Abstract
Background
We evaluated the cost-effectiveness of a hepatitis C (HCV) screening and active linkage to care intervention in US methadone maintenance treatment (MMT) patients using data from a randomized trial conducted in New York City and San Francisco.
Methods
We used a decision analytic model to compare 1) no intervention; 2) HCV screening and education (control); and 3) HCV screening, education, and care coordination (active linkage intervention). We also explored an alternative strategy wherein HCV/HIV co-infected participants linked elsewhere. Trial data include population characteristics (67% male, mean age 48, 58% HCV infected) and linkage rates. Data from published sources include treatment efficacy and HCV re-infection risk. We projected quality-adjusted life years (QALYs) and lifetime medical costs using an established model of HCV (HEP-CE). Incremental cost-effectiveness ratios (ICERs) are in 2015 US$/QALY discounted 3% annually.
Results
The control strategy resulted in a projected 35% linking to care within 6 months and 31% achieving sustained viral response (SVR). The intervention resulted in 60% linking and 54% achieving SVR with an ICER of $24,600/QALY compared to no intervention from the healthcare sector perspective and was a more efficient use of resources than the control strategy. The intervention had an ICER of $76,500/QALY compared to the alternative strategy. From a societal perspective, the intervention had a net monetary benefit of $511,000–$975,600.
Conclusions
HCV care coordination interventions that include screening, education and active linkage to care in MMT settings are likely cost-effective at a conventional $100,000/QALY threshold for both HCV mono-infected and HIV co-infected patients.
Keywords: Hepatitis C, Methadone Maintenance Therapy, Cost-Effectiveness
1. Introduction
Hepatitis C virus (HCV) is now the leading cause of infectious disease deaths in the United States, exceeding HIV-related deaths and the top 60 infectious diseases combined (Ly et al., 2016). HCV is often transmitted through injection drug use and is highly prevalent among methadone maintenance treatment program (MMT) patients (Hagan et al., 2011). The National Viral Hepatitis Strategy has identified people who inject drugs as a priority population for HCV treatment to reduce HCV prevalence and prevent re-infection (Wolitski and Dan, 2016). The strategy calls for developing programs that test and educate people who use drugs and are at risk for viral hepatitis, and link those who are positive for HCV to viral hepatitis care and treatment. The strategy also identifies people living with HIV as a priority population for HCV testing and diagnosis due to higher liver-related mortality rate among HCV/HIV co-infected populations. Onsite screening for HCV in substance use disorder treatment programs can be cost-effective (Schackman et al., 2015), but onsite testing is rare (Frimpong, 2013) and many programs that test onsite rely on passive referrals to HCV treatment and evaluation; few evidence-based models exist for active linkage to care after receiving a positive test result in this setting. A hepatitis care coordination program, evaluated in a randomized trial conducted in MMT programs in San Francisco, CA and New York City, NY, was found to show efficacy for linkage to HCV care (Masson et al., 2013). Among HCV-antibody positive participants, those receiving the screening, education, and care coordination intervention were significantly more likely to receive an HCV evaluation within 6 months than those receiving screening and education alone (Masson et al., 2013).
We evaluated the cost-effectiveness of this screening, education, and care coordination intervention using data from this trial, including intervention efficacy and resources used to deliver the intervention, an established computer simulation model of HCV disease to project lifetime quality-adjusted life year (QALY) and cost outcomes. We report cost-effectiveness results from the healthcare sector perspective and the societal perspective, following recent cost-effectiveness guidelines (Neumann et al., 2016).
2. Methods
2.1 Analytic overview
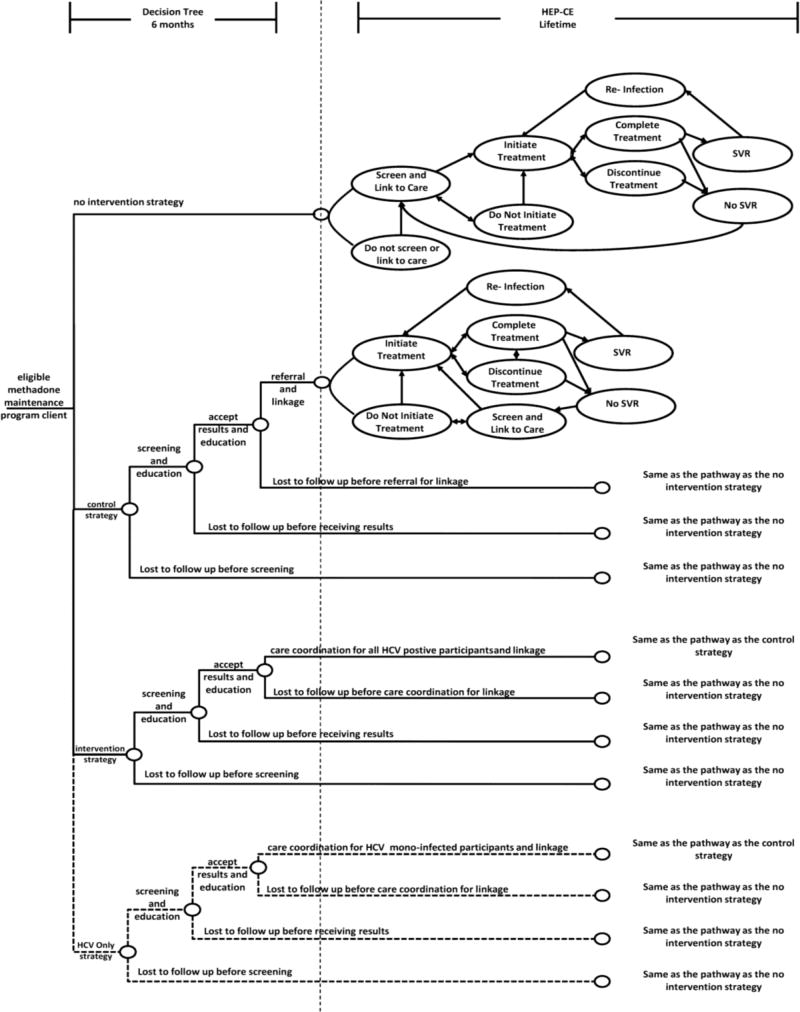
We compared the cost-effectiveness of HCV screening and care linkage strategies in an MMT setting for individuals meeting the HCV-related trial entry criteria. We initially evaluated three strategies: (i) no intervention; (ii) HCV screening and education (control); and (iii) HCV screening and education for all plus care coordination (i.e., active linkage to care) for all HCV-infected patients (intervention). In a secondary analysis, we added a fourth strategy, HCV screening and education for all and care coordination only for HCV mono-infected patients (HCV only strategy). The fourth strategy was evaluated to explore trial results, indicating that many HCV/HIV co-infected participants in the control arm were successfully linked to HCV care, presumably through other systems of care available to HIV-infected individuals. For each strategy, a decision tree decision analytic model (Petrou and Gray, 2011) describes test acceptance, receipt of results, and linkage to care (Figure 1). HCV antibody test and RNA test sensitivity and specificity are included in the model (Table 1). The decision analytic model was programmed in TreeAge Pro version 2016 (Williamstown, MA, USA).
Figure 1.
Analysis Overview
Table 1.
Cohort characteristics and analysis inputs
| Input | Base Case | Sensitivity Analysis | Source | ||
|---|---|---|---|---|---|
| Cohort characteristics | |||||
| Mean age (SD), years | 48 (9) | 38 (9) – 58 (9) | Linkage Trial | ||
| Mean age of infection (SD), years | 26 (16) | 16 (16) – 36 (16) | (Freeman, Dore et al. 2001) | ||
| Proportion male | 0.673 | 0 – 1 | Linkage Trial | ||
| Proportion HIV-infecteda | 0.104 | 0 – 1 | Linkage Trial | ||
| Proportion HCV antibody-positiveb | 0.581 | 0.240 – 0.760 | Linkage Trial (2004; Masson, Delucchi et al. 2013; Nolan, Dias Lima et al. 2014) | ||
| Proportion of HCV antibody-positive with chronic HCV-infection | |||||
| HIV uninfected | 0.740 | 0.710 – 0.780 | (Micallef, Kaldor et al. 2006; Denniston, Jiles et al. 2014) | ||
| HIV-infected | 0.870 | 0.800 – 0.870 | (Soriano, Mocroft et al. 2008; Schnuriger, Dominguez et al. 2009) | ||
| Proportion by HCV genotype | |||||
| Genotype 1 | 0.770 | -- | (Messina, Humphreys et al. 2015) | ||
| Genotype 2 | 0.130 | -- | (Messina, Humphreys et al. 2015) | ||
| Genotype 3 | 0.100 | -- | (Messina, Humphreys et al. 2015) | ||
| Proportion with current IDU risk behavior | |||||
| HCV- uninfected | 0.200 | 0 – 1 | Linkage Trial | ||
| HCV- and/or HIV-infected | 0.560 | 0.230 – 1 | Linkage Trial (Masson, Delucchi et al. 2013; Butner, Gupta et al. 2017) | ||
| Linkage to HCV care by onsite screening and linkage strategy | |||||
| Control | |||||
| HIV uninfected | 0.336 | 0.247 – 0.426 | Linkage Trial | ||
| HIV co-infected | 0.786 | 0.540 – 1 | Linkage Trial | ||
| Intervention | |||||
| HIV uninfected | 0.661 | 0.586 – 0.766 | Linkage Trial | ||
| HIV co-infected | 0.800 | 0.732 – 1 | Linkage Trial | ||
| Background Testing and Subsequent Linkage to Care by HIV status | |||||
| Off-site HCV testing, monthlyc | 0.0420 | 0.0160 – 0.1680 | Linkage Trial | ||
| Linkage, monthlyd | |||||
| HIV uninfected | 0.0014 | 0.0011 – 0.0020 | Linkage Trial | ||
| HIV-infected | 0.0042 | 0.0026 – 0.0058 | Linkage Trial | ||
| Onsite Screening, Education and Linkage costs ($) | |||||
| Control Arm | |||||
| Program | 201 | -- | Linkage Trial | ||
| Patiente | 14 | -- | Linkage Trial | ||
| Intervention Arm | |||||
| Program | 885 | -- | Linkage Trial | ||
| Patiente | 28 | -- | Linkage Trial | ||
| HCV Disease progression and Re-infection | |||||
| Median time to cirrhosis from time of HCV infection, years | 25 | 10 – 40 | (Poynard, Bedossa et al. 1997; Thein, Yi et al. 2008) | ||
| Median time to first liver-related event after developing cirrhosis, years | 11 | 6 – 19 | (Bruno, Zuin et al. 2009) | ||
| Liver-related mortality with compensated cirrhosis, deaths per 100 PYs | 1.39 | 0.96 – 1.82 | (Bruno, Zuin et al. 2009) | ||
| Liver-related mortality with decompensated cirrhosis, deaths per 100 PYs | 12.00 | 8.28 – 15.72 | (Bruno, Zuin et al. 2009) | ||
| Multiplier for liver cirrhosis progression for HIV-infected | 2.1 | 1.0 – 3.0 | (Them, Yi et al. 2008) | ||
| Reduction in liver-mortality after SVR, % | 94 | 81 – 98 | (van der Meer, Veldt et al. 2012) | ||
| HCV reinfection rate among current IDUs, reinfections per 100 PYs | 4.6 | 1.7 – 10.0 | (Dore, Altice et al. 2016) | ||
| Test characteristics | |||||
| HCV-antibody, laboratory-based test | |||||
| Sensitivity | 0.989 | 0.916 – 1 | (Colin, Lanoir et al. 2001; Smith, Teshale et al. 2011) | ||
| Specificity | 0.998 | 0.929– 1 | (Morota, Fujinami et al. 2009; Smith, Teshale et al. 2011) | ||
| HCV RNA | |||||
| Sensitivity | 0.960 | 0.950 – 1 | (Gerken, Pontisso et al. 1996; Albadalejo, Alonso et al. 1998) | ||
| Specificity | 0.993 | 0.980 – 1 | (Gerken, Pontisso et al. 1996; Albadalejo, Alonso et al. 1998) | ||
| HCV treatment efficacy and cost | |||||
| Treatment efficacy | |||||
| without cirrhosis | 0.91 | 0.86 – 0.95 | (Dore, Altice et al. 2016) | ||
| with cirrhosis | 0.93 | 0.80 – 0.98 | (Dore, Altice et al. 2016) | ||
| Total treatment cost, $f | |||||
| ribavirin | 400 | 300 – 500 | (Truven Health Analytics 2016; Veterans Affairs Office of Acquisition and Logistics (OAL) 2016) | ||
| elbasvir/grazoprevir | 54,600 | 39,500 – 74,800 | (Truven Health Analytics 2016; Veterans Affairs Office of Acquisition and Logistics (OAL) 2016) | ||
| sofosbuvir/velpatasvir | 74,800 | 49,900 – 74,800 | (Truven Health Analytics 2016; Veterans Affairs Office of Acquisition and Logistics (OAL) 2016) | ||
| Quality of life weights (0 = death, 1 = best possible health) | |||||
| Methadone Maintenance Treatment | 0.72 | 0.69 – 0.76 | (Wittenberg, Bray et al. 2016) | ||
| HCV-infected | |||||
| No to moderate fibrosis | 0.89 | 0.75 – 1 | (Stein, Dalziel et al. 2002; Chong, Gulamhussein et al. 2003; Grieve, Roberts et al. 2006) | ||
| Cirrhosis | 0.62 | 0.55 – 0.75 | (Stein, Dalziel et al. 2002; Chong, Gulamhussein et al. 2003; Grieve, Roberts et al. 2006) | ||
| Decompensated cirrhosis | 0.48 | 0.40 – 0.60 | (Stein, Dalziel et al. 2002; Chong, Gulamhussein et al. 2003; Grieve, Roberts et al. 2006) | ||
| HIV-infected | 0.87 | 0.74 – 1 | (Schackman, Goldie et al. 2002) | ||
| On HCV treatment | |||||
| Receiving interferon-sparing therapyg | 0.99 | 0.95 – 1 | (Stepanova, Nader et al. 2014) | ||
| Major toxicity decrementh | 0.16 | 0.09 – 0.25 | (Schackman, Teixeira et al. 2008) | ||
| Non-HCV, non-HIV mortality risk | |||||
| Standardized Mortality Rate | 1.8 | 1.0 – 5.0 | (Evans, Li et al. 2015) | ||
PY= person years; SVR= sustained virological response; SD= standard deviation; IDU=injection drug use
Note: all costs are in 2015 US dollars.
78% of whom are also HCV-antibody positive in the base case (varied in sensitivity analysis between 0% and 100%)
14% of whom are also HIV-infected in the base case (varied in sensitivity analysis between 0% and 100%)
Assumes approximately 66% are tested by 2.08 years in the base case (varied in sensitivity analysis between 33% and 99%)
Monthly probability is calculated in order to reach half of the linkage rate observed in the control group within 10 years; sensitivity analysis probability range reflects sensitivity analysis ranges for control group linkage rates in one-way sensitivity analyses.
Patient costs are only included in the societal perspective
The medication costs from the Federal Supply Schedule fall within the range of the sensitivity analyses: ribavirin $400; elbasvir/grazoprevir $52,100; sofosbuvir/velpatasvir $56,000
Multiplied by patient's quality of life weight during the months the patient receives HCV therapy
Subtracted from patient's health state utility during the month of a major toxicity event
Subsequent outcomes including sustained viral response (SVR), the possibility of HCV re-infection (Figure 1), and lifetime outcomes were projected using an established computer simulation model of HCV disease and treatment (Hepatitis C Cost-Effectiveness model: HEP-CE) (Linas et al., 2017). The HEP-CE model simulates chronic HCV disease progression through three stages of liver disease: mild to moderate fibrosis, cirrhosis, and decompensated cirrhosis. Each disease stage is associated with a decrease in quality of life and an increase in healthcare costs (Table 1). If simulated individuals with chronic HCV become cirrhotic, they have an increased risk of mortality attributable to their liver disease. Simulated individuals without chronic HCV infection at baseline have a probability of HCV infection; simulated individuals who achieve SVR after treatment have a probability of re-infection if they engage in injection drug use risk behavior. Simulated individuals who are HIV-infected or HCV/HIV co-infected are assigned HIV-attributable mortality, HIV-related health-care costs and quality of life weights (Table 1). All simulated individuals in the model have an elevated mortality risk (standardized mortality rate) compared to the general population (Evans et al., 2015). The model has been validated by comparing HCV natural history all-cause mortality from a simulated cohort of patients to data from long-term observational studies (Linas et al., 2016). To ensure stability of results, the HEPCE model was simulated with cohorts of 1 million individuals.
Outcomes include lifetime costs (2015 US$) and quality-adjusted life years (QALYs), both discounted at 3% annually. Costs estimated from the health sector perspective include costs to MMT programs (which may or may not be reimbursed), downstream costs for HCV and HIV healthcare, and unrelated healthcare costs (Supplemental Tables 1–4)1. Incremental cost-effectiveness ratios (ICERs) are calculated from the healthcare sector perspective as the additional cost per QALY gained compared to the next least expensive strategy after eliminating strategies due to dominance (when one strategy is more effective and costs less) or extended dominance (when a combination of alternative strategies is a more efficient use of resources than the dominated strategy) (Neumann et al., 2017).
As recommended by recent cost-effectiveness guidelines (Neumann et al., 2017), we used an impact inventory to consider potential impacts of the intervention outside of the healthcare sector (Supplemental Table 5)2. We assigned a societal willingness-to-pay of $100,000/QALY, a commonly used threshold in the United States that more appropriately reflects contemporary medical costs than the $50,000/QALY threshold used in earlier studies (Braithwaite et al., 2008; Neumann et al., 2014). We calculated net monetary benefit for each strategy from the societal perspective. Net monetary benefit uses the willingness to pay threshold to convert QALY benefits to monetary units, then subtracts the cost of the intervention to determine the net monetary benefit (Neumann et al., 2016). For the societal perspective analysis, we also assigned costs to the participants for their time spent during the intervention (education, testing, and care coordination) (Table 1), and included future productivity and consumption effects. Results are reported calculating productivity effects, both assuming national labor force participation rates (U.S. Bureau of Labor Statistics, 2015a) for all individuals and assuming the average labor force participation rate reported by trial participants (16.3%) for individuals under age 65. The Weill Cornell Medical College institutional review board (IRB) approved the cost-effectiveness analysis; sites obtained approval from their IRBs to conduct the randomized controlled trial and to share data.
2.2 Trial data
Eligible participants were at least 18 years old, reported being either HCV antibody negative, of unknown HCV status or, if HCV positive, with no prior medical care or diagnostic evaluation for HCV (liver biopsy, viral load test, genotype test, liver imaging) and willing to participate in all study-related activities (Masson et al., 2013). The trial was conducted in February 2008 to June 2011. A total of 489 participants were randomized across both sites; 244 were assigned to the intervention group and 245 were assigned to the control group. Only results from individuals with complete HIV and HCV status information were included in this analysis resulting in a total sample of 480 (232 from the intervention group; 226 from the control group).
Consenting participants were tested for Hepatitis A, B, and C, and for HIV. If the participant tested positive for HCV antibodies, HCV linkage referrals were made to an affiliated clinical setting where an HCV RNA test and liver staging would be conducted (Masson et al., 2013). Participants who, on the basis of serological tests results, were susceptible to Hepatitis A or Hepatitis B or both were offered combination vaccine onsite at the MMT program (Masson et al., 2013). Both the intervention and control group received individual 2-session manual guided HIV and viral hepatitis counseling and education administered by research staff (Masson et al., 2013). The intervention group received education sessions delivered with motivational interviewing and motivational interviewing-enhanced case management assistance with off-site HCV evaluation for 6 months (Masson et al., 2013). Case management sessions were held weekly.
2.3 Model inputs
Cohort characteristics including age, proportion male, proportion HIV-infected, proportion with HCV and proportion currently injecting drugs were from trial participants (Table 1). Individuals in a hypothetical no intervention strategy were assigned a probability of being tested for HCV outside of the intervention (background testing) based on the median time reported since the most recent HCV test by HCV-uninfected trial participants. Individuals in the no intervention strategy who were tested outside of the intervention and tested positive were then assigned the same probabilities of linking to care and successful treatment as in the control strategy. In the remaining strategies, model inputs were derived from the trial in which all participants received an HCV antibody test, 97% returned for an educational session and receipt of results, and 58% tested positive (50% HCV mono-infected, 8% HCV/HIV co-infected). For those testing positive, model inputs for average age at the time of HCV infection, proportions with chronic HCV for HCV mono-infected and HCV/HIV co-infected individuals, and proportions of individuals with chronic HCV by genotype were derived from the literature (Table 1).
The proportions linking to HCV care within 6 months in the control and intervention strategies for HCV mono-infected and HCV/HIV co-infected individuals with chronic infection were derived from the trial data (Table 1). Linkage to care within 6 months was measured from the date of receiving a referral to care. One individual had a referral date after the linkage to care date; this was remedied by measuring linkage to care from the second education session date. We assumed that individuals who successfully linked to care would be treated with direct acting antivirals. Treatment success (sustained viral suppression, SVR) and risk of HCV re-infection for those currently injecting drugs were derived from a recent trial of HCV treatment in persons receiving opioid agonist therapy (Dore et al., 2016). If individuals did not link to HCV care, they were assigned a monthly probability of subsequently engaging in care (Table 1).
The cost of phlebotomy, HCV antibody tests, HIV antibody tests and an HCV evaluation visit were estimated using the appropriate Medicare reimbursement codes (Supplemental Table 13; Tumeh et al., 2005). Using session time and utilization data from the trial databases, we calculated the cost of the average educational session and the average case management session (Supplemental Table 2)3. We determined the labor cost of each session by assigning the relevant wage and fringe benefit rates from the Bureau of Labor Statistics to the time estimates for each encounter (Supplemental Table 13; U.S. Bureau of Labor Statistics, 2015b). The cost of patient time from the societal perspective for each encounter was calculated similarly using the national minimum wage. Additional costs were estimated based on interviews with investigators and site staff including labor costs for visit documentation, supervision, weekly case conferencing, and training (Supplemental Table 3)3. Site start-up costs were also estimated separately, including training, equipment, and inventory (participant support materials including transportation tokens) (Supplemental Table 6)3.
The cost of HCV medications in the base case was derived from the wholesale acquisition cost (WAC; Truven Health Analytics, 2016), with the recommended Federal Supply Schedule cost included in the sensitivity analysis ranges (Neumann et al., 2017). In calculating these costs, individuals with HCV genotype 1 were assumed to be treated with elbasvir/grazoprevir, those with HCV genotypes 2 and 3 were assumed to be treated with sofosbuvir/velpatasvir, and those with decompensated cirrhosis were assumed to be treated with additional ribavirin (Table 1; Supplemental Table 13). Treatment duration depended on presence of cirrhosis. We conservatively assigned the same treatment efficacies reported in the trial of elbasvir/grazoprevir in persons receiving opioid agonist therapy (Dore et al., 2016) to all assumed regimens across all genotypes even though other trials report higher efficacies for the sofosbuvir/velpatasvir treatment regimen (Grebely et al., 2016).
2.4 Sensitivity analysis
We evaluated the impact of two alternative scenarios on our results. First, we considered whether the quality-of-life impact of HCV treatment might differ for individuals in methadone maintenance treatment, whose overall quality of life is rated lower than that of individuals in the general population (Wittenberg et al., 2016). In an alternative scenario, we applied the minimum quality of life estimator, which may be a more accurate assessment of quality of life for multiple co-morbid conditions in this population (Wittenberg et al., 2017). In this scenario, the quality-of-life benefit from HCV treatment is observed only in the cirrhosis health state and subsequent stages of chronic HCV disease. Second, we considered that individuals who are linked to HCV care may not be able to initiate treatment due to treatment access restrictions based on disease stage (Barua et al., 2015; Roundtable, 2016). In this alternative scenario, HCV mono-infected individuals were assumed to be ineligible for treatment unless they have cirrhosis.
In one-way sensitivity analyses, we varied the following parameters: age, age of infection, sex, HCV prevalence, HIV prevalence, elevated mortality risk compared to the general population, chronic HCV disease progression rate, liver-related mortality rate, probability of off-site HCV testing, intervention efficacy (linkage to care), SVR rate, HCV treatment efficacies and costs, and HCV re-infection rate. In two-way sensitivity analyses, we simultaneously varied case management costs and linkage to care inputs.
We also constructed cost-effectiveness acceptability curves to graphically represent the uncertainty around our cost-effectiveness estimates for each linkage model (Fenwick et al., 2004). To calculate these results, we varied decision analytic model inputs simultaneously across relevant ranges (Table 1). Beta distributions were used for probability inputs derived from the randomized trial such as linkage and background testing. We used lognormal distributions for the costs of the education sessions for the intervention groups and for case management, and an exponential distribution for the costs of the education sessions for the control group; these distributions were determined by using the Akaike information criterion (AIC) to determine the best fit distributions. We used gamma distributions for treatment costs and quality of life inputs derived from the HEP-CE model. We applied uniform distributions for other inputs derived from the literature (Briggs et al., 2012; Neumann et al., 2017).
3. Results
3.1 Continuum of care
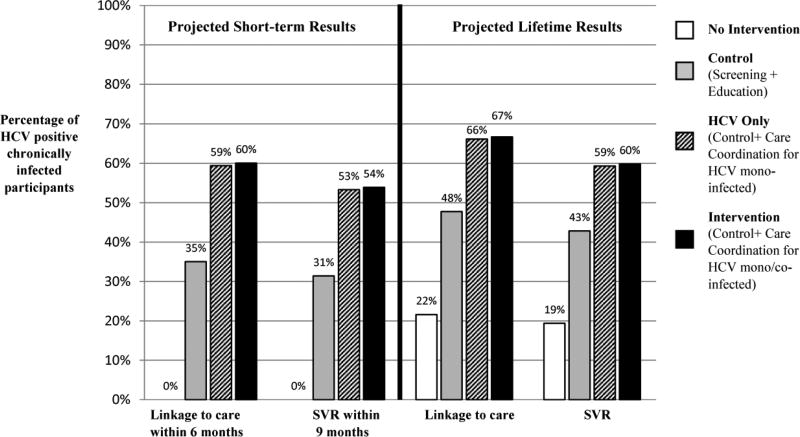
We projected short-term (within 6 months) and lifetime linkage to care outcomes and SVR outcomes within 9 months and over a lifetime (Figure 2). Although individuals not receiving the intervention have no immediate benefits, over a lifetime, 22% link to HCV care and 19% achieve SVR. In the control arm, 35% of individuals with chronic HCV infection link to care within 6 months and 31% achieved SVR within 9 months; ultimately 48% link to care over a lifetime, and 43% achieve SVR over a lifetime (Figure 2). In the intervention strategy, 60% of individuals with chronic HCV infection link to HCV care within 6 months and 54% achieve SVR within 9 months (Figure 2). Over a lifetime, 67% link to care and 60% achieve SVR. In the HCV only strategy, slightly fewer link to HCV care and achieve SVR at each time point.
Figure 2.
Continuum of Care Results
3.2 Cost-effectiveness
The control strategy results in a mean discounted lifetime cost of $139,900 per person and quality adjusted life expectancy of 7.299 QALYs, corresponding to an incremental lifetime $10,100 cost and 0.377 QALYS compared to no intervention (Table 2). The incremental costs include the costs of HCV testing ($66), pre-test education session 1 ($32) and post-test education session 2 ($30) (Supplemental Table 2). The intervention strategy results in an incremental lifetime cost of $7,300 and 0.328 QALYs compared to the control strategy. The intervention strategy is more efficient than the control strategy (as a result of extended dominance), and has an ICER of $24,600/QALY compared to no intervention. When the HCV only strategy is considered as a fourth alternative, this strategy is more efficient than the control strategy (also as a result of extended dominance) and has an ICER of $24,400/QALY compared to no intervention. The HCV mono/co-infected intervention strategy (i.e., intervention) has an ICER of $76,500/QALY compared to the HCV only strategy. Results are similar in the scenarios using the minimum quality of life estimator and the scenario restricting treatment access (Table 2).
Table 2.
Base Case and Scenario Analyses Results
| Strategy | Total cost per person ($) |
Total QALY per person |
Incremental cost per person ($) |
Incremental QALY per person |
Incremental cost-effectiveness ratio ($/QALY) |
|
|---|---|---|---|---|---|---|
| Base Case Scenario: 3 linkage models | ||||||
| Base Case Results | ||||||
| No Intervention | 129, 800 | 6.922 | --- | --- | --- | |
| Control Strategy | 139, 900 | 7.299 | 10, 100 | 0.377 | dominated | |
| Intervention | 147, 200 | 7.627 | 7, 300 | 0.328 | 24, 600 | |
| Quality of Life Minimum Estimator Scenario | ||||||
| No Intervention | 129, 800 | 7.310 | --- | --- | --- | |
| Control Strategy | 139, 900 | 7.602 | 10, 100 | 0.293 | dominated | |
| Intervention | 147, 200 | 7.852 | 7, 300 | 0.250 | 29,200 | |
| Early Stage Disease Treatment Restriction Scenarioa | ||||||
| No Intervention | 129,300 | 6.912 | --- | --- | --- | |
| Control Strategy | 138, 900 | 7.264 | 9,600 | 0.352 | dominated | |
| Intervention | 145, 600 | 7.569 | 6, 700 | 0.305 | 24,800 | |
| Scenario Analysis: 4 linkage models | ||||||
| Base Case Results | ||||||
| No Intervention | 129, 800 | 6.923 | --- | --- | --- | |
| Control Strategy | 139, 900 | 7.299 | 10, 100 | 0.377 | dominated | |
| HCV Only Strategy | 146,900 | 7.624 | 7, 000 | 0.324 | 24, 400 | |
| Intervention | 147,200 | 7.627 | 300 | 0.003 | 76,500 | |
| Quality of Life Minimum Estimator Scenario | ||||||
| No Intervention | 129, 800 | 7.310 | --- | --- | --- | |
| Control Strategy | 139, 900 | 7.602 | 10, 100 | 0.292 | dominated | |
| HCV Only Strategy | 146,900 | 7.849 | 7, 000 | 0.247 | 31,700 | |
| Intervention | 147,200 | 7.852 | 300 | 0.003 | 88,300 | |
| Early Stage Disease Treatment Restriction Scenarioa | ||||||
| No Intervention | 129, 300 | 6.912 | --- | --- | --- | |
| Control Strategy | 138, 800 | 7.264 | 9,500 | 0.352 | dominated | |
| HCV Only Strategy | 145, 300 | 7.565 | 6,500 | 0.302 | 24,500 | |
| Intervention | 145, 600 | 7.569 | 300 | 0.003 | 81,000 | |
QALY = Quality-Adjusted Life Year
Note: all costs are in 2015 US dollars.
Early treatment restriction represents delaying access to HCV treatment medication until developing cirrhosis (F3 metavir liver staging)
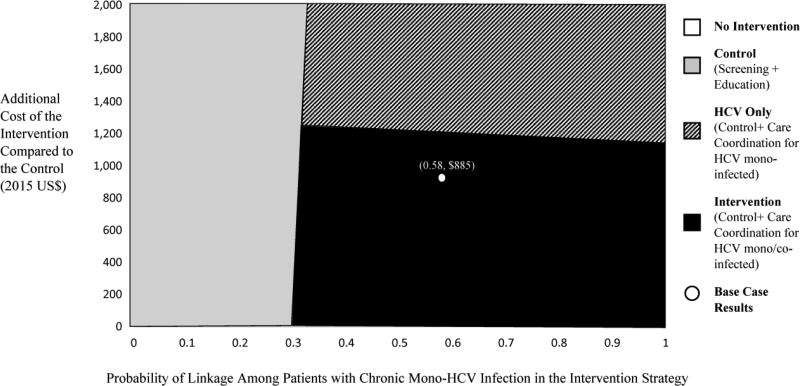
The control strategy remains inefficient in almost all one-way sensitivity analyses. In these analyses, the ICER for the intervention strategy compared to the no intervention strategy varies between $19,000/QALY when the HCV treatment cost is reduced and $34,400/QALY when the HCV re-infection rate is increased (Supplemental Table 8)4. When the HCV only strategy is considered, the ICER for providing the intervention to both HCV mono-infected and HCV/HIV co-infected individuals compared to this strategy remains <$100,000/ QALY in all one-way sensitivity analyses except when we assume the maximum increase in the standardized mortality rate for MMT patients. In one-way sensitivity analysis varying HCV prevalence, the intervention resulted in 1.23 additional QALYs and 2.55 unadjusted life years gained per HCV positive individual compared the no intervention strategy. In a two-way sensitivity analysis, when the proportion of individuals linking to care and intervention costs are varied for HCV mono-infected patients, the ICER for the intervention compared to no intervention remains below the $100,000/QALY as long as the intervention cost does not exceed approximately $1,200 (compared to $885 in the base case) and >31% of patients with chronic HCV infection link to care (compared to 58% in the base case) (Figure 3).
Figure 3.
Two Way Sensitivity Analysis of HCV Linkage to Care and Incremental Cost of the Intervention for HCV Mono-Infected Patients (Cost-Effectiveness Threshold $100,000/QALY)
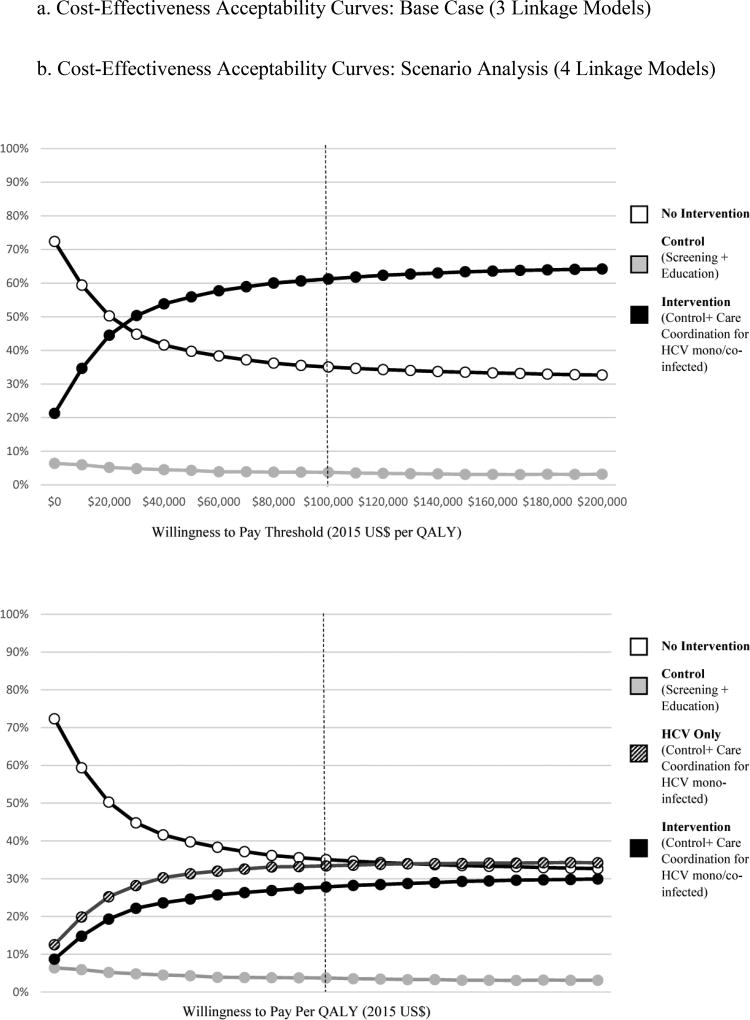
At a willingness-to-pay threshold of $100,000/QALY, the intervention is the preferred strategy in 61% of probabilistic sensitivity analyses comparing intervention, control and no intervention strategies (Figure 4a). When the HCV only strategy is also considered, the intervention strategy is preferred in 28% of probabilistic sensitivity analyses and the HCV only strategy is preferred in 33% of analyses (Figure 4b).
Figure 4.
a. Cost-Effectiveness Acceptability Curves: Base Case (3 Linkage Models)
b. Cost-Effectiveness Acceptability Curves: Scenario Analysis (4 Linkage Models)
The net monetary benefit from the societal perspective of the intervention is $975,600, assuming national labor force participation rates when determining productivity effects, and $511,000, assuming the average labor force participation rate reported by trial participants. The net monetary benefit for the control strategy using each of these approaches is $939,100 and $487,200 respectively, and for the HCV only strategy is $975,300 and $510,900.
4. Discussion
We evaluated the cost-effectiveness of a HCV screening and active linkage to care intervention in MMT patients using data from a randomized trial conducted in New York City and San Francisco. We found that the HCV screening, education, and care coordination intervention evaluated in the trial had a cost-effectiveness ratio of $24,600/QALY from the healthcare sector perspective, which is very attractive compared to a conventional $100,000/QALY threshold, and a more efficient use of resources than providing HCV testing and education alone. Although cost and QALY differences between individual strategies in some cases were small, results were consistent when we considered alternative scenarios regarding quality of life assumptions and access to HCV care, in one-way, two-way, and probabilistic sensitivity analyses, and when we considered the societal perspective. The low incremental costs and quality-adjusted life expectancies reflect the small proportion of individuals who are HCV-infected, accept the referral, link to care, initiate treatment and achieve SVR. In one-way sensitivity analyses varying HCV prevalence, we find that the benefit of the testing and linkage intervention among those with HCV is meaningful.
When we explored including an HCV only strategy based on trial results indicating that many HCV/HIV co-infected participants in the control arm were successfully linked to HCV care, implementing the intervention for all MMT patients without consideration of HIV co-infection status had a higher cost/effectiveness ratio but was still attractive at a $100,000/QALY threshold. This result should be interpreted with caution, however, because the other systems of care available to HIV-infected individuals in New York City and San Francisco at the time the trial was conducted that presumably assisted the control arm participants in linking to HCV care may not be available in other jurisdictions. In other locations, linkage results for HCV/HIV co-infected patients may be more similar to those of HCV mono-infected patients in the absence of a comprehensive linkage intervention. Trial data were also used to inform characteristics in the model, which may be different from the HCV patient population in other treatment settings. Our conclusions do not change, however, in sensitivity analyses varying age, and quality of life weights (all of which may vary with patient demographics).
Our analysis is subject to limitations. The clinical trial was conducted during the era of interferon-containing treatment regimens, which could have adversely affected linkage rates in both groups. Although trial results may not reflect linkage rates that can be achieved using current therapies, the subsequent steps along the continuum of care were derived from existing literature and our modeled outcomes reflect the efficacy of current directly-acting antiviral treatment. Although actual negotiated HCV medication costs are unknown, a low-cost scenario using prices for current direct-acting antivirals from the Federal Supply Schedule was included in sensitivity analysis ranges (Neumann et al., 2016). Our conclusions were not sensitive to treatment costs in one-way sensitivity analyses (Supplemental Table 7)5.
We conclude that HCV care coordination interventions that include screening, education and linkage to care in MMT settings are likely cost-effective at a conventional $100,000/QALY threshold for both HCV mono-infected and HIV co-infected patients and is preferred to screening and education alone. Funds invested in programs in these settings that only test and educate are better spent on programs that also assist with active linkage to HCV care. These interventions should be a priority for implementation of the National Viral Hepatitis Strategy.
Supplementary Material
Highlights.
Data are from a randomized trial of hepatitis C virus (HCV) care coordination in US methadone maintenance treatment (MMT) clinics.
HCV care coordination in MMT with active linkage to care is likely cost-effective.
This finding holds true for HCV mono-infected and HCV/HIV co-infected patients.
Acknowledgments
Role of Funding Source
This research was supported by the National Institute on Drug Abuse (P30 DA040500; R01 DA027379; R01 DA031059; R01 DA020781; R01 DA020841; P30 DA011041; P50 DA009253) and the National Institute of Allergy and Infectious Diseases (P30 AI042853). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies or the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi: ...
Contributors
Dr. Schackman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Schackman and Ms. Gutkind drafted the manuscript. Drs. Schackman, Perlman, Masson, and Linas devised the study concept and design. Data acquisition was conducted by Dr. Delucchi, Dr. McKnight, Dr. Perlman, and Dr. Masson. All authors conducted data analysis and interpretation, and conducted critical revisions of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Conflict of Interest
None
References
- Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann. Intern. Med. 2015;163:215–223. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Meltzer DO, King JTJ, Douglas L, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med. Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health. 2012;15:835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, Leutkemeyer A, Nahass R, Peng C-Y, Conway B, Grebely J, Howe AYM, Gendrano IN, Chen E, Huang H-C, Dutko FJ, Nickle DC, Nguyen B-Y, Wahl J, Barr E, Robertson MN, Platt HL. Elbasvir–Grazoprevir to treat hepatitis c virus infection in persons receiving opioid agonist therapy: A randomized trial. Ann. Intern. Med. 2016;165:625–634. doi: 10.7326/M16-0816. [DOI] [PubMed] [Google Scholar]
- Evans E, Li L, Min J, Huang D, Urada D, Liu L, Hser YI, Nosyk B. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction. 2015;110:996–1005. doi: 10.1111/add.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves--facts, fallacies and frequently asked questions. Health Econ. 2004;13:405–415. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- Frimpong JA. Missed opportunities for hepatitis C testing in opioid treatment programs. Am. J. Public Health. 2013;103:1028–1030. doi: 10.2105/AJPH.2012.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Dore GJ, Zeuzem S, Aspinall RJ, Fox R, Han L, McNally J, Osinusi A, Brainard DM, Subramanian GM, Natha M, Foster GR, Mangia A, Sulkowski M, Feld JJ. Efficacy and safety of Sofosbuvir/Velpatasvir in patients with chronic hepatitis C virus infection receiving opioid substitution therapy: Analysis of phase 3 ASTRAL trials. Clin. Infect. Dis. 2016;63:1479–1481. doi: 10.1093/cid/ciw579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J. Infect. Dis. 2011;204:74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linas BP, Morgan JR, Pho MT, Leff JA, Schackman BR, Assoumou SA, Horsburgh CR, Weinstein MC, Freedberg KA, Kim AY. Cost effectiveness and cost containment in the era of interferon-free therapies to treat hepatitis c virus genotype 1. Open Forum Infect. Dis. 2016:1–11. doi: 10.1093/ofid/ofw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linas BP, Morgan JR, Pho MT, Leff JA, Schackman BR, Horsburgh CR, Assoumou SA, Salomon JA, Weinstein MC, Freedberg KA, Kim AY. Cost effectiveness and cost containment in the era of interferon-free therapies to treat hepatitis C virus genotype 1. Open Forum Infect. Dis. 2017;4:ofw266–ofw266. doi: 10.1093/ofid/ofw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis c virus in the United States, 2003–2013. Clin. Infect. Dis. 2016;10:1287–1288. doi: 10.1093/cid/ciw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson CL, Delucchi KL, McKnight C, Haettema J, Khalili M, Min A, Jordan AE, Pepper N, Hall J, Hengle NS, Young C, Shopshire MS, Manuel JK, Coffin L, Hammer H, Shapiro B, Seewald RM, Bodenheimer HC, Sorenson JL, Des Jarliais DC, Perlman DC. A randomized trial of a hepatitis care coordination model in methadone maintenance treatment. Am. J. Public Health. 2013;103:e81–e85. doi: 10.2105/AJPH.2013.301458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness — The curious resilience of the $50,000-per-QALY threshold. N. Engl. J. Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- Neumann PJ, Ganiats TG, Russell LB, Sanders GD, Siegel JE. Cost-effectiveness in health and medicine. second. Oxford University Press; New York, NY: 2017. [Google Scholar]
- Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine. second. Oxford University Press; New York, NY USA: 2016. [Google Scholar]
- Petrou S, Gray A. Economic evaluation using decision analytical modelling: Design, conduct, analysis, and reporting. BMJ. 2011;11 doi: 10.1136/bmj.d1766. [DOI] [PubMed] [Google Scholar]
- Roundtable NVH. [Accessed January 19, 2018];Heptatis C: The state of medicaid access preliminary findings national summary report. 2016 Online. http://www.chlpi.org/wp-content/uploads/2013/12/HCV-Report-Card-National-Summary_FINAL.pdf.
- Schackman BR, Leff JA, Barter DM, DiLorenzo MA, Feaster DJ, Metsch LR, Freedberg KA, Linas BP. Cost-effectiveness of rapid hepatitis C virus (HCV) testing and simultaneous rapid HCV and HIV testing in substance abuse treatment programs. Addiction. 2015;110:129–143. doi: 10.1111/add.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbia Basin College Library, editor. Truven Health Analytics. Hepatitis C: Drug Therapy, In micromedex. Truven Health Analytics; Greenwood Village, CO: 2016. [Accessed January 19, 2018]. Online. http://www.micromedexsolutions.com/micromedex2/librarian/ [Google Scholar]
- Tumeh JW, Moore SG, Shapiro R, Flowers CR. Practical approach for using Medicare data to estimate costs for cost–effectiveness analysis. Expert Rev. Pharmacoecon. Outcomes Res. 2005;5:153–162. doi: 10.1586/14737167.5.2.153. [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of Labor Statistics. Civilian labor force participation rate by age, gender, race and ethnicity. [Accessed January 19, 2018];The Bureau of Labor Statistics. 2015a Online. https://www.bls.gov/emp/
- U.S. Bureau of Labor Statistics. Occupational employment statistics, May 2015 ed. [Accessed January 19, 2018];The Bureau of Labor Statistics. 2015b Online. https://www.bls.gov/oes/
- Wittenberg E, Bray JW, Aden B, Gebremariam A, Nosyk B, Schackman BR. Measuring benefits of opioid misuse treatment for economic evaluation: Health-related quality of life of opioid-dependent individuals and their spouses as assessed by a sample of the US population. Addiction. 2016;111:675–684. doi: 10.1111/add.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg E, Bray JW, Gebremariam A, Aden B, Nosyk B, Schackman BR. Joint utility estimators in substance use disorders. Value Health. 2017;20:458–465. doi: 10.1016/j.jval.2016.09.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitski R, Dan C. Updated National Viral Hepatitis Action Plan 2017–2020. [Accessed January 19, 2018];Health and Human Services. 2016 Online. https://www.hhs.gov/sites/default/files/National%20Viral%20Hepatitis%20Action%20Plan%202017-2020.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.