Abstract
Background
Under-reporting of drug use in the perinatal period is well-documented, and significantly limits the reach of proactive intervention approaches. The Wayne Indirect Drug Use Screener (WIDUS) focuses on correlates of drug use rather than use itself. This trial tested a computer-delivered, brief intervention designed for use with indirect screen-positive cases, seeking to motivate reductions in drug use without presuming its presence.
Methods
Randomized clinical trial with 500 WIDUS-positive postpartum women recruited between August 14, 2012 and November 19, 2014. Participants were randomly assigned to either a time control condition or a single-session, tailored, indirect, brief intervention. The primary outcome was days of drug use over the 6-month follow-up period; secondary outcomes included urine and hair analyses results at 3- and 6-month follow-up. All outcomes were measured by blinded evaluators.
Results
Of the 500 participants (252 intervention and 248 control), 36.1% of participants acknowledged drug use in the 3 months prior to pregnancy, but 89% tested positive at the 6-month follow-up. Participants rated the intervention as easy to use (4.9/5) and helpful (4.4/5). Analyses revealed no between-group differences in drug use (52% in the intervention group, vs. 53% among controls; OR 1.03). Exploratory analyses also showed that intervention effects were not moderated by baseline severity, WIDUS score, or readiness to change.
Conclusions
The present trial showed no evidence of efficacy for an indirect, single-session, computer-delivered, brief intervention designed as a complement to indirect screening. More direct approaches that still do not presume active drug use may be possible and appropriate.
Keywords: postpartum, drug use, alcohol use, brief intervention, disclosure
1. Introduction
Parental substance abuse is associated with a range of negative child outcomes, including increased risk of child maltreatment (Besinger et al., 1999; Hanson et al., 2006b; Ondersma, 2002; Wells, 2009), violence exposure (Hanson et al., 2006a; Ondersma et al., 2006), and behavioral problems (Manly et al., 2013). Substance use tends to decrease during pregnancy and increase following childbirth (Office of Applied Studies, 2009), suggesting that the immediate post-partum period may be an opportunity to encourage maintenance of natural change. Notably, computer-delivered, brief interventions with postpartum women are associated with reductions in drug use (Ondersma et al., 2007; Ondersma et al., 2014).
However, under-reporting of drug use during the perinatal period is well documented (Beatty, Chase, and Ondersma, 2014; Ondersma et al., 2012; Ostrea et al., 2001). In one study with postpartum women, only 20.3% of participants testing positive for drug use in the last trimester of pregnancy admitted to use of drugs in the past year (Grekin et al., 2010). This underreporting makes identification a challenge.
Although direct disclosure of drug use may be limited, a great deal is known about correlates of drug use. This allowed construction of an index consisting of items that are associated with drug use but which individually are less likely to be under-reported, i.e., indirect measurement (Ondersma et al., 2012). The Wayne Indirect Drug Use Screener (WIDUS) consists of six true-false items such as “Most of my friends smoke cigarettes,” “There have been times in my life, for at least 2 weeks straight, where I felt like everything was an effort,” and “I get mad easily and feel a need to blow off some steam,” answers to which are summed to create an index of indirect risk ranging from 0 to 6. These six items were derived from a longer list of items using a rigorous and objective item selection process. In a cross-validation sample, the WIDUS predicted a positive urine or hair test with an area under the curve (AUC) of .74 (Ondersma et al., 2012). In that same study, sensitivity and negative predictive value (NPV) for the WIDUS (.88 and .83) were substantially higher than for direct measurement of drug use in identifying the results of hair and urine analysis. Specificity of the WIDUS in the cross-validation sample (.42) was poor, but was improved by introduction of an additional item regarding drug use prior to pregnancy (yielding sensitivity, specificity, and NPV of .77, .71, and .92, respectively).
Indirect identification of risk is only useful if tied to an effective response. This raises a unique challenge: how to intervene regarding drug use without presuming its existence. Two lines of research suggest possible responses. First, there is growing awareness of the extent to which non-treatment-related activities can facilitate change. For example, Epstein et al. (2005) found that drinking among alcohol-dependent women decreased dramatically before the treatment phase of a randomized trial even began, such that 44% of participants became abstinent without treatment. Epstein et al. further showed that this decrease in drinking was associated with each successive step of the pre-treatment research process. Kypri and colleagues (Kypri et al., 2007) randomly assigned problem drinkers to either a screening only or a screening plus 10-minute assessment condition, and found the assessment process itself to affect drinking one year later. Ondersma et al. found a significant pre-treatment decrease in substance use among pregnant women following the study baseline session (Ondersma et al., 2012). Such findings suggest that perhaps mere attention to drug use and its consequences, even if not directly targeted at the individual, may promote change.
Research also suggests that human behavior change may be less linear than previously thought. For example, spontaneous change attempts appear more likely to succeed long-term than planned change attempts (West and Sohal, 2006), and the majority of persons who do successfully change an alcohol use disorder do so without professional help (Bischof et al., 2003; Burman, 1997; Sobell et al., 2000). In studies randomly assigning persons with substance use disorders to either brief or extended interventions, the extended intervention condition often shows no added benefit (Burke et al., 2003; Kaner et al., 2007; Moyer et al., 2002).
These lines of research suggest that even brief, oblique approaches to drug abuse could potentially serve as a trigger for self-directed change. Further, by identifying and engaging a higher proportion of individuals with drug use disorders, such an approach could have a meaningful population impact with even a small effect size (defining population impact as the product of effect size and proportion receiving the intervention; e.g., Smeeth and Ebrahim, 2000). Further, the indirect intervention approach may be more acceptable than typical brief interventions, in that it carries no stigma and requires no willingness to directly discuss drug use.
This study evaluated a single-session, computer-delivered, brief intervention for use with WIDUS-positive postpartum women in three areas. First, we evaluated feasibility and acceptability of this novel intervention approach. Second, we evaluated the extent to which exposure to the intervention was associated with immediate changes in state motivation. Third, we evaluated intervention effects on drug use at 3- and 6-months. We predicted that the brief intervention would be associated with reductions in drug use as measured by self-reported days of use in the past 90 days and by urine drug screen results. We also predicted that HIV risk behaviors would be lower among participants who received the intervention.
2. Method
2.1 Participants
Recruitment took place at the labor and delivery unit of a hospital in Detroit, Michigan; recruitment began on August 14, 2012, and ended on November 19, 2014 when the planned sample of 500 was attained. Participants were women recruited during their postpartum hospital stay, who were age 18-45, able to understand spoken English, and who scored three or above on the WIDUS (Ondersma et al., 2012). Participants were excluded if they had received opioid pain medication in the past 3 hours, had not slept since giving birth, or were unable to provide consent. Study procedures were approved by the Wayne State University IRB and the trial was registered with clinicaltrials.gov (NCT01650675). Protocol available from the first author upon request.
2.2 Procedure
Women were approached in their hospital rooms, all of which were private. The study was described as being about parent factors that can influence child health. Those who expressed interest were screened for eligibility, following consent via information sheet, using Audio-enhanced Computer-Assisted Self-Interview (ACASI) software. Those who were eligible were asked to provide consent for the trial, which described the study broadly as focused on factors that can influence child health, including substance use. Follow-up urine and hair sampling was also described. Those who provided consent immediately went on to complete the baseline assessment and were then randomized to either the indirect intervention or a time control (infant nutrition) condition, each of which was completed during the initial encounter. Participants were given a gift for their baby worth $3 for completing screening. Those who were eligible and provided consent were given a $50 gift card for the baseline assessment and randomization session. The entire process took approximately 40 minutes for eligible participants. Of 1,251 women approached regarding this study, 1,220 (97.5%) consented to screening, 600 (49.2%) were eligible, and 502 (41.1%) enrolled (Fig 2). Participants were randomly assigned to either intervention or control conditions by the intervention software using adaptive randomization in a 1:1 allocation ratio. In part because of the software-based automatic randomization, research staff were blind to condition at baseline as well as follow-up.
Figure 2.
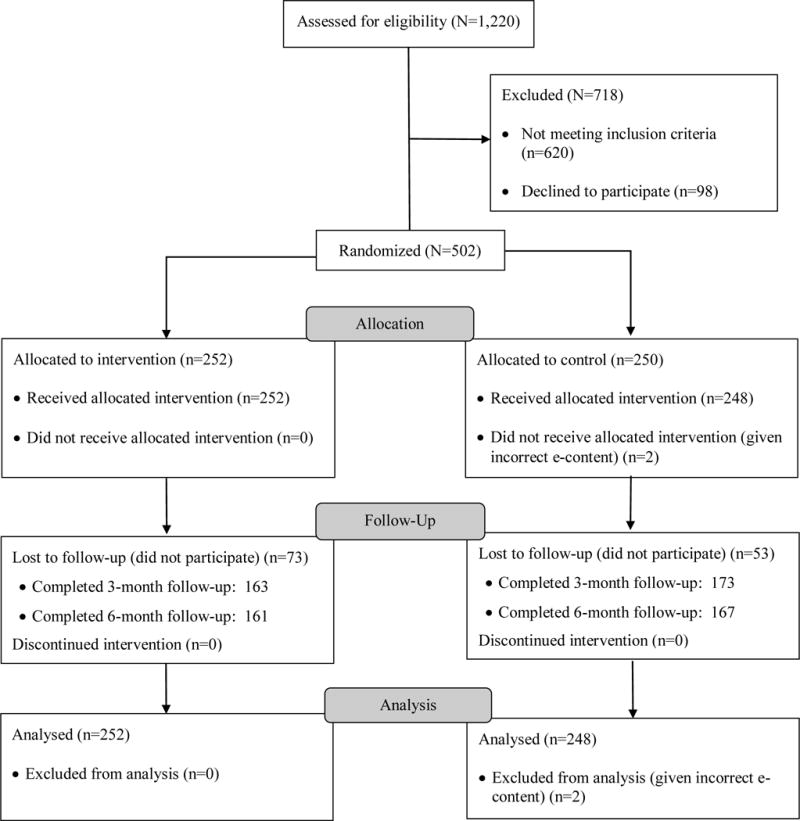
Participant flow
Participants completed 3- and 6-month follow-up sessions in our lab. Follow-up sessions took approximately two hours; participants received gift cards worth $75 for the 3-month session and $100 for the 6-month session. Participants received an additional $25 gift card at the 3- and 6-month follow-ups for providing urine and hair samples. As seen in Figure 2, 374 participants (74.8%) completed at least one follow-up session.
2.3 Conditions
All participants, regardless of group assignment, completed a 20-minute assessment regarding depression, intimate partner violence, violence exposure, and perceived need for and intention to make changes in a range of areas (see below).
2.3.1. Indirect intervention
The indirect intervention was developed using a software platform designed to facilitate interactivity, in part through an animated talking narrator and natural-language reflections. The intervention used tunneling (Fogg, 2002), meaning that participants followed a specific path that was tailored to their input. This approach enhances ease of use, since participants never need to learn to navigate within the software. It was delivered using touch-screen tablet PCs and headphones, and required participants only to tap the screen to indicate their responses. The indirect intervention was patterned after Motivational Interviewing (MI) principles (Miller and Rollnick, 2002) and additionally used tailoring on multiple elements to present each participant with a unique and highly relevant content (consistent with the Elaboration Likelihood Model, Petty and Cacioppo, 1986).
The flow of the indirect intervention is presented in Figure 1. Participants began by completing a brief assessment of substance use, mental health, relationship safety, violence exposure, risky sexual behavior, ethnic identity (the extent to which the participant identifies and is involved with her racial or ethnic group; Langford et al., 2010), and religiosity. They then received a video-based orientation to the intervention, described as “The Parent Check-Up (PCU).” This orientation described the PCU as an opportunity, at this important juncture in their lives, to step back and think about “what to keep, and what to leave behind.” The video itself was tailored on ethnic identity and religiosity.
Figure 1. Parent Check-up indirect intervention flow.
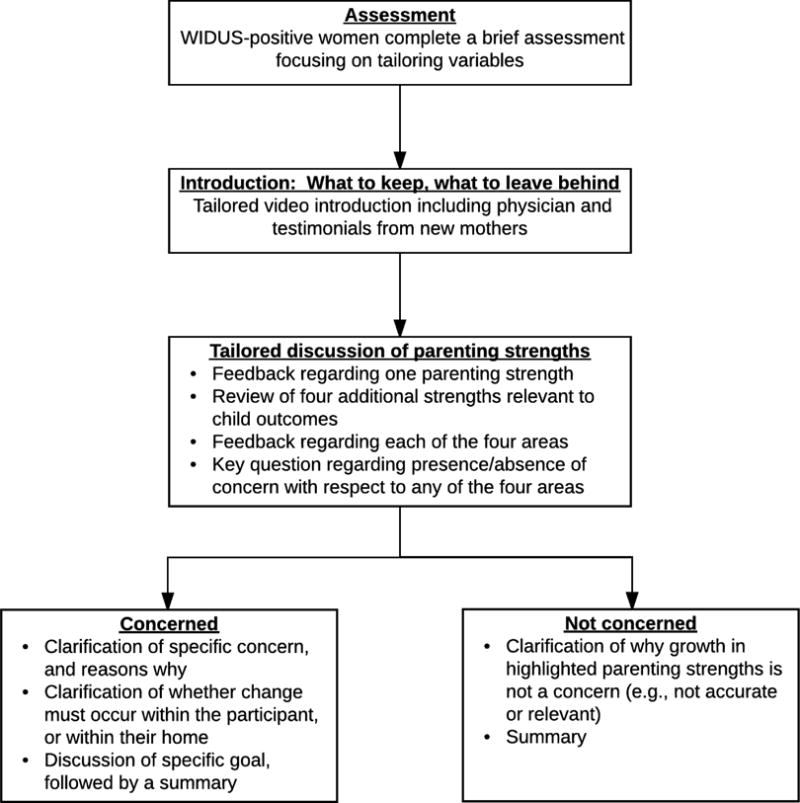
Note. The WIDUS is a brief screener consisting of non-face valid items that are correlated with drug use (e.g., cigarette smoking, chronic pain, trauma).
Next, using a gain-framed approach, the intervention focused on key parenting strengths. It first described a strength that the participant demonstrated through the assessment process, followed by a discussion of four key strengths that can facilitate infant growth and development: emotional health, safety, physical health, and a healthy home. A video then defined these strengths and described the ways that they can facilitate infant health. Importantly, this video touched on substance use (as part of the “healthy home” section), but did not focus on it exclusively. Also of importance is the fact that the key strengths noted here are all themselves associated with drug use, such that change in any one of them could potentially be ameliorative regarding drug use. Participants then received a feedback report indicating which of these areas were already strengths, and which might be considered a growth area. Participants were asked for their thoughts regarding that feedback, and were offered the option of changing in one of the four areas or ending the PCU. Those who chose to make a change were helped to identify a specific goal and strategies. Notably, participants who chose to make a change of some kind were offered the chance to select whether they wanted to focus on change in themselves, or in their home. A total of 43 additional videos, tailored on participant choices, characteristics, race, and level of concern, were incorporated within the PCU.
2.3.2. Infant nutrition control
The infant nutrition intervention was similar in duration to the indirect drug use intervention. It provided education regarding infant nutrition from birth to age one (i.e., breastfeeding, formula feeding, when to introduce solids) with no mention of safety, emotional health, or substance use. Like the indirect intervention, it included videos and questions and was designed to be interactive and easy to use. However, it specifically avoided expressions of empathy or affirmations. The information and videos presented in the intervention were obtained from the American Academy of Pediatrics “Bright Futures” Nutrition program (https://brightfutures.aap.org).
2.4 Measures
The primary outcome was days of drug use in the 6 months following randomization, as measured by Timeline Follow-back interviews (Sobell and Sobell, 1996). Secondary outcomes included the results of urine drug screening (Redwood Toxicology Labs, Inc.), hair testing (Psychemedics, Inc.), the HIV Risk-taking Behavior Scale (Darke et al., 1991), the Short Inventory of Problems-Revised (a measure of drug use consequences; Kiluk et al., 2013), the CESD-10 depression screener (Kohout et al., 1993; Radloff, 1977), the Conflict Tactics Scale (a measure of intimate partner violence; Straus et al., 1996), and a measure of community violence exposure (the Survey on Exposure to Community Violence; Richters and Martinez, 1993). All participants completed baseline measures of age, race, ethnicity, employment, receipt of government assistance, substance use prior to pregnancy, and marital status. Those randomized to the indirect intervention also completed a measure of intervention acceptability using a scale asking participants to rate the extent to which the intervention was helpful, easy to work with, respectful, etc. on a 1-5 scale in which 1 = “not at all” and 5 = “very much” (Ondersma et al., 2004; Ondersma et al., 2007). Finally, all intervention participants were asked a series of questions—before and after exposure to the brief intervention—regarding the extent to which they believed that change was necessary and likely, either specifically within themselves or more broadly “within their home” (thus not necessarily implicating themselves) in the areas addressed by the intervention.
2.5 Statistical analysis
Initial analyses included checks for out of range and missing values. Missing data on outcome variables were due to loss to follow-up. Participants lost to follow-up were younger (23.7 versus 25.1 years old, p=0.001), more likely to currently smoke (46% versus 33%, p=0.004), and less likely to have worked in the last 6 months (26.6% versus 40.4%, p=0.002). The HRBS injection drug use subscale had very few positive cases (97.6% and 98.8% no risk at three and six-month follow-up respectively) and is not reported. Only the HRBS sexual risk subscale is reported. The sample size of 500 was based on an effect size of d = .2, a retention rate of 80%, and correlation between repeated measures of r = .5, yielding power of .84 to detect a significant between-group difference.
2.5.1 Missing values
At the three-month follow-up, 328 (65.6%) of the 500 participants provided urine samples and 290 (58%) provided hair samples. At the six-month follow-up, 319 (63.8%) provided urine samples and 270 (54%) provided hair samples. Missing values were imputed using predictive mean matching (PMM) via the Multiple Imputation by Chained Equations (MICE) package in R. Twenty imputed datasets were generated. PMM was chosen due to the lack of normality in study outcomes (Horton and Lipsitz, 2001). For all outcomes, two sets of analyses were performed: 1) using only cases with complete data and 2) using all cases with imputed data. Efficacy was assessed at three and six months separately and across the entire six-month interval. The overall three- and six-month score was based on prevalence and number of days of use across the entire six-month interval for substance use and based on a total score for the HRBS sexual risk subscale.
Analysis of intervention efficacy was based on originally assigned groups following intent to treat, and accounted for the level of measurement and distribution of each of the outcomes. Logistic regression was used for dichotomous outcomes (e.g., past 90-day prevalence of drug use using timeline follow-back, and prevalence of drug use from urine drug screen). Timeline follow-back data on of number of days using drugs were extremely positively skewed, with an inflated number of participants reporting no use. Consequently, generalized linear modeling using a negative binomial error structure with a log link was employed to test for group differences on these outcomes. Measures of risk (e.g., HRBS) were also positively skewed. As an ordinal variable, sex risk was recoded into four levels and tested via ordinal regression. For all outcomes, secondary analyses evaluated whether an a priori set of variables moderated differences between the control and intervention groups. For the moderator analyses, main effects for group and the moderator were entered into the model followed by the group-by-moderator interaction term. Analyses of pre-post brief intervention change in problem recognition used matched-pairs t-tests.
3. Results
3.1 Participants
Participants were 500 primarily African-American and non-Hispanic women; nearly 85% reported receipt of government food assistance (Table 1). Participants ranged from 18 to 37 years of age. Most pregnancies were unplanned. There were no study-related serious adverse events.
Table 1.
Participant characteristics
| Characteristic | Full Sample (N=500) |
Control (N = 248) |
Intervention (N= 252) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 24.6 (4.9) | 24.4 (4.8) | 24.8 (5.1) |
| Range | 18 to 37 | 18 to 37 | 18 to 37 |
| Race | |||
| Black | 366 (73.2%) | 184 (74.2%) | 182 (72.2%) |
| Multiple/Other/Not Given | 121 (24.2%) | 58 (23.4%) | 63 (25.0%) |
| White | 13 (2.6%) | 6 (2.4%) | 7 (2.8%) |
| Hispanic Ethnicity (any race) | 18 (3.6%) | 9 (3.6%) | 9 (3.6%) |
| Employment | |||
| Employed, part-time and odd jobs | 91 (18.2%) | 4* 54 (21.8%) | 37 (14.7%) |
| Employed full Time | 85 (17.0%) | 43 (17.3%) | 42 (16.7%) |
| Legally Married | 15 (3.0%) | 10 (4.0%) | 5 (2.0%) |
| Planned pregnancy | 125 (25.0%) | 57 (23.0%) | 68 (27.0%) |
| Government Support | |||
| Any | 480 (96.0%) | 237 (95.6%) | 243 (96.4%) |
| Food Assistance through WIC | 417 (83.4%) | 210 (84.7%) | 207 (82.1%) |
| Bridge Card for Food | 422 (84.4%) | 207 (83.5%) | 215 (85.3%) |
| Housing Assistance | 30 (6.0%) | 13 (5.2%) | 17 (6.7%) |
| Depression screen positive | 184 (36.8%) | 96 (38.7%) | 88 (34.9%) |
| Pre-pregnancy prescription opioid misuse | 60 (12%) | 25 (10%) | 35 (13.9%) |
3.2 Intervention acceptability
Ratings of indirect intervention acceptability were high, ranging from a low of 4.4 (sd=.93) for helpfulness to a high of 4.9 (sd=.57) for ease of use (N=252 for all acceptability items). Other ratings included 4.8 (sd=.58) for perceived respectfulness; 4.7 (sd=.68) for the extent to which other moms would be helped by the program, and 4.5 (sd=.94) for enjoying their interactions with the computer.
3.3 Changes in state intention to change
For the intervention group, overall ratings of the extent to which change was needed, either before or after exposure to the intervention, were low. As seen in Table 2, participant responses to the question regarding the necessity of change “within their home” (which thus could include themselves, but could be interpreted as referring to another family member) showed significant increases from pre- to post-intervention in all three specific areas measured (substance use, emotional health, and violence) and a significant reduction in the perception that no change was needed. In contrast, these areas showed no significant improvements in participants’ reports that she herself needed to change; however, there was a significant increase in recognition of the need to reduce risky sexual behaviors.
Table 2.
Pre-post fluctuation in state change motivation in key risk areas among intervention group participants (N = 252)
| Risk area | % indicating change in home needed | % indicating change in self needed | ||||
|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | |
| Marijuana use | 15.5 | 21.1 | .024 | 11.6 | 15.1 | .057 |
| Emotional health | 14.3 | 25.5 | <.001 | 24.7 | 28.3 | .243 |
| Violence | 8.8 | 10.8 | <.001 | 6.0 | 7.2 | .508 |
| No change needed | 46.6 | 31.1 | <.001 | 21.9 | 17.1 | .111 |
| Sexual risk behavior | NA | NA | NA | 35.5 | 44.2 | .004 |
Note. Scores reflect responses to visual analogue scales ranging from 0 to 100. “Change in home” refers to the extent to which participants reported that something needed to change within their home in each area, in order to benefit their baby; “change in self” refers to the extent to which participants reported that they themselves needed to make a change in each area in order to benefit their baby.
Similarly, participant-rated intentions to change, self-efficacy, and importance of change were also rated separately for change “within the home” and by the participant herself. Summary variables representing the total score on all three home-change focused items at pre- and post-test, as well as the three self-change focused items at pre- and post-test, showed significant increases in paired-samples t-tests (t[247] = −2.6, p = .011 for change in the home, and t[247] = −2.2, p = .030 for self-change).
3.4 Intervention effects on primary and secondary outcomes
Outcome analyses are presented in Tables 3 (continuous outcomes) and 4 (dichotomous outcomes). Overall drug use in this sample at the 3- and 6-month post-delivery follow-ups was high, with over 80% of hair samples testing positive for an illicit drug—primarily marijuana—at one or both follow-up points. No between-group differences were detected on the primary outcome of days of drug use or on secondary outcomes including point-prevalence abstinence at 3- or 6-month observations (as measured by urine and hair testing as well as 90-day period prevalence based on the TLFB), and the HRBS sex risk scale. Group differences were also not observed for exploratory outcomes including consequences of drug use, alcohol use, depression, self-reported intimate partner violence, community violence exposure, or treatment utilization (data not shown). Results for imputed, intent to treat analyses and completer analyses did not differ.
Table 3.
Drug use days and sexual risk behavior scores by condition and time point
| Complete Data | Imputed Data | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Continuous outcomes | Control* | Intervention* | exp (B)±SE | 95% CI exp(B) |
Control N=248 |
Intervention N=252 |
exp (B) ±SE | 95% CI exp (B) |
|
| # days drug use | 3 month | 1 (0 – 12) | 1 (0 – 7) | 0.91±0.20 | 0.61 – 1.34 | 1 (0 – 13.7) | 1 (0 – 14.9) | 0.98±0.18 | 0.68 – 1.4 |
| in past 90 days | 6 month | 0 (0 – 17) | 0 (0 – 14) | 0.91±0.20 | 0.62 – 1.35 | 1.4 (0 – 23.1) | 0.8 (0 – 22.7) | 0.97±0.17 | 0.69 – 1.35 |
| Combined | 3 (0 – 31.3) | 2 (0 – 27.5) | 0.90±0.20 | 0.61 – 1.33 | 4.5 (0 – 46.4) | 4 (0 – 41.9) | 0.97±0.16 | 0.71 – 1.34 | |
| HRBS sexual risk | 3 month | 4 (1–6) | 4 (2–6) | 0.87±0.20 | 0.59–1.27 | 3.9 (1.1 – 6) | 3.3 (1–6) | 0.86±0.19 | 0.59 to 1.26 |
| 6 month | 5 (1–6) | 4 (2–6) | 0.88±0.20 | 0.60–1.30 | 4.3 (1.1 – 6.1) | 3.9 (1.56) | 0.94±0.20 | 0.63 to 1.39 | |
| Combined | 4.5 (2.5–6) | 4 (1.56) | 0.82±0.23 | 0.53–1.28 | 4.1 (2.4 – 6) | 3.8 (2.1 – 6) | 0.88±0.20 | 0.59 to 1.31 | |
Note:
Median (25th and 75th percentiles). Days of drug use measured via Timeline Follow-back interview; HRBS = HIV Risk-Taking Behavior Scale.
Table 4.
Any drug use, by condition and time point
| Prevalence | Control* | Intervention* | Odds Ratio | 95% CI OR | Risk Differenceb | 95% CI RD | Relative Risk | 95% CI RR | |
|---|---|---|---|---|---|---|---|---|---|
| Any drug use by | 3 month | 0.52 | 0.53 | 1.03 | 0.69 – 1.55 | 0.008 | −0.09 – 0.11 | 1.02 | 0.84 – 1.23 |
| self-report (TLFB) | 6 month | 0.49 | 0.48 | 0.95 | 0.63 – 1.43 | −0.014 | −0.12 – 0.09 | 0.97 | 0.79 – 1.20 |
| Combined | 0.60 | 0.61 | 1.04 | 0.69 – 1.57 | 0.009 | −0.09 – 0.11 | 1.01 | 0.86 – 1.19 | |
| Any MJ use by | 3 month | 0.52 | 0.52 | 1.03 | 0.70 – 1.52 | 0.007 | −0.09 – 0.10 | 1.01 | 0.84 – 1.22 |
| self-report (TLFB) | 6 month | 0.47 | 0.47 | 0.95 | 0.64 – 1.51 | −0.003 | −0.11 – 0.10 | 0.99 | 0.79 – 1.24 |
| Combined | 0.59 | 0.60 | 1.07 | 0.72 – 1.59 | 0.015 | −0.08 – 0.11 | 1.03 | 0.87 – 1.20 | |
| Urine analysis, | 3 month | 0.47 | 0.45 | 0.90 | 0.58 – 1.41 | −0.025 | −0.14 – 0.08 | 0.95 | 0.74 – 1.20 |
| any drug use | 6 month | 0.47 | 0.45 | 0.94 | 0.62 – 1.42 | −0.015 | −0.12 – 0.09 | 0.97 | 0.77 – 1.21 |
| Combined | 0.58 | 0.55 | 0.91 | 0.59 – 1.39 | −0.024 | −0.13 – 0.08 | 0.96 | 0.80 – 1.15 | |
| Urine analysis, | 3 month | 0.44 | 0.41 | 0.88 | 0.57 – 1.35 | −0.031 | −0.14 – 0.07 | 0.93 | 0.73 – 1.18 |
| MJ drug use | 6 month | 0.43 | 0.41 | 0.92 | 0.61 – 1.39 | −0.021 | −0.12 – 0.08 | 0.95 | 0.75 – 1.21 |
| Combined | 0.54 | 0.51 | 0.88 | 0.58 – 1.34 | −0.031 | −0.13 – 0.07 | 0.94 | 0.77 – 1.15 | |
| Hair analysis, | 3 month | 0.80 | 0.78 | 0.91 | 0.55 – 1.51 | −0.015 | −0.10 – 0.07 | 0.98 | 0.88 – 1.09 |
| any drug use | 6 month | 0.72 | 0.71 | 0.96 | 0.59 – 1.54 | −0.009 | −0.11 – 0.09 | 0.99 | 0.86 – 1.13 |
| Combined | 0.87 | 0.86 | 0.89 | 0.50 – 1.58 | −0.014 | −0.08 – 0.05 | 0.98 | 0.91 – 1.06 | |
| Hair analysis, | 3 month | 0.60 | 0.57 | 0.88 | 0.57 – 1.37 | −0.030 | −0.14 – 0.08 | 0.95 | 0.79 – 1.14 |
| MJ drug use | 6 month | 0.59 | 0.56 | 0.89 | 0.59 – 1.33 | −0.028 | −0.13 – 0.07 | 0.95 | 0.80 – 1.13 |
| Combined | 0.71 | 0.69 | 0.92 | 0.59 – 1.43 | −0.018 | −0.11 – 0.07 | 0.97 | 0.85 – 1.11 |
Note:
Proportion positive. MJ = marijuana.
Risk Difference calculated as Intervention – Control.
3.5 Exploratory analysis of potential moderators of intervention efficacy
Exploratory analyses of potential moderators, including WIDUS score (median split) and self-report of drug use prior to pregnancy, showed no significant moderation for any outcomes.
4. Discussion
This trial evaluated the efficacy of a brief, indirect intervention designed to facilitate reevaluation of drug use without presuming its presence. Under-reporting of drug use in the perinatal period dramatically reduces the potential population impact of any brief intervention with this population, and underscores the potential utility of an indirect intervention that does not rely on disclosure. Even small effects from a technology-delivered, indirect intervention might be justified by its low cost, broad applicability, and ability to protect women from perceived or actual risk as a result of drug use disclosure.
However, this trial found no support for an indirect intervention among a sample that was accurately identified as high risk. The intervention led to significant increases in reporting of problems within the home but not to disclosure by the participant herself. This could simply reflect unwillingness to disclose and, in fact, was designed to allow this possibility. However, this finding could also reflect a failure to achieve the intended increase in recognition of one’s drug use as a potential target for change. This finding is consistent with evidence from large-scale trials that shows even direct brief interventions for drug use are ineffective (Bogenschutz et al., 2014; Roy-Byrne et al., 2014; Saitz et al., 2014). Such findings suggest that there are challenges for all forms of brief interventions hoping to impact drug use, and that the additional barrier of not being able to directly address drug use may only exacerbate those challenges.
Research in this area includes reports that assessment and/or attention to substance use can facilitate change (e.g., McCambridge and Kypri, 2011). However, one trial noted above (Bogenschutz et al., 2014) included a screen-only control condition and found no evidence for assessment reactivity. Drug use in these studies declined significantly for all conditions, suggesting that regression to the mean or other forms of reactivity (for example, to being identified as using at levels sufficient to be of interest to scientists, or to awareness of future follow-up; e.g., Epstein et al., 2005) may have been present. The consistency with which substance use falls in all conditions in brief intervention trials suggests that interrupted time series designs (e.g., Ambroggio et al., 2012) should be considered.
4.1 Limitations
This trial has several limitations. First, although 74.8% of participants completed at least one follow-up session, loss to follow-up was 32.8% at 3 months and 34.4% at 6 months. Further, the primarily African-American and low-income sample is not broadly representative. Drug use was also primarily marijuana, making it difficult to generalize to samples with different patterns of use. Further, although direct brief interventions for drug use among postpartum women have been successful (Ondersma et al., 2014), it is also possible that the immediate postpartum period was not ideal for an intentionally thought-provoking intervention of this type. Finally, because of the demonstrated and substantial under-reporting of drug use among postpartum women, and because of the strong tendency of women to reduce drug use during pregnancy, there is no valid way to directly compare drug use from prior to enrollment to follow-up; analyses were limited to between-group comparisons at each follow-up.
4.2 Implications
A substantial proportion of women using drugs during or following pregnancy are neither seeking treatment nor willing to disclose their drug use. Reducing the overall burden of drug use on women in the perinatal period—and consequently on their children—will require careful attention to this large subgroup. Although the approach tested in the present study failed to show promise, the public health implications of drug use among women who neither seek treatment nor disclose their use are too significant to ignore. Future efforts should consider the extent to which careful, prolonged messaging, or indirect interventions that are slightly more direct than that tested in this study, might promote self-change and/or treatment seeking. Alternately, individual brief interventions may need to be combined with, or replaced by, other approaches such as those that seek to exert population-level influence through regulatory, community-based, and prevention-focused means.
Highlights.
Under-reporting of perinatal drug use significantly reduces reach
A new indirect screener has shown promise in identifying risk
Computer-delivered, indirect brief intervention was not efficacious
Acknowledgments
Role of Funding Source
This work was supported by the National Institutes of Health (DA029050) and by Helene Lycacki/Joe Young Sr. funds from the State of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
SJO conceptualized this study, was the PI of the grant that funded this trial, was the primary author of the indirect intervention, and wrote the first draft of this paper. DSS provided substantive guidance and feedback in the design of the trial and of the indirect intervention, and contributed to revisions of this paper. CT served as the initial project coordinator and also contributed to revisions of this paper. KR provided substantive guidance and feedback in the design of the intervention and contributed revisions of this paper. JRB served as the second project coordinator and assisted with data analysis and revisions to this paper. JJ was the primary statistician and also contributed revisions. KP contributed to conceptualization of this study as well as to the design of the intervention, assisted with study management and participant recruitment, and contributed revisions to this paper. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed and the manuscript has been approved by all of us.
Conflict of Interest
Dr. Ondersma is part-owner of the company that markets the intervention authoring software used to develop the computer-delivered indirect intervention used in this trial. The remaining authors have no conflicts of interest to disclose.
References
- Ambroggio L, Smith MJ, Shah SS. Editorial commentary: Quasi-experimental and interrupted time-series design. J Pediatric Infect Dis Soc. 2012;1:187–189. doi: 10.1093/jpids/pis059. [DOI] [PubMed] [Google Scholar]
- Beatty JR, Chase SK, Ondersma SJ. A randomized study of the effect of anonymity, quasi-anonymity, and Certificates of Confidentiality on postpartum women’s disclosure of sensitive information. Drug Alcohol Depend. 2014;134:280–284. doi: 10.1016/j.drugalcdep.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Besinger BA, Garland AF, Litrownik AJ, Landsverk JA. Caregiver substance abuse among maltreated children placed in out-come-home care. Child Welfare. 1999;78:221–239. [PubMed] [Google Scholar]
- Bischof G, Rumpf HJ, Hapke U, Meyer C, John U. Types of natural recovery from alcohol dependence: A cluster analytic approach. Addiction. 2003;98:1737–1746. doi: 10.1111/j.1360-0443.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Donovan DM, Mandler RN, Perl HI, Forcehimes AA, Crandall C, Lindblad R, Oden NL, Sharma G, Metsch L, Lyons MS, McCormack R, Macia-Konstantopolous W, Douaihy A. Brief intervention for patients with problematic drug use presenting in emergency departments: A randomized clinical trial. JAMA Intern Med. 2014;174:1736–1745. doi: 10.1001/jamainternmed.2014.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Burman S. The challenge of sobriety: natural recovery without treatment and self-help groups. J Subst Abuse. 1997;9:41–61. doi: 10.1016/s0899-3289(97)90005-5. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS. 1991;5:181–185. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Epstein EE, Drapkin ML, Yusko DA, Cook SM, McCrady BS, Jensen NK. Is alcohol assessment therapeutic? Pretreatment change in drinking among alcohol-dependent women. J Stud Alcohol. 2005;66:369–378. doi: 10.15288/jsa.2005.66.369. [DOI] [PubMed] [Google Scholar]
- Fogg BJ. Persuasive Technology: Using Computers to Change What We Think and Do. San Francisco: Morgan Kaufmann; 2002. [Google Scholar]
- Grekin ER, Svikis DS, Lam P, Connors V, Lebreton JM, Streiner DL, SMoth C, Ondersma SJ. Drug use during pregnancy: Validating the Drug Abuse Screening Test against physiological measures. Psychol Addict Behav. 2010;24:719–723. doi: 10.1037/a0021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RF, Self-Brown S, Fricker-Elhai A, Kilpatrick DG, Saunders BE, Resnick H. Relations among parental substance use, violence exposure and mental health: The national survey of adolescents. Addict Behav. 2006a;31:1988–2001. doi: 10.1016/j.addbeh.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Hanson RF, Self-Brown S, Fricker-Elhai AE, Kilpatrick DG, Saunders BE, Resnick HS. The relations between family environment and violence exposure among youth: Findings from the national survey of adolescents. Child Maltreat. 2006b;11:3–15. doi: 10.1177/1077559505279295. [DOI] [PubMed] [Google Scholar]
- Horton NJ, Lipsitz SR. Multiple Imputation in Practice: Comparison of Software Packages for Regression Models with Missing Variables. The American Statistician. 2001;55:244–254. http://dx.doi.org/10.1198/000313001317098266. [Google Scholar]
- Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, Heather N, Saunders J, Burnand B. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007:CD004148. doi: 10.1002/14651858.CD004148.pub3. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Dreifuss JA, Weiss RD, Morgenstern J, Carroll KM. The Short Inventory of Problems – Revised (SIP-R): Psychometric properties within a large, diverse sample of substance use disorder treatment seekers. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2013;27(1):307–314. doi: 10.1037/a0028445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D Depression Symptoms Index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Kypri K, Langley JD, Saunders JB, Cashell-Smith ML. Assessment may conceal therapeutic benefit: Findings from a randomized controlled trial for hazardous drinking. Addiction. 2007;102:62–70. doi: 10.1111/j.1360-0443.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- Langford AT, Resnicow K, Davis RE, Alexander GL, Calvi J, Weise C, Tolsma D. Ethnic Identity predicts loss-to-follow-up in a health promotion trial. Contemp Clin Trials. 2010;31:414–418. doi: 10.1016/j.cct.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JT, Oshri A, Lynch M, Herzog M, Wortel S. Child neglect and the development of externalizing behavior problems: Associations with maternal drug dependence and neighborhood crime. Child Maltreat. 2013;18:17–29. doi: 10.1177/1077559512464119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J, Kypri K. Can Simply Answering Research Questions Change Behaviour? Systematic Review and Meta Analyses of Brief Alcohol Intervention Trials. In: McCulloch P, editor. PLoS ONE. 10. Vol. 6. 2011. p. e23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing People for change. Second. New York: Guilford; 2002. [Google Scholar]
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: A meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies. Substance use among women during pregnancy and following childbirth. The NSDUH Rep; 2009. Retrieved from http://www.samhsa.gov/data/2k9/135/PregWoSubUse.htm. [Google Scholar]
- Ondersma SJ. Predictors of neglect within low-SES families: The importance of substance abuse. Am J Orthopsychiatry. 2002;72:383–391. doi: 10.1037/0002-9432.72.3.383. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Preliminary evaluation of the acceptability and efficacy of a computer-based brief motivational intervention for perinatal drug use. Poster presentation, 66th Annual Scientific Meeting of the College of Problems on Drug Dependence; San Juan, Puerto Rico. June 2004.2004. [Google Scholar]
- Ondersma SJ, Delaney-Black V, Covington CY, Nordstrom B, Sokol RJ. The association between caregiver substance abuse and self-reported violence exposure among young urban children. J Trauma Stress. 2006;19:107–118. doi: 10.1002/jts.20105. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, LeBreton JM, Streiner DL, Grekin ER, Lam PK, Connors-Burge V. Development and preliminary validation of an indirect screener for drug use in the perinatal period. Addiction. 2012;107:2099–2106. doi: 10.1111/j.1360-0443.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med. 2007;32:231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Thacker LR, Beatty JR, Lockhart N. Computer-delivered screening and brief intervention (e-SBI) for postpartum drug use: A randomized trial. J Subst Abuse Treat. 2014;46:52–59. doi: 10.1016/j.jsat.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Winhusen T, Lewis DF. Pre-treatment change in a randomized trial with pregnant substance-abusing women in community-based outpatient treatment. Contemp Clin Trials. 2012;33:1074–1079. doi: 10.1016/j.cct.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Jr, Knapp DK, Tannenbaum L, Ostrea AR, Romero A, Salari V, Ager J. Estimates of illicit drug use during pregnancy by maternal interview, hair analysis, and meconium analysis. J Pediatr. 2001;138:344–348. doi: 10.1067/mpd.2001.111429. [DOI] [PubMed] [Google Scholar]
- Petty RE, Cacioppo JT. Communication and persuasion: Central and peripheral routes to attitude change. New York: Springer-Verlag; 1986. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- Richters JE, Martinez P. The NIMH community violence project: I. Children as victims of and witnesses to violence. Psychiatry. 1993a;56:7–21. doi: 10.1080/00332747.1993.11024617. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, West I, Maynard C, Atkins DC, Graves MC, Joesch JM, Zarkin GA. Brief intervention for problem drug use in safety-net primary care settings: A randomized clinical trial. JAMA. 2014;312:492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, Samet JH. Screening and brief intervention for drug use in primary care: The ASPIRE randomized clinical trial. JAMA. 2014;312:502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeeth L, Ebrahim S. Commentary: DINS, PINS, and things–clinical and population perspectives on treatment effects. Br Med J. 2000;321:952–953. [Google Scholar]
- Sobell LC, Ellingstad TP, Sobell MB. Natural recovery from alcohol and drug problems: Methodological review of the research with suggestions for future directions. Addiction. 2000;95:749–764. doi: 10.1046/j.1360-0443.2000.95574911.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline FollowBack: A calendar method for assessing alcohol and drug use. Toronto, Ontario: Addiction Research Foundation; 1996. [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2) - Development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- Suglia SF, Ryan L, Wright RJ. Creation of a Community Violence Exposure Scale: Accounting for What, Who, Where, and How Often. Journal of traumatic stress. 2008;21(5):479–486. doi: 10.1002/jts.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K. Substance abuse and child maltreatment. Pediatr Clin North Am. 2009;56:345–362. doi: 10.1016/j.pcl.2009.01.006. [DOI] [PubMed] [Google Scholar]
- West R, Sohal T. “Catastrophic” pathways to smoking cessation: Findings from national survey. BMJ. 2006;332:458–460. doi: 10.1136/bmj.38723.573866.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]


