Abstract
AIM
To investigate the clinicopathological features of the patients testing negative for high titer serum anti-Helicobacter pylori (H. pylori) antibody.
METHODS
The antibody titers were measured using antigens derived from Japanese individuals. 13C-urea breath test-positive individuals were defined as having H. pylori infection. We investigated the demographic characteristics, laboratory data, endoscopic findings including Kyoto classification of gastritis, and histology in negative-high titer patients without H. pylori eradication therapy. Kyoto classification consisted of scores for gastric atrophy, intestinal metaplasia, enlarged folds, nodularity, and redness.
RESULTS
Of the 136 subjects enrolled, 23 (17%) had H. pylori infection. Kyoto classification had an excellent area under the receiver operating characteristics curve (0.886, 95% confidence interval: 0.803-0.968, P = 3.7 × 10-20) for predicting H. pylori infection with a cut-off value of 2. Further, Kyoto classification, H. pylori density, and neutrophil activity had high accuracies (89.7%, 96.3%, and 94.1%, respectively). Kyoto classification was independent of the demographic and laboratory parameters in multivariate analysis.
CONCLUSION
Endoscopic Kyoto classification of gastritis is a useful predictor of H. pylori infection in negative-high titer antibody patients.
Keywords: Kyoto classification, Gastritis, Helicobacter pylori, Antibody, Endoscopy
Core tip: Compared with negative-low titer (< 3 U/mL on E-plate Eiken kit), negative-high titer (3-9.9 U/mL) have been reported to be at higher risk for intestinal gastric cancer. Helicobacter pylori (H. pylori)-infected patients accounted for 94% of gastric cancer patients with an antibody titer of < 10 U/mL. Seventeen percent of subjects with negative-high titer serum anti-H. pylori antibody tested positive for H. pylori infection. Endoscopic Kyoto classification of gastritis was an excellent predictor of H. pylori infection with large area under the receiver operating characteristics curve (0.886), cut-off value of 2, and high accuracy (89.7%), indicating its high confidence.
INTRODUCTION
Helicobacter pylori (H. pylori) is a group 1 carcinogen for gastric cancer. Therefore, the International Agency for Research on Cancer has recommended screening for and eradication of H. pylori for preventing gastric cancer[1]. The main non-invasive methods for diagnosing H. pylori infection are the serum immunoglobulin G antibody test, 13C-urea breath test (UBT), and stool antigen test. Endoscopy, histology, culture, and rapid urease test have been used as the main invasive methods. The Maastricht V/Florence consensus report states that the urea breath test using 13C-urea is the best test to diagnose H. pylori infection[2,3]. However, some of the available serum antibody kits including E-plate Eiken are excellent kits, with sensitivity and specificity above 90%[4,5]. Serology is hardly affected by the changes in the stomach that result in a low bacterial load, including gastrointestinal bleeding, atrophic gastritis, gastric mucosa-associated lymphoid tissue lymphoma, and gastric carcinoma[2,6]. Additionally, proton pump inhibitors and antibiotics have little influence on serological tests as well[7]. A serological test, with levels of serum anti-H. pylori antibody and pepsinogen I and II, is useful for identifying patients at increased risk of gastric cancer[2,8,9]. These are some of the merits of serological testing. However, subjects with an E-plate antibody titer of < 10 U/mL include patients with spontaneous disappearance of H. pylori from the gastric mucosa, who are known to have extremely severe gastritis and high risk for gastric cancer[10].
In clinical practice, in addition to evaluating the results of H. pylori serology as a categorical variable (i.e., positive or negative), it is also important to consider the titer of H. pylori antibodies because there is a relationship between the antibody titer and the risk of gastric cancer. We mainly use the E-plate Eiken kit as an anti-H. pylori antibody test in Japan. The cut-off titer of this kit for diagnosing H. pylori infection is ≥ 10 U/mL, while the lower sensitivity limit of this kit is 3 U/mL. Previous reports have defined the titer between 3 and 9.9 U/mL as negative-high titer, and the titer < 3 U/mL as a negative-low titer. Compared with the negative-low titer, the negative-high titer has been reported to carry a higher risk, especially for intestinal gastric cancer in subjects with gastric atrophy[10-12].
There are some false negative results when screening for current H. pylori infection in patients with an E-plate antibody titer of < 10 U/mL. H. pylori-infected patients accounted for 94% of patients with gastric cancer with an E-plate antibody titer of < 10 U/mL. Additionally, in patients with gastric cancer with an E-plate antibody titer of < 10 U/mL, H. pylori infection was associated with higher titers of antibodies[13].
Thus, seronegative-high titer antibody is associated with gastric cancer. However, the clinicopathological characteristics of negative-high titer patients, including the prevalence of H. pylori infection, have not been studied extensively. This study focused on serum negative-high titer antibody subjects without history of H. pylori eradication therapy and investigated the features of H. pylori-infected patients in the category.
MATERIALS AND METHODS
Subjects
We conducted this retrospective case-control study in patients with negative-high titer serum anti-H. pylori antibodies, who underwent esophagogastroduodenoscopy (EGD) and histological evaluation based on the updated Sydney system at Toyoshima Endoscopy Clinic between September 2016 to May 2017. EGDs were performed for screening, surveillance for gastrointestinal diseases, and investigation of some symptoms or abnormal results of the other assessments. We did not include subjects with history of gastric cancer, gastrectomy, H. pylori eradication therapy, and severe concomitant illnesses, and those who did not consent to this study. The following demographic characteristics were collected from the medical records: age, sex, body mass index (BMI), first-degree family history of gastric cancer, smoking history, and habitual drinking[14]. A score of at least 400 on the Brinkman index was defined as positive smoking history. Consumption of at least one drink of alcohol per day was defined as habitual.
This retrospective study was approved by the Ethical Review Committee of Hattori Clinic on September 7, 2017. Written informed consents were obtained from the participants. All clinical investigations were conducted according to the ethical guidelines of the Declaration of Helsinki.
Diagnosis of H. pylori and related findings
The H. pylori antibody titer was measured in the blood samples obtained at the time of the first visit or EGD. The antibody titer was measured using an enzyme immunoassay kit using antigens derived from Japanese individuals (E-plate Eiken H. pylori antibody II; Eiken Chemical, Tokyo, Japan). A negative-high titer was defined as 3-9.9 U/mL of anti-H. pylori antibodies.
UBT-positive individuals were defined as subjects with H. pylori infection[2,15,16]. We performed UBT using a 100 mg 13C-urea tablet (Pylonic; Sumitomo Dainippon Pharma, Osaka, Japan) after at least 2 wk of cessation of proton pump inhibitors or antibiotics. The result was declared negative if it was lower than 3 per mil.
Kyoto classification of gastritis is based on the sum of scores of the following five endoscopic findings, which are scored from 0 to 8: atrophy, intestinal metaplasia (IM), enlarged folds, nodularity, and redness. A high score represents increased risk for gastric cancer[13,17]. Gastric atrophy was classified according to the extent of mucosal atrophy as described by Kimura and Takemoto[14,18,19]. C-II and C-III of Kimura-Takemoto classification were scored as 1, and O-I to O-III as 2. IM is observed as grayish-whitish and slightly opalescent patches. IM within the antrum was scored as 1, and IM extending into the corpus as 2. The presence of folds enlarged over 5 mm or more was scored as 1. Nodularity is characterized by the appearance of multiple whitish elevated lesions mainly in the pyloric gland mucosa. The presence of nodularity was scored as 1. Diffuse redness refers to uniform redness involving the entire fundic gland mucosa. The presence of redness with regular arrangements of collecting venules was scored as 1, and that without regular arrangement of collecting venules as 2. We also considered the presence of gastric sticky mucus and gastroduodenal ulcer as positive findings of H. pylori infection. On the contrary, gastroesophageal reflux disease, hiatal hernia, and fundic gland polyp were considered as findings of absence of H. pylori infection. Sticky mucus refers to grayish or yellowish mucus that adheres to the mucosal surface prior to washing with water. Gastroduodenal ulcer scars were included in the positive group. Grade A or more severe of Los Angeles classification in gastroesophageal reflux disease was defined as positive. We defined hiatal hernia of 2 cm or more as positive. Figure 1A-F shows the representative endoscopic findings related to H. pylori infection in negative-high titer patients of this study.
Figure 1.
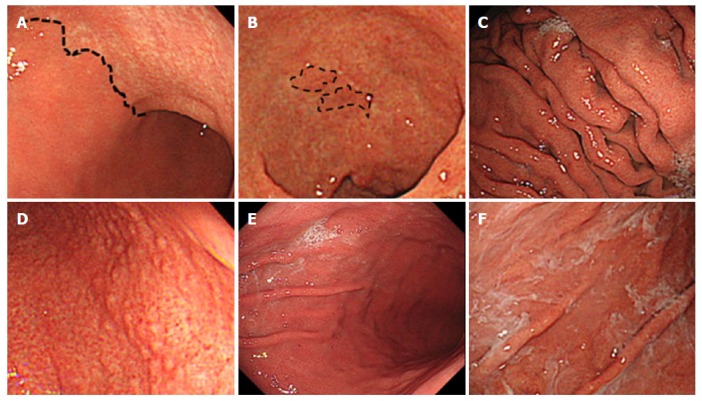
Endoscopic findings related to Helicobacter pylori infection. A: Atrophy is diagnosed based on the vascular pattern and rugal atrophy. The dotted line indicates an atrophic border in the anterior wall of the body (43-year-old woman; antibody titer: 4.3 U/mL; UBT: 55.3 per mil; Kyoto classification score: 2); B: Intestinal metaplasia is visible as grayish-whitish, slightly opalescent patches. The dotted line indicates the extent of the lesions in the lesser curvature of the antrum (81-year-old woman; antibody titer: 4.7 U/mL; UBT: 7.3 per mil; Kyoto classification score: 5); C: An enlarged fold is defined as that which is 5 mm or more in diameter. Enlarged folds are present in the greater curvature of the body (56-year-old man; antibody titer: 3.8 U/mL; UBT: 7.0 per mil; Kyoto classification score: 3); D: Nodularity is characterized by the appearance of multiple whitish elevated lesions mainly in the pyloric gland mucosa. Nodularity is present in the antrum (28-year-old man; antibody titer: 9.4 U/mL; UBT: 3.6 per mil; Kyoto classification score: 2); E: Redness refers to uniform redness involving the entire fundic gland mucosa. Redness is visible in the greater curvature of the body (44-year-old man; antibody titer: 8.7 U/mL; UBT: 26.5 per mil; Kyoto classification score: 3); F: Sticky mucus refers to grayish or yellowish mucus adhering to the mucosal surface. There is sticky mucus in the greater curvature of the body (70-year-old woman; antibody titer: 6.5 U/mL; UBT: 26.4 per mil; Kyoto classification score: 4). UBT: Urea breath test.
EGDs were performed by 14 expert physicians using Olympus Evis Lucera Elite system with endoscope: GIF-HQ290 or GIF-H290Z (Olympus Corporation, Tokyo, Japan). We carried out EGDs under conscious sedation with midazolam and/or pethidine hydrochloride. The EGD images were retrospectively reviewed by the chief investigator (OT). Any disagreements were resolved by consulting a third reviewer (TN). Discrepancies in diagnoses between the two sets of physicians were resolved through discussions.
Pathological findings were evaluated using the updated Sydney system score, including H. pylori density, neutrophil activity, chronic inflammation, IM, and glandular atrophy, with hematoxylin and eosin stains[20-22]. The biopsy samples were collected from the greater curvature of the corpus and antrum. We defined one or more score in either of the two points as present. The histological diagnosis was performed by an expert gastrointestinal pathologist, who was not an endoscopist, and was from another organization.
Statistical analysis
First, we evaluated the effects of age, sex, BMI, family history of gastric cancer, smoking, habitual drinking, serum anti-H. pylori antibody titer, endoscopic findings, and histological findings on H. pylori infection in univariate analysis using Fisher’s exact test or Cochran-Armitage test for categorical variables and Mann-Whitney U test for quantitative variables. Next, the values of the area under the receiver operating characteristic curve (AUC) for predicting H. pylori infection were compared with the value of 0.5 using the chi-squared test. The cut-off values for predicting H. pylori infection were estimated using the Youden index, which is the farthest point on the receiver operating characteristic curve from the positive diagonal[23]. We compared AUC values with the use of a chi-square test. Then, the performances of the endoscopic and histological findings for H. pylori infection, including accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were investigated. The predictors associated with H. pylori infection were subsequently assessed using multiple logistic regression analysis to distinguish the independent factors from other demographic and laboratory variables. A two-sided P value less than 0.05 was considered as significant. The data were analyzed using Ekuseru-Toukei 2015 software (Social Survey Research Information, Tokyo, Japan).
RESULTS
The characteristics of the participants of the present study are shown in Table 1. A total of 136 subjects were enrolled. The median age of the subjects was 45 (range: 17-82, interquartile range: 37-56) years, and 39% were males. The median titer of H. pylori antibody was 4.7 (interquartile range, 3.7-6.6) U/mL. Seventeen percent (n = 23) were diagnosed as H. pylori-infected based on UBT.
Table 1.
Characteristics of enrolled subjects
| Total | H. pylori infected1 | H. pylori uninfected | P value3 | |
| n (%) | 136 | 23 (17) | 113 (83) | |
| Demographic characteristics | ||||
| Age median (IQR), yr | 45 (37-56) | 53 (44-68) | 42 (35-53) | 0.0057 |
| Male sex (%) | 53 (39) | 7 (30) | 46 (41) | 0.48 |
| Body mass index median (IQR), kg/m2 | 21.2 (19.6-23.7) | 22.7 (20.4-25.6) | 21.2 (19.5-23.3) | 0.028 |
| Family history of gastric cancer, present/absent | 12/124 | 3/20 | 9/104 | 0.43 |
| Smoking, present/absent | 4/132 | 0/23 | 4/109 | 1.0 |
| Drinking, present/absent | 25/111 | 4/19 | 21/92 | 1.0 |
| Laboratory data | ||||
| Anti-H. pylori antibody median (IQR), U/mL | 4.7 (3.7-6.6) | 5.4 (4.2-7.9) | 4.7 (3.7-6.4) | 0.048 |
| 13C-urea breath test result median (IQR), per mil | 0.3 (0.1-0.8) | 19.3 (9.3-26.3) | 0.3 (0.1-0.4) | 3.6 × 10-14 |
| Endoscopic findings | ||||
| Kyoto classification of gastritis4, 5/4/3/2/1/0 | 1/3/14/9/13/96 | 1/3/8/6/2/3 | 0/0/6/3/11/93 | 3.8 × 10-13 |
| Atrophy, 2/1/0 | 15/20/101 | 10/4/2009 | 6/10/97 | 5.8 × 10-12 |
| Intestinal metaplasia, 1/0 | 14/122 | 9/14 | 5/108 | 2.9 × 10-5 |
| Enlarged folds, 1/0 | 5/131 | 4/19 | 1/112 | 0.0029 |
| Nodularity, 1/0 | 2/134 | 2/21 | 0/113 | 0.028 |
| Redness, 1/0 | 19/117 | 12/11 | 7/106 | 7.3 × 10-7 |
| Gastric sticky mucus, present/absent | 22/114 | 8/15 | 14/99 | 0.013 |
| Gastric ulcer, present/absent | 1/135 | 1/22 | 0/113 | 0.17 |
| Duodenal ulcer, present/absent | 3/133 | 1/22 | 2/111 | 0.43 |
| Gastroesophageal reflux disease, present/absent | 20/116 | 2/21 | 18/95 | 0.53 |
| Hiatal hernia, present/absent | 18/118 | 2/21 | 16/97 | 0.74 |
| Fundic gland polyp, present/absent | 41/95 | 1/22 | 40/73 | 0.0022 |
| Histological findings5 | ||||
| H. pylori density, present/absent | 18/118 | 18/5 | 0/113 | 2.8 × 10-18 |
| Chronic inflammation, present/absent | 46/90 | 21/2 | 25/88 | 4.5 × 10-10 |
| Neutrophil activity, present/absent | 15/121 | 15/8 | 0/113 | 1.4 × 10-14 |
| Intestinal metaplasia, present/absent | 4/132 | 1/22 | 3/110 | 0.53 |
| Glandular atrophy, present/absent | 4/132 | 1/22 | 3/110 | 0.53 |
1 13C-urea breath test-positive subjects were defined as H. pylori-infected patients;
Fisher’s exact test, Cochran-Armitage test, or Mann-Whitney U test was used as appropriate;
Kyoto classification of gastritis was estimated by gastric atrophy, intestinal metaplasia, enlarged folds, nodularity, and redness[13];
We defined one or more score classified by the updated Sydney system in either the great curvature of the corpus or the antrum as present. H. pylori: Helicobacter pylori; IQR: Interquartile range.
On comparing H. pylori-infected and -uninfected patients regarding the demographic characteristics and laboratory data, H. pylori-infected patients were older (53 years vs 42 years, P = 0.0057), and had higher BMI (22.7 kg/m2 vs 21.2 kg/m2, P = 0.028) and serum antibody titer (5.4 U/mL vs 4.7 U/mL, P = 0.048). No significant differences due to sex, family history of gastric cancer, habitual smoking, or habitual drinking were demonstrated. Regarding endoscopic findings, we found significant differences between them in Kyoto classification of gastritis score (P = 3.8 × 10-13), gastric sticky mucus (P = 0.013), and fundic gland polyp (P = 0.0022). Histologically, H. pylori density (P = 2.8 × 10-18), chronic inflammation (P = 4.5 × 10-10), and neutrophil activity (P = 1.4 × 10-14) were significantly different between the two groups (Table 1).
Then, we analyzed AUC for predicting H. pylori infection based on the variables that had significant differences between H. pylori-infected and -uninfected patients (Table 2). AUC of H. pylori density (0.891, 95%CI: 0.805-0.977, P = 5.6 × 10-19) was the largest followed by those of Kyoto classification (0.886, 95%CI: 0.803-0.968, P = 3.7 × 10-20) and endoscopic atrophy (0.848, 95%CI: 0.760-0.936, P = 7.7 × 10-15). There was no significant difference between the three AUC values. The cut-off value of Kyoto classification of gastritis score for correlation with H. pylori infection was 2 and that of endoscopic atrophy was 1. The receiver operating characteristic curves based on Kyoto endoscopic classification, serum antibody titer, and age in 136 patients with negative-high titer antibody are shown in Figure 2.
Table 2.
Area under the receiver operating characteristic curve for predicting Helicobacter pylori infection
| AUC | 95%CI | P value | |
| Age | 0.684 | 0.564-0.804 | 0.0027 |
| Body mass index | 0.646 | 0.518-0.774 | 0.026 |
| Serum antibody titer | 0.631 | 0.500-0.763 | 0.051 |
| Kyoto classification of gastritis | 0.886 | 0.803-0.968 | 3.7 × 10-20 |
| Endoscopic atrophy | 0.848 | 0.760-0.936 | 7.7 × 10-15 |
| Endoscopic intestinal metaplasia | 0.674 | 0.570-0.777 | 0.0010 |
| Enlarged fold | 0.583 | 0.503-0.662 | 0.042 |
| Nodularity | 0.543 | 0.485-0.602 | 0.15 |
| Redness | 0.730 | 0.623-0.837 | 2.4 × 10-5 |
| Gastric sticky mucus | 0.612 | 0.508-0.716 | 0.035 |
| Fundic gland polyp | 0.655 | 0.594-0.717 | 7.4 × 10-7 |
| H. pylori density | 0.891 | 0.805-0.977 | 5.6 × 10-19 |
| Chronic inflammation | 0.846 | 0.776-0.916 | 5.3 × 10-22 |
| Neutrophil activity | 0.826 | 0.727-0.926 | 1.3 × 10-10 |
Positive urea breath test was defined as H. pylori infection. The values of the AUC were compared with the value of 0.5 using the chi-square test. AUC: Area under the receiver operating characteristics curve; CI: Confidence interval; H. pylori: Helicobacter pylori.
Figure 2.
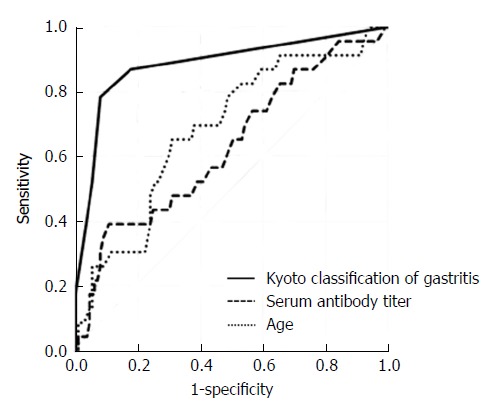
Receiver operating characteristics curves for predicting Helicobacter pylori infection. Receiver operating characteristics curves were based on endoscopic Kyoto classification of gastritis score, serum antibody titer, and age in 136 patients with negative-high titer antibody. Positive UBT was defined as H. pylori infection. UBT: Urea breath test.
The performances of endoscopic and histological findings for H. pylori infection are shown in Table 3. The highest accuracy was found in histological H. pylori density (96.3%), and its specificity and PPV were 100%. H. pylori density also had the second highest NPV (95.8%). The second highest accuracy was in neutrophil activity (94.1%), and its specificity and PPV were 100%. With regards to endoscopic findings, Kyoto classification of gastritis showed the highest accuracy (89.7%). The accuracies of redness, IM, atrophy (1 or more score as positive), enlarged folds, and nodularity followed that of Kyoto classification in order. The highest sensitivity (91.3%) and highest NPV (97.8%) were shown with histological chronic inflammation.
Table 3.
Performance of endoscopic and histological findings for Helicobacter pylori infection
| Accuracy | Sensitivity | Specificity | PPV | NPV | |
| Endoscopic findings | |||||
| Kyoto classification of gastritis1 | 89.7 | 78.3 | 92.0 | 66.7 | 95.4 |
| Atrophy2 | 85.3 | 82.6 | 85.8 | 54.3 | 96.0 |
| Intestinal metaplasia | 86.0 | 39.1 | 95.6 | 64.3 | 88.5 |
| Enlarged folds | 85.3 | 17.4 | 99.1 | 80.0 | 85.5 |
| Nodularity | 84.6 | 8.7 | 100 | 100 | 84.3 |
| Redness | 86.8 | 52.2 | 93.8 | 63.2 | 90.6 |
| Gastric sticky mucus | 78.7 | 34.8 | 87.6 | 36.4 | 86.8 |
| Gastric ulcer | 83.8 | 4.3 | 100 | 50.0 | 83.7 |
| Duodenal ulcer | 82.4 | 4.3 | 98.2 | 33.3 | 83.5 |
| Gastroesophageal reflux disease | 71.3 | 8.7 | 84.1 | 10.0 | 81.9 |
| Hiatal hernia | 72.8 | 8.7 | 85.8 | 11.1 | 82.2 |
| Fundic gland polyp | 54.4 | 4.3 | 64.6 | 2.4 | 76.8 |
| Histological findings | |||||
| H. pylori density | 96.3 | 78.3 | 100 | 100 | 95.8 |
| Chronic inflammation | 80.1 | 91.3 | 77.9 | 45.7 | 97.8 |
| Neutrophil activity | 94.1 | 65.2 | 100 | 100 | 93.4 |
| Intestinal metaplasia | 81.6 | 4.3 | 97.3 | 25.0 | 83.3 |
| Glandular atrophy | 81.6 | 4.3 | 97.3 | 25.0 | 83.3 |
A score of 2 or more was defined as positive;
A score of 1 or more was defined as positive. The data are presented as %. Positive urea breath test was defined as H. pylori infection. PPV: Positive predictive value; NPV: Negative predictive value; H. pylori: Helicobacter pylori.
Lastly, Kyoto classification was assessed using multivariate logistic regression analysis to identify any association with the variables such as age, BMI, and serum antibody titer. Kyoto classification was identified as an independent predictor of H. pylori infection (P = 2.2 × 10-6, Table 4).
Table 4.
Multivariate analysis for independent predictors of Helicobacter pylori infection
| Odds ratio | 95%CI | P value | |
| Age | 0.98 | 0.93-1.03 | 0.49 |
| Body mass index | 1.06 | 0.90-1.24 | 0.50 |
| Serum antibody titer | 1.21 | 0.87-1.68 | 0.26 |
| Kyoto classification of gastritis | 4.23 | 2.33-7.67 | 2.2 × 10-6 |
Representative endoscopic findings of negative-high titer cases with or without H. pylori infection are demonstrated in Figure 3A-F.
Figure 3.
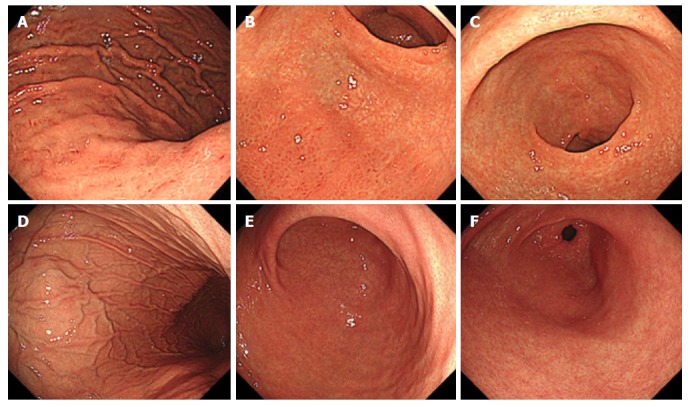
Representative endoscopic findings of negative-high titer antibody cases. A case with Helicobacter pylori infection; 81-year-old woman with antibody titer of 4.7 U/mL, UBT of 7.3 per mil, and Kyoto classification score of 5 (A-C). A: Greater curvature of the body of the stomach. Enlarged folds and redness are present; B: Lower body of the stomach. Endoscopic atrophic border lies in the anterior wall and greater curvature. Redness is present in the greater curvature; C: Antrum. Intestinal metaplasia is present in the lesser curvature. The mucosa is atrophic. A case without H. pylori infection; 31-year-old man with antibody titer of 5.7 U/mL, UBT of 1.2 per mil, and Kyoto classification score of 0 (D-F); D: The greater curvature of the body of the stomach. Regular arrangement of collecting venules and fundic gland polyps are present; E: Lower body of the stomach. Atrophy and redness are absent; F: Antrum. Intestinal metaplasia and atrophy are absent. UBT: Urea breath test.
DISCUSSION
We found that 17% of subjects with negative-high titer serum anti-H. pylori antibody were positive for H. pylori infection. Higher bacterial counts induce intense immune responses, resulting in subsequent higher antibody titers, while genetic differences between human hosts may affect the antibody levels in response to pathogens[24]. Precise diagnosis in patients with seronegativity is necessary to reduce the false negative estimation of gastric cancer risk[13]. We should identify H. pylori-infected cases in negative-high titer patients and carefully examine them.
Endoscopic Kyoto classification of gastritis proved to be an excellent predictor of H. pylori infection with large AUC (0.886), cut-off value of 2, high accuracy (89.7%), and was comparable to histological H. pylori density, indicating its high confidence. Kyoto classification also demonstrated to be independent of demographic and laboratory data. These results show that Kyoto classification is useful in the diagnosis of H. pylori infection among negative high-titer serum antibody patients. Endoscopic atrophy and nodularity have been attributed to H. pylori infection consistently, as was also seen with our results[25-28].
Kyoto classification score is believed to provide an estimate of the risk of gastric cancer. Sugimoto et al[29] showed that the mean Kyoto classification score in gastric cancer group was 4.6 ± 1.2, which was significantly higher than in gastritis-alone group (3.8 ± 1.1; P < 0.001). In subgroup analysis within the cancer group, the mean Kyoto classification score in the H. pylori-uneradicated subgroup was 4.8 ± 1.1, which was significantly higher than that in the eradicated subgroup (4.2 ± 1.2; P < 0.001). Our study showed that Kyoto classification score might be useful for not only estimating the risk of gastric cancer but also the prediction of H. pylori infection in negative-high titer patients.
Cases with negative-high titer antibodies with negative UBT could include not only subjects who have never been infected but also patients in whom infections resolved spontaneously[10,30]. Patients with spontaneous resolution are known to be at very high risk for gastric cancer. In this study, nine patients with Kyoto classification score 2 or more had negative results with UBT. These cases might be after spontaneous disappearance of H. pylori infection. Such patients would need careful surveillance.
Histological H. pylori density was the strongest contributing factor to H. pylori infection with the largest AUC and highest accuracy (96.3%). Neutrophil activity had the second highest accuracy (94.1%). Several investigators have inferred significant associations of anti-H. pylori antibody titers with H. pylori density and neutrophil activity[25,31-33]. Our findings are in accordance with their reports. Chronic inflammation had the highest sensitivity and NPV. Chronic inflammation has been reported to progress in parallel with increases in serum anti-H. pylori antibodies, and our results are consistent with this observation[25,28,32].
H. pylori-infected patients were older than the uninfected patients among negative-high titer antibody participants. Kiso et al[13] reported that in serum H. pylori antibody-negative subjects, those with H. pylori infection and gastric cancer were older than those with gastric cancer but without the infection. Our results were concordant with their results. In this study, the BMI of H. pylori-infected patients was higher than that of -uninfected patients. Our results are in agreement with the results of a report that concluded a positive association between being overweight and serum H. pylori antibody[34].
There are some limitations to this study. We used UBT as the gold standard for H. pylori infection; however, its accuracy is not 100%. Better performance in serological screening depends on the use of the appropriate antigens and adjustment of cut-off values[35]. As we used antibodies against the Japanese strain, further investigation of the other antibodies is needed. Non-H. pylori Helicobacter species, including H. suis and H. felis, could provoke serum anti-H. pylori antibody positivity[36], and anti-H. pylori antibody correlates with the presence of cytotoxin associated gene A-positive strains[37]; however, we did not assess them. Furthermore, we did not analyze the long-term outcomes in 17% of the patients with negative-high titer anti-H. pylori antibodies without history of eradication therapy who had H. pylori infection. Further studies should be performed to analyze the long-term outcomes and the association between the presence of CagA positive H. pylori infection and Kyoto classification.
In conclusion, 17% of the patients with negative-high titer serum anti-H. pylori antibodies without history of eradication therapy had H. pylori infection. Endoscopic Kyoto classification of gastritis with a score of 2 or more could predict H. pylori infection in negative high-titer patients. Further examination including UBT should be considered in these patients with Kyoto classification score 2 or more.
ARTICLE HIGHLIGHTS
Research background
Patients who test negative but in the negative-high titer range of serum anti-Helicobacter pylori (H. pylori) antibodies are at a high risk for gastric cancer, especially the intestinal type, and sometimes have H. pylori infection. Patients with negative-high titers with H. pylori infection have higher risk for gastric cancer than do those without H. pylori infection.
Research motivation
The clinicopathological features including H. pylori infection rate in the negative-high titer patients are unclear.
Research objectives
The objective of this research was to elucidate the clinicopathological features of the negative-high titer patients.
Research methods
The antibody titers were measured using antigens derived from Japanese individuals, E-plate Eiken. 13C-urea breath test (UBT)-positive individuals were defined as having H. pylori infection. We investigated the demographic characteristics, laboratory data, endoscopic findings including Kyoto classification of gastritis, and histology in negative-high titer patients without history of H. pylori eradication therapy.
Research results
Of the 136 subjects enrolled, 23 (17%) had H. pylori infection. Kyoto classification had an excellent area under the receiver operating characteristics curve (0.886) for predicting H. pylori infection, with a cut-off value of 2. Further, Kyoto classification had high accuracy (89.7%). Kyoto classification was independent of the demographic and laboratory parameters in multivariate analysis.
Research conclusions
In this study, 17% of patients with negative-high titer had H. pylori infection. Endoscopic Kyoto classification of gastritis with a score of 2 or more could predict H. pylori infection in negative high-titer patients. Further investigations including UBT should be considered in these patients.
Research perspectives
Long-term prospective studies are expected to investigate the role of serum antibody titer and Kyoto classification of gastritis in predicting not only H. pylori infection but also the risk of gastric cancer.
ACKNOWLEDGEMENTS
We would also like to thank Kanazawa T, Matsumoto S, Isomura Y, Arano T, Kinoshita H, Ohki D, Fukagawa K, and Sekiba K for performing esophagogastroduodenoscopy and Sugita K, Sakurai C, and Yamakawa T for collecting data.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This retrospective study was approved by the Ethical Review Committee of Hattori Clinic on September 7, 2017.
Informed consent statement: Written informed consents were obtained from the participants.
Conflict-of-interest statement: During the last five years, Toyoshima O received personal fees from Otsuka Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. outside of the submitted work; Suzuki H received scholarship funds for the research from Astellas Pharma Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd. and received service honoraria from Astellas Pharma, Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Peer-review started: February 25, 2018
First decision: March 9, 2018
Article in press: March 18, 2018
P- Reviewer: Engin AB, Papamichail K, Park WS S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Osamu Toyoshima, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan. t@ichou.com.
Toshihiro Nishizawa, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan.
Masahide Arita, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan.
Yosuke Kataoka, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan.
Kosuke Sakitani, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan.
Shuntaro Yoshida, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan.
Hiroharu Yamashita, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan.
Keisuke Hata, Department of Gastroenterology, Toyoshima Endoscopy Clinic, Tokyo 1570066, Japan.
Hidenobu Watanabe, Department of Pathology, Pathology and Cytology Laboratory Japan, Tokyo 1660003, Japan.
Hidekazu Suzuki, Medical Education Center, Keio University School of Medicine, Tokyo 1608582, Japan.
References
- 1.Cancer IAfRo. Helicobacter pylori eradication as a strategy for preventing gastric cancer. IARC Working Group Reports. Volume 8 [Google Scholar]
- 2.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 3.Nishizawa T, Suzuki H, Fujimoto A, Kinoshita H, Yoshida S, Isomura Y, Toyoshima A, Kanai T, Yahagi N, Toyoshima O. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr. 2017;60:208–210. doi: 10.3164/jcbn.16-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burucoa C, Delchier JC, Courillon-Mallet A, de Korwin JD, Mégraud F, Zerbib F, Raymond J, Fauchère JL. Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter. 2013;18:169–179. doi: 10.1111/hel.12030. [DOI] [PubMed] [Google Scholar]
- 5.Ueda J, Okuda M, Nishiyama T, Lin Y, Fukuda Y, Kikuchi S. Diagnostic accuracy of the E-plate serum antibody test kit in detecting Helicobacter pylori infection among Japanese children. J Epidemiol. 2014;24:47–51. doi: 10.2188/jea.JE20130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:1–8. doi: 10.1111/j.1523-5378.2012.00975.x. [DOI] [PubMed] [Google Scholar]
- 7.Parente F, Sainaghi M, Sangaletti O, Imbesi V, Maconi G, Anderloni A, Bianchi Porro G. Different effects of short-term omeprazole, lansoprazole or pantoprazole on the accuracy of the 13C-urea breath test. Aliment Pharmacol Ther. 2002;16:553–557. doi: 10.1046/j.1365-2036.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- 8.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaji Y, Mitsushima T, Ikuma H, Okamoto M, Yoshida H, Kawabe T, Shiratori Y, Saito K, Yokouchi K, Omata M. Inverse background of Helicobacter pylori antibody and pepsinogen in reflux oesophagitis compared with gastric cancer: analysis of 5732 Japanese subjects. Gut. 2001;49:335–340. doi: 10.1136/gut.49.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishikawa H, Kimura K, Takarabe S, Kaida S, Nishida J. Helicobacter pylori Antibody Titer and Gastric Cancer Screening. Dis Markers. 2015;2015:156719. doi: 10.1155/2015/156719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaji Y, Mitsushima T, Ikuma H, Okamoto M, Yoshida H, Kawabe T, Shiratori Y, Saito K, Yokouchi K, Omata M. Weak response of helicobacter pylori antibody is high risk for gastric cancer: a cross-sectional study of 10,234 endoscoped Japanese. Scand J Gastroenterol. 2002;37:148–153. doi: 10.1080/003655202753416795. [DOI] [PubMed] [Google Scholar]
- 12.Tatemichi M, Sasazuki S, Inoue M, Tsugane S; JPHC Study Group. Clinical significance of IgG antibody titer against Helicobacter pylori. Helicobacter. 2009;14:231–236. doi: 10.1111/j.1523-5378.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiso M, Yoshihara M, Ito M, Inoue K, Kato K, Nakajima S, Mabe K, Kobayashi M, Uemura N, Yada T, et al. Characteristics of gastric cancer in negative test of serum anti-Helicobacter pylori antibody and pepsinogen test: a multicenter study. Gastric Cancer. 2017;20:764–771. doi: 10.1007/s10120-016-0682-5. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa T, Suzuki H, Sakitani K, Yamashita H, Yoshida S, Hata K, Kanazawa T, Fujiwara N, Kanai T, Yahagi N, et al. Family history is an independent risk factor for the progression of gastric atrophy among patients with Helicobacter pylori infection. United European Gastroenterol J. 2017;5:32–36. doi: 10.1177/2050640616642341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gisbert JP, Calvet X. Helicobacter Pylori “Test-and-Treat” Strategy for Management of Dyspepsia: A Comprehensive Review. Clin Transl Gastroenterol. 2013;4:e32. doi: 10.1038/ctg.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, Altayar O, Limburg PJ, Murad MH, Knawy B. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21:1305–1314. doi: 10.3748/wjg.v21.i4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017;32:1581–1586. doi: 10.1111/jgh.13764. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87–97. [Google Scholar]
- 19.Toyoshima O, Yamaji Y, Yoshida S, Matsumoto S, Yamashita H, Kanazawa T, Hata K. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc. 2017;31:2140–2148. doi: 10.1007/s00464-016-5211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 22.Toyoshima O, Tanikawa C, Yamamoto R, Watanabe H, Yamashita H, Sakitani K, Yoshida S, Kubo M, Matsuo K, Ito H, et al. Decrease in PSCA expression caused by Helicobacter pylori infection may promote progression to severe gastritis. Oncotarget. 2017;9:3936–3945. doi: 10.18632/oncotarget.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubicz R, Leach CT, Kraig E, Dhurandhar NV, Duggirala R, Blangero J, Yolken R, Göring HH. Genetic factors influence serological measures of common infections. Hum Hered. 2011;72:133–141. doi: 10.1159/000331220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamada T, Sugiu K, Hata J, Kusunoki H, Hamada H, Kido S, Nagashima Y, Kawamura Y, Tanaka S, Chayama K, et al. Evaluation of endoscopic and histological findings in Helicobacter pylori-positive Japanese young adults. J Gastroenterol Hepatol. 2006;21:258–261. doi: 10.1111/j.1440-1746.2006.04128.x. [DOI] [PubMed] [Google Scholar]
- 26.Adachi K, Mishiro T, Tanaka S, Kinoshita Y. Analysis of negative result in serum anti-H. pylori IgG antibody test in cases with gastric mucosal atrophy. J Clin Biochem Nutr. 2016;59:145–148. doi: 10.3164/jcbn.16-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M, Kato J, Inoue I, Yoshimura N, Yoshida T, Mukoubayashi C, Deguchi H, Enomoto S, Ueda K, Maekita T, et al. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131:2632–2642. doi: 10.1002/ijc.27514. [DOI] [PubMed] [Google Scholar]
- 28.Bruden DL, Bruce MG, Miernyk KM, Morris J, Hurlburt D, Hennessy TW, Peters H, Sacco F, Parkinson AJ, McMahon BJ. Diagnostic accuracy of tests for Helicobacter pylori in an Alaska Native population. World J Gastroenterol. 2011;17:4682–4688. doi: 10.3748/wjg.v17.i42.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579–586. doi: 10.2169/internalmedicine.56.7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergey B, Marchildon P, Peacock J, Mégraud F. What is the role of serology in assessing Helicobacter pylori eradication? Aliment Pharmacol Ther. 2003;18:635–639. doi: 10.1046/j.1365-2036.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 31.Kreuning J, Lindeman J, Biemond I, Lamers CB. Relation between IgG and IgA antibody titres against Helicobacter pylori in serum and severity of gastritis in asymptomatic subjects. J Clin Pathol. 1994;47:227–231. doi: 10.1136/jcp.47.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheu BS, Shiesh SC, Yang HB, Su IJ, Chen CY, Lin XZ. Implications of Helicobacter pylori serological titer for the histological severity of antral gastritis. Endoscopy. 1997;29:27–30. doi: 10.1055/s-2007-1004057. [DOI] [PubMed] [Google Scholar]
- 33.Park CH, Kim EH, Jung DH, Chung H, Park JC, Shin SK, Lee SK, Lee YC. The new modified ABCD method for gastric neoplasm screening. Gastric Cancer. 2016;19:128–135. doi: 10.1007/s10120-015-0473-4. [DOI] [PubMed] [Google Scholar]
- 34.Thjodleifsson B, Olafsson I, Gislason D, Gislason T, Jögi R, Janson C. Infections and obesity: A multinational epidemiological study. Scand J Infect Dis. 2008;40:381–386. doi: 10.1080/00365540701708293. [DOI] [PubMed] [Google Scholar]
- 35.Hoang TT, Wheeldon TU, Bengtsson C, Phung DC, Sörberg M, Granström M. Enzyme-linked immunosorbent assay for Helicobacter pylori needs adjustment for the population investigated. J Clin Microbiol. 2004;42:627–630. doi: 10.1128/JCM.42.2.627-630.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flahou B, Haesebrouck F, Smet A, Yonezawa H, Osaki T, Kamiya S. Gastric and enterohepatic non-Helicobacter pylori Helicobacters. Helicobacter. 2013;18 Suppl 1:66–72. doi: 10.1111/hel.12072. [DOI] [PubMed] [Google Scholar]
- 37.Loffeld RJ, Werdmuller BF, Kusters JG, Kuipers EJ. IgG antibody titer against Helicobacter pylori correlates with presence of cytotoxin associated gene A-positive H. pylori strains. FEMS Immunol Med Microbiol. 2000;28:139–141. doi: 10.1111/j.1574-695X.2000.tb01468.x. [DOI] [PubMed] [Google Scholar]


