Abstract
In the present work, magnetic nanoparticles (MNPs) were prepared by chemical precipitation of trivalent and divalent iron ions which were functionalized using citric acid. The bacterial isolate Staphylococcus epidermidis KX781317 was isolated from oil-contaminated site. The isolate produced lipase, which was purified and immobilized on magnetic nanoparticles (MNPs) for ester synthesis from waste frying oil (WFO). The characterization of MNPs employed conventional TEM, XRD and FTIR techniques. TEM analysis of MNPs showed the particle size in the range of 20–50 nm. FTIR spectra revealed the binding of citric acid to Fe3O4 and lipase on citric acid-coated MNPs. The citric acid-coated MNPs and lipase-conjugated citric acid-coated MNPs had similar XRD patterns which indicate MNPs could preserve their magnetic properties. The maximum immobilization efficiency 98.21% of lipase-containing citric acid-coated MNPs was observed at ratio 10:1 of Cit-MNPs:lipase. The pH and temperature optima for lipase conjugated with Cit-MNPs were 7 and 35 °C, respectively. Isobutanol was found to be an effective solvent for ester synthesis and 1:2 ratio of oil:alcohol observed significant for ester formation. The ester formation was determined using TLC and the % yield of ester conversion was calculated. The rate of ester formation is directly proportional to the enzyme load. Formed esters were identified as isobutyl laurate ester and isobutyl myristate ester through GC–MS analysis.
Keywords: Lipase, Citric acid-coated nanomagnetic particles (Cit-MNPs), Waste frying oil, Ester
Introduction
Lipase (triacylglycerol acyl hydrolase; EC 3.1.1.3) is a water-soluble and serine hydrolase enzyme (Eggert et al. 2000) which catalyzes the hydrolysis of triacylglycerol to release free fatty acids and glycerol. In immiscible or anhydrous solvents, lipase can accelerate other reactions such as esterification, inter-esterification, acidolysis, alcoholysis and aminolysis. (Sharma and Kanwar 2014). Therefore, lipase has emerged as one of the leading biocatalysts with proven potential and utilize in in situ lipid metabolism as well as ex situ multifaceted industrial applications (Joseph et al. 2008). Recently, enzymatic trans-esterification of vegetable oils and waste oils have appealed more attention due to the advantages of environmental friendliness and effectively converting both triglycerides and free fatty acids into fatty acid methyl/ethyl esters. There are extensive studies related to free lipase-mediated alcoholysis of various kinds of oils. But one of the major problems of enzyme-catalyzed process is the high cost of the lipases used as catalyst and the cost of raw material which accounts for about 70% of the total cost (Halim et al. 2009). Therefore, researchers are always looking for other alternatives which reduce the production cost of the process. Waste cooking oil, originated from restaurants and household disposals, is creating serious problems of environmental control. Production of biodiesel from usage of a waste cooking oil as feedstock not only will reduce disposal problems, but more importantly, would decrease the production cost of biodiesel. Several studies have shown that the enzymatic methanolysis with waste cooking oil is a promising alternative as a feedstock for biodiesel production (Panadare and Rathod 2015; Yu et al. 2013; Karmee et al. 2015). Conventional chemical technology uses either acid or base as chemical catalysts for the biodiesel production. However, difficulties exist in terms of recovery of glycerol, the removal of inorganic salts, certain undesirable side reactions (Mehrasbi et al. 2017). Free lipase can efficiently catalyze production of biodiesel in oil/water two-phase system but several problems such as low stability, poor reusability, and physical denaturation are associated with free enzyme (Mehrasbi et al. 2017; Bezbradica et al. 2006). Therefore, the use of immobilized enzymes can be considered as one of the promising and suitable alternatives which allow easy recovery and reusability (Ozyilmaz and Gezer 2010). However, immobilized enzyme systems also face certain challenges such as identification of new matrix materials with appropriate structural characteristics, surface functionality, and availability of surface area (Lettera et al. 2015). Thus, nowadays nanoscale materials such as Fe3O4, ZrO2, chitosan and silica nanoparticles are considered ideal substrate materials for the immobilization of enzymes used for biocatalytic reactions. Among these, nanoscale magnetite (Fe3O4) and maghemite (γ-Fe2O3) are of great interest due to their biocompatibility, stability, large surface. Super-paramagnetic properties allow selective separation of immobilized enzymes from a reaction mixture by applying a magnetic field (Yu et al. 2013). So, overall the use of magnetic nanoparticles as supports for enzyme immobilization is endowed with the following advantages: (1) provide higher specific surface area for efficient binding, (2) lower mass transfer resistance and less fouling, (3) the selective separation of immobilized enzymes under magnetic field and hence reduce overall operational cost, and (4) the application of a continuous biocatalysis system. However, Fe3O4 nanoparticles often need modification or functionalization by certain chemical methods to improve immobilization of enzyme through physical adsorption or covalent interaction (Thangaraj et al. 2016). Magnetic nanoparticles are functionalized/modified by compounds such as 3-aminopropyltriethoxysilane (APTES), p-aminophenyltrimethoxysilane (APTS), mercaptopropyltriethoxysilane (MPTES), and citric acid. Mehrasbi et al. (2017) reported immobilized lipase from Candida antarctica (CALB) with silica core shell Fe3O4 nanoparticles were functionalized with (3-glycidoxypropyl)trimethoxylsilane (GPTMS) and then applied for biodiesel production. Similarly, magnetic nanoparticles were functionalized by APTES followed by coupling with glutaraldehyde immobilized with esterase from Pseudozyma sp. NII 08165, further evaluated for synthesis of ethyl acetate and trans-esterification of vegetable oil for biodiesel production (Alex et al. 2014). Moreover, Liu et al. (2011) synthesized magnetic Fe3O4–chitosan nanoparticles, cross-linking with glutaraldehyde used to immobilize lipase. Sui et al. 2012 coated super-paramagnetic magnetite nanoparticles (SMN) by gluconic acid to improve their bio-affinity followed by coupling with carbodiimide (EDC), which were used for immobilization of lipase. Chen et al. (2009) used carboxylic acids with different alkyl chain lengths to modify ZrO2 nanoparticles, serving as the support for immobilization of lipase from Pseudomonas cepacia which were applied for kinetic resolution of (R,S)-1-phenylethanol by vinyl acetate in isooctane. Therefore, in recent days, Fe3O4 nanoparticle applications have become wider and are applied for immunoassay, as biosensor, for bioseparation and for targeted drug delivery (Huang et al. 2003).
In the present study, lipase from S. epidermidis KX781317 was immobilized on citric acid-coated magnetic nanoparticles (Cit-MNPs). The synthesized magnetic nanoparticles were characterized by TEM analysis. The binding of citric acid to Fe3O4 and lipase on citric acid-coated MNPs were analyzed by FTIR and XRD. These lipase immobilized on Cit-MNPs were applied for ester formation using waste frying oil (WFO) and further ester formation analyzed by GC–MS.
Materials and methods
Materials
Tris-base, sodium hydroxide (NaOH), olive oil, citric acid, hydrochloric acid (HCL), acetone, methanol, ethanol, propanol, isopropanol, isobutanol, butanol, ferric chloride (FeCl3), ferrous chloride (FeCl2), n-hexane, petroleum ether, iodine crystals, diethyl ether and acetic acid were procured from Hi-media labs. (Mumbai, India). Methyl oleate was purchased from Sigma-Aldrich (St. Louis, USA) and TLC plate-containing silica gel 60 F254 was purchased from Merck (Mumbai, India).
Isolation and screening of lipase producer
Lipase-producing microorganisms were isolated using tributyrin agar plate from different oil contaminated sites such as petrol pumps, temple, and kitchen waste dumping sites. The plates were observed for the zone of hydrolysis and potential lipase-producing microorganisms were further screened for their solvent tolerance ability. Among them, seven isolates (C2, C3, C4, C7, K25, K31 and K35) have shown zone of hydrolysis on tributyrin agar plate in the presence of organic solvents. Out of these seven isolates, bacterial isolate K31 has shown maximum growth in the presence of almost all the organic solvents, which were further identified as Staphylococcus epidermidis KX781317 using 16S rRNA nucleotide sequence analysis.
Lipase production and purification
The isolate was grown in the basal media containing component (g/l) yeast extract (20), MgSO4 (2), NaCl (5) and olive oil (10 ml) which was incubated under shaking condition at 120 rpm at 37 °C for 16 h. The actively grown culture Staphylococcus epidermidis KX781317 was used as inoculum having OD 1.0 at 600 nm. The flasks were inoculated with 1% inoculum and incubated at 28 °C. Upon 24 h, incubation samples were centrifuged at 8000 rpm for 5 min and the supernatant was taken for lipase assay. The obtained supernatant was precipitated using acetone precipitation saturation method and it was precipitated at 50%. Furthermore, precipitated protein was purified using gel filtration chromatography (Sephadex G-75) at pH 7.2 (Tris buffer, 50 mM).
Determination of lipase activity
Lipase activity was performed as the method described by Cadirci and Yasa (2010) using p-nitrophenyl palmitate (p-NPP) as a substrate. The substrate p-NPP (30 mg) dissolved in 10 ml of isopropanol and 90 ml of Tris buffer (pH 7.2) in conjunction with Triton X-100 [0.4% (w/v)]. The assay system consisted of 2.4 ml of substrate and 0.1 ml of enzyme which incubated at 37 °C for 15 min. The absorbance was measured at 410 nm. One unit (U) of lipase activity is defined as the amount of enzyme required to convert 1 μmol of p-NPP to p-nitrophenol per min under specific conditions.
Preparation of magnetic particles
Synthesis of magnetic particles is based on chemical precipitation of mixed trivalent and divalent iron ions as method suggested by (Yamaura et al. 2004) The ferric chloride (FeCl3) and ferrous chloride (FeCl2) were mixed at molar ratio (1:2) in deionized water under alkaline condition (pH 11). The chemical precipitation was achieved at 25 °C under vigorous stirring by adding 1 N NaOH until black precipitate formed and subsequently the precipitates were heated at 80 °C for 30 min. Further, the magnetic black particles were washed several times with deionized water till neutral pH developed, washed with ethanol and finally dried in oven at 70 °C.
Functionalization of Fe3O4 magnetic nanoparticles with citric acid
The MNPs were collected and washed three times with deionized water. The MNPs were functionalized through citric acid coating which was achieved by addition of 10 ml 0.05 M citric acid to 100 ml aqueous solution under stirring condition for 15 min at 60 °C. Modified citric acid-coated Fe3O4 magnetic nanoparticles were magnetically separated and washed three times for the removal of impurities as suggested by Florian (2015).
Immobilization of lipase on citric acid-coated MNPs
Purified lipase was dissolved in 50 mM Tris–HCl buffer pH 7.2. The citric acid-coated MNPs (Cit-MNPs) were added in 10 ml of buffer (0.05 M Tris–HCl, pH 7.2, 0.1 M NaCl) which were further sonicated for 10 min with frequency 10 Hz. The Cit-MNPs were washed with 0.05 M Tris–HCl, pH 7.2 buffer, incubated overnight with 2 ml of lipase at a concentration of 1 mg ml−1 at 4 °C. The lipase-bound magnetic particles were recovered from the reaction mixture by placing under magnetic field. The supernatant was used to determine immobilization efficacy and activity of recovery as per equations described by (Thangaraj et al. 2016)
where E represents the lipase immobilization efficiency; C1 is the amount of the lipase protein existing in solution before immobilization; C0 is the amount of the lipase protein existing in solution after immobilization.
where R represents the activity recovery of immobilized lipase, A is the activity of the immobilized lipase, and A0 is the activity of free lipase in solution before immobilization.
Effect of initial concentration of Cit-MNPs on immobilization efficiency and activity recovery
To determine the effect of initial concentration of Cit-MNPs on immobilization efficacy of lipase-containing magnetic nanoparticles, variable concentration of Cit-MNPs was added to 10 mL of buffer (0.05 M Tris–HCl, pH 7.2, 0.1 M NaCl) which was further sonicated for 10 min with frequency 10 Hz. The Cit-MNPs were washed with 0.05 M Tris–HCl, pH 7.2 buffer, incubated overnight with 2 ml of lipase at a concentration of 1 mg ml−1 at 4 °C. The lipase-bound magnetic particles were recovered from the reaction mixture by placing under magnetic field. The supernatant was used to determine immobilization efficacy and % recovery (Thangaraj et al. 2016). The protein estimation was carried out by Lowry’s method (Lowry et al. 1951).
Effect of temperature and pH on free enzyme and immobilized enzyme on Cit-MPs
The effect of temperature on free enzyme and immobilized enzyme on Cit-MNPs was examined by determining percent relative activity of enzyme at temperature range 25–65 °C. For this, free enzyme and immobilized enzyme were incubated in 50 mM Tris–HCl buffer (pH 7.2) at temperature ranging from 25 to 65 °C for 1 h, followed by determination of percent relative activity of enzyme at 37 °C as mentioned in lipase assay section. The effect of pH on free and immobilized enzyme was investigated by pre-incubating at variable pH range from 5 to 9 for 1 h, followed by measurement of percent relative activity of enzyme as described in lipase assay section. The percent relative activity of free and immobilized enzyme was standardized to the initial value determined at 37 °C, pH 7.2 which was taken as 100%.
Physical characterization of Cit-MNPs with and without lipase immobilization
The MNPs coated with citric acid (Cit-MNPs) were characterized by various analytical techniques which include X-ray diffraction (XRD), Fourier transform infrared spectrum (FTIR) and transmission electron microscopy (TEM). Cit-MPs and Cit-MNPs immobilized with lipase were confirmed by FTIR spectrum measurements. The FTIR spectrum range (4000–400 cm−1) of Cit-MNPs and Cit-MNPs with lipase dispersed in KBr revealed bands from the iron citrate spectrum. X-ray diffraction patterns of dried powder of Cit-MNPs and Cit-MNPs with lipase were recorded with BRUKER X-ray powder diffractometer using Cu-Kα1 (1.54060 Å) radiation. The size and shape of the magnetic nanoparticles were analyzed using TEM (JOEL, JEM 2100, USA).
Alcoholysis of waste frying oils to form esters
For the ester formation, waste frying oil (2.63 ml) and methanol (0.33 ml) were taken in screw-cap glass tubes, mixed with lipase immobilized Cit-MNPs (1 mg) and incubated at 37 °C for 24 h (Ugur et al. 2014). The control set was prepared which contained Cit-MNPs without lipase, oil and alcohol. The samples (500 µl) were collected from control and experimental tubes, mixed with n-hexane (1 ml). The 5 µl upper layer from both the tubes and methyl oleate (as reference) were loaded on TLC plate. The TLC plate was developed with solvent system of petroleum ether:diethyl ether:acetic acid (85:15:1) and spot were visualized with iodine vapor.
Screening of organic solvent for ester production
To determine the effect of organic solvent on ester production, an ester synthesis was carried out using different organic solvents (methanol, ethanol, butanol, propanol, isopropanol). For each reaction system, control and experimental tubes were prepared as per earlier description, where control reaction mixture contained Cit-MNPs, oil and alcohol while experimental mixture contained immobilized lipase, Cit-MNPs, oil and alcohol. The ester formation was determined using TLC with solvent system of petroleum ether:diethyl ether:acetic acid (85:15:1) and the % yield of ester conversion was calculated (Neta et al. 2012).
| 1 |
| 2 |
| 3 |
where VNaOH is the volume of sodium hydroxide, Wsample is the weight of the sample and Wcontrol is the weight of the control.
Determination of oil:alcohol ratio for ester production
Lipase from S. epidermidis KX781317 was immobilized on Cit-MNPs and for each reaction system, control and experimental tubes were prepared as described above. The effect of oil:alcohol ratio on ester synthesis using lipase immobilized on Cit-MNPs was determined by analyzing various ratios of oil:alcohol (4:1, 3:1, 2:1, 1:1, 1:2, 1:3 and 1:4) and the % yield of ester conversion was calculated for each ratio (Neta et al. 2012).
Effect of enzyme load on ester production
To determine significant concentration of lipase on Cit-MNPs, in the reaction mixture, variable units of lipase 15U, 30U, 45U, 60U, 75U and 90U were added on Cit-MNPs. The ester formation was analyzed by TLC using same solvent system mentioned above and the % yield of ester conversion was calculated (Neta et al. 2012).
GC–MS analysis of ester
The control and test sample were extracted in n-hexane which were analyzed by GC–MS (Capillary GC–MS Auto System XL, Perkin Elmer Turbo Mass, USA). Helium was used as a carrier gas with a flow rate of 1 ml per minute. The column oven temperature was programmed from 150 to 250 °C (at the rate of 10 °C min) with injector and detector temperature were at 240 and 250 °C, respectively. The compounds were identified on the basis of mass spectra and using NIST library provided by the manufacturer along with instrument.
Results and discussion
Synthesis and characterization of magnetite nanoparticles coated with citric acid
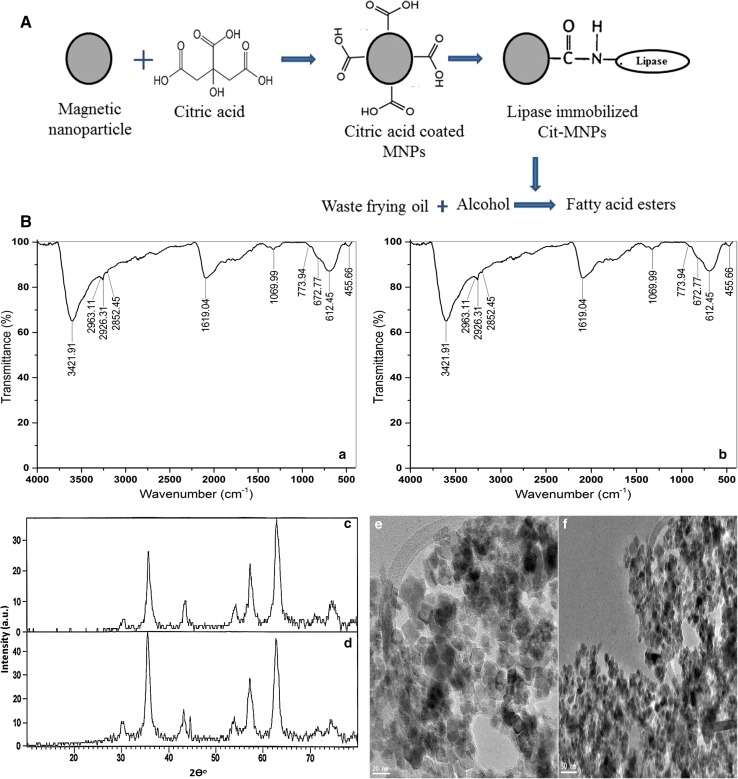
The magnetic nanoparticles were prepared as per the method suggested by Yamaura et al. (2004), which is based on the chemical precipitation of trivalent and divalent iron ions. First, the ferric and ferrous chloride hydrolysis was occurred, which was precipitated in the presence of sodium hydroxide. Second, the ferric hydroxide decomposes to FeOOH, due to the low water activity of the resulting NaOH solution. Finally, a solid state reaction between FeOOH and Fe (OH)2 takes place, which produces magnetite when final pH reaches around 11 and the deposition changes to black. The solid state reaction takes place between 10 and 15 min at room temperature subsequently; the precipitates were heated at 80 °C for 30 min. Further, the magnetic particles were washed several times with deionized water till neutral pH developed, washed with ethanol and finally dried in oven at 70 °C. The overall reaction mechanism is a dynamic equilibrium equation in which the concentration and size of Fe3O4 nanoparticles are influenced by [Fe3+], [Fe2+] and [OH−], as well as the water activity of the solution. MNPs were functionalized using citric acid, and the lipase was immobilized. The particles were further applied for ester formation using waste frying oil Fig. 1A.
Fig. 1.
(A) Schematic illustration of magnetic nanoparticle synthesis and functionalization using citric acid. Lipase was immobilized on Cit-MNPs and applied for ester formation from waste frying oil. (B) a FTIR spectra of citric acid-coated magnetic particles (Cit-MPs), b FTIR spectra of Cit-MPs with lipase, c XRD patterns of Cit-MPs, d XRD patterns of Cit-MPs with lipase, e TEM images of MNPs 20 nm MNPs and f TEM images of MNPs 50 nm MNPs
The Fourier transform infrared spectroscopy (FTIR) of Cit-MNPs
FTIR analysis of Cit-MNPs and lipase-conjugated Cit-MNPs was carried out using GX-FTIR model, Perkin Elmer, USA spectrophotometer. The samples were mixed with spectroscopically pure KBr (1:99) and pelleted by pressed pellet technique.
A large and intense band at 3421 cm−1 may be assigned to the structural OH groups as well as to the traces of molecular water. Moreover, the peak at 1700 cm−1 assignable to the C=O vibration (symmetric stretching) from the COOH group of citric acid shifts to an intense band at about 1619 cm−1 for the Fe3O4 coated with citric acid (Cit-MNPs) reveals the binding of a citric acid radical to the magnetite surface (Racuciu et al. 2006) as shown in Fig. 1(B)a. The peak at 1538.6 cm−1 is the bending vibration peaks of CO=N–H amide I which indicate lipase is immobilized on Cit-MNPs (Jafari et al. 2015) (Fig. 1(B)b). The low-intensity bands between 400 and 600 cm−1 may be associated with the stretching and torsional vibration modes of the magnetite (Yamaura et al. 2004).
X-ray diffraction (XRD) of Cit-MNPs
The crystalline structure of Cit-MNPs and lipase immobilized Cit-MNPs particles is characterized further by XRD analysis. The Cit-MNPs particles, six characteristic peaks were obtained at 2θ of 30.6°, 32.3°, 43.6°, 54°, 57.2°, and 62.7°, respectively, suggest the particles are Fe3O4 kind (JCPDS database file, no. 00-003-0863) and have cubic spinal structure (Singh et al. 2014) as shown in Fig. 1(B)c, while for lipase-coated Cit-MNPs, same six diffraction peaks were obtained as shown in Fig. 1(B)d. This indicates Cit-MNPs and Cit-MNPs immobilized with lipase had similar XRD patterns which suggest the binding process did not result in the phase change of Fe3O4. As a result, the magnetic particles could preserve their magnetic properties during the separation process.
Transmission electron microscope (TEM) of magnetic particles
MNPs micrographs showed cubic-shaped particles with an average size of 20–50 nm (Fig. 1(B)e). The aggregation of particles was observed due to magnetic dipole moment interaction between the particles (Wang et al. 2009). The magnetic particles were synthesized by co-precipitation method therefore their size was not obtained uniformly (Lu et al. 2007).
Determination of immobilization efficiency of lipase on Cit-MNPs
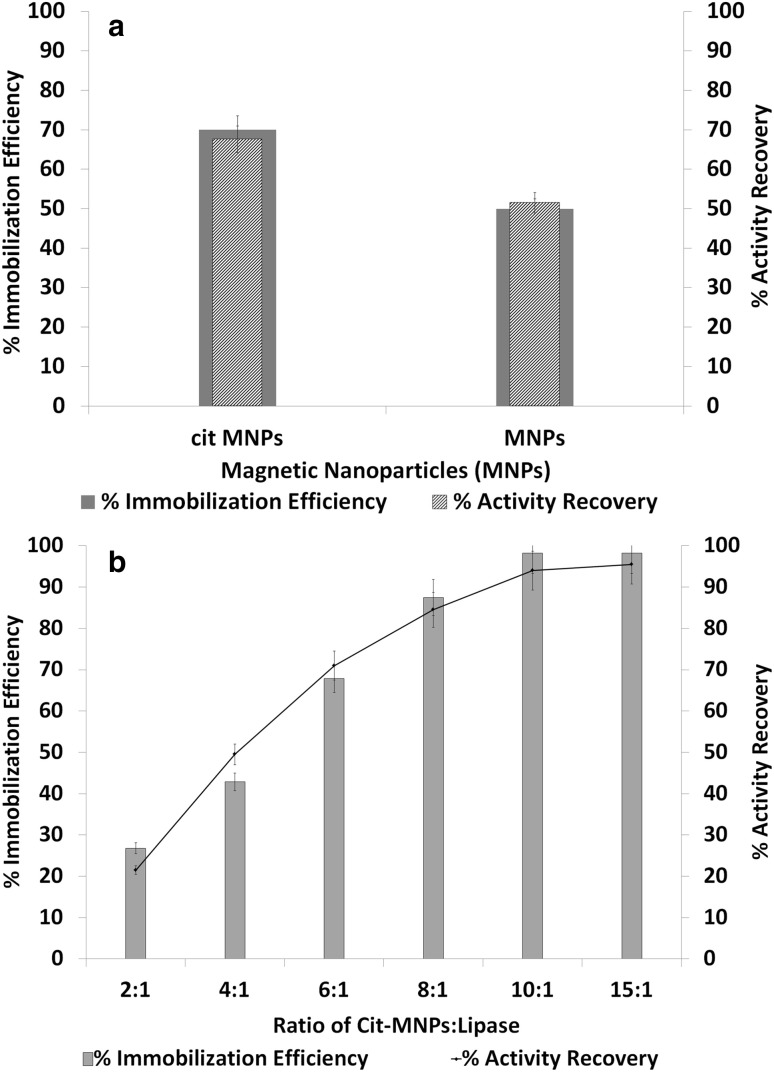
Enzyme immobilization on magnetic particles was accomplished through the surface coating with different polymers, such as coupling agents, cross-linking reagents, and encapsulation or directly immobilized on carbodiimide-activated magnetic particles (Huang et al. 2003). In the present study, magnetic nanoparticles coated with citric acid show immobilization efficiency and activity recovery 70 ± 0.5 and 67 ± 0.6%, respectively, whereas the particles without citric acid show efficiency 50 ± 0.3% and recovery 51 ± 0.54%, respectively (Fig. 2a). The results obtained indicate modification and functionalization of naked-magnetic particles is essential for the direct enzyme immobilization which allow covalent bonding of enzymes, thereby increase stability, activity and reusability of enzymes (Alex et al. 2014). The lipase-immobilized Fe3O4 magnetic nanoparticles functionalized with organosilane compounds such as (3-aminopropyl)triethoxysilane (APTES) and (3-mercaptopropyl)trimethoxysilane) MPTMS and applied for biodiesel production (Thangaraj et al. 2016).
Fig. 2.
a Immobilization efficiency and % activity recovery of MNPs and Cit-MNPs. b Effect of concentration of Cit-MNPs on immobilization efficiency and % recovery of enzyme
Effect of initial concentration of Cit-MNPs on immobilization efficiency and activity recovery
In case of enzyme load and Cit-MNPs ratio study, maximum lipase immobilization efficiency and activity recovery were 98 ± 0.3 and 95 ± 0.5%, respectively, with 10:1 ratio (Fig. 2b). Initially enzyme immobilization efficacy increased with enzyme load per amount of Cit-MNPs. However, further increase in Cit-MNPs concentration did not show any improvement in efficiency of immobilization. Similar observation was reported where initially immobilization of enzyme increased per amount of magnetic nanoparticles (MNPs) which gradually decreased per amount of MNP concentration which may occur due to the competition for binding with other proteins (Alex et al. 2014). A direct correlation between enzyme activity and amount of MNPs cannot be expected because enzymes which are bound and active can only give positive results. However, loss in enzyme activity may be attributed to the non-specific binding of amino acid which allows and conformational changes.
Effect of temperature and pH on free and immobilized enzyme on Cit-MNPs
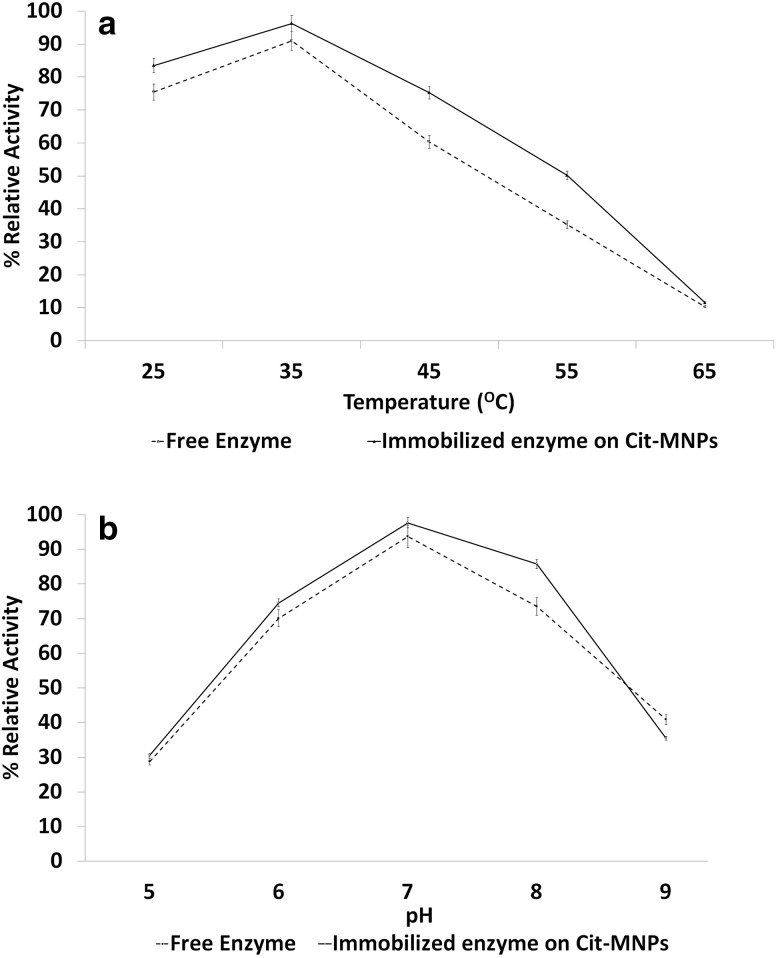
The effect of variable temperature on free and immobilized enzyme on Cit-MNPs is shown in Fig. 3a. The relative activity of free and immobilized lipase is not adversely affected at temperatures below 45 °C, particles retained highest activity (90 ± 1%) at 35 °C but activity of free and immobilized lipase was declined significantly as temperature increase above 45 °C. However, immobilized lipase particles retained 75 ± 0.5% and 50 ± 1% activity at 45 °C and 55 °C, respectively. It is observed that the multipoint interaction between lipase and magnetic particles leads to reinforcement of the intra-molecular forces and prevents the autolysis of lipase, and therefore immobilized particle stability is higher than free enzymes (Qi et al. 2017). The effect of variable pH on free and immobilized enzyme is shown in Fig. 3b. The relative activity of immobilized lipase on Cit-MNPs was retained 74±0.7, 97 ± 0.5 and 87 ± 0.2% at pH 6, 7 and 8, respectively. However, free lipase retained 93 ± 0.5 and 75 ± 0.5% relative activity at pH 7 and 8, respectively. These results indicate that immobilized enzyme was stable at wide range of pH due to covalent bonding of lipase and Cit-MNPs which may prevent the denaturation of lipase (Ren et al. 2011; Kumar et al. 2013).
Fig. 3.
a Effect of temperature on free enzyme and immobilized enzyme on Cit-MNPs and b effect of pH on free enzyme and immobilized enzyme on Cit-MNPs
Alcoholysis of waste frying oils to form esters
Annually, over 15 million waste cooking oil are produced and creating serious environmental problems (Mehrasbi et al. 2017), if used as raw material for alkyl esters (biodiesel) formation as it may reduce 85% cost of the total biodiesel production (Cesarini et al. 2015). In present study, lipase from Staphylococcus epidermidis KX781317 immobilized Cit-MNPs applied for ester production using waste frying oil. In the present study, two reaction system test and control were prepared. Test system contained waste frying oil, methanol and lipase-immobilized Cit-MNPs while control system contained same reaction mixture but Cit-MNPs without lipase. As shown in Fig. 4 in the control lane, three spots were observed while in test lane four spots were developed. Upon further analysis, Rf value of new spot matches with Rf value of methyl oleate which is utilized as a reference compound in the system. Therefore, immobilized lipase on Cit-MNPs has the ability to form esters from waste frying oil. Similarly, lipase immobilized onto a novel microporous polymer used for biodiesel production using sunflower, soybean and waste cooking oils (Dizge et al. 2009). Fatty acid alkyl ester production by trans-esterification of waste cooking oil using lipase from Streptomyces sp. was also reported by Mander et al. (2014).
Fig. 4.
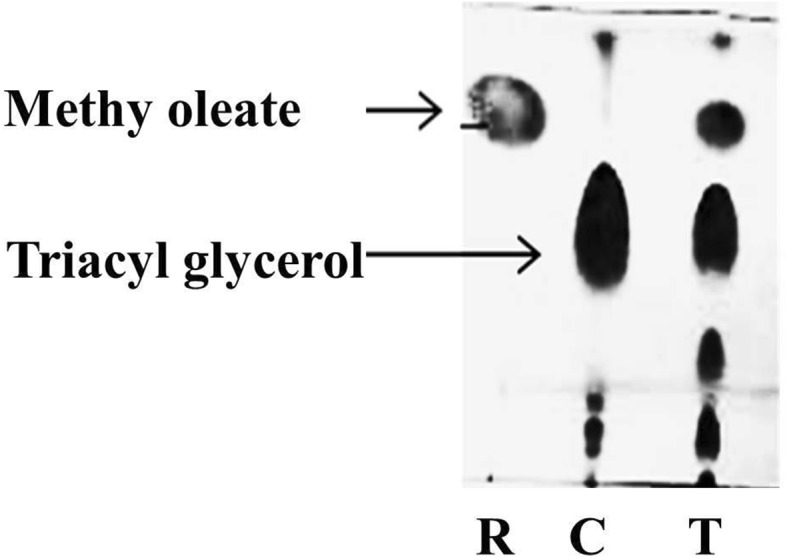
Alcoholysis of waste frying oil; R-methyl oleate, C-reaction mixture without immobilized lipase Cit-MNPs, T-reaction mixture with immobilized lipase Cit-MNPs
Screening of organic solvent for ester production
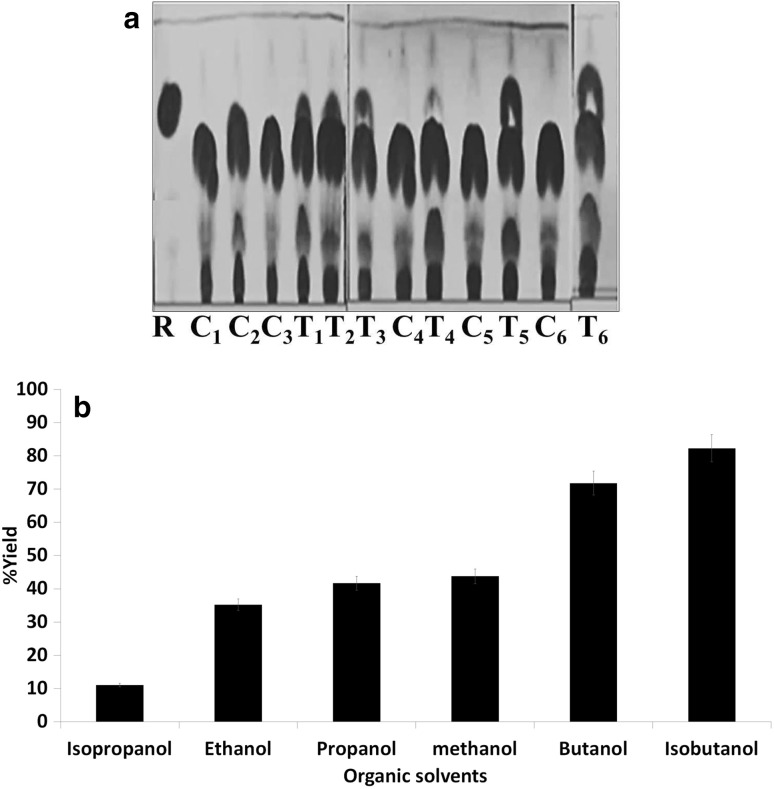
The ester synthesis using waste frying oil (WFO) was carried out in the presence of different organic solvents which were analyzed by TLC. The test systems (T1–T6) were prepared with waste frying oil, lipase-immobilized Cit-MNPs and different organic solvents (Ethanol, Propanol, Methanol, Isopropanol, Butanol and Isobutanol) while controls (C1–C6) were prepared with waste frying oil, Cit-MNPs without lipase and different organic solvents. As shown in Fig. 5a, ester spot was observed in the all the test T1–T6 lanes. However, maximum ester yield (81 ± 2%) was obtained with isobutanol followed by butanol (71 ± 0.8%), methanol (43 ± 1%), propanol (41 ± 2%), ethanol (35 ± 0.5%) and isopropanol (11 ± 0.25%) (Fig. 5b). Similarly, immobilized Pseudomonas cepacia lipase onto magnetic nanoparticles (MNPs) was used for ester production from waste cooking oil. Lipase from Rhizomucor miehei immobilized on ion-exchange macroporus hydrophilic resins also used for trans-esterification of waste oil for ester production (de Lima et al. 2016).
Fig. 5.
Effect of organic solvents on ester synthesis using waste frying oils a TLC of ester formation [R-methyl oleate, C1 and T1 ester formation using ethanol, C2 and T2 ester formation using propanol, C3 and T3 ester formation using methanol, C4 and T4 ester formation using isopropanol, C5 and T5 ester formation using butanol, C6 and T6 ester formation using isobutanol] and b % yield of ester in different solvents
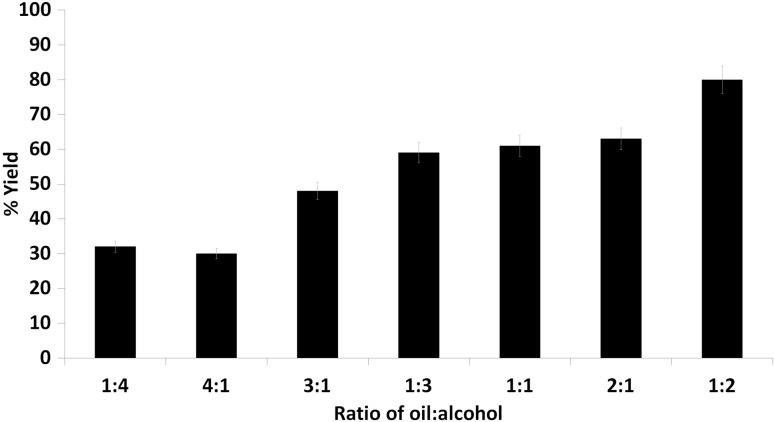
Determination of oil:alcohol ratio for ester production
Different oil:alcohol (isobutanol) ratios were taken, among them maximum percent yield of ester was obtained with oil:alcohol ratio of 1:2 (Fig. 6), whereas ester yield was decreased with further rise in concentration of isobutanol due to inactivation of lipase at high amount of isobutanol. Most of enzymes, including lipases, are not stable in organic solvents, especially hydrophilic organic solvents, as organic solvent strip water molecule which inactivates the enzyme (Romero et al. 2012). Similarly, esterification reaction was carried out with ratio of methanol to soybean oil from 3:1 to 9:1 and 62.3, 63.6, and 63.5%, of ester conversion was observed, for substrate molar ratios of 3:1, 4:1, and 5:1, respectively, upon 12 h incubation. However, ester conversion ratio was only 47.5%, for molar ratio 9:1 as the lipase was apparently inactivated irreversibly, in high methanol concentration (Guan et al. 2010). Functionalized magnetic nanoparticles (MNPs) immobilized with lipase from Candida antarctica (CALB) applied for biodiesel synthesis and optimum production observed with 1:3 ratio of oil to methanol (Mehrasbi et al. 2017).
Fig. 6.
Ester yield in the presence of different oil:alcohol (isobutanol) ratios
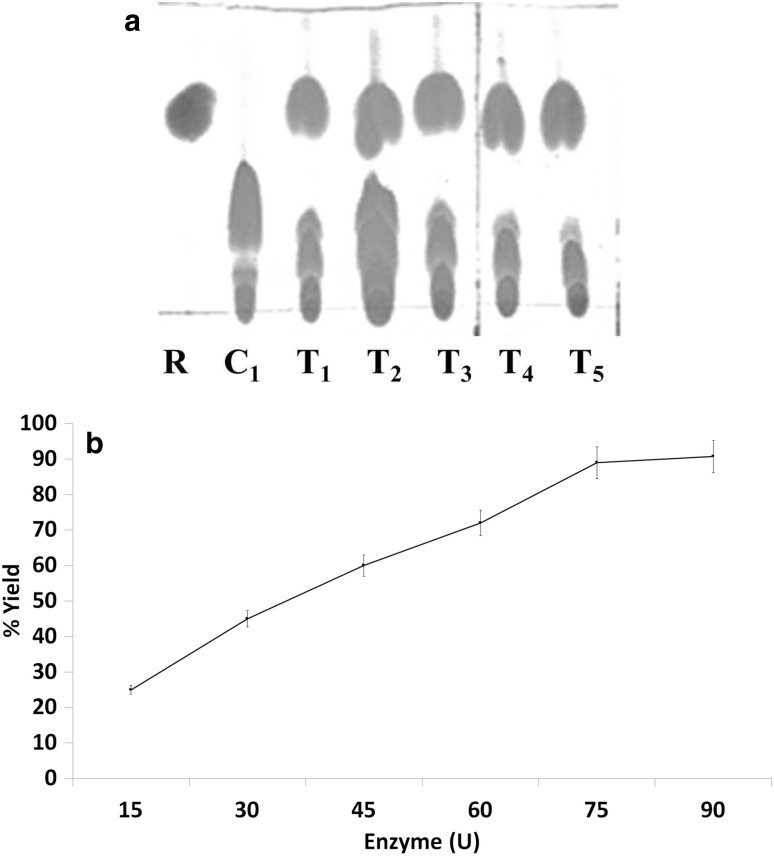
Effect of initial concentration of enzyme on ester production
To determine the effect of initial concentration of enzyme on ester synthesis, variable lipase concentrations (U) were added in the reaction mixture. The control system was prepared with waste frying oil, isobutanol and Cit-MNPs without lipase while the test T1–T5 systems were prepared with varying lipase concentration. The ester was formed but triacylglycerol spot was disappeared rapidly as lipase load increased (Fig. 7a). Maximum yield of ester (90 ± 1%) was obtained with 75U enzyme load and no rise was observed in ester yield with further increase of enzyme load (Fig. 7b). Apparently, a higher initial loading of enzyme had resulted in an increased bound enzyme per unit amount of MNPs. In case of acidolysis of canola oil with caprylic acid with lipase load (1–12%), the ester formation enhanced up to 10% but a significant increase was not observed when the enzyme load was more than 10% (Wang et al. 2012). Immobilized crude lipase from Rhizopus oryzae also applied for 1-butyl oleate synthesis and 73% conversion was obtained with 60 IU immobilized lipase (Ghamgui et al. 2004).
Fig. 7.
Effect of enzyme load on esterification. a TLC of esters (R-methyl oleate and C-reaction mixture without immobilized lipase Cit-MNPs) and variable concentration of enzyme T1:15U, T2:30U, T3:45U, T4:60U and T5:75U, b % yield of ester at variable lipase concentration
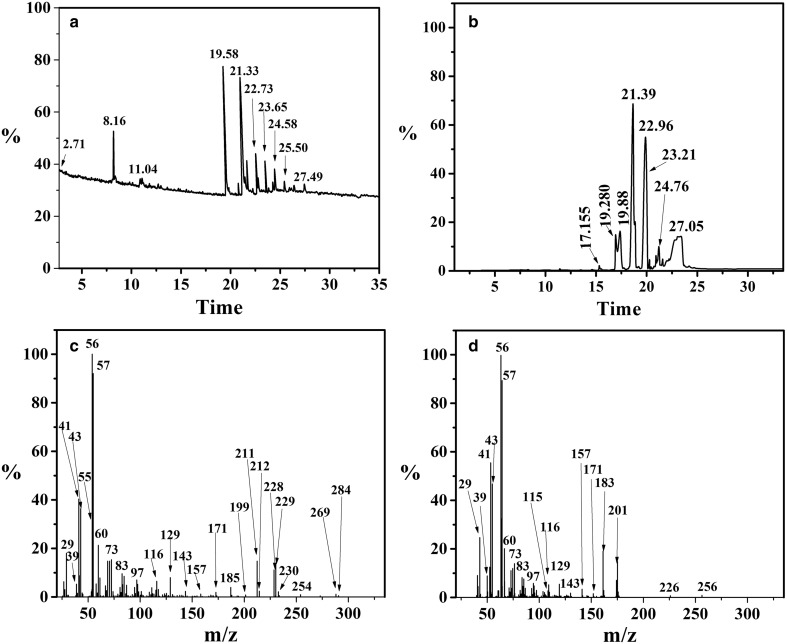
GC–MS analysis of ester
The control and test sample were extracted in n-hexane in which ester formation was analyzed using gas chromatography. It was observed that few peaks disappeared from control (Fig. 8a) and new peaks appeared in the test samples (Fig. 8b). The peaks from test sample with RT 17.159 and 19.285 min were selected and further analyzed through MS which were identified as isobutyl laurate ester (Fig. 8c) and isobutyl myristate ester (Fig. 8d).
Fig. 8.
GC–MS analysis of ester. a GC analysis of control, b GC analysis of test, c MS of isobutyl laurate ester and d MS of isobutyl myristate ester
Conclusion
Lipase from S. epidermidis KX781317 immobilized on citric acid-coated magnetic nanoparticles which were synthesized by chemical co-precipitation with size range of 20–50 nm. The XRD analysis revealed structure of MNPs had no significant change upon lipase binding and the FTIR analysis showed the citric acid coating and the existence of amino group on the particles which necessary for conjugation with biomolecules when they were applied in biological applications. Enzyme-binding efficiency on Cit-MNPs was raised due to covalent bond (amide-I) formation between enzyme and citric acid-coated on MNPs. The lipase-immobilized Cit-MNPs were applied for alcoholysis of waste frying oil and maximum ester yield was obtained with 1:2 ratio of waste frying oil:isobutanol. Through GC–MS analysis isobutyl laurate and isobutyl myristate were identified. Therefore, immobilization of enzyme on functionalized MNPs is of great interest as it allows selective separation by applying a magnetic field due which it is easy to recover and reuse.
Acknowledgements
The authors wish to acknowledge SICART, Vallabh Vidyanagar, Anand, Gujarat, for providing the necessary instrumentation facilities. This research did not receive any specific grant from funding agencies in the public, commercial or not for profit sectors.
Compliance with ethical standards
Conflict of interest
The authors hereby want to declare that there is no conflict of interest whatsoever in this publication.
References
- Alex D, Mathew A, Sukumaran RK. Esterases immobilized on aminosilane modified magnetic nanoparticles as a catalyst for biotransformation reactions. Bioresour Technol. 2014;167:547–550. doi: 10.1016/j.biortech.2014.05.110. [DOI] [PubMed] [Google Scholar]
- Bezbradica D, Mijin D, Siler-Marinkovic S, Knezevic Z. The Candida rugosa lipase catalyzed synthesis of amyl isobutyrate in organic solvent and solvent-free system: a kinetic study. J Mol Catal B Enzym. 2006;38:11–16. doi: 10.1016/j.molcatb.2005.10.004. [DOI] [Google Scholar]
- Cadirci BH, Yasa I. An organic solvents tolerant and thermotolerant lipase from Pseudomonas fluorescens P21. J Mol Catal B Enzym. 2010;64(3–4):155–161. doi: 10.1016/j.molcatb.2009.09.009. [DOI] [Google Scholar]
- Cesarini S, Pastor F, Nielsen P, Diaz P. Moving towards a competitive fully enzymatic biodiesel process. Sustainability. 2015;7:7884–7903. doi: 10.3390/su7067884. [DOI] [Google Scholar]
- Chen YZ, Ching CB, Xu R. Lipase immobilization on modified zirconia nanoparticles: studies on the effects of modifiers. Process Biochem. 2009;44:1245–1251. doi: 10.1016/j.procbio.2009.06.023. [DOI] [Google Scholar]
- De Lima LN, Vieira GNA, Kopp W, et al. Mono- and heterofunctionalized silica magnetic microparticles (SMMPs) as new carriers for immobilization of lipases. J Mol Catal B Enzym. 2016 [Google Scholar]
- Dizge N, Aydiner C, Imer DY, et al. Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour Technol. 2009;100:1983–1991. doi: 10.1016/j.biortech.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Eggert T, Pencreac HG, Douchet I, et al. A novel extracellular esterase from Bacillus subtilis and its conversion to a monoacylglycerol hydrolase. Eur J Biochem. 2000;267:6459–6469. doi: 10.1046/j.1432-1327.2000.01736.x. [DOI] [PubMed] [Google Scholar]
- Florian TN (2015) Iron oxide nanoparticles with citric acid coating: properties and studies on experimental models. University of Medicine and Pharmacy of Craiova Doctoral School, PhD Thesis Summary
- Ghamgui H, Karra-Chaâbouni M, Gargouri Y. 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: a comparative study between n-hexane and solvent-free system. Enzyme Microb Technol. 2004;35:355–363. doi: 10.1016/j.enzmictec.2004.06.002. [DOI] [Google Scholar]
- Guan F, Peng P, Wang G, et al. Combination of two lipases more efficiently catalyzes methanolysis of soybean oil for biodiesel production in aqueous medium. Process Biochem. 2010;45:1677–1682. doi: 10.1016/j.procbio.2010.06.021. [DOI] [Google Scholar]
- Halim SFA, Kamaruddin AH, Fernando WJN. Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: optimization using response surface methodology (RSM) and mass transfer studies. Bioresour Technol. 2009;100:710–716. doi: 10.1016/j.biortech.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Huang SH, Liao MH, Chen DH. Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnol Prog. 2003;19:1095–1100. doi: 10.1021/bp025587v. [DOI] [PubMed] [Google Scholar]
- Jafari A, Salouti M, Shayesteh SF, et al. Synthesis and characterization of Bombesin-superparamagnetic iron oxide nanoparticles as a targeted contrast agent for imaging of breast cancer using MRI. Nanotechnology. 2015;26:075101. doi: 10.1088/0957-4484/26/7/075101. [DOI] [PubMed] [Google Scholar]
- Joseph B, Ramteke PW, Thomas G. Cold active microbial lipases: some hot issues and recent developments. Biotechnol Adv. 2008;26:457–470. doi: 10.1016/j.biotechadv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Karmee S, Patria R, Lin C. Techno-economic evaluation of biodiesel production from waste cooking oil—a case study of Hong Kong. Int J Mol Sci. 2015;16:4362–4371. doi: 10.3390/ijms16034362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Nagar S, Bhushan I, et al. Covalent immobilization of organic solvent tolerant lipase on aluminum oxide pellets and its potential application in esterification reaction. J Mol Catal B Enzym. 2013;87:51–61. doi: 10.1016/j.molcatb.2012.10.002. [DOI] [Google Scholar]
- Lettera V, Pezzella C, Cicatiello P, et al. Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem. 2015;196:1272–1278. doi: 10.1016/j.foodchem.2015.10.074. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jia S, Wu Q, et al. Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal Commun. 2011;12:717–720. doi: 10.1016/j.catcom.2010.12.032. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu AH, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chemie Int Ed. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- Mander P, Yoo HY, Kim SW, et al. Transesterification of waste cooking oil by an organic solvent-tolerant alkaline lipase from Streptomyces sp. CS273. Appl Biochem Biotechnol. 2014;172:1377–1389. doi: 10.1007/s12010-013-0610-7. [DOI] [PubMed] [Google Scholar]
- Mehrasbi MR, Mohammadi J, Peyda M, Mohammadi M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew Energy. 2017;101:593–602. doi: 10.1016/j.renene.2016.09.022. [DOI] [Google Scholar]
- Neta NDAS, Dos Santos JCS, Sancho SDO, et al. Enzymatic synthesis of sugar esters and their potential as surface-active stabilizers of coconut milk emulsions. Food Hydrocoll. 2012;27:324–331. doi: 10.1016/j.foodhyd.2011.10.009. [DOI] [Google Scholar]
- Ozyilmaz G, Gezer E. Production of aroma esters by immobilized Candida rugosa and porcine pancreatic lipase into calcium alginate gel. J Mol Catal B Enzym. 2010;64:140–145. doi: 10.1016/j.molcatb.2009.04.013. [DOI] [Google Scholar]
- Panadare DC, Rathod VK. Applications of waste cooking oil other than biodiesel: a review. Iran J Chem Eng. 2015;12:55–76. [Google Scholar]
- Qi H, Du Y, Hu G, Zhang L. Poly(carboxybetaine methacrylate)-functionalized magnetic composite particles: a biofriendly support for lipase immobilization. Int J Biol Macromol. 2017 doi: 10.1016/j.ijbiomac.2017.10.150. [DOI] [PubMed] [Google Scholar]
- Racuciu M, Creanga DE, Airinei A. Citric-acid-coated magnetite nanoparticles for biological applications. Eur Phys J E. 2006;21:117–121. doi: 10.1140/epje/i2006-10051-y. [DOI] [PubMed] [Google Scholar]
- Ren Y, Rivera JG, He L, et al. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol. 2011;11:63. doi: 10.1186/1472-6750-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero CM, Pera LM, Loto F, et al. Purification of an organic solvent-tolerant lipase from Aspergillus niger MYA 135 and its application in ester synthesis. Biocatal Agric Biotechnol. 2012;1:25–31. [Google Scholar]
- Sharma S, Kanwar SS. Organic solvent tolerant lipases and applications. Sci World J. 2014;2014:1–15. doi: 10.1155/2014/625258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Gautam RK, Kumar R, et al. Citric acid-coated magnetic nanoparticles: synthesis, characterization and application in removal of Cd(II) ions from aqueous solution. J Water Process Eng. 2014;4:233–241. doi: 10.1016/j.jwpe.2014.10.005. [DOI] [Google Scholar]
- Sui Y, Cui Y, Nie Y, et al. Surface modification of magnetite nanoparticles using gluconic acid and their application in immobilized lipase. Colloids Surf B Biointerf. 2012;93:24–28. doi: 10.1016/j.colsurfb.2011.11.054. [DOI] [PubMed] [Google Scholar]
- Thangaraj B, Jia Z, Dai L, et al. Lipase NS81006 immobilized on Fe3O4 magnetic nanoparticles for biodiesel production. Ovidius Univ Ann Chem. 2016;27:13–21. [Google Scholar]
- Ugur A, Sarac N, Boran R, et al. New lipase for biodiesel production: partial purification and characterization of LipSB 25-4. ISRN Biochem. 2014;2014:1–7. doi: 10.1155/2014/289749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dou P, Zhao P, et al. Immobilization of lipases onto magnetic Fe3O4 nanoparticles for application in biodiesel production. Chemsuschem. 2009;2:947–950. doi: 10.1002/cssc.200900174. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xia L, Xu X, et al. Lipase-catalyzed acidolysis of canola oil with caprylic acid to produce medium-, long- and medium-chain-type structured lipids. Food Bioprod Process. 2012;90:707–712. doi: 10.1016/j.fbp.2012.02.003. [DOI] [Google Scholar]
- Yamaura M, Camilo RL, Sampaio LC, et al. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J Magn Magn Mater. 2004;279:210–217. doi: 10.1016/j.jmmm.2004.01.094. [DOI] [Google Scholar]
- Yu CY, Huang LY, Kuan IC, Lee SL. Optimized production of biodiesel from waste cooking oil by lipase immobilized on magnetic nanoparticles. Int J Mol Sci. 2013;14:24074–24086. doi: 10.3390/ijms141224074. [DOI] [PMC free article] [PubMed] [Google Scholar]