). The test involves presentation of two identical bottles, one containing water and the other containing sucrose. One of the most common uses of the sucrose preference test is to assess stress-induced anhedonia in animal models of depression, where reduced consumption of sucrose is considered indicative of depressive-like behavior (Willner et al., 1996). While it can be argued that choosing between two containers and consuming the fluid from one requires aspects of learning and approach motivation, Hoffman (2015) has argued that the sucrose preference test is more specific to liking due to lack of work that the animal has to perform.
4.1.2 Approach Motivation
Two tasks from the ELS literature that probe more motivational components of reward processing are conditioned place preference and operant responses on a progressive ratio schedule. In the conditioned place preference task, a reward is repeatedly paired with a certain environment (i.e. getting food in a white painted cage), and then the animal is tested on the how much time it spends in the environment in the absence of primary reward. The conditioned place preference task is thought to measure incentive motivation to seek out cues previously associated with reward (Bardo and Bevins, 2000). As this measures the extent to which an environment acquires incentive salience for the animal, conditioned place preference can be conceptualized as probing the “reward valuation” subconstruct within approach motivation, but expectancy is arguably also involved, given the presence of Pavlovian conditioned approach behavior (Flagel et al., 2009; NIMH Research Domain Criteria). In progressive ratio responding, the animal is required to complete an increasing number of operant responses to obtain reward, thus testing its willingness to put forth effort for reward, and mapping onto the “willingness to work” subconstruct of approach motivation. While both of these tasks are thought to assess primarily the approach motivation components of reward processing, it should be noted that an element of reward learning is also required, particularly for conditioned place preference (Huston et al., 2013).
4.1.3 Learning
Beyond the simple associative learning processes that are likely involved in conditioned place preference and operant tasks, reward learning studies have utilized more complex paradigms that probe the animal’s ability to adjust behavior according to the value of a reward. For example, in contrast experiments, increasing reward value (i.e., increasing the concentration of sucrose) is expected to increase responding (Matthews et al., 1996b), and thus can reveal the extent to which an animal is able to appreciate changes in reward contingency. In addition, some studies utilize tasks that require reversal learning, testing the ability to change behaviors based on new reward contingencies. For example, in a reversal learning task, an animal that had learned to press the left of two levers to obtain reward must now begin pressing the right lever. Such reversal is known to depend on an intact orbitofrontal cortex to inhibit the prior learned response (Placek et al., 2013; Rolls, 2016).
4.2 Effects of ELS on Reward Processing in Animal Models
4.2.1 Maternal Separation
While maternal separation can refer to a range of postnatal manipulations, these paradigms usually involve separation of neonatal animals from the mother for at least three hours. Maternal separation interferes with the postnatal regulatory influence that the mother has on the animal’s physiology and development, and is considered by many to be an analog of human child neglect (Carlyle et al., 2012; Pryce et al., 2005). However, it should be noted that the age at which maternal separation occurs in most animal experiments corresponds to a gestational age in humans, at least in terms of some aspects of brain development (Semple et al., 2013). For example, development of the limbic region and cortex on postnatal day (PND) 10 in the rat equates to that of a human fetus in the second to third trimester (Clancy et al., 2001). This might make it tempting to view maternal separation as relating more to a prenatal stressor in humans. However, development of the human brain is protracted compared to other species (Clancy et al., 2001; Miller et al, 2012; Semple et al., 2013), so despite having a more “mature” brain at birth compared to rats, vulnerability to insult remains.
Experiments assessing the effects of maternal separation on various components of reward are displayed in Table 1. One difficulty in characterizing the maternal separation literature is the wide variety of separation methods, control groups, and statistical methods that have been utilized (Lehmann and Feldon, 2000; Tractenberg et al., 2016). For example, studies of the effect of maternal separation on liking/responsiveness to reward are notable for such methodological differences as duration and frequency of maternal separation, temperature during separation, control group comparison, and sex of the animals. Not surprisingly, observed effects have been conflicting.
Numerous studies of the effects of maternal separation on sucrose preference have reported decreased preference (Amiri et al., 2016; Bolton et al., 2018; Hui et al., 2011; Sadeghi et al., 2016; Shu et al., 2015) or no change in sucrose preference (Matthews et al., 1996a; Mrdalj et al., 2016; Øines et al., 2012; Rüedi-Bettschen et al., 2005; Shalev and Kafkafi, 2002; Uchida et al., 2010), while others have reported increases in sucrose preference (Ferreira et al., 2013; Vazquez et al., 2005). Table 1 shows the methods employed in these studies. Review of the methodological approaches in relation to the pattern of findings did not reveal factors responsible for the variable outcomes, other than a likely role of strain of animal. Specifically, 4 out of the 5 studies using Sprague Dawley male rats demonstrated decreased sucrose preference following maternal separation. In Wistar male rats, decreased sucrose preference was found in only 1 out of 6 studies. Other factors, such as sex of animal or duration/length of separation, did not account for variation in results. That there were limited methodological factors underlying divergent results suggests the possible influence of a combination of subtle differences in methods and differences in laboratory conditions that tend not to be reported (e.g., sound levels and/or experience of the animal handlers).
Several studies found that while maternal separation by itself did not result in changes in sucrose preference, occurrence of a second stressor or challenge in adolescence or adulthood resulted in reduced sucrose preference (Hill et al., 2014; Klug and van den Buuse, 2012; Uchida et al., 2010). These studies support a “two hit” model in which ELS, such as maternal separation, confers vulnerability to symptoms of psychiatric illness, but with symptom onset only occurring after exposure to a subsequent stressor (Bayer et al., 1999; Maynard et al., 2001).
Even when indices of liking are not altered by maternal separation, indices of approach motivation may be impaired. In one frequently cited study in marmoset monkeys, daily bouts of isolation from parents for the first month of life resulted in unchanged free consumption of a sweet solution at seven months, but decreased effort expenditure on a progressive reinforcement schedule (Pryce et al., 2004). Thus, it appears that separation preferentially affected the willingness to work subconstruct of approach motivation. A similar pattern in rodents was identified by two groups, in which there was decreased willingness to work via progressive reinforcement but unchanged consumption of sucrose when it was given freely (Rüedi-Bettschen et al., 2005) or provided on a fixed ratio (Campbell et al., 2017). Maternal separation was also associated with decreased conditioned locomotor activity to food-related cues (Matthews et al., 1996b, 1996a) as well as decreased conditioned place preference to chocolate (Sasagawa et al., 2017) indicative of a decreased reward valuation/incentive salience function within the approach motivation construct.
In addition to changes to approach motivation and reward responsiveness, evidence exists for alterations of various types of reward learning as a result of maternal separation. As mentioned above, reduced conditioning to predictive appetitive cues, while possibly reflecting decreased motivation, might also indicate impairments in associative learning (Matthews et al., 1996a, 1996b). Deficits in the ability to alter behavior based on changing reward cues, known as reversal learning, have also been reported with parental deprivation in monkeys (Pryce et al., 2004) and maternal separation in rats (Matthews et al., 1996a).
4.2.1.1 Neurobiology of Maternal Separation and Its Implications for Reward Processing
The neonatal rat relies upon the dam for protection from predators, nutrition, thermoregulation, and proper elimination of waste (Hofer, 1996). In addition to these basic protective and physiological functions, maternal care in the form of pup retrieval, nursing, licking and grooming have potent and long-lasting effects on the developing brain that translate into variations in adult behavior. In general, maternal care appears to promote decreased stress reactivity on both physiological and behavioral levels. Meaney and colleagues amply demonstrated this using rats with natural variations in maternal care. Pups raised by dams who exhibit high levels of maternal care demonstrate increases in hippocampal synaptogenesis (Liu et al., 2000), increased glucocorticoid receptor (GR) mRNA, decreased hypothalamic CRF mRNA (Liu et al., 1997), and increased benzodiazepine receptor binding in brain regions regulating emotion (Caldji et al., 2000b). Subsequent work revealed that such changes occurred via an epigenetic mechanism in which maternal care altered levels of gene methylation (Weaver et al., 2004). These changes suggest that high levels of maternal care enhance HPA axis regulation and allow more control of the endocrine stress cascade and better regulation of emotional behavior. Indeed, when adult rats exposed to high levels of maternal care as pups were challenged with an acute stressor as adults, they demonstrated decreased adrenocorticotrophic hormone (ACTH) and corticosterone levels, suggesting enhanced glucocorticoid negative feedback regulation (Liu et al., 1997). These same rats were also found to demonstrate lower behavioral indications of fear and anxiety (Caldji et al., 2000a; Caldji et al., 2000b).
Given that maternal separation deprives rat pups from opportunities for maternal care, this paradigm would be expected to result in changes opposite to that seen in rats exposed to high levels of maternal care, and this does appear to be the case (Ladd et al., 2000). Similar to rats exposed to low levels of maternal care, maternal separation results in a greater peak and duration of glucocorticoid release in response to stress, decreased GR expression, increased CRF expression, and reduced benzodiazepine receptor binding (Sánchez et al., 2001). Further evidence of stress sensitivity is indicated by a decrease in alpha-2 adrenergic autoreceptor binding in the locus coeruleus, which promotes enhanced noradrenergic activity in response to stress (Liu et al., 2000; Swinny et al., 2010). Overall, these changes tend to result in a phenotype that is more reactive to stressful stimuli on a physiological level.
In addition to the association of stress-related pathways with fearful and depressive behavioral phenotypes, these pathways are closely linked to reward circuitry. This is an important concept in the field of addiction (Burke and Miczek, 2014b; George et al., 2012; Sinha, 2008), that is hypothesized to mediate the relationship between ELS and increased risk of substance use disorders (Andersen and Teicher, 2009). Glucocorticoid exposure in utero affects the development of dopaminergic neurons in the midbrain (Leão et al., 2007). Postnatally, CRF and glucocorticoids can potentiate dopamine release (Bagosi et al., 2006; Kalivas et al., 1987; Piazza et al., 1996). However, as with studies of reward-related behaviors, contradictory results exist on the effects of maternal separation on reward-related neurochemistry, as discussed below.
Maternal separation can result in both hypersensitive and hyposensitive mesolimbic dopamine function. For example, Matthews and colleagues have demonstrated decreased sensitivity to the enhancing effects of amphetamine and increased sensitivity to the blunting effects of D2 antagonists on behaviors related to approach motivation (Matthews et al., 1996b,Matthews and Robbins, 2003). However, other studies demonstrate enhanced mesolimbic dopamine function, as evidenced by increased nucleus accumbens dopamine release in response to stress (Brake et al., 2004) or amphetamine (Hall et al., 1999), as well as increased locomotion in response to cocaine (Brake et al., 2004). The enhanced sensitivity to psychostimulants found in the latter studies might be due to decreases in dopamine transporter levels within the nucleus accumbens of maternally separated rats, limiting dopamine clearance (Brake et al., 2004; Meaney et al., 2002; Zhu et al., 2010). One reason for seemingly divergent findings may be variation in methodology, with Matthews et al. using a 6-hour separation procedure and Brake et al. using a 3-hour separation. Changes to the opioid system are also apparent with maternal separation, but conflicting results also exist based on specific methodology, demonstrating both increases and decreases in levels of endogenous peptides (Ploj et al., 2003; Vazquez et al., 2005) as well as sensitivity to ligands (Kalinichev et al., 2001; Vazquez et al., 2005). Such variations in the substrates of reward processing following maternal separation likely contribute to the differential findings in reward processing described in the previous section.
4.2.2 Social Isolation
While pre-weaning maternal separation is often conceptualized as early life neglect and results in a depressive-like phenotype (Pryce et al., 2005), post-weaning social isolation during the juvenile and adolescent period produces sensory processing deficits and increases in fear and anxiety-like behavior (Fone and Porkess, 2008; Lukkes et al., 2009). By depriving animals of the play experiences that normally guide cortical development, post-weaning social isolation is conceptualized as a rodent model of adolescent adversity with long-lasting effects on neurobiology and behavior (Fone and Porkess, 2008; Lukkes et al., 2009; Robbins, 2016).
As shown in Table 1, a preponderance of the evidence suggests that rodents subjected to post-weaning isolation demonstrate increased hedonic behavior. In tasks measuring liking, social isolation generally results in increased sucrose preference (Brenes and Fornaguera, 2008, 2009; Colonnello et al., 2011; Hall et al., 1998a; Van den Berg et al., 2000), although there are some discrepant reports (McCool and Chappell, 2009; Pisu et al., 2011). One explanation for these divergent findings might be the existence of differences in the timing and duration of separation. For example, while the majority of studies started isolation around postnatal day (PND) 21, McCool and Chappell (2009) started isolation when rats were between PND 28 and PND 32, thus allowing the rats to have experience with peers during a longer duration within their peak play period (Panksepp, 1981).
Increased indices of approach motivation are also found following social isolation, with evidence of enhanced responding for increasing concentrations of sucrose (Cuenya et al., 2015; Hall et al., 1997), as well as greater conditioned responses to sucrose-related stimuli (Jones et al., 1990). However, a study using a briefer isolation period during the juvenile/periadolescent period found the opposite effect for conditioned locomotor response to sucrose (Van den Berg et al., 1999).
In line with the developmental sensitivity of the prefrontal cortex during adolescence, post-weaning social isolation results in deficits in cognitive flexibility that impair reward learning processes (Fone and Porkess, 2008; Robbins, 2016). For example, despite enhanced approach motivation compared to socially housed controls, when a reward task requires fewer lever presses, socially isolated rats end up receiving less reward due to perseveration (Morgan and Einon, 1975). This paradigm also results in deficits in reversal learning (Amitai et al., 2014; Li et al., 2007), as well as in learning new strategies based on a previously unrelated cue (attentional set shifting) (Schrijver and Würbel, 2001).
Table 1 also includes additional models of ELS such as social defeat, as well as those that use a combination of stressors (e.g., in which the animal is exposed to different stressors, such as restraint, social defeat, isolation, and temperature changes). In terms of developmental timing, these paradigms are commonly utilized with juvenile or adolescent animals. While there are fewer studies of reward processing in studies using these other models of ELS compared to maternal separation and social isolation, there is some indication that female rats exposed to variable stressors in adolescence (restraint, social defeat) demonstrate subsequent deficits in sucrose consumption (Bourke and Neigh, 2011; Pohl et al., 2007) while male animals do not (Bourke and Neigh, 2011; Novick et al., 2013; Pohl et al., 2007; Toth et al., 2008).
4.2.2.1 Neurobiology of Social Isolation
As with maternal separation, post-weaning social isolation results in enhanced sensitivity of the HPA axis, especially in male rats (Holson et al., 1991; Viveros et al., 1988; Weiss et al., 2004), as well as an overall anxiogenic phenotype. The effects of post-weaning social isolation on reward related circuitry, while not without discrepancies, are much more consistent than those found with maternal separation. Specifically, the literature supports that there is an overall increase in nucleus accumbens dopamine function following social isolation, as evidenced by increased basal dopamine in the nucleus accumbens, increased firing of midbrain dopaminergic neurons (Fabricius et al., 2010), and increased dopamine release in the nucleus accumbens in response to psychostimulants (Hall et al., 1998b; Heidbreder et al., 2000; Yorgason et al., 2016, 2013).
Opposite to the effects on dopamine in the nucleus accumbens, PFC dopamine is decreased following social isolation, as evidenced by decreased turnover as well as decreased sensitivity to dopamine agonists (Baarendse et al., 2013; Heidbreder et al., 2000). This decreased dopamine activity in PFC may have a disinhibiting effect on dopamine release in nucleus accumbens, as prefrontal dopamine has an inhibitory function on subcortical release (del Arco and Mora, 2008). The combination of high accumbens dopamine activity with low cortical dopamine activity likely contributes to the reward processing findings in socially isolated animals which demonstrate increased behaviors indicative of approach motivation, but deficiency in aspects of reward learning that require greater executive control.
5. Human Neuroimaging Studies (Table 2)
Compared to the animal literature, there are fewer studies investigating reward processing in humans with ELS. While there is a literature on automatic emotional processing in ELS, many of these studies focus on threat processing and amygdala activity rather than reward (McCrory et al., 2017). Within the studies that do assess reward, variation in terms of type and developmental time point of ELS, age of testing, and method used for testing also presents challenges for drawing definitive conclusions.
While numerous tasks have been used to measure reward processing in humans (reviewed in Rivzi et al. 2016), the two most commonly employed in the literature relevant to ELS are the monetary incentive delay task and the reward guessing task, or variants thereof. Both tasks have two phases, an anticipation phase, in which the subject is given information about the potential for reward, and a delivery phase, in which the reward is received. By measuring brain activity during the anticipation and delivery phases, a motivational component and a reward responsiveness component can be inferred, respectively (Knutson et al., 2000; Lutz and Widmer, 2014; Wang et al., 2016). In line with reward circuitry, studies utilizing fMRI to measure reward processing revealed increased brain activation signals in response to the anticipation and receipt of reward (Wang et al., 2016).
Using the monetary incentive delay task, four studies demonstrated decreased activity in regions of basal ganglia during the anticipation phase, suggesting reduced approach motivation in individuals who were exposed to institutionalization or early life adversity prior to adolescence (Boecker et al., 2014; Boecker-Schlier et al., 2016; Dillon et al., 2009; Mehta et al., 2010). However, in one study, while activation in the ventral striatum and putamen was decreased during anticipation, activity was increased during the delivery phase, but only for positive verbal feedback as opposed to monetary reward (Boecker et al., 2014). The authors suggested that individuals experiencing ELS might be particularly sensitive to social feedback.
Despite the capacity to differentiate phases corresponding to approach motivation and reward responsiveness on the reward guessing tasks, data on both components were not available for all studies. In two studies by the same group, ELS was associated with decreased ventral striatal activity to positive feedback (Hanson et al, 2015; Hanson et al, 2016). This relationship was significant when the stress was experienced earlier in life (kindergarten through grade 3) but not later (Hanson et al., 2016). Similarly, individuals with a history of reactive attachment disorder and early life maltreatment demonstrated reduced activation of caudate and nucleus accumbens during the reward guessing task (delivery versus anticipation was not differentiated) (Takiguchi et al., 2015). In these studies, stressors experienced in early childhood most powerfully modulated the relationship between ELS and activation in reward-related brain regions.
There are divergent findings in studies of responses to viewing positive emotional stimuli. While institutionally reared children had decreased ventral striatal response to positive emotional faces (Goff et al., 2013), adolescents with a history of physical and sexual abuse demonstrated increased reactivity to positively valenced social stimuli (Dennison et al., 2016), suggesting a potential difference in the effects of neglect vs abuse. Despite this difference, in both groups decreased activation of the ventral striatum correlated with increased depressive symptoms.
While most studies demonstrate decreased activation of the striatal regions in tasks engaging motivational and reward responsiveness processes, this was not the case when the specific stressor investigated was variations in parental warmth. In young adult males, low maternal warmth in early childhood was associated with increased medial prefrontal cortex and striatal activation during anticipation of monetary reward (Morgan et al., 2014). A similar association was found for adolescent females with low maternal warmth in early adolescence, although increased activity was associated with increased depressive symptoms (Casement et al., 2014). These two studies suggest that, depending on gender and developmental timing of stressor and testing, low maternal warmth may increase the brain’s sensitivity to anticipation of reward.
A major difference between the human fMRI studies and the animal studies is that while the animal studies rely almost solely on behavioral measures to infer reward processes, behavioral measures (reaction times, performance) are reported much less frequently in the human literature. The monetary incentive delay task and the reward guessing task, while well adapted for use with imaging, are less suited to capturing differences in reward behavior. Reaction times on the monetary incentive delay task could be interpreted as a function of motivation or reward sensitivity. Dillon et al. (2009) and Boeker et al. (2014) found increased reaction times in individuals with prior ELS in the monetary incentive delay task, while decreased reaction times were noted in children with reactive attachment disorder in a gambling task (Takiguchi et al., 2015), although the remainder of studies either did not measure behavior (Casement et al., 2015, 2014; Goff et al., 2013; Hanson et al., 2016, 2015; Morgan et al., 2014) or reported a lack of differences (Boecker-Schlier et al., 2016; Mehta et al., 2010). It should be noted that in various studies utilizing fMRI and the monetary incentive delay task, changes in ventral striatal activation are often found in the absence of changes in reaction time (Jansma et al., 2013; Saji et al., 2013; Smoski et al., 2011), suggesting that reaction time may lack sensitivity as a marker for reward processing.
Differences in reward behavior have been found using a probabilistic reward task. For example, women with a history of both childhood sexual abuse and MDD (Pechtel and Pizzagalli, 2013), adolescents exposed to physical abuse (Hanson et al., 2017), and young adults who had high levels of various adversities in early life (Birn et al., 2017) demonstrate decreased reinforcement learning. Hanson (2017) suggests that growing up in adverse environments where there is inconsistency in caregiver behavior, the individual is taught that positive and negative outcomes happen randomly. In such environments, there would be less value in utilizing information to predict reward, and more value in a generalized state of emotional arousal, as evidenced by enhanced activation of the subgenual cingulate cortex (Pechtel and Pizzagalli, 2013). Such increased emotional hyperarousal might explain why children with a history of ELS failed to modulate their reaction time based on the probability of reward (Guyer et al., 2006).
Overall, a limited literature in humans does suggest that ELS is associated with decreased neural responsiveness to reward on both measures of approach motivation and reward responsiveness, although studies evaluating decreased maternal warmth appear to be an exception. There is also indication that the earlier in life that maltreatment or neglect is experienced, the more likely it is to result in decreased striatal responses to reward. Interestingly, this is similar to the developmental pattern that is seen in the animal literature, with maternal separation more likely to result in deficits in approach motivation and responsiveness to reward compared to post-weaning social isolation. While it does appear that decreased striatal responsiveness in humans is linked to anhedonia and depressive symptoms, studies measuring how this impacts actual behavior are lacking.
6. Discussion
Compared to work on reward processing in specific psychiatric disorders, interest in the effects of ELS on reward processing is relatively recent. Despite limitations in the available human and animal literature, decreased indices of approach motivation and responsiveness to reward appear to be more common when the stressor is experienced earlier in life (i.e., maternal separation in animals, or early institutionalization in humans). Stressors experienced closer to the periadolescence period, such as social isolation in rats or lack of parental warmth in teenage girls, can have the opposite effect. This may be due to the fact that stressors later in adolescence could potentially have greater effects on the prefrontal cortex (Andersen et al., 2008; Leussis and Andersen, 2008), facilitating a central disinhibition of subcortical reward function.
When interpreting these findings, it is important to consider that differences in physiology and behavior should not simply be viewed as pathological insults, but can also be seen as adaptations to facilitate survival in an adverse environment. Viewed from such a lens, one could speculate that decreased approach motivation might be adaptive in environments where reward seeking behavior might be particularly risky. Similarly, shunting cognitive resources away from reward learning and towards hypervigilant attention to threat might also be adaptive. This might be especially true in the case of early life physical and sexual abuse, where inconsistency in caregiver behavior would preclude early experiences of using cues to predict reward outcome (Hanson et al., 2017; Pollak, 2015). In addition, in situations such as social isolation in juvenile animals or lack of parental warmth in humans, enhanced sensitivity to motivated behavior and sensitivity to reward might be adaptive in order to facilitate acquisition of needs in a deprived environment.
Findings of alterations in reward processing following ELS have several implications. As posited in the introduction, ELS likely alters core aspects of affect and cognition that in turns makes individuals susceptible to psychopathology. In support of this idea, in many of the human studies reviewed, decreased activation in regions of the basal ganglia to the anticipation or receipt of reward was not only associated with depressive symptoms (Dillon et al., 2009; Goff et al., 2013), but often found to mediate the relationship between ELS and depression (Corral-Frías et al., 2015; Hanson et al., 2015). A similar mediating effect of reduced activity in the mPFC during reward anticipation was found with alcohol dependence (Casement et al., 2015). While changes to reward processing did not mediate the association between ELS and ADHD, similar patterns of ventral striatal activity were found (Boecker et al., 2014). All of these studies suggest that changes to reward processing represents a transdiagnostic marker for susceptibility to psychiatric illness.
Viewing deficits in reward processing as a risk factor for the development of psychopathology in individuals who have experienced abuse, maltreatment, or neglect opens the possibility for early interventions prior to the development of diagnosable psychopathology (Casement et al., 2014; McCrory and Viding, 2015). To our knowledge, interventions meant to target subclinical anhedonia or impairments in reinforcement learning in at risk populations have yet to be tested, however there is evidence for potential efficacy. When individuals who have completed behavioral activation therapy are challenged with a monetary incentive delay task, they demonstrate increased activation of the ventromedial PFC during anticipation of a loss, which is interpreted as allowing for ongoing engagement in rewarding activity despite negative emotions (Mori et al., 2016). Another example is specialized mindfulness training for addiction, which has been hypothesized to restructure associative learning and valuation of natural rewards via alterations to anterior cingulate and ventral striatal activity (Garland, 2016). Given the role of dopamine in various aspects of reward processing, dopaminergic agents might have utility in enhancing reward learning and approach motivation when used in conjunction with behavioral training (Acheson et al., 2013; Admon et al., 2016). While the available literature in this area is still small, this represents an exciting area of research with the potential to reduce or ameliorate the lifelong burden of risk for psychiatric illness faced by individuals exposed to ELS.
References
- Acheson DT, Twamley EW, Young JW. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: a roadmap for preclinical development. Front Neurosci. 2013;7 doi: 10.3389/fnins.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP, Vitaliano G, Pizzagalli DA. Dopaminergic Enhancement of Striatal Response to Reward in Major Depression. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.16010111. appiajp201616010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Curr Opin Psychol, Depression. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S, Amini-Khoei H, Mohammadi-Asl A, Alijanpour S, Haj-Mirzaian A, Rahimi-Balaei M, Razmi A, Olson CO, Rastegar M, Mehdizadeh M, Zarrindast MR. Involvement of D1 and D2 dopamine receptors in the antidepressant-like effects of selegiline in maternal separation model of mouse. Physiol Behav. 2016;163:107–114. doi: 10.1016/j.physbeh.2016.04.052. [DOI] [PubMed] [Google Scholar]
- Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, Powell SB. Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cogn Affect Behav Neurosci. 2014;14:388–406. doi: 10.3758/s13415-013-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2008 doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synap N Y N. 2000;37:167–9. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, Limpens JHW, Vanderschuren LJMJ. Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology (Berl) 2013;231:1695–1704. doi: 10.1007/s00213-013-3362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagosi Z, Jászberényi M, Bujdosó E, Telegdy G. The Effects of Corticoptropin-Releasing Factor and the Urocortins on Striatal Dopamine Release Induced by Electrical Stimulation—An in vitro Superfusion Study. Neurochem Res. 2006;31:209–213. doi: 10.1007/s11064-005-9010-x. [DOI] [PubMed] [Google Scholar]
- Bai M, Zhang L, Zhu X, Zhang Y, Zhang S, Xue L. Comparison of depressive behaviors induced by three stress paradigms in rats. Physiol Behav. 2014;131:81–86. doi: 10.1016/j.physbeh.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Ball JS, Links PS. Borderline personality disorder and childhood trauma: Evidence for a causal relationship. Curr Psychiatry Rep. 2009;11:63–68. doi: 10.1007/s11920-009-0010-4. [DOI] [PubMed] [Google Scholar]
- Baranger DAA, Ifrah C, Prather AA, Carey CE, Corral-Frías NS, Drabant Conley E, Hariri AR, Bogdan R. PER1 rs3027172 Genotype Interacts with Early Life Stress to Predict Problematic Alcohol Use, but Not Reward-Related Ventral Striatum Activity. Front Psychol. 2016;7:464. doi: 10.3389/fpsyg.2016.00464. https://doi.org/10.3389/fpsyg.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “Two hit hypothesis”. J Psychiatr Res. 1999;33:543–548. doi: 10.1016/S0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. https://doi.org/10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Birn RM, Roeber BJ, Pollak SD. Early childhood stress exposure, reward pathways, and adult decision making. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1708791114. https://doi.org/10.1073/pnas.1708791114. [DOI] [PMC free article] [PubMed]
- Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Baumeister S, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M. Impact of Early Life Adversity on Reward Processing in Young Adults: EEG-fMRI Results from a Prospective Study over 25 Years. PLoS ONE. 2014;9:e104185. doi: 10.1371/journal.pone.0104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker-Schlier R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Jennen-Steinmetz C, Wolf I, Baumeister S, Treutlein J, Rietschel M, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M. Interaction between COMT Val(158)Met polymorphism and childhood adversity affects reward processing in adulthood. NeuroImage. 2016;132:556–570. doi: 10.1016/j.neuroimage.2016.02.006. https://doi.org/10.1016/j.neuroimage.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, Yang DZ, Obenaus A, Baram TZ. Anhedonia Following Early-Life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-Releasing Hormone Gene. Biol Psychiatry. 2018;83:137–147. doi: 10.1016/j.biopsych.2017.08.023. https://doi.org/10.1016/j.biopsych.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J. The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav Brain Res. 2009;198:199–205. doi: 10.1016/j.bbr.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: Associations with depressive-like behavior and ventral striatum dopamine. Neurosci Lett. 2008;436:278–282. doi: 10.1016/j.neulet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Bryce CA, Floresco SB. Perturbations in Effort-Related Decision-Making Driven by Acute Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology. 2016;41:2147–2159. doi: 10.1038/npp.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 2014a;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 2014b;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry, ADAA/NIMH Genetics. 2000a;48:1164–1174. doi: 10.1016/S0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2000b;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, Papademetris X, Hyder F, Taylor JR, Simen AA. Maternal separation with early weaning: a rodent model providing novel insights into neglect associated developmental deficits. Dev Psychopathol. 2012;24:1401–1416. doi: 10.1017/S095457941200079X. https://doi.org/10.1017/S095457941200079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Mitchell CS, Adams CD, Yeoh JW, Hodgson DM, Graham BA, Dayas CV. Chemogenetic activation of the lateral hypothalamus reverses early life stress-induced deficits in motivational drive. Eur J Neurosci. 2017;46:2285–2296. doi: 10.1111/ejn.13674. https://doi.org/10.1111/ejn.13674. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CP, Martins CMS, Stingel AM, Lemgruber VB, Juruena MF. The Role of Early Life Stress in Adult Psychiatric Disorders: A Systematic Review According to Childhood Trauma Subtypes. J Nerv Ment Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev Cogn Neurosci. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement MD, Shaw DS, Sitnick SL, Musselman SC, Forbes EE. Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. Soc Cogn Affect Neurosci. 2015;10:416–423. doi: 10.1093/scan/nsu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Rubia K, Conrod PJ. Response Inhibition and Reward Response Bias Mediate the Predictive Relationships Between Impulsivity and Sensation Seeking and Common and Unique Variance in Conduct Disorder and Substance Misuse. Alcohol Clin Exp Res. 2011;35:140–155. doi: 10.1111/j.1530-0277.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Colonnello V, Iacobucci P, Anderson MP, Panksepp J. Brief periods of positive peer interactions mitigate the effects of total social isolation in young Octodon degus. Dev Psychobiol. 2011;53:280–290. doi: 10.1002/dev.20520. [DOI] [PubMed] [Google Scholar]
- Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DAA, Hariri AR, Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 2015;45:2605–2617. doi: 10.1017/S0033291715000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craenenbroeck KV, Bosscher KD, Berghe WV, Vanhoenacker P, Haegeman G. Role of glucocorticoids in dopamine-related neuropsychiatric disorders. Mol Cell Endocrinol. 2005;245:10–22. doi: 10.1016/j.mce.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Cuenya L, Mustaca A, Kamenetzky G. Postweaning isolation affects responses to incentive contrast in adulthood. Dev Psychobiol. 2015;57:177–188. doi: 10.1002/dev.21273. [DOI] [PubMed] [Google Scholar]
- del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Delavari F, Sheibani V, Esmaeili-Mahani S, Nakhaee N. Maternal Separation and the Risk of Drug Abuse in Later Life. Addict Health. 2016;8:107–114. [PMC free article] [PubMed] [Google Scholar]
- Dennison MJ, Sheridan MA, Busso DS, Jenness JL, Peverill M, Rosen ML, McLaughlin KA. Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. J Abnorm Psychol. 2016;125:1201–1212. doi: 10.1037/abn0000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood Adversity Is Associated with Left Basal Ganglia Dysfunction During Reward Anticipation in Adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583:186–193. doi: 10.1016/j.ejphar.2007.11.069. https://doi.org/10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Curr Psychiatry Rep. 2012;14:150–158. doi: 10.1007/s11920-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, Helboe L, Fink-Jensen A, Wörtwein G, Steiniger-Brach B, Sotty F. Increased dopaminergic activity in socially isolated rats: an electrophysiological study. Neurosci Lett. 2010;482:117–122. doi: 10.1016/j.neulet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Ferreira CF, Bernardi JR, Krolow R, Arcego DM, Fries GR, de Aguiar BW, Senter G, Kapczinski FP, Silveira PP, Dalmaz C. Vulnerability to dietary n-3 polyunsaturated fatty acid deficiency after exposure to early stress in rats. Pharmacol Biochem Behav. 2013;107:11–19. doi: 10.1016/j.pbb.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. https://doi.org/10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev, The long-term consequences of stress on brain function: from adaptation to mental diseases. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield JBB, Lubman DI, Yücel M. Anhedonia in substance use disorders: A systematic review of its nature, course and clinical correlates. Aust N Z J Psychiatry. 2014;48:36–51. doi: 10.1177/0004867413508455. [DOI] [PubMed] [Google Scholar]
- Garland EL. Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Ann N Y Acad Sci. 2016;1373:25–37. doi: 10.1111/nyas.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and Addiction: Role of the Dopamine and Corticotropin-Releasing Factor Systems. Physiol Behav. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Reid I, Hall J, Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–1764. doi: 10.1093/brain/awr059. https://doi.org/10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- GUYER AE, KAUFMAN J, HODGDON HB, MASTEN CL, JAZBEC S, PINE DS, ERNST M. Behavioral Alterations in Reward System Function: The Role of Childhood Maltreatment and Psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998a;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Hall FS, Humby T, Wilkinson LS, Robbins TW. The Effects of Isolation-Rearing on Sucrose Consumption in Rats. Physiol Behav. 1997;62:291–297. doi: 10.1016/S0031-9384(97)00116-9. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation Rearing in Rats: Pre- and Postsynaptic Changes in Striatal Dopaminergic Systems. Pharmacol Biochem Behav. 1998b;59:859–872. doi: 10.1016/S0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Albert D, Iselin AMR, Carré JM, Dodge KA, Hariri AR. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc Cogn Affect Neurosci. 2016;11:405–412. doi: 10.1093/scan/nsv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE. Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biol Psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, van den Bos W, Roeber BJ, Rudolph KD, Davidson RJ, Pollak SD. Early adversity and learning: implications for typical and atypical behavioral development. J Child Psychol Psychiatry. 2017;58:770–778. doi: 10.1111/jcpp.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF. Love in infant monkeys. Sci Am. 1959;200:68–74. doi: 10.1038/scientificamerican0659-68. [DOI] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, Shaw DS, Reiss D, Thapar A. Biological and Rearing Mother Influences on Child ADHD Symptoms: Revisiting the Developmental Interface between Nature and Nurture. J Child Psychol Psychiatry. 2013;54:1038–1046. doi: 10.1111/jcpp.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol, Special Issue: Stress and neurological disease. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport D, Heit S, et al. PItuitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. https://doi.org/10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24:1197–1211. doi: 10.1002/hipo.22302. [DOI] [PubMed] [Google Scholar]
- Hofer MA. On the nature and consequences of early loss. Psychosom Med. 1996;58:570–81. doi: 10.1097/00006842-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Hoffman KL. Modeling Neuropsychiatric Disorders in Laboratory Animals. Woodhead Publishing; 2015. [Google Scholar]
- Holson RR, Scallet AC, Ali SF, Turner BB. “Isolation stress” revisited: isolation-rearing effects depend on animal care methods. Physiol Behav. 1991;49:1107–1118. doi: 10.1016/0031-9384(91)90338-o. [DOI] [PubMed] [Google Scholar]
- Holz NE, Boecker-Schlier R, Buchmann AF, Blomeyer D, Jennen-Steinmetz C, Baumeister S, Plichta MM, Cattrell A, Schumann G, Esser G, Schmidt M, Buitelaar J, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M. Ventral striatum and amygdala activity as convergence sites for early adversity and conduct disorder. Soc Cogn Affect Neurosci. 2017;12:261–272. doi: 10.1093/scan/nsw120. https://doi.org/10.1093/scan/nsw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Flashner B, Chiu M, ver Hoeve E, Luz S, Bhatnagar S. Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not in male rats. Physiol Behav. 2012;105:269–275. doi: 10.1016/j.physbeh.2011.08.036. https://doi.org/10.1016/j.physbeh.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J, Zhang Z, Liu S, Xi G, Zhang X, Teng GJ, Chan KC, Wu EX, Nie B, Shan B, Li L, Reynolds GP. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: A magnetic resonance study. Behav Brain Res. 2011;217:122–127. doi: 10.1016/j.bbr.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Huston JP, Silva MA de S, Topic B, Müller CP. What’s conditioned in conditioned place preference? Trends Pharmacol Sci. 2013;34:162–166. doi: 10.1016/j.tips.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Jansma JM, van Hell HH, Vanderschuren LJMJ, Bossong MG, Jager G, Kahn RS, Ramsey NF. THC reduces the anticipatory nucleus accumbens response to reward in subjects with a nicotine addiction. Transl Psychiatry. 2013;3:e234. doi: 10.1038/tp.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology (Berl) 1990;102:364–372. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- Kalebasi N, Kuelen E, Schnyder U, Schumacher S, Mueller-Pfeiffer C, Wilhelm FH, Athilingam J, Moergeli H, Martin-Soelch C. Blunted responses to reward in remitted post-traumatic stress disorder. Brain Behav. 2015;5 doi: 10.1002/brb3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in morphine tolerance and dependence. Psychopharmacology (Berl) 2001;157:305–312. doi: 10.1007/s002130100806. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- Klug M, van den Buuse M. Chronic cannabinoid treatment during young adulthood induces sex-specific behavioural deficits in maternally separated rats. Behav Brain Res. 2012;233:305–313. doi: 10.1016/j.bbr.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. https://doi.org/10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-Specific and Strain-Dependent Effects of Early Life Adversity on Behavioral and Epigenetic Outcomes. Front Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00078. https://doi.org/10.3389/fpsyt.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Chapter 7 – Long-term behavioral and neuroendocrine adaptations to adverse early experience. In: Saper EAM, C B, editors. Progress in Brain Research, The Biological Basis for Mind Body Interactions. Elsevier; 2000. pp. 81–103. [DOI] [PubMed] [Google Scholar]
- Leão P, Sousa JC, Oliveira M, Silva R, Almeida OFX, Sousa N. Programming effects of antenatal dexamethasone in the developing mesolimbic pathways. Synapse. 2007;61:40–49. doi: 10.1002/syn.20341. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synap N Y N. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Li N, Wu X, Li L. Chronic administration of clozapine alleviates reversal-learning impairment in isolation-reared rats. Behav Pharmacol. 2007;18:135–145. doi: 10.1097/FBP.0b013e3280d3ee83. [DOI] [PubMed] [Google Scholar]
- Li X, Xiao YH, Zou LQ, Li HH, Yang ZY, Shi HS, Lui SSY, Cheung EFC, Chan RCK. The effects of working memory training on enhancing hedonic processing to affective rewards in individuals with high social anhedonia. Psychiatry Res. 2016;245:482–490. doi: 10.1016/j.psychres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS, Wassum KM. Basolateral Amygdala to Orbitofrontal Cortex Projections Enable Cue-Triggered Reward Expectations. J Neurosci. 2017;37:8374–8384. doi: 10.1523/JNEUROSCI.0486-17.2017. https://doi.org/10.1523/JNEUROSCI.0486-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Caldji, Sharma, Plotsky, Meaney Influence of Neonatal Rearing Conditions on Stress-Induced Adrenocorticotropin Responses and Norepinepherine Release in the Hypothalamic Paraventricular Nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Rana SA, McCutcheon D, Parker LA, Wainwright PE. Artificial rearing alters the response of rats to natural and drug-mediated rewards. Dev Psychobiol. 2006;48:301–314. doi: 10.1002/dev.20139. https://doi.org/10.1002/dev.20139. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz K, Widmer M. What can the monetary incentive delay task tell us about the neural processing of reward and punishment? [WWW Document] Neurosci Neuroeconomics. 2014 URL https://www.dovepress.com/what-can-the-monetary-incentive-delay-task-tell-us-about-the-neural-pr-peer-reviewed-fulltext-article-NAN (accessed 3.10.17)
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances “liking” of a sweet reward. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010;35:717–728. doi: 10.1016/j.psyneuen.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS, Robbins TW. Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of prefeeding,d. Psychopharmacology (Berl) 1996a;126:75–84. doi: 10.1007/BF02246414. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev, Brain Development, Sex Differences and Stress: Implications for Psychopathology. 2003;27:45–55. doi: 10.1016/S0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Wilkinson LS, Robbins TW. Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol Behav. 1996b;59:99–107. doi: 10.1016/0031-9384(95)02069-1. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170:278–284. doi: 10.1016/j.schres.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early Social Isolation in Male Long-Evans Rats Alters Both Appetitive and Consummatory Behaviors Expressed During Operant Ethanol Self-Administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research Review: The neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Gerin MI, Viding E. Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry – the contribution of functional brain imaging. J Child Psychol Psychiatry. 2017;58:338–357. doi: 10.1111/jcpp.12713. https://doi.org/10.1111/jcpp.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, Viding E. The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Dev Psychopathol. 2015;27:493–505. doi: 10.1017/S0954579415000115. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/S0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Neonatal stress and litter composition alter sucrose intake in both rat dam and offspring. Physiol Behav. 2006;89:735–741. doi: 10.1016/j.physbeh.2006.08.015. https://doi.org/10.1016/j.physbeh.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AMM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. https://doi.org/10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The Developmental Mismatch in Structural Brain Maturation during Adolescence. Dev Neurosci. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Mintz M, Rüedi-Bettschen D, Feldon J, Pryce CR. Early social and physical deprivation leads to reduced social motivation in adulthood in Wistar rats. Behav Brain Res. 2005;156:311–320. doi: 10.1016/j.bbr.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, Forbes EE. Maternal Depression and Warmth During Childhood Predict Age 20 Neural Response to Reward. J Am Acad Child Adolesc Psychiatry. 2014;53:108–117.e1. doi: 10.1016/j.jaac.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Einon D. Incentive motivation and behavioral inhibition in socially-isolated rats. Physiol Behav. 1975;15:405–409. doi: 10.1016/0031-9384(75)90250-4. [DOI] [PubMed] [Google Scholar]
- Mori A, Okamoto Y, Okada G, Takagaki K, Jinnin R, Takamura M, Kobayakawa M, Yamawaki S. Behavioral activation can normalize neural hypoactivation in subthreshold depression during a monetary incentive delay task. J Affect Disord. 2016;189:254–262. doi: 10.1016/j.jad.2015.09.036. [DOI] [PubMed] [Google Scholar]
- Mrdalj J, Murison R, Soulé J, Kinn Rød AM, Milde AM, Pallesen S, Grønli J. Mild daily stressors in adulthood may counteract behavioural effects after constant presence of mother during early life. Physiol Behav. 2016;165:313–321. doi: 10.1016/j.physbeh.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted Neural Response to Rewards as a Prospective Predictor of the Development of Depression in Adolescent Girls. Am J Psychiatry appi.ajp. 2016;2016:15121524. doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- NIMH. Research Domain Criteria (RDoC) [WWW Document] URL https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml (accessed 12.4.17)
- Novick AM, Miiller LC, Forster GL, Watt MJ. Adolescent social defeat decreases spatial working memory performance in adulthood. Behav Brain Funct BBF. 2013;9:39. doi: 10.1186/1744-9081-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øines E, Murison R, Mrdalj J, Grønli J, Milde AM. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol Behav. 2012;105:1058–1066. doi: 10.1016/j.physbeh.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. https://doi.org/10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Palma-Gudiel H, Fañanás L. An integrative review of methylation at the serotonin transporter gene and its dialogue with environmental risk factors, psychopathology and 5-HTTLPR. Neurosci Biobehav Rev. 2016;72:190–209. doi: 10.1016/j.neubiorev.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Paul IA, English JA, Halaris A. Sucrose and quinine intake by maternally-deprived and control rhesus monkeys. Behav Brain Res. 2000;112:127–134. doi: 10.1016/s0166-4328(00)00173-x. https://doi.org/10.1016/S0166-4328(00)00173-X. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: A high-density event-related potential study. JAMA Psychiatry. 2013;70:499–507. doi: 10.1001/jamapsychiatry.2013.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci Off J Soc Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic Mutant Mice Have Higher “Wanting” But Not “Liking” for Sweet Rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips NK, Hammen CL, Brennan PA, Najman JM, Bor W. Early Adversity and the Prospective Prediction of Depressive and Anxiety Disorders in Adolescents. J Abnorm Child Psychol. 2005;33:13–24. doi: 10.1007/s10802-005-0930-3. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deroche V, Maccari S, Simon H, Moal ML. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu MG, Dore R, Mostallino MC, Loi M, Pibiri F, Mameli R, Cadeddu R, Secci PP, Serra M. Down-regulation of hippocampal BDNF and Arc associated with improvement in aversive spatial memory performance in socially isolated rats. Behav Brain Res. 2011;222:73–80. doi: 10.1016/j.bbr.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Placek K, Dippel WC, Jones S, Brady AM. Impairments in set-shifting but not reversal learning in the neonatal ventral hippocampal lesion model of schizophrenia: Further evidence for medial prefrontal deficits. Behav Brain Res. 2013;256:405–413. doi: 10.1016/j.bbr.2013.08.034. https://doi.org/10.1016/j.bbr.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides. 2003;37:149–156. doi: 10.1016/S0143-4179(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety-and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Pollak SD. Multilevel developmental approaches to understanding the effects of child maltreatment: Recent advances and future challenges. Dev Psychopathol. 2015;27:1387–1397. doi: 10.1017/S0954579415000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004;56:72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev, Animal Models of Depression and Antidepressant Activity. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. https://doi.org/10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Neurobehavioural sequelae of social deprivation in rodents revisited: Modelling social adversity for developmental neuropsychiatric disorders. J Psychopharmacol Oxf Engl. 2016;30:1082–1089. doi: 10.1177/0269881116664450. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A non-reward attractor theory of depression. Neurosci Biobehav Rev. 2016;68:47–58. doi: 10.1016/j.neubiorev.2016.05.007. https://doi.org/10.1016/j.neubiorev.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, Brammer MJ. Disorder-Specific Dissociation of Orbitofrontal Dysfunction in Boys With Pure Conduct Disorder During Reward and Ventrolateral Prefrontal Dysfunction in Boys With Pure ADHD During Sustained Attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res. 2005;156:297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal Cortex and Reward-Guided Learning and Decision-Making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Peeri M, Hosseini MJ. Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol Behav. 2016;163:177–183. doi: 10.1016/j.physbeh.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Saji K, Ikeda Y, Kim W, Shingai Y, Tateno A, Takahashi H, Okubo Y, Fukayama H, Suzuki H. Acute NK₁ receptor antagonist administration affects reward incentive anticipation processing in healthy volunteers. Int J Neuropsychopharmacol. 2013;16:1461–1471. doi: 10.1017/S1461145712001678. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/S0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sasagawa T, Horii-Hayashi N, Okuda A, Hashimoto T, Azuma C, Nishi M. Long-term effects of maternal separation coupled with social isolation on reward seeking and changes in dopamine D1 receptor expression in the nucleus accumbens via DNA methylation in mice. Neurosci Lett. 2017;641:33–39. doi: 10.1016/j.neulet.2017.01.025. https://doi.org/10.1016/j.neulet.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Würbel H. Early social deprivation disrupts attentional, but not affective, shifts in rats. Behav Neurosci. 2001;115:437–442. doi: 10.1037/0735-7044.115.2.437. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine reward prediction error coding. Dialogues Clin Neurosci. 2016;18:23–32. doi: 10.31887/DCNS.2016.18.1/wschultz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;0:1–16. doi: 10.1016/j.pneurobio.2013.04.001. https://doi.org/10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol Biochem Behav. 2002;73:115–122. doi: 10.1016/S0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- Shu C, Xiao L, Tang J, Wang G, Zhang X, Wang X. Blunted behavioral and molecular responses to chronic mild stress in adult rats with experience of infancy maternal separation. Tohoku J Exp Med. 2015;235:81–87. doi: 10.1620/tjem.235.81. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Rittenberg A, Dichter GS. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Res. 2011;194:263–270. doi: 10.1016/j.pscychresns.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz RA, Wolf KM. Anaclitic depression; an inquiry into the genesis of psychiatric conditions in early childhood, II. Psychoanal Study Child. 1946;2:313–342. [PubMed] [Google Scholar]
- Strüber N, Strüber D, Roth G. Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci Biobehav Rev. 2014;38:17–37. doi: 10.1016/j.neubiorev.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, van der Horst FCP, Van der Veer R. Rigorous experiments on monkey love: an account of Harry F. Harlow’s role in the history of attachment theory. Integr Psychol Behav Sci. 2008;42:354–369. doi: 10.1007/s12124-008-9072-9. https://doi.org/10.1007/s12124-008-9072-9. [DOI] [PubMed] [Google Scholar]
- Swinny JD, O’Farrell E, Bingham BC, Piel DA, Valentino RJ, Beck SG. Neonatal rearing conditions distinctly shape locus coeruleus neuronal activity, dendritic arborization, and sensitivity to corticotrophin-releasing factor. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP. 2010;13:515–525. doi: 10.1017/S146114570999037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi S, Fujisawa TX, Mizushima S, Saito DN, Okamoto Y, Shimada K, Koizumi M, Kumazaki H, Jung M, Kosaka H, Hiratani M, Ohshima Y, Teicher MH, Tomoda A. Ventral striatum dysfunction in children and adolescents with reactive attachment disorder: functional MRI study. Br J Psychiatry Open. 2015;1:121–128. doi: 10.1192/bjpo.bp.115.001586. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. doi:17365. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Preller KH, Campbell-Meiklejohn DK, Kirschner M, Kraehenmann R, Stämpfli P, Herdener M, Seifritz E, Quednow BB. Shared neural basis of social and non-social reward deficits in chronic cocaine users. Soc Cogn Affect Neurosci. 2016;11:1017–1025. doi: 10.1093/scan/nsw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tractenberg SG, Levandowski ML, de Azeredo LA, Orso R, Roithmann LG, Hoffmann ES, Brenhouse H, Grassi-Oliveira R. An overview of maternal separation effects on behavioural outcomes in mice: Evidence from a four-stage methodological systematic review. Neurosci Biobehav Rev. 2016;68:489–503. doi: 10.1016/j.neubiorev.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller N, Shelton RC, Zald DH. Effort-Based Decision-Making in Major Depressive Disorder: A Translational Model of Motivational Anhedonia. J Abnorm Psychol. 2012;121:553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatr Scand. 2013;128:434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. Early Life Stress Enhances Behavioral Vulnerability to Stress through the Activation of REST4-Mediated Gene Transcription in the Medial Prefrontal Cortex of Rodents. J Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Pijlman FTA, Koning HAM, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res. 1999;106:133–142. doi: 10.1016/S0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Van Ree JM, Spruijt BM. Morphine attenuates the effects of juvenile isolation in rats. Neuropharmacology. 2000;39:969–976. doi: 10.1016/S0028-3908(99)00216-6. [DOI] [PubMed] [Google Scholar]
- van Hulst BM, de Zeeuw P, Bos DJ, Rijks Y, Neggers SFW, Durston S. Children with ADHD symptoms show decreased activity in ventral striatum during the anticipation of reward, irrespective of ADHD diagnosis. J Child Psychol Psychiatry. 2016 doi: 10.1111/jcpp.12643. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. Childhood Adversities Increase the Risk of Psychosis: A Meta-analysis of Patient-Control, Prospective- and Cross-sectional Cohort Studies. Schizophr Bull. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Daugé V. Maternal Deprivation Increases Vulnerability to Morphine Dependence and Disturbs the Enkephalinergic System in Adulthood. J Neurosci. 2005;25:4453–4462. doi: 10.1523/JNEUROSCI.4807-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Hernández R, Martínez I, González P. Effects of social isolation and crowding upon adrenocortical reactivity and behavior in the rat. Rev Esp Fisiol. 1988;44:315–321. [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH. Stress gates neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KS, Smith DV, Delgado MR. Using fMRI to study reward processing in humans: past, present, and future. J Neurophysiol. 2016;115:1664–1678. doi: 10.1152/jn.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. https://doi.org/10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-Accumbens Amphetamine Increases the Conditioned Incentive Salience of Sucrose Reward: Enhancement of Reward “Wanting” without Enhanced “Liking” or Response Reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Calipari ES, Ferris MJ, Karkhanis AN, Fordahl SC, Weiner JL, Jones SR. Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum. Neuropharmacology. 2016;101:471–479. doi: 10.1016/j.neuropharm.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37 doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Sanchez H, Kehoe P, Kosten TA. Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology (Berl.) 2004;177:391–399. doi: 10.1007/s00213-004-1963-y. https://doi.org/10.1007/s00213-004-1963-y. [DOI] [PubMed] [Google Scholar]
- Zhu X, Peng S, Ma X, Li T. Effect of maternal deprivation on emotion and expression of dopamine transporter in adult rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:32–37. doi: 10.3969/j.issn.1672-7347.2010.01.005. [DOI] [PubMed] [Google Scholar]


