Abstract
Background
Little is known regarding the combined influence of psychosocial and neural protective mechanisms against substance use. The present study examined the extent to which neuroimaging measures of disinhibition predicted resilience against binge drinking and marijuana use among youth with a family history of substance use disorder (SUD; FH+), accounting for psychosocial measures of behavioral control.
Methods
Participants were 57 FH+ youth from the Michigan Longitudinal Study categorized into resilient and high-risk groups based on patterns of weekly binge drinking and monthly marijuana use during early adulthood. Psychosocial measures of behavioral control (reactive control and externalizing behavior during early and late adolescence) and neural measures of disinhibition (Go/No-Go task and Monetary Incentive Delay Task (MIDT) measured through functional magnetic resonance imaging (fMRI)) were entered sequentially in hierarchical logistic regression models to predict resilient versus high-risk groups.
Results
Greater activation in the right dorsolateral prefrontal cortex (DLPFC) during correctly inhibited trials on the Go/No-Go task was a significant predictor of resilience (OR = 2.46, p < 0.05), over and above greater reactive control in early adolescence (OR = 4.96, p < 0.05) and lower externalizing behavior in late adolescence (OR = 0.64, p < 0.05). Neural activation in the ventral striatum associated with reward anticipation during the MIDT was not a significant predictor of resilience.
Conclusions
Brain function in the right DLPFC associated with inhibitory control may be a neural indicator of resilience against elevated substance use among FH+ youth, even after accounting for psychosocial measures of behavioral control.
Keywords: Family history, Substance use trajectories, Resilience, fMRI, Inhibitory control, Reward
1. Introduction
Youth with a family history of substance use disorder (SUD; FH+) are vulnerable to drug and alcohol problems (Zucker et al., 2014), including recurrent patterns of disordered use, impaired functioning, and greater mental health service utilization through adulthood (Milne et al., 2009). Multiple, often complex, factors confer risk for FH+ youth to develop substance use problems, such as conflict and chaos in the home environment and negative parent-child interactions (Arria et al., 2012; Smith et al., 2016). In addition, deficits in self-regulation displayed by heightened impulsivity and externalizing behavior are believed to be an inherited behavioral phenotype that predisposes FH+ youth for later substance use (Dougherty et al., 2015). Yet, certain FH+ youth are resilient. Resilience has been defined as the ability to avoid a pathological outcome, or achieve a successful one, despite experiences of adversity (e.g., Hurd and Zimmerman, 2016; Masten et al., 1990; Windle and Zucker, 2010). Despite genetic and environmental risks, subgroups of FH+ youth have been found to display low or developmentally limited rates of substance use from adolescence through early adulthood (Chassin et al., 2002; Jester et al., 2015; Park and Schepp, 2015; Warner et al., 2007), an age period typically coinciding with escalating substance use and SUD onset (Schulenberg et al., 2017; Substance Abuse and Mental Health Services Administration (SAMHSA), 2015, respectively). Thus, a pattern of low substance use through the transition to adulthood is a useful measure of resilience among FH+ youth.
Factors within an individual, familial, and other social domain have been found to be protective against heavy substance use among FH+ youth (Hurd and Zimmerman, 2016; Park et al., 2015). For example, individual level protective factors include greater self-regulation, temperament characteristics of being flexible and optimistic, and positive affect (Martel et al., 2007; Pearson et al., 2011). Family level factors associated with resilience are having one versus both parents with a SUD (Hussong et al., 2007) and greater family cohesion and parental monitoring (Shorey et al., 2013). Additional protective factors within the social domain are positive adult role models and non-substance using peers (e.g., Moe et al., 2007).
The few studies that have examined neural processes involved in resilience among FH+ youth have focused on differences between FH+ and FH− youth rather than within the ostensibly vulnerable FH+ group. No studies to-date have specifically examined how neural function is associated with resilience among FH+ youth over and above other sociodemographic and psychosocial factors. Such information would add uniquely to understanding the constellation of protective factors related to resilience among FH+ youth. Indeed, investigating neural mechanisms associated with resilience has been cited as an important direction for research on factors impacting substance use outcomes among FH+ youth (Cservenka, 2016).
To study the complex, multi-level mechanisms involved in resilience against substance use problems, it is beneficial to employ a developmental psychopathology framework (Hussong et al., 2011). This theoretical framework posits that resilience should be considered in addition to risk, that both psychological and neurobiological components are essential to examine in predicting behavioral outcomes such as substance use, and that the cascading influence of distal and proximal developmental factors should be considered (Cicchetti and Rogosch, 1999; Cox et al., 2010). In line with a developmental psychopathology framework, and given that an externalizing behavioral phenotype has been linked to familial risk for substance dependence, the under-control/disinhibition pathway to SUDs (Zucker et al., 2011) may be a useful target to identify psychosocial and neural mechanisms underlying resilience. Behavioral under-control and disinhibition are related constructs, with the prior describing psychosocial function and the latter characterized by the neural function associated with self-regulation.
1.1. Behavioral Under-Control
Behavioral under-control, defined as the inability, unwillingness, or failure to inhibit behaviors despite experiencing negative consequences of those behaviors, is associated with two related yet distinct psychosocial constructs—reactive control and externalizing behavior (Wong et al., 2006; Zucker et al., 2011). Reactive control is characterized by the capability to inhibit impulsive responding to immediate rewards (Eisenberg, 2015). Individuals with low reactive control are sensitive to immediate gratification and rewarding stimuli, and therefore, at heightened risk for substance use problems (Martel et al., 2009; Wong et al., 2006). Externalizing behavior, including aggression and delinquency, is also an important component of behavioral under-control (Zucker et al., 2011). Reactive control and externalizing behavior have an impact across the spectrum of risk and resilience (Park and Schepp, 2015). Thus, higher levels of reactive control and lower levels of externalizing behavior may be associated with resilience against heavy substance use among FH+ youth.
1.2. Disinhibition
Inhibitory control and reward responsivity are two key neural functions involved in disinhibition, which are related more broadly to dual-systems models of risk-taking (Zucker et al., 2011). These models posit that heightened risk behaviors during adolescence and early adulthood, such as substance use, are attributable to a developmental mismatch between two brain systems—a bottom-up subcortical brain system involved in reward responsivity that develops in early adolescence and a later maturing top-down prefrontal brain system associated with inhibitory control (Casey et al., 2008; Shulman et al., 2016). While a useful heuristic, dual-systems models of risk-taking often fail to account for heterogeneity in risk outcomes even among the putatively homogeneous high or low-risk groups (Pfeifer and Allen, 2012). This is particularly relevant to FH+ youth, who are believed to share an underlying vulnerability to SUDs but in fact display variation in their levels of substance use (Heitzeg et al., 2008).
1.3. Present Study
Examining both psychosocial and neural measures of behavioral control/under-control and disinhibition may not only predict resilience versus risk among FH+ youth but may also demonstrate the incremental predictive utility of neural function measures beyond psychosocial measures of the same underlying construct. Toward this aim, the present longitudinal study investigated the extent to which neural function involved in disinhibition (inhibitory control measured by a Go/No-Go task and reward responsivity measured by a Monetary Incentive Delay Task (MIDT)) predicted resilience in terms of low substance use across the transition to adulthood among FH+ youth, over and above the psychosocial influence of behavioral control (reactive control and externalizing behavior). Resilient and high-risk FH+ groups were categorized based on 1) binge drinking and marijuana use trajectory classes through the transition to adulthood (ages 17 to 26); and 2) more conservative groupings based on heavy past-year binge drinking (i.e., weekly) and/or marijuana use (i.e., monthly) reported during two or more time points from ages 17 to 26. Findings from prior literature were used to determine heavy binge drinking (Hasin and Beseler, 2009; Schulenberg et al., 1996) and marijuana use (Harper et al., 2012; Hides et al., 2009; Schulenberg et al., 2005) thresholds. This latter step was taken to improve the face validity of characterization of resilient and high-risk groups estimated through trajectory classes. As the most commonly used drugs of abuse among youth during the transition to adulthood (Schulenberg et al., 2017), developmental patterns of alcohol and marijuana use during early adulthood were used to determine resilient and high-risk groups. To account for other substance use, cigarette and any other drug use within the age range of 17 to 26 were controlled for in the regression analyses predicting resilient versus high-risk groups. Since earlier experiences often have downstream effects on later functioning (Dodge et al., 2009; Schulenberg and Maggs, 2008), reactive control and externalizing behavior were assessed both in early adolescence (ages 12 to 14) when substance use tends to begin (SAMHSA, 2015) and also in late adolescence (ages of 17 to 18) when substance use begins to peak (Schulenberg et al., 2017) and disordered use begins to emerge (Windle and Zucker, 2010). Neuroimaging data were collected during early adulthood (approximately age 20).
The resilient group was hypothesized to have greater reactive control and less externalizing behavior both in early adolescence and late adolescence compared to the high-risk group. Compared to the high-risk group, resilient youth were hypothesized to have greater activation in prefrontal brain regions during the Go/No-Go task and lower activation in subcortical brain regions during the MIDT task measuring reward responsivity. Greater activation associated with inhibitory control and lower activation associated with reward responsivity were hypothesized to predict resilient versus high-risk group membership after accounting for sociodemographic characteristics, cigarette and other illicit drug use, and both early and late adolescent psychosocial functioning involving reactive control and externalizing problems.
2. Methods
2.1. Participants
Two hundred and thirty-five youth (75.32% male; 96.60% White) followed annually from ages 12 to 26 from the Michigan Longitudinal Study (MLS), a prospective study of community-recruited youth from families at high risk for SUD (Zucker et al., 2000), were included in initial analyses to determine resilient and high-risk groups through growth mixture modeling (GMM). Fifty-seven youth who participated in the neuroimaging component of MLS at approximately 20 years of age and had at least one parent with SUD were then included in hierarchical multivariable logistic regression analyses. A family history of SUD was defined as having a biological father and/or mother with a lifetime diagnosis of any alcohol or drug use disorder; diagnosis was assessed by a clinical psychologist using the Diagnostic Interview Schedule—Version 4 (DIS-IV) (Robins et al., 2000). Signs of fetal alcohol syndrome at the time of study enrollment was exclusionary. Additional information on MLS recruitment and assessment can be found elsewhere (Zucker et al., 2000).
Exclusionary criteria for neuroimaging assessments included: being left-handed or ambidextrous, determined by the Edinburgh Handedness Inventory (Oldfield, 1971); neurologic, acute, uncorrected, or chronic medical illness; treatment with psychoactive medication within the past 6 months; history of psychosis or schizophrenia in a first degree relative; presence of Axis I psychiatric or developmental disorders, except for conduct and attention-deficit/hyperactivity disorders (ADHD) or SUD, as these disorders would eliminate participants at high risk for SUD; and pregnancy. All participants were instructed to abstain from alcohol and illicit substances and, if applicable, stop taking medication for ADHD at least 48 hours prior to scanning. Participants were given a drug urine screen before scanning. Due to Δ9-tetrahydrocannabinol (THC) metabolites being detectable in urine for a week or longer, participants who tested positive for marijuana but self-reported abstinence within 48 hours prior to the scan were not excluded from the study. Self-reported drug use within the past 48 hours and/or a positive test for drugs not including marijuana were exclusionary. All participants provided informed consent approved by the University of Michigan Medical School Institutional Review Board.
2.2. Measures
2.2.1. Substance use
Substance use was measured annually from ages 12 through 26 using the Drinking and Drug History Questionnaire (Zucker et al., 1990) for participants in the present study. Binge drinking was measured by the number of days during the past year participants reported consuming five or more standard drinks. Marijuana use was measured by the number of occasions participants used marijuana or hashish during the past year. Response options for marijuana use occasions were 0 = Never, 1 = 1 to 2 occasions, 2 = 3 to 5 occasions, 3 = 6 to 9 occasions, 4 = 10 to 19 occasions, 5 = 20 to 39 occasions, 6 = 40 to 99 occasions, 7 = 100 to 249 occasions, 8 = 250 to 499 occasions, or 9 = 500 or more occasions during the past year. Cigarette and other drug use were measured by the sum of past year days when participants reported cigarette and/or illicit drug use other than marijuana.
2.2.2. Sociodemographic characteristics
Sociodemographic characteristics were sex and number of parents diagnosed with a SUD. Sex was coded as 1= male or 0 = female. The number of parents diagnosed with a SUD was coded as to whether one or two biological parents were diagnosed with a SUD based on DIS-IV lifetime criteria for alcohol or drug use disorder.
2.2.3. Reactive control
Reactive control was measured in early adolescence (ages 12 to 14) and late adolescence (ages 17 to 18) by the reactive control scale developed by Eisenberg et al. (1996; 2003), which was derived from the California Child Q-Sort (CCQ; Block and Block, 1980) and the Revised Adult California Q-Sort (CAQ; Block and Block, 1980). Both the CCQ and CAQ are standardized clinician-evaluations categorizing 100 statement cards about child temperament (CCQ) or adult personality (CAQ) and behavioral functioning. After several hours spent interacting directly with the participant and observing parent-child interactions during the MLS assessment, all cards were arranged by a trained masters’ level clinician in a fixed, normally distributed set to describe the participant from 1 = “extremely uncharacteristic” to 9 = “extremely characteristic” (Martel et al., 2009). In early adolescence, 14 items (α = 0.81) were included in the reactive control scale. Sample items were “Is inhibited and constricted” and “Is reflective; deliberates before speaking or acting.” In late adolescence, 12 items (α = 0.81) were included in the reactive control scale. Example items (reverse coded) were “Unable to delay gratification” and “Is self-indulgent”.
2.2.4. Externalizing behavior
Self-reported externalizing behavior during early adolescence (ages 12–14) was measured by the sum of 30 items (α = 0.85) from the aggressive behavior and delinquency subscales of the Youth Self Report (YSR; Achenbach, 1991). Self-reported externalizing behavior during late adolescence (ages 17 to 18) was measured by the sum of 35 items (α = 0.80) from the aggressive behavior, rule-breaking, and intrusiveness subscales of the Adult Self Report (ASR; Achenbach, 1991). For both the YSR and ASR, behaviors were based on rating the accuracy of statements on a 3-point scale where 0 = “not at all true”, 1 = “somewhat true”, and 2 = “very true”. To avoid confounds with binge drinking and marijuana use, items on the YSR and ASR pertaining to substance use were removed from the delinquency subscale.
2.2.5. fMRI paradigms
An event-related fMRI Go/No-Go task (Durston et al., 2002; Hardee et al., 2014; Heitzeg et al., 2014) was used to measure blood oxygen level-dependent (BOLD) response associated with inhibitory control when participants were approximately 20 years old. Participants were instructed to respond as quickly and accurately as possible to target stimuli (letters other than “X”) by pressing a button, but not to respond via button press during infrequent non-target stimuli (“X”). Target stimuli were categorized as Go trials, and non-target stimuli were categorized as No-Go trials. Stimulus duration lasted 500 ms, followed by 3500 ms of a fixation cross. There were a total of 5 runs of 49 trials, each lasting 3.5 minutes. To focus on inhibitory control, analyses in the present study were conducted using the contrast for correct inhibition (correct No-Go trials) versus baseline trials.
To assess neural response during reward anticipation, participants (approximately 20 years of age) performed a modified version of the MIDT (Knutson et al., 2001; Martz et al., 2016). For each trial, participants first saw an incentive cue for 2000 milliseconds (ms), indicating whether on that trial they could win $5.00 (large reward), lose $5.00 (large loss), win $0.20 (small reward), or lose $0.20 (small loss), or no money was at stake (neutral condition). They then saw a white fixation cross for 2000 ms, followed by a variable-duration target, during which they were instructed to press a button as quickly as possible. Pressing the button while the target was on the screen signified a correct response. Finally, participants were shown feedback indicating whether they won money, failed to win money, lost money, avoided losing money, or no money was at stake. Analyses in the present study were conducted using the contrast for combined large and small reward cue anticipation (reward anticipation) versus neutral cue anticipation (neutral anticipation) trials.
2.3. fMRI Acquisition
Participants were scanned using a 3.0 Tesla GE Signa scanner (GE Healthcare). Whole-brain BOLD images were acquired using a T2*-weighted single-shot combined spiral in/out sequence (repetition time [TR] = 2000 ms; echo time [TE] = 30 ms; flip angle = 90°; field of view [FOV] = 200 mm; 64 × 64 matrix; in-plane resolution = 3.12 × 3.12 mm2; slice thickness = 4 mm). For spatial normalization, a high-resolution anatomical T1-weighted scan was obtained (TR = 25 ms; minimum TE; FOV = 25 cm; 256 × 256 matrix; slice thickness = 1.4 mm). Foam padding around the head secured with a forehead strap minimized participant motion and participants were instructed to remain still during scanning.
2.4. Analytic Plan
The analytic plan for the present study involved three steps: (1) Identify resilient and high-risk groups; (2) Examine whole-brain task activation during the Go/No-Go task and MIDT among combined resilient and high-risk groups in order to identify ROIs for hierarchical multivariable logistic regression analyses; and (3) Conduct hierarchical multivariable logistic regression to examine the extent to which neural mechanisms of inhibitory control and reward responsivity predict resilient versus high-risk group membership, over and above what the psychosocial measures of reactive control and externalizing behavior can accomplish.
2.4.1. Resilient and high-risk group classifications
Binge drinking and marijuana use trajectories from ages 17 to 26 were modeled separately using LGM and then GMM in Mplus. LGM and GMM were conducted among the full MLS sample (i.e., both non-neuroimaging and neuroimaging subsamples of the larger MLS) due to limitations of GMM with small samples (Ram and Grimm, 2009). The robust maximum likelihood (MLR) estimator with the full information maximum likelihood (FIML) was used in GMM analyses. FIML accounts for missing data by using all available data from at least one-time point and produces unbiased parameter estimates and standard errors. As the first step in GMM analyses, intercept, linear, quadratic, and piecewise LGMs were compared for model fit. Piecewise models were based on normative age trends for binge drinking and marijuana use (Schulenberg et al., 2017). Model fit criteria were based on: 1) Root-mean-square error of approximation (RMSEA) ≤ 0.06; 2) Comparative fit index (CFI) ≥ .90); 3) Tucker-Lewis index (TLI) ≥ .90); and 4) Chi-square difference test based on log-likelihood values and scaling correction factors. Using the best fitting LGM, GMM was then performed to determine the most likely number of latent subgroups for binge drinking and marijuana from ages 17 to 26. The two-class GMM was tested, followed by an increasing number of classes until model fit declined. Once the appropriate number of classes were determined through GMM, the estimated class for each subject was outputted to SPSS.
Participants categorized in the resilient group were: 1) In the neuroimaging subsample of MLS; 2) FH+; 3) Classified within the low binge drinking and marijuana use trajectory groups; and 4) Reported no weekly binge drinking or monthly marijuana use occasions from ages 17 to 26. The high-risk group met the first two aforementioned criteria and was also classified within either the late-onset increasing or chronic high binge drinking trajectory group and/or the moderate or chronic high use marijuana use trajectory group. In order to ensure that the high-risk group displayed a consistent pattern of frequent substance use through the transition to adulthood that was not developmentally limited, classification into the high-risk group was also contingent upon reporting at least two occasions of weekly binge drinking (i.e., greater than 52 days during the past year) and/or monthly marijuana use (i.e., greater than 20–39 occasions of use during the past year) from ages 17 to 26. Because comparisons between resilient and high-risk FH+ groups were the focal point of the present study, only these groups were included in all following analyses.
2.4.2. fMRI data preprocessing
Functional image reconstruction was performed using an iterative algorithm (Noll et al., 2005; Sutton et al., 2003) and head motion corrected using the FSL 5.0.2.2 analysis tools library (FMRIB, Oxford, United Kingdom). Motion at each volume was compared to the previous volume and runs were excluded if they exceed 3 mm translation, 3° rotation in any direction or two volumes showed a shift greater than 3mm. Slice timing correction was completed using statistical parametric mapping (Wellcome Institute of Cognitive Neurology, London, United Kingdom). Functional images were spatially normalized to a standard stereotactic space as defined by the Montreal Neurological Institute (MNI) and smoothed with a 6 mm full-width at half-maximum Gaussian spatial smoothing kernel applied to improve signal-to-noise ratio and account for individual anatomic differences.
2.4.3. Whole-brain task activation
One-sample t-tests were completed in SPM to examine whole-brain activation associated with the contrasts of interest for the Go/No-Go task and MIDT. For the Go/No-Go task, three regressors of interest were convolved with the canonical hemodynamic response function: correct No-Go trials, failed No-Go trials, and Go trials. Remaining data not modeled into these three events was classified as the implicit baseline, as in DeVito et al. (2013) and Heitzeg et al. (2014). For the MIDT, regressors of interest for all events were convolved with the canonical hemodynamic response function. For both the Go/No-Go task and MIDT, motion parameters and white matter signal intensity were modeled as nuisance regressors to remove residual motion artifacts and capture non-task-related noise, respectively. Areas of activation during contrasts of interest were considered significant if they reached a minimum false-discovery rate (FDR) corrected, a voxel-wise threshold of p < 0.05, where a cluster-forming threshold of p < 0.05 family-wise error (FWE) corrected was used. Given our interest in frontostriatal circuitry, we used only regions from the one-sample t-test that were localized in the frontal lobes or striatum for further analyses. In line with study hypotheses, beta values for significant prefrontal areas of activation during the correct inhibition versus baseline contrast (Go/No-Go task) and subcortical areas of activation during the reward anticipation versus neural contrast (MIDT) were extracted using MarsBaR (Brett et al., 2002) and then imported into SPSS for further analysis in hierarchical multivariable logistic regression models.
2.4.4. Hierarchical multivariable logistic regression
Models were examined in temporal order, with Model 1 consisting of early adolescent reactive control and externalizing behavior, controlling for sex, number of biological parents with SUD, and a sum measure of past-year cigarette and other drug use from ages 17 to 26, Model 2 adding reactive control and externalizing behavior during late adolescence, and Model 3 adding neural activation from the Go/No-Go task and MIDT during early adulthood. Due to the relatively small sample size included in the present study and to isolate the influence of each contrast of interest, ROIs from the Go/No-Go task and MIDT were included in separate models. Nagelkerke R2 and the change in likelihood ratio test, as indicated by the model χ2 test, were used to assess model fit at each step.
3. Results
3.1. Identification of Resilient and High-Risk Groups
The linear LGM model fit the data best for both binge drinking (RMSEA = 0.07, CFI = 0.90, TLI = 0.85) and marijuana use (RMSEA = 0.06, CFI = 0.97, TLI = 0.96). Thus, the linear LGM was used in all subsequent GMM models. The best fitting GMM for both binge drinking and marijuana use estimated three trajectory classes (Table 1; Figure 1).
Table 1.
Growth mixture model fit for binge drinking and marijuana use
| BIC | Entropy | Class proportions | L-M-R test | L-M-R p-value | |
|---|---|---|---|---|---|
| Binge drinking model | |||||
| 2 classes | 15389.15 | 0.99 | 3.78 96.22 | 274.96 | <0.01 |
| 3 classes | 15242.77 | 0.97 | 11.21 3.52 85.27 | 155.28 | <0.01 |
| 4 classes | 15175.08 | 0.95 | 2.82 78.58 9.91 8.69 | 80.79 | 0.68 |
| Marijuana use model | |||||
| 2 classes | 5841.40 | 0.95 | 73.96 26.04 | 300.58 | <0.001 |
| 3 classes | 5691.71 | 0.95 | 10.89 18.15 70.96 | 158.41 | <0.001 |
| 4 classes | 5653. 50 | 0.94 | 17.96 69.34 6.63 6.10 | 52.89 | 0.36 |
Note. Model fit is shown for the linear binge drinking model and linear marijuana use model; Model fit was based on the following criteria: 1) Bayesian Information Criterion (BIC), with lower numbers indicating better model fit; 2) Entropy closer to 1, which identifies better fitting classification of posterior probability class values; 3) Class estimates based on posterior probabilities consisting of no less than 5% of the total sample, which supports improved replicability; 4) Lo-Mendell-Rubin (LMR) adjusted likelihood ratio test, which compares the fit of the k class model to the k-1 (i.e., 4 versus 3 class model); and 5) Class interpretability.
Figure 1.
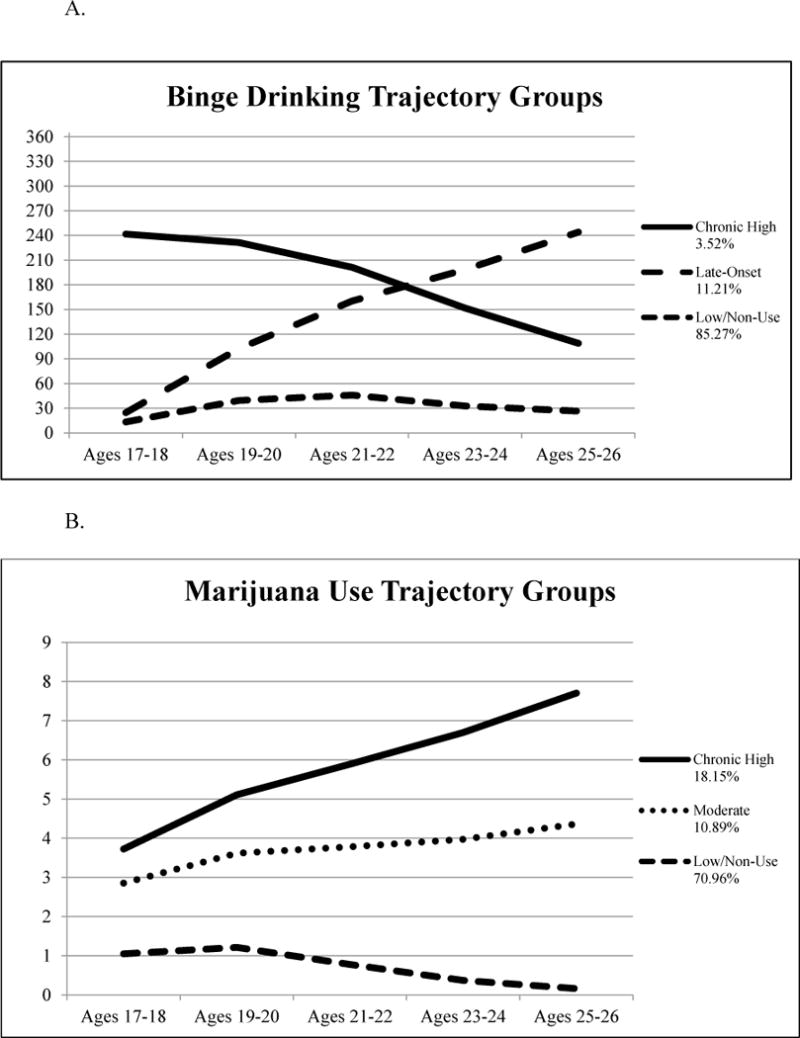
Binge drinking (A.) and marijuana use (B.) trajectory groups. Estimated model means for the best fitting models for binge drinking and marijuana use during the past year. In Figure 1 A., the vertical axis indicates number of days. In Figure 1 B., response options for marijuana use are 0 = 0 occasions, 1 = 1 to 2 occasions, 2 = 3 to 5 occasions, 3 = 6 to 9 occasions, 4 = 10 to 19 occasions, 5 = 20 to 39 occasions, 6 = 40 to 99 occasions, 7 = 100 to 249 occasions, 8 = 250 to 499 occasions, or 9 = 500+ occasions during the past year.
3.2. Descriptive Characteristics of Resilient and High-Risk Groups
Thirty-six participants were classified as high-risk and 21 classified as resilient. One participant from the resilient group and two participants from the high-risk group were excluded from the analytic sample, due to meeting grouping criteria based on trajectory analyses but not cut-points based on weekly binge drinking and/or marijuana use during at least two-time points from ages 17 to 26. Comparisons between resilient and high-risk groups on sociodemographic characteristics, substance use, reactive control and externalizing behavior, and mean ages when Go/No-Go task and MIDT scan data were collected are shown in Table 2. Participant characteristics for the full MLS sample are provided in Supplementary Material Table 11.
Table 2.
Participants’ characteristics by resilient and high-risk groups
| Resilient | High-risk | X2 or t-value | |
|---|---|---|---|
|
| |||
| n = 21 | n = 36 | ||
| Sociodemographic characteristics | |||
| Malea | 66.67% | 75.00% | 0.50 |
| Two parents with SUDb | 42.90% | 52.80% | −0.47 |
| Mean age at Go/No-Go scan | 20.68 (1.74) | 19.88 (1.78) | 1.64 |
| Mean age at MIDT scan | 20.83 (1.45) | 20.51 (1.20) | 0.86 |
| Substance usec | |||
| Binge drinking – ages 12–16 | 0.11 (0.33) | 4.01 (10.45) | −2.24* |
| Binge drinking – ages 17–18 | 5.00 (15.31) | 49.74 (77.57) | −2.60* |
| Binge drinking – ages 19–20 | 5.06 (9.72) | 96.21 (101.16) | −3.69** |
| Binge drinking – ages 21–22 | 3.97 (9.57) | 112.02 (100.20) | −4.91*** |
| Binge drinking – ages 23–24 | 3.13 (6.36) | 68.77 (66.09) | −4.52*** |
| Binge drinking – ages 25–26 | 2.85 (77.10) | 77.10 (71.34) | −3.86*** |
| Marijuana use – ages 12–16 | 0.00 (0.00) | 2.21 (4.99) | −2.57* |
| Marijuana use – ages 17–18 | 1.13 (3.62) | 67.74 (156.64) | −2.48* |
| Marijuana use – ages 19–20 | 1.33 (4.16) | 77.18 (147.17) | −2.77* |
| Marijuana use – ages 21–22 | 0.20 (0.49) | 67.91 (125.70) | −3.19** |
| Marijuana use – ages 23–24 | 0.08 (0.24) | 46.81 (98.20) | −2.82** |
| Marijuana use – ages 25–26 | 0.04 (0.18) | 59.88 (130.60) | −2.67* |
| Cigarette and other drug use – ages 12–16 | 1.35 (5.87) | 27.68 (67.87) | −2.31* |
| Cigarette and other drug use – ages 17–26 | 162.80 (456.87) | 596.83 (567.83) | −3.06** |
| Early adolescent factors – ages 12–14 | |||
| Reactive control | 5.61 (1.28) | 4.77 (1.04) | 2.60* |
| Externalizing behavior | 8.14 (5.18 | 13.11 (8.24) | −2.49* |
| Late adolescent factors – ages 17–18 | |||
| Reactive control | 5.19 (1.06) | 4.65 (0.91) | 1.75 |
| Externalizing behavior | 7.38 (3.96) | 12.45 (5.56) | −3.66** |
Note. N = 57; SUD = substance use disorder; MIDT = monetary incentive delay task; Mean differences between categorical variables are shown by Χ2 tests; Standard deviations are shown in parentheses;
Reference group female;
Reference group one parent with SUD;
Binge drinking is displayed as the mean number of days during the past year during each age range when participants reported consuming 5 or more drinks. Marijuana use and the sum of cigarette and other drug use are displayed as the mean number of use occasions during the past year for each age range. The original 0-9 scale for the marijuana use measure was converted to the following in order to improve interpretability: 0(0 occasions) = 0 occasions, 1(1–2 occasions) = 1.5 occasions, 2(3–5 occasions) = 4 occasions, 3(6–9 occasions) = 7.5 occasions; 4(10–19 occasions) = 14.5 occasions; 5(20–39 occasions) = 29.5 occasions, 6(40–99) = 69.5 occasions, 7(100–249 occasions) = 174.5 occasions, 8(250–499 occasions) = 365 occasions, 9(500+ occasions) = 500 occasions.
p < 0.05
p < 0.01
p < 0.001
3.3. fMRI Task Results
Results from independent samples t-tests showed no significant differences between resilient and high-risk groups on Go/No-Go and MIDT task performance (see Supplementary Material Table 2).1 Expected whole-brain task effects for the Go/No-Go task and MIDT were observed, with ROIs relevant to study hypotheses (Table 3, bold) tested in subsequent regression models.
Table 3.
Whole-brain task activation during go/no-go task and monetary incentive delay task
| Activations | MNI coordinates
|
k | t | PFDR |
|---|---|---|---|---|
| x, y, z | ||||
| Go/no-go task | ||||
| Correct inhibition versus baseline | ||||
| Right dorsolateral prefrontal cortex | 40, 44, 26 | 412 | 7.23 | 0.004 |
| Right inferior orbitofrontal gyrus | 48, 46, −10 | 56 | 6.43 | 0.04 |
| Left postcentral gyrus | −52, −8, 42 | 497 | 7.89 | 0.001 |
| Right postcentral gyrus | 54, −8, 38 | 448 | 7.01 | 0.008 |
| Right inferior parietal lobe | 58, −50, 42 | 280 | 6.58 | 0.03 |
| Right inferior temporal gyrus | 60, −32, −18 | 94 | 6.70 | 0.02 |
| Right supplemental motor area | 6, 8, 50 | 532 | 7.05 | 0.007 |
| MIDT | ||||
| Reward anticipation versus neutral | ||||
| Left ventral striatuma | −10, 4, 2 | 769 | 11.86 | <0.001 |
| Right ventral striatuma | 8, 10, 0 | 645 | 11.51 | <0.001 |
| Left supplemental motor area | −4, −4, 56 | 702 | 8.26 | <0.001 |
| Left precentral gyrus | −38, −16, 52 | 504 | 7.91 | 0.001 |
| Right cerebellum | 14, −44, −24 | 101 | 6.56 | 0.044 |
Note. All values shown for a cluster-forming threshold of p < 0.05 family-wise error (FWE) corrected; MNI = Montreal Neurological Institute; MIDT = monetary incentive delay task;
Left and right ventral striatum differentiated at FWE-corrected p < 0.00005.
3.4. Hierarchical Multivariable Logistic Regression Results
Correlations between all variables included in regression analyses are provided in Supplementary Material Table 3.1 As shown in Table 4, results from Model 1 (sociodemographic characteristics, early substance use, and early adolescent psychosocial predictors) indicated no significant differences by resilient versus high-risk group membership. Adding Model 2 (late adolescent psychosocial predictors) resulted in a trend toward resilient youth being less likely than the high-risk group to report externalizing behavior in late adolescence, although this finding was at the p = 0.05 threshold. Nagelkerke R2 indicated improved model fit over Model 1. Adding Model 3 (neural activation in the right dorsolateral prefrontal cortex (DLPFC) associated with inhibitory control during the Go/No-Go task) showed that resilient youth were significantly more likely to report higher reactive control during adolescence, lower externalizing behavior during late adolescence, and greater activation in the right DLPFC during correct inhibition. Nagelkerke R2 indicated improved model fit over Model 2 and the model χ2 was significant.
Table 4.
Hierarchical multivariable logistic regression models predicting resilient versus high-risk groups
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Sociodemographic characteristics and other substance use | ||||||
| Malea | 0.41 | (0.51, 3.31) | 0.37 | (0.04, 3.59) | 0.60 | (0.04, 9.06) |
| Number of parents with SUDb | 0.81 | (0.14, 4.84) | 0.46 | (0.06, 3.84) | 0.01 | (0.00, 1.19) |
| Cigarette/other drug usec | 0.99 | (0.99, 1.00) | 0.99 | (0.99, 1.00) | 0.99 | (0.99, 1.00) |
| Early adolescence (ages 12–14) | ||||||
| Reactive control | 1.68 | (0.78, 3.61) | 1.80 | (0.77, 4.22) | 4.96* | (1.19, 20.67) |
| Externalizing behavior | 0.95 | (0.82, 1.10) | 0.99 | (0.85, 1.16) | 1.00 | (0.85, 1.18) |
| Late adolescence (ages 17–18) | ||||||
| Reactive control | 0.74 | (0.21, 2.61) | 0.20 | (0.02, 1.72) | ||
| Externalizing behavior | 0.77† | (0.59, 1.00) | 0.64* | (0.43, 0.96) | ||
| Neural activation go/no-go taskd | ||||||
| Right dorsolateral prefrontal cortex | 2.46* | (1.03, 5.84) | ||||
| Nagelkerke R2 | 0.28 | 0.42 | 0.61 | |||
| Model χ2 | 8.17 | 13.33 | 20.29** | |||
Note. OR = odds ratio; CI = 95% confidence intervals; SUD = substance use disorder;
Reference group female;
Reference group one parent with SUD;
Sum of cigarette and other drug use occasions during the past year from ages 17 to 26;
Correct inhibition versus baseline contrast.
p < 0.05
p < 0.01
p < 0.001
p = 0.05
Additional findings (not tabled) showed that neural activation in the right inferior orbitofrontal gyrus (iOFG; odds ratio (OR) = 1.32, 95% confidence interval (CI) = 0.91, 1.93, p = ns) during correct inhibition that was tested in Model 3 did not significantly predict resilient versus high-risk group membership. The only significant predictor of resilience in Model 3 was lower externalizing behavior during late adolescence (OR = 0.74, CI = 0.56, 0.98, p < 0.05). In addition, testing Model 3 with left VS (OR = 1.66, 95% CI = 0.12, 22.45, p = ns) and right VS (OR = 0.72, 95% CI = 0.05, 10.07, p = ns) activation associated with reward anticipation during the MIDT did not significantly predict resilient versus high-risk groups over and above measures included in Models 1 and 2.
To test whether activations in the ROIs examined in the regression models described above were attributable to the effects of earlier substance use, a series of post hoc analyses were performed. Correlations between ROIs tested in regression analyses, and the sum measure of cigarette and other drug use from ages 17 to 26 and all available binge drinking and marijuana use data measured from ages 12 to 26 are shown in Supplementary Material Tables 3 and 4,2 respectively. There were no significant correlations between ROIs related to inhibitory control during the Go/No-Go task tested in regression analyses and substance use among the study sample. These results indicate that prior substance use was not driving significant differences between resilient and high-risk groups in terms of right DLPFC activation. There was a positive correlation between VS activation associated with reward anticipation and higher frequencies of binge drinking (only in the right VS) and marijuana use from ages 17 to 18 (both left and right VS). Despite this correlation, VS activation during reward anticipation did not significantly predict resilient versus high-risk group membership. To further test the impact of early substance use on differences in fMRI results between resilient and high-risk groups, we tested hierarchical logistic regression models examined in the present study (described above) with the inclusion of a sum measure of binge drinking and marijuana use from ages 12 to 16. Adding this measure did not impact the main findings of the hierarchical logistic regression models. Activation in the right DLPFC (OR = 4.47, CI = 1.25, 16.03, p < 0.05) during the Go/No-Go task remained significant, whereas activation in the right iOFG (OR = 1.56, CI = 0.96, 2.54, p = ns) did not. For the MIDT, left VS (OR = 4.88, CI = 0.29, 82.36, p = ns) and right VS (OR = 0.30, CI = 0.02, 4.79, p = ns) were not significant predictors of resilient versus risk groups. No other measures in the regression models (sociodemographic characteristics, early substance use, or early and late adolescent reactive control and externalizing behavior) were significant predictors of resilient versus high-risk groups. We also tested regression models that included both the sum measure of early binge drinking and marijuana use from ages 12 to 16 and cigarette and other drug use from ages 17 to 26; however, no results could be computed due to errors in model estimation. Model estimation errors were also found when testing models with sum levels of all prior substance use from ages 12 to 16, including binge drinking, marijuana use, cigarette use, and other illicit drug use. These findings suggest that prior substance use from ages 12 to 16 predicting levels of substance use from ages 17 to 26 (outcome measure) produces an uninterpretable, and possibly over-controlled, analytic model.
4. Discussion
The present study examined the extent to which neural function associated with disinhibition assessed in early adulthood predicted resilient versus high-risk substance use trajectories across the transition to adulthood among FH+ youth over and above that produced by psychosocial measures of behavioral control/under-control during early and late adolescence. There were two key findings from this work, which were partially supportive of the study hypotheses. First, the full analytic model that included both psychosocial and neural measures of behavioral under-control/disinhibition indicated that resilient FH+ youth had greater early adolescent reactive control and less externalizing behavior during late adolescence compared to the high-risk group. Second, among all neuroimaging measures tested in the full analytic model, only the right DLPFC associated with inhibitory control was a significant predictor of resilient versus high-risk group membership, over and above the differentiation achieved by sociodemographic characteristics, cigarette and other drug use, and psychosocial indicators in early and late adolescence. Main findings remained during post hoc analyses that controlled for early substance use, which indicates that prior substance use did not exert a meaningful impact on fMRI results differentiating resilient and high-risk groups.
Given that early adolescence is often when substance use begins (SAMHSA, 2015) and late adolescence is when substance use escalates (Schulenberg et al., 2017), it is possible that a greater amount of early reactive control among resilient FH+ youth in the present study served as a foundation for effective self-regulation that was protective against later externalizing problems and substance use. Whereas distal factors (i.e., during early adolescence) may contribute to more downstream outcomes (i.e., substance use during the transition to adulthood), temporally proximal factors (i.e., during late adolescence) can exert a powerful impact on functioning to increase, decrease, or even reverse effects of prior distal influences (Schulenberg et al., 2016).
Results from the present study also provide support for individual differences in neural function associated with the inhibitory control that may be protective against elevated substance use through the transition to adulthood. Related to dual-systems models of risk-taking, the functioning of the DLPFC is involved in top-down regulation, including inhibitory control (see Luijten et al., 2014 for review) and response inhibition (Chaarani et al., 2017; Garavan et al., 2002). The DLPFC also shares connections with brain regions associated with reward responsivity, including the orbitofrontal cortex, amygdala, and hippocampus (Fiel et al., 2010). Therefore, neural activation in the DLPFC may help to regulate inappropriate responding to goal-directed behaviors, such as engaging in substance use and failing to weigh negative consequences in relation to positive outcomes (Feil et al., 2010). Thus, increased activation in the right DLPFC associated with inhibitory control may be an indicator of resilience among FH+ youth.
Another important finding was that adding VS activation during reward anticipation did not significantly predict resilient and high-risk groups. Due to these groups both having a family history of SUD, it is possible that both resilient and high-risk FH+ youth share a similar underlying predisposition to greater reward responsivity. Indeed, FH+ youth have been found to have heightened NAcc responsivity during reward anticipation compared to youth without this vulnerability (Andrews et al., 2011). Among individuals with heightened reward responsivity, Khurana et al. (2015) found that those who also had weak self-control experienced early progression into heavy substance use. However, individuals sensitive to rewards which also had higher self-control only engaged in occasional substance use. Thus, a greater level of inhibitory control may be a characteristic of resilience among individuals with similar levels of reward responsivity, such as FH+ youth.
4.1. Strengths and Limitations
Key strengths of the present study are its focus on resilient, FH+ youth and its innovative approach to examine both psychosocial and neural mechanisms involved in substance use resilience and risk. Resilience was defined developmentally in terms of consistently low binge drinking and marijuana use across the transition to adulthood, a time when substance use often escalates, and problems emerge. Despite limited generalizability due to the sample being relatively homogenous in terms of race/ethnicity and family history of SUDs, the MLS is one of only a few longitudinal studies of FH+ youth that also include a neuroimaging component. This pairing allows for more comprehensive analyses of complex psychosocial and neural processes involved in producing control over elevated substance use among this vulnerable population. Results from the present study may also help inform substance use prevention and intervention programs. Neuroimaging techniques, such as fMRI, may be useful in pre-test/post-test interventions to acquire a baseline measure of inhibitory control that could then be tested again after completion of interventions aimed at boosting self-regulation. The present study also provides support for the instrumental predictive utility of neuroimaging measures in relation to behavioral measures. For example, the Go/No-Go task is used to assess relatively automatic top-down processing to inhibit a prepotent response. Unlike behavioral assessments, neuroimaging analyses measure both behavioral task performance and neural function. Thus, neuroimaging results may show the underlying neural processing associated with self-regulation that behavioral characterization is unable to capture. As evident in the present study, no significant differences in fMRI task performance data existed between resilient and high-risk groups.
Despite its strengths, there were limitations of the present study. Resilience was defined only in relation to family history of SUD and patterns of binge drinking and marijuana use through early adulthood. Future studies are needed to include other aspects of resilience, such as adaptive functioning in academic, work, and social domains. The present study was also limited by a relatively small sample size. Although the sample size is consistent with other fMRI studies and was adequate to yield significant effects, larger sample sizes in future such studies will permit consideration of more nuanced effects and subsample differences. Findings should be replicated among larger and more diverse samples. An additional limitation of the present study is that the questionnaire used in the MLS defines binge drinking as five or more standard alcoholic drinks for both men and women, which is beyond the typical four drink cut-point for binge drinking among women.
4.2. Conclusions
Although characterization of risk factors for elevated substance use is crucial to inform prevention and intervention programs aimed at youth most vulnerable to developing SUDs, identifying protective factors associated with low use among FH+ youth is beneficial to understand the underlying mechanisms of this resilience. Specifically, neural activation involved in inhibitory control may help identify brain function unique to resilient FH+ youth. Examining the link between developmental trajectories of substance use and brain function associated with resilience provides a more comprehensive understanding of neurodevelopmental processes protective against problematic substance use during a developmental period when longer-term patterns of substance use are becoming established.
Supplementary Material
Highlights.
Resilient youth showed heightened prefrontal activation during inhibitory control.
Reward responsivity did not differentiate resilient versus high-risk groups.
Psychosocial and neuroimaging measures each uniquely characterize resilience.
Acknowledgments
Role of Funding Sources
This study was supported by the National Institute on Drug Abuse (R01 DA027261 to M. M. Heitzeg and R. A. Zucker, and R01 DA00141 to R. Miech and L. Johnston) and the National Institute on Alcohol Abuse and Alcoholism (R01 AA07065 and R01 AA012217 to R. A. Zucker and M. M. Heitzeg; T32 AA07477 to F. Blow). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: …
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: …
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: …
Contributors
M.M.H. and R.A.Z. designed the study and wrote the protocol. M.E.M. conducted the literature review, statistical analyses, and the first draft of the manuscript. J.E.S. provided critical feedback on the analytic approach and manuscript revisions. All authors have approved the final manuscript.
Conflict of Interest
No conflict declared.
References
- Achenbach TM. Manual for the Youth Behavior Self-Report and 1991 Profile. University of Vermont; Burlington VT: 1991. [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O’Malley S, Book GA, Reynolds B, Pearlson GD. Individuals family history positive for alcoholism show fMRI differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, Mericle AA, Meyers K, Winters KC. Parental substance use impairment, parenting and substance use disorder risk. J Subst Abus Treat. 2012;43:114–122. doi: 10.1016/j.jsat.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block JH, Block J. The role of ego-control and ego-resiliency in the organization of behavior. In: Collins WA, editor. Development of Cognition, Affect, and Social Relations: The Minnesota Symposia on Child Psychology. Erlbaum; Hillsdale, NJ: 1980. pp. 39–101. [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM Toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaarani B, Spechler PA, Hudson KE, Foxe JJ, Potter AS, Garavan H. The Neural basis of response inhibition and substance abuse. In: Enger T, editor. The Wiley Handbook of Cognitive Control. John Wiley and Sons, Ltd; Chichester, UK: 2017. pp. 581–601. [Google Scholar]
- Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. J Consult Clin Psychol. 2002;70:67–78. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Psychopathology as risk for adolescent substance use disorders: A developmental psychopathology perspective. J Clin Child Psychol. 1999;28:355–365. doi: 10.1207/S15374424jccp280308. [DOI] [PubMed] [Google Scholar]
- Colder CR, Scalco MD, Trucco EM, Read JP, Lengua LJ, Wieczorek WF, Hawk L. Prospective associations of internalizing and externalizing problems and their co-occurrence with early adolescent substance use. J Abnorm Child Psychol. 2013;41:667–677. doi: 10.1007/s10802-012-9701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Mills-Koonce R, Propper C, Gariépy JL. Systems theory and cascades in developmental psychopathology. Dev Psychopathol. 2010;22:497–506. doi: 10.1017/S0954579410000234. [DOI] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Meda SA, Jiantonio R, Potenza MN, Krystal JH, Pearlson GD. Neural correlates of impulsivity in healthy males and females with family histories of alcoholism. Neuropsychopharmacology. 2013;38:1854–63. doi: 10.1038/npp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Malone PS, Lansford JE, Miller S, Pettit GS, Bates JE. A dynamic cascade model of the development of substance-use onset. Monogr Soc Res Child Dev. 2009;74:1–119. doi: 10.1111/j.1540-5834.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Mathias CW, Ryan SR, Bray BC, Charles NE, Acheson A. Behavioral impulsivity and risk-taking trajectories across early adolescence in youths with and without family histories of alcohol and other drug use disorders. Alcohol Clin Exp Res. 2015;39:1501–1509. doi: 10.1111/acer.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Murphy BC, Maszk P, Holmgren R, Suh K. The relations of regulation and emotionality to problem behavior in elementary school children. Dev Psychopathol. 1996;8:141–162. [Google Scholar]
- Eisenberg N, Valiente C, Fabes RA, Smith CL, Reiser M, Shepard SA, Losoya SH, Guthrie IK, Murphy BC, Cumberland AJ. The relations of effortful control and ego control to children’s resiliency and social functioning. Child Dev. 2003;39:761–776. doi: 10.1037/0012-1649.39.4.761. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Self-regulation: Conceptual issues and relations to developmental outcomes in childhood and adolescence. In: Oettingen G, Gollwitzer PM, editors. Self-Regulation in Adolescence. Cambridge University Press; New York, NY: 2015. pp. 55–77. [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche R, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Hardee JE, Weiland BJ, Nichols TE, Welsh RC, Soules ME, Steinberg DB, Zubieta JK, Zucker RA, Heitzeg MM. Development of impulse control circuitry in children of alcoholics. Biol Psychiatry. 2014:1–9. doi: 10.1016/j.biopsych.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S, Strumpf EC, Kaufman JS. Do medical marijuana laws increase marijuana use? Replication study and extension. Ann Epidemiol. 2012;22:207–212. doi: 10.1016/j.annepidem.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Beseler CL. Dimensionality of lifetime alcohol abuse, dependence and binge drinking. Drug Alcohol Depend. 2009;10:53–61. doi: 10.1016/j.drugalcdep.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, Zucker RA. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend. 2014;141:51–7. doi: 10.1016/j.drugalcdep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WYW, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin Exp Res. 2008;32:414–26. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hides L, Lubman DI, Buckby J, Yuen HP, Cosgrave E, Baker K, Yung AR. The association between early cannabis use and psychotic-like experiences in a community adolescent sample. Schizophr Res. 2009;112:130–135. doi: 10.1016/j.schres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Hurd N, Zimmerman M. Adolescent resilience: Promoting more positive outcomes among youth at risk of using and abusing substances. In: Zucker R, Brown S, editors. The Oxford Handbook of Adolescent Substance Abuse. Oxford University Press; Oxford, UK: 2016. pp. 1–44. [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH. An internalizing pathway to alcohol and substance use disorders. Psychol Addict Behav. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Wirth RJ, Edwards MC, Curran PJ, Chassin LA, Zucker RA. Externalizing symptoms among children of alcoholic parents: Entry points for an antisocial pathway to alcoholism. J Abnorm Psychol. 2007;3:529–542. doi: 10.1037/0021-843X.116.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JM, Buu A, Zucker RA. Longitudinal phenotypes for alcoholism: Heterogeneity of course, early identifiers, and life course correlates. Dev Psychopathol. 2015;10:1–16. doi: 10.1017/S0954579415001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Kessler RC, Margulies RS. Antecedents of adolescent initiation into stages of drug use: A developmental analysis. J Youth Adolesc. 1978;7:13–40. doi: 10.1007/BF01538684. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Experimentation versus progression in adolescent drug use: A test of an emerging neurobehavioral imbalance model. Dev Psychopathol. 2015;27:1–13. doi: 10.1017/S0954579414000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Luijten M, Machielsen MWJ, Veltman DJ, Hester R, de Haan L, Franken IHA. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci. 2014;39:149–169. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon VV, Agrawal A, Kuo SIC, Su J, Dick DM, Meyers JL, Edenberg HJ, Nurnberger JI, Kramer JR, Kuperman S, Schuckit MA, Hesselbrock VM, Brooks A, Porjesz B, Bucholz KK. Associations of parental alcohol use disorders and parental separation with offspring initiation of alcohol, cigarette and cannabis use and sexual debut in high-risk families. Addiction. 2018;113:336–345. doi: 10.1111/add.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nigg JT, Wong MM, Fitzgerald HE, Jester JM, Puttler LI, Glass JM, Adams KM, Zucker RA. Childhood and adolescent resiliency, regulation, and executive functioning in relation to adolescent problems and competence in a high-risk sample. Dev Psychopathol. 2007;19:541–563. doi: 10.1017/S0954579407070265. [DOI] [PubMed] [Google Scholar]
- Martel MM, Pierce L, Nigg JT, Jester JM, Adams K, Puttler LI, Buu A, Fitzgerald H, Zucker RA. Temperament pathways to childhood disruptive behavior and adolescent substance abuse: Testing a cascade model. J Abnorm Child Psychol. 2009;37:363–73. doi: 10.1007/s10802-008-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, Heitzeg MM. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. 2016;370:2219–2227. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne BJ, Caspi A, Harrington H, Poulton R, Rutter M, Moffitt TE. Predictive value of family history on severity of illness. Arch Gen Psychiatry. 2009;66:738–747. doi: 10.1001/archgenpsychiatry.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe J, Johnson JL, Wade W. Resilience in children of substance users: In their own words. Subst Use and Misuse. 2007;42:381–398. doi: 10.1080/10826080601142147. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Donovan JE, Belendiuk KA. Familial loading for alcoholism and offspring behavior: Mediating and moderating influences. Alcohol Clin Exp Res. 2010;34:1972–1984. doi: 10.1111/j.1530-0277.2010.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll DC, Fessler JA, Sutton BP. Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Trans Med Imaging. 2005;24:325–336. doi: 10.1109/tmi.2004.842452. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park S, Schepp KG. A systematic review of research on children of alcoholics: Their inherent resilience and vulnerability. J Child Fam Stud. 2015;24:1222–1231. [Google Scholar]
- Pearson MR, D’Lima GM, Kelley ML. Self-regulation as a buffer of the relationship between parental alcohol misuse and alcohol-related outcomes in first-year college students. Addict Behav. 2011;36:1309–1312. doi: 10.1016/j.addbeh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16:322–9. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke K. Diagnostic Interview Schedule for the DSM-IV (DSM-IV) Washington University School of Medicine; St. Louis, MO: 2000. [Google Scholar]
- Sanchez-Roige S, Stephens DN, Duka T. Heightened impulsivity: Associated with family history of alcohol misuse, and a consequence of alcohol intake. Alcohol Clin Exp Res. 2016;40:2208–2217. doi: 10.1111/acer.13184. [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the Future national survey results on drug use, 1975-2016. Volume II: College students and adults ages 19-55. Institute for Social Research, The University of Michigan; Ann Arbor, MI: 2017. [Google Scholar]
- Schulenberg JE, Maggs JL. Destiny matters: Distal developmental influences on adult alcohol use and abuse. Addiction. 2008;103(Suppl):1–6. doi: 10.1111/j.1360-0443.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Maslowsky J, Patrick ME, Martz ME. Substance use in the context of adolescent development. In: Zucker R, Brown S, editors. The Oxford Handbook of Adolescent Substance Abuse. Oxford University Press; Oxford, UK: 2016. pp. 1–34. [Google Scholar]
- Schulenberg JE, Merline AC, Johnston LD, O’Malley PM, Bachman JG, Virginia B. Trajectories of marijuana use during the transition to adulthood: The big picture based on national panel data. J Drug Issues. 2005;35:255–279. doi: 10.1177/002204260503500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg J, O’Malley PM, Bachman JG, Wadsworth KN, Johnston LD. Getting drunk and growing up: Trajectories of frequent binge drinking during the transition to young adulthood. J Stud Alcohol. 1996;57:289–304. doi: 10.15288/jsa.1996.57.289. [DOI] [PubMed] [Google Scholar]
- Shorey RC, Fite PJ, Elkins SR, Frissell KC, Tortolero SR, Stuart GL, Temple JR. The association between problematic parental substance use and adolescent substance use in an ethnically diverse sample of 9th and 10th graders. J Prim Prev. 2013;34:381–393. doi: 10.1007/s10935-013-0326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VC, Wilson CR, Committee on Substance Use Prevention Families affected by parental substance use. Pediatrics. 2016;138:e1–e13. doi: 10.1542/peds.2016-1575. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (HHS Publication No. SMA 15-4927, NSDUH Series H-50).Behavioral health trends in the United States: Results from the 2014 national survey on drug use and health. 2015 Retrieved from http://www.samhsa.gov/data/. (accessed February 3, 2018)
- Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field in homogeneities. IEEE Trans Med Imaging. 2003;22:178–188. doi: 10.1109/tmi.2002.808360. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The brain on drugs: From reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Warner LA, White HR, Johnson V. Alcohol initiation experiences and family history of alcoholism as predictors of problem-drinking trajectories. J Stud Alcohol Drugs. 2007;68:56–65. doi: 10.15288/jsad.2007.68.56. [DOI] [PubMed] [Google Scholar]
- Windle M, Zucker RA. Reducing underage and young adult drinking. Alcohol Res Heal. 2010;33:29–44. [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, Adams K. Behavioral control and resiliency in the onset of alcohol and illicit drug use: A prospective study from preschool to adolescence. Child Dev. 2006;77:1016–33. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA. Alcohol use and the alcohol use disorders: A developmental-biopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Vol 3 Risk, Disorder, and Adaption. Wiley; New York: 2006. pp. 620–656. [Google Scholar]
- Zucker RA. Genes, brain, behavior, and context: The developmental matrix of addictive behavior. In: Stoltenberg SF, editor. Genes and the Motivation to use Substances Nebraska Symposium on Motivation, 61. New York: Springer; 2014. pp. 51–69. Chapter 4. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald HE, Noll RB. Drinking and drug history. 4th. Michigan State University; East Lansing, MI: 1990. Unpublished manuscript. [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Fitzgerald HE, Lester BM, Zuckerman BS, editors. Children of Addiction: Research, Health, and Public Policy Issues. RoutledgeFalmer; New York: 2000. pp. 109–141. [Google Scholar]
- Zucker RA, Heitzeg M, Nigg JT. Parsing the undercontrol/disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Dev Perspect. 2011;5:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


