Abstract
AIM
To investigate the prognostic value of the combination of preoperative plasma fibrinogen and CA199 in patients with gallbladder carcinoma (GBC).
METHODS
The clinicopathological data of 154 GBC patients were retrospectively reviewed after surgery. A receiver operating characteristic (ROC) curve was plotted to verify the optimum cut-off values for plasma fibrinogen and CA199. Univariate and multivariate survival analyses were performed to identify the factors associated with GBC prognosis. Based on the HRs calculated via multivariate survival analyses, patients with elevated plasma fibrinogen and CA199 levels were allocated a score of 2.1; those with an elevated plasma fibrinogen level only were allocated a score of 1, those with an elevated CA199 level only were allocated a score of 1.1, and those with neither of these abnormalities were allocated a score of 0.
RESULTS
ROC curve analysis showed that the optimum cut-off values for preoperative plasma fibrinogen and CA199 were 3.47 g/L and 25.45 U/mL, respectively. Multivariate analysis indicated that elevated preoperative plasma fibrinogen and CA199 levels were significantly correlated with worse overall survival (OS) (HR = 1.711, 95%CI: 1.114-2.627, P = 0.014, and HR = 1.842, 95%CI: 1.111-3.056, P = 0.018). When we combined these two parameters, the area under the ROC curve increased from 0.735 (for preoperative plasma fibrinogen only) and 0.729 (for preoperative CA199 only) to 0.765. When this combined variable was added to the multivariate analysis, the combination of plasma fibrinogen and CA199 (P < 0.001), resection margin (P < 0.001) and TNM stage (P = 0.010) were independent prognostic factors for GBC.
CONCLUSION
The combination of plasma fibrinogen and CA199 may serve as a more efficient independent prognostic biomarker for postoperative GBC patients than either parameter alone.
Keywords: Prognostic factor, Plasma fibrinogen, CA199, Survival, Gallbladder cancer
Core tip: Elevated plasma fibrinogen and CA199 levels are associated with poor prognosis in patients with gallbladder carcinoma (GBC). The prognostic value of the combination of plasma fibrinogen and CA199 for GBC has not been reported. The most important finding in this study was that the combination of preoperative plasma fibrinogen and CA199 levels was a better independent prognostic indicator for GBC than either parameter alone.
INTRODUCTION
Primary gallbladder carcinoma (GBC) is relatively rare worldwide, but is the most common malignancy of the biliary tract system[1]. GBC is the seventh most common gastrointestinal cancer[2] and is attributable to approximately 1% of all cancer cases in China[3]. The incidence of this malignancy was recently reported to be approximately 2.5 per 100000 persons[4]. The prognosis of GBC is still typically poor due to nonspecific symptoms, late diagnosis, lack of treatment options, and the absence of effective prognostic markers. According to epidemiological studies, the overall survival (OS) of GBC patients is 6 mo, with a 5-year survival rate of less than 10%[1,5-7]. Therefore, investigations on the prognostic factors of GBC are especially important.
The association between hemostasis and cancer, and the influence of hemostatic factors on cancer development, growth, and metastasis are evident[8,9]. Fibrinogen is a 340-kDa plasma glycoprotein that is upregulated during systemic inflammation and tissue injury. Fibrinogen is synthesized in the liver and transformed into fibrin through the activity of activated thrombin, which is a key coagulation factor in platelet aggregation, clot formation, wound healing, and coagulation[10-12]. A number of studies have shown that plasma fibrinogen levels are upregulated in various cancer types, such as respiratory system tumors[13,14], digestive system tumors[15-18], gynecological tumors[19-22], head and neck cancer[23,24] and genitourinary tumors[25,26], and may indicate cancer progression, metastasis and recurrence[17,22,27-29]. However, to our knowledge, studies on the prognostic value of plasma fibrinogen levels in GBC are very rare[30].
In addition, CA199 has been traditionally used for the diagnosis and prognosis of GBC[31]; however, the reported results on its prognostic value in GBC patients are inconsistent and controversial[30,32,33]. Therefore, we hypothesized that the combination of plasma fibrinogen and CA199 levels may avoid inconsistent results and increase the prognostic accuracy for GBC.
Hence, the aim of the current study was to investigate the prognostic value of the combination of plasma fibrinogen and CA199 levels in patients with GBC. Additionally, we aimed to determine whether the combination of plasma fibrinogen and CA199 levels can serve as a more efficient predictive factor than either parameter alone in patients with GBC.
MATERIALS AND METHODS
Study population
From January 2005 to May 2017, a retrospective analysis of 154 GBC patients was conducted following surgery in the Department of Liver Surgery at the Peking Union Medical College Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC). The patients included in the analysis met the following criteria: (1) GBC diagnosis confirmed by histopathology and cancer stage determined in accordance with the American Joint Committee on Cancer staging system, 8th Edition (AJCC-8), and the histopathologic postoperative pathologic tumor-node-metastasis (pTNM) categorization system (International Union against Cancer Staging Manual, 7th Edition; UICC-7); and (2) no adjuvant chemotherapy and/or radiotherapy before gallbladder resection surgery. Patients with the following characteristics were excluded: (1) other tumors; (2) inflammatory conditions, including infections, collagen diseases, anemia, other diseases concerning the hematological system, and absolute cardiovascular and cerebrovascular disorders; (3) liver disease; (4) oral administration of anticoagulants or acetylsalicylic acids within 3 mo before surgery; (5) lack of adequate clinical data or loss to follow-up. This study was approved by the Medical Ethics Committee of the Peking Union Medical College Hospital of the CAMS & PUMC, and all participants signed written informed consent forms.
Data collection
Patient characteristics were obtained via a retrospective medical record review using a standardized data collection form. Based on the medical records, the following data were collected for each patient: age, gender, plasma fibrinogen concentration, CA199 level, tumor size (defined as the longest diameter of the general postoperative pathological specimens), gallstone history, jaundice, comorbidity (diabetes), resection margin, tumor differentiation (categorized as poorly differentiated, moderately differentiated and well differentiated), T stage, N stage, M stage, pTNM stage (as defined by AJCC-8), pathological type and other miscellaneous characteristics.
Plasma fibrinogen concentration and CA199 level
The plasma fibrinogen concentration and CA199 level were measured within 3 d before surgery as part of a routine workup in these patients. The fibrinogen concentration was measured based on the Clauss method as previously described[34], and the CA199 level was determined via an electrochemiluminescence immunoassay at the Department of Liver Surgery of the Peking Union Medical Hospital affiliated to Peking Union Medical College, Beijing, China. According to the assay protocols, the normal reference values were as follows: serum fibrinogen concentration ≤ 4.0 g/L and CA199 level ≤ 39 U/mL.
Clinical treatment and follow-up assessments
All patients were treated by modified radical cholecystectomy or radical cholecystectomy and received systemic therapy in the adjuvant setting. All patients were followed via telephone interviews. The patients were carefully followed at 3-mo intervals for the first 2 years after surgery, at 6-mo intervals during the third year, and at 1-year intervals thereafter. The date of surgery marked the beginning of the follow-up period, which ended at the last follow-up visit (December 2017) or death.
Statistical analysis
Continuous variables are expressed as means ± standard deviation for normally distributed variables (Kolmogorov-Smirnov test, P > 0.05) or as medians (range) for non-normally distributed variables, and categorical variables were expressed as frequencies and percentages. OS was defined as the time from surgery to death from any cause or the last follow-up. A receiver operating characteristic (ROC) curve for OS prediction was constructed to estimate the optimal cut-off value for plasma fibrinogen, which allowed us to treat this parameter as a binary variable. The optimal cut-off value was determined as the point on the ROC curve that maximizes the Youden index. The area under the ROC curve (AUC) was used to calculate discrimination ability. The associations between clinicopathological variables and pretreatment plasma fibrinogen levels were assessed using either the chi-square test or the trend version of the chi-square test, as appropriate. Survival curves were generated using the Kaplan-Meier method, and the log-rank test was used to evaluate survival differences between groups. A univariate analysis using the log-rank test was performed to screen variables that could potentially predict prognosis. The statistically significantly predictive variables were then included in a multivariate Cox regression model to determine the independent prognostic risk factors. Statistical analysis of the data was performed using Statistical Package for the Social Sciences (SPSS®, version 24.0; IBM Corp., Armonk, NY, United States). Statistical significance was defined as a two-sided P < 0.05.
RESULTS
Patient characteristics
The detailed baseline clinicopathological characteristics of the 154 GBC patients are displayed in Table 1. There were 91 (59.1%) women and 63 (40.9%) men, and 98 (63.6%) of the patients were > 60 years old. The median age at diagnosis was 64 years (range: 29-85 years). There were 75 (48.7%) patients with a history of gallstones before surgery. Thirty-eight (24.7%) patients had diabetes before surgery. The entire cohort comprised 150 (97.4%) adenocarcinoma carcinoma patients, 3 (1.9%) adenosquamous carcinoma patients and 1 (0.6%) papillary carcinoma patient. The majority of patients had moderately or well differentiated cancer [94 (61.0%) patients with moderately or well differentiated cancer, 60 (39.0%) patients with poorly differentiated cancer]. Fifty-eight (37.7%) patients had a positive resection margin. Tumor invasion depths of Tis-T1a, T1b-T2b, T3, and T4 were observed in 10 (6.5%), 29 (18.8%), 103 (66.9%), and 12 (7.8%) patients, respectively. In terms of lymph node metastasis, 98 (63.6%) patients were N0, 47 (30.5%) patients were N1 (1-3 positive lymph nodes), and 9 (5.8%) patients were N2 (≥ 4 positive lymph nodes). The majority [142 (92.2%)] of patients did not have distant metastasis. Of the 154 patients, 16 (10.4%) had stage 0-I disease, 16 (10.4%) had stage IIA-IIB, 92 (59.7%) had stage IIIA-IIIB, and 30 (19.5%) had stage IVA-IVB.
Table 1.
Baseline characteristics of 154 patients who underwent potential curative cholecystectomy n (%)
| Characteristic | Patients (n = 154) |
| Age (yr) | 64 (29-85) |
| ≤ 60 | 56 (36.4) |
| > 60 | 98 (63.6) |
| Sex | |
| Male | 63 (40.9) |
| Female | 91 (59.1) |
| Cholecystolithiasis | |
| Absent | 79 (51.3) |
| Present | 75 (48.7) |
| Diabetes | |
| Absent | 116 (75.3) |
| Present | 38 (24.7) |
| Jaundice | |
| Absent | 129 (83.8) |
| Present | 25 (8.9) |
| Blood groups | |
| A | 43 (27.9) |
| B | 56 (36.4) |
| AB | 9 (5.8) |
| O | 46 (29.9) |
| Pathological types | |
| Adenosquamous carcinoma | 3 (1.9) |
| Adenocarcinoma | 150 (97.4) |
| Papillocarcinoma | 1 (0.6) |
| Degree of differentiation | |
| Poor | 60 (39.0) |
| Moderate-well | 94 (61.0) |
| Resection margin status | |
| Negative | 96 (62.3) |
| Positive | 58 (37.7) |
| Maximum tumor diameter (cm) | 3 (0.2-13) |
| ≤ 2.45 | 68 (44.2) |
| > 2.45 | 86 (55.8) |
| T stage | |
| Tis-T1a | 10 (6.5) |
| T1b-T2b | 29 (18.8) |
| T3 | 103 (66.9) |
| T4 | 12 (7.8) |
| N stage | |
| 0 | 98 (63.6) |
| 1 | 47 (30.5) |
| 2 | 9 (5.8) |
| Distant metastasis | |
| Absent | 142 (92.2) |
| Present | 12 (7.8) |
| TNM stage | |
| 0-I stage | 16 (10.4) |
| IIA-IIB stage | 16 (10.4) |
| IIIA-IIIB stage | 92 (59.7) |
| IVA-IVB stage | 30 (19.5) |
| CA199 (U/mL) | 69.3 (0.6-10524) |
| ≤ 25.45 | 57 (37.0) |
| > 25.45 | 97 (63.0) |
| Fibrinogen concentration (g/L) | 3.54 (1.71-7.47) |
| ≤ 3.47 | 75 (48.7) |
| > 3.47 | 79 (51.3) |
Association between plasma fibrinogen levels and patient clinicopathological characteristics
The median plasma fibrinogen concentration in all patients was 3.54 g/L (range: 1.71-7.47 g/L). The optimum cut-off value for plasma fibrinogen according to the ROC curve was 3.47 g/L, with a sensitivity of 0.709 and a specificity of 0.721 (Figure 1A); the AUC was 0.735 (95%CI: 0.654-0.816). The entire cohort was stratified into 2 groups for further analysis: group A, with a plasma fibrinogen concentration > 3.47 g/L, included 79 patients (51.3%); group B, with a plasma fibrinogen concentration ≤ 3.47 g/L, included 75 patients (48.7%) (Table 1). As shown in Table 2, an elevated plasma fibrinogen level was significantly correlated with resection margin (P = 0.003), degree of differentiation (P = 0.048), jaundice (P = 0.003), T stage (P < 0.001), CA199 level (P = 0.003) and TNM stage (P = 0.011), but was not significantly correlated with gender, age, gallstone history, comorbidity (diabetes), pathological type, N stage, distant metastasis, ABO blood group, or tumor size (P > 0.05).
Figure 1.
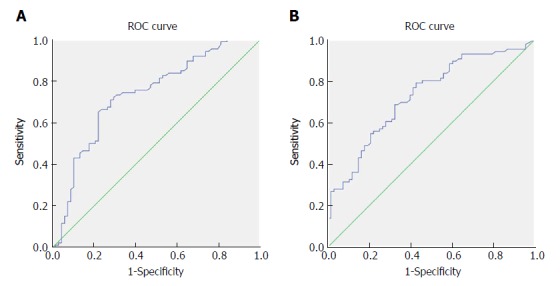
Receiver operating characteristic curve analysis based on fibrinogen for overall survival. A: The area under the ROC curve (AUC) indicates the diagnostic power of preoperative plasma fibrinogen concentration. In this model, the optimum cut-off point for fibrinogen concentration was 3.47 g/L, AUC was 0.735 (95%CI: 0.654-0.816), with a sensitivity of 0.709 and a specificity of 0.721 by the Youden index. B: AUC indicates the diagnostic power of preoperative CA199 level. In this model, the optimum cut-off point for CA199 level was 25.45 U/mL, AUC was 0.729 (95%CI: 0.650-0.808), with a sensitivity of 0.791 and a specificity of 0.574 by the Youden index. AUC: Area under curve. ROC: Receiver operating characteristic curve.
Table 2.
Correlation between fibrinogen concentration and clinicopathological characteristics in gallbladder carcinoma patients n (%)
| Characteristics |
Fibrinogen concentration |
P value | |
| ≤ 3.47 g/L (n = 75) | > 3.47 g/L (n = 79) | ||
| Age (yr) | |||
| ≤ 60 | 31 (20.1) | 25 (16.2) | 0.243 |
| > 60 | 44 (28.6) | 54 (35.1) | |
| Sex | |||
| Male | 33 (21.4) | 30 (19.5) | 0.513 |
| Female | 42 (27.3) | 49 (31.8) | |
| Cholecystolithiasis | |||
| Absent | 38 (24.7) | 41 (26.6) | 0.878 |
| Present | 37 (24.0) | 38 (24.7) | |
| Diabetes | |||
| Absent | 57 (37.0) | 59 (38.3) | 0.850 |
| Present | 18 (11.7) | 20 (13.0) | |
| Jaundice | |||
| Absent | 68 (44.2) | 61 (39.6) | 0.029 |
| Present | 7 (4.5) | 18 (11.7) | |
| Blood groups | |||
| A | 19 (12.3) | 24 (15.6) | 0.145 |
| B | 33 (21.4) | 23 (14.9) | |
| AB | 2 (1.3) | 7 (4.5) | |
| O | 21 (13.6) | 25 (16.2) | |
| Pathological types | |||
| Adenosquamous carcinoma | 0 (0) | 3 (1.9) | 0.142 |
| Adenocarcinoma | 75 (48.7) | 75 (48.7) | |
| Papillocarcinoma | 0 (0) | 1 (0.6) | |
| Degree of differentiation | |||
| Poor | 23 (14.9) | 37 (24.0) | 0.048 |
| Moderate-well | 52 (33.8) | 42 (27.3) | |
| Resection margin status | |||
| Negative | 56 (36.4) | 40 (26.4) | 0.003 |
| Positive | 19 (12.3) | 39 (25.3) | |
| Maximum tumor diameter (cm) | |||
| ≤ 2.45 | 34 (22.1) | 34 (22.1) | 0.871 |
| > 2.45 | 41 (26.6) | 45 (29.2) | |
| T stage | |||
| Tis-T1a | 8 (5.2) | 2 (1.3) | < 0.001 |
| T1b-T2b | 22 (14.3) | 7 (4.5) | |
| T3 | 43 (27.9) | 60 (39.0) | |
| T4 | 2 (1.3) | 10 (6.5) | |
| N stage | |||
| N0 | 50 (32.5) | 48 (31.2) | 0.748 |
| N1 | 21 (13.6) | 26 (16.9) | |
| N2 | 4 (2.6) | 5 (3.2) | |
| Distant metastasis | |||
| Absent | 69 (44.8) | 73 (47.4) | 0.925 |
| Present | 6 (3.9) | 6 (3.9) | |
| TNM stage | |||
| 0-I stage | 12 (7.8) | 4 (2.6) | 0.011 |
| IIA-IIB stage | 12 (7.8) | 4 (2.6) | |
| IIIA-IIIB stage | 39 (25.3) | 53 (34.4) | |
| IVA-IVB stage | 12 (7.8) | 18 (11.7) | |
| CA199 (U/mL) | |||
| ≤ 25.45 | 37 (24.0) | 20 (13.0) | 0.003 |
| > 25.45 | 38 (24.7) | 59 (38.3) | |
Association between CA199 levels and patient clinicopathological characteristics
The median CA199 level in all patients was 69.3 U/mL (range: 0.6-10524 U/mL). The optimum cut-off value for CA199 according to the ROC curve was 25.45 U/mL, with a sensitivity of 0.791 and a specificity of 0.574 (Figure 1B); the AUC was 0.729 (95%CI: 0.650-0.808). The entire cohort was stratified into 2 groups for further analysis: group A, with a CA199 level > 25.45 U/mL, included 97 patients (63.0%); group B, with a CA199 level ≤ 25.45 U/mL, included 57 patients (37.0%) (Table 1). As shown in Table 3, an elevated CA199 level was significantly correlated with resection margin (P = 0.001), jaundice (P = 0.022), T stage (P < 0.001), plasma fibrinogen concentration (P = 0.003) and TNM stage (P < 0.001), but was not significantly correlated with other factors (P > 0.05).
Table 3.
Correlation between CA199 level and clinicopathological characteristics in gallbladder carcinoma patients n (%)
| Characteristics |
CA199 level |
P value | |
| ≤ 25.45 U/mL (n = 57) | > 25.45 U/mL (n = 97) | ||
| Age (yr) | |||
| ≤ 60 | 24 (15.6) | 32 (20.8) | 0.299 |
| > 60 | 33 (21.4) | 65 (42.2) | |
| Sex | |||
| Male | 23 (14.9) | 40 (26.0) | 0.914 |
| Female | 34 (21.1) | 57 (37.0) | |
| Cholecystolithiasis | |||
| Absent | 32 (20.8) | 47 (30.5) | 0.406 |
| Present | 25 (16.2) | 50 (32.5) | |
| Diabetes | |||
| Absent | 45 (29.2) | 71 (46.1) | 0.447 |
| Present | 12 (7.8) | 26 (16.9) | |
| Jaundice | |||
| Absent | 53 (34.4) | 76 (49.4) | 0.022 |
| Present | 4 (2.6) | 21 (13.6) | |
| Blood groups | |||
| A | 19 (12.3) | 24 (15.6) | 0.303 |
| B | 21 (13.6) | 35 (22.7) | |
| AB | 1 (0.6) | 8 (5.2) | |
| O | 16 (10.4) | 30 (19.5) | |
| Pathological types | |||
| Adenosquamous carcinoma | 0 (0) | 3 (1.9) | 0.299 |
| Adenocarcinoma | 57 (37.0) | 93 (60.4) | |
| Papillocarcinoma | 0 (0) | 1 (0.6) | |
| Degree of differentiation | |||
| Poor | 33 (21.4) | 61 (39.6) | 0.069 |
| Moderate-well | 24 (15.6) | 36 (23.4) | |
| Resection margin status | |||
| Negative | 45 (29.2) | 51 (33.1) | 0.001 |
| Positive | 12 (7.8) | 46 (29.9) | |
| Maximum tumor diameter (cm) | |||
| ≤ 2.45 | 30 (19.5) | 38 (24.7) | 0.131 |
| > 2.45 | 27 (17.5) | 59 (38.3) | |
| T stage | |||
| Tis-T1a | 7 (4.5) | 3 (1.9) | < 0.001 |
| T1b-T2b | 18 (11.7) | 11 (7.1) | |
| T3 | 32 (20.8) | 71 (46.1) | |
| T4 | 0 (0.0) | 12 (7.8) | |
| N stage | |||
| N0 | 43 (27.9) | 55 (35.7) | 0.056 |
| N1 | 11 (7.1) | 36 (23.4) | |
| N2 | 3 (1.9) | 6 (3.9) | |
| Distant metastasis | |||
| Absent | 53 (34.4) | 89 (57.8) | 0.783 |
| Present | 4 (2.6) | 8 (5.2) | |
| TNM stage | |||
| 0-I stage | 11 (7.1) | 5 (3.2) | < 0.001 |
| IIA-IIB stage | 12 (7.8) | 4 (2.6) | |
| IIIA-IIIB stage | 27 (17.5) | 65 (42.2) | |
| IVA-IVB stage | 7 (4.5) | 23 (14.9) | |
| Fibrinogen concentration (g/L) | |||
| ≤ 3.47 | 37 (24.0) | 38 (24.7) | 0.003 |
| > 3.47 | 20 (13.0) | 59 (38.3) | |
Analysis of factors influencing prognosis
The median follow-up time was 17 mo. One hundred and three patients died during the follow-up period, with an estimated median OS duration of 14.5 mo (range: 0.5-153.0 mo). The 1-year and 2-year survival rates were 55.8% and 35.7%, respectively.
A Cox univariate analysis of OS showed that resection margin (HR: 3.683, 95%CI: 2.468-5.496, P < 0.001), distant metastasis (HR = 2.550, 95%CI: 1.388-4.684, P = 0.003), jaundice (HR = 2.598, 95%CI: 1.644-4.106, P < 0.001), CA199 level (HR = 3.570, 95%CI: 2.213-5.760, P < 0.001), lymph node metastasis (P < 0.001), degree of differentiation (HR = 1.527, 95%CI: 1.031-2.261, P = 0.035), T stage (P < 0.001), TNM stage (P < 0.001), and plasma fibrinogen level (HR = 2.795, 95%CI: 1.853-4.214, P < 0.001) were significantly associated with unfavorable OS (Table 4). The OS curve stratified by plasma fibrinogen level showed that GBC patients with a plasma fibrinogen level ≤ 3.47 g/L had longer OS durations than those with a plasma fibrinogen level > 3.47 g/L (Figure 2A). In addition, the OS curve stratified by CA199 showed that GBC patients with a CA199 level ≤ 25.45 U/mL had longer OS durations than those with a CA199 level > 25.45 U/mL (Figure 2B). Next, we selected the risk factors identified by the univariate analysis described above for multivariate Cox regression analysis of survival. Resection margin (HR = 1.971, 95%CI: 1.288-3.017, P = 0.002), TNM stage (P = 0.003), CA199 level (HR = 1.842, 95%CI: 1.111-3.056, P = 0.018) and plasma fibrinogen level (HR = 1.711, 95%CI: 1.114-2.627, P = 0.014) were identified as independent prognostic factors for GBC patient survival (Table 5).
Table 4.
Univariate analysis of overall survival in gallbladder cancer patients
| Characteristics | HR (95%CI) | P value |
| Age (yr) | 1.473 (0.973-2.230) | 0.067 |
| ≤ 60 | ||
| > 60 | ||
| Sex | 0.995 (0.670-1.477) | 0.981 |
| Male | ||
| Female | ||
| Cholecystolithiasis | 1.198 (0.814-1.764) | 0.360 |
| Absent | ||
| Present | ||
| Diabetes | 1.028 (0.651-1.623) | 0.906 |
| Absent | ||
| Present | ||
| Jaundice | 2.598 (1.644-4.106) | < 0.001 |
| Absent | ||
| Present | ||
| Blood groups | - | 0.113 |
| A | ||
| B | ||
| AB | ||
| O | ||
| Pathological types | - | 0.165 |
| Adenosquamous carcinoma | ||
| Adenocarcinoma | ||
| Papillocarcinoma | ||
| Degree of differentiation | 1.527 (1.031-2.261) | 0.035 |
| Poor | ||
| Moderate-well | ||
| Resection margin status | 3.683 (2.468-5.496) | < 0.001 |
| Negative | ||
| Positive | ||
| Maximum tumor diameter (cm) | 1.101 (0.744-1.630) | 0.631 |
| ≤ 2.45 | ||
| > 2.45 | ||
| T stage | - | < 0.001 |
| Tis-T1a | ||
| T1b-T2b | ||
| T3 | ||
| T4 | ||
| N stage | - | < 0.001 |
| N0 | ||
| N1 | ||
| N2 | ||
| Distant metastasis | 2.550 (1.388-4.684) | < 0.003 |
| Absent | ||
| Present | ||
| TNM stage | - | < 0.001 |
| 0-I stage | ||
| IIA-IIB stage | ||
| IIIA-IIIB stage | ||
| IVA-IVB stage | ||
| CA199 (U/mL) | 3.570 (2.213-5.760) | < 0.001 |
| ≤ 25.45 | ||
| > 25.45 | ||
| Fibrinogen concentration (g/L) | 2.790 (1.853-4.214) | < 0.001 |
| ≤ 3.47 | ||
| > 3.47 |
Figure 2.
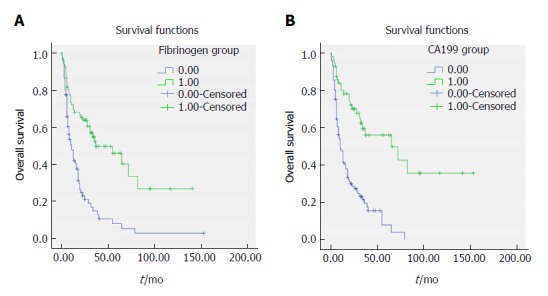
Survival curve according to the preoperative fibrinogen concentration (A) and CA199 level (B). A: Data compares fibrinogen concentration > 3.47 g/L vs ≤ 3.47 g/L group (P < 0.05). The number 1 for ≤ 3.47 g/L group, number 2 for > 3.47 g/L group. B: Data compares CA199 level > 25.45 U/mL vs ≤ 25.45 U/mL (P < 0.05). The number 1 for ≤ 25.45 U/mL group, number 2 for > 25.45 U/mL group.
Table 5.
Multivariate analysis of overall survival in gallbladder cancer patients
| Characteristics | HR (95%CI) | Wald | P value |
| Resection margin status | 1.971 (1.288-3.017) | 0.002 | |
| Negative | |||
| Positive | |||
| TNM stage | 11.299 | 0.003 | |
| IIA-IIB stage/0-1 stage | 1.336 (0.317-5.627) | 0.156 | 0.693 |
| IIIA-IIIB stage/0-1 stage | 3.831 (1.167-12.571) | 4.907 | 0.027 |
| IVA-IVB stage/0-1 stage | 5.204 (1.497-18.093) | 6.730 | 0.009 |
| Fibrinogen concentration (g/L) | 1.711 (1.114-2.627) | 0.014 | |
| ≤ 3.47 | |||
| > 3.47 | |||
| CA199 (U/mL) | 1.842 (1.111-3.056) | 0.018 | |
| ≤ 25.45 | |||
| > 25.45 |
Prognostic significance of the combination of plasma fibrinogen and CA199 in predicting the long-term survival of GBC patients
As shown by the above results of multivariate analysis, plasma fibrinogen and CA199 were independent prognostic biomarkers in GBC patients, but whether the combination of plasma fibrinogen and CA199 had the same efficacy remained unclear. As the HR for CA199/the HR for plasma fibrinogen = 1.842/1.711 approximately 1.10, patients with elevated plasma fibrinogen and CA199 levels were allocated a score of 2.1, those with an elevated plasma fibrinogen level only were allocated a score of 1, those with an elevated CA199 level only were allocated a score of 1.1, and those with neither of these abnormalities were allocated a score of 0. We then used the Kaplan-Meier method and a Cox regression model to investigate the prognostic significance of the combination of plasma fibrinogen and CA199 in these GBC patients.
Both the univariate and multivariate Cox regression analyses of survival revealed that the combination of plasma fibrinogen and CA199 was an independent prognostic factor for survival in GBC patients following surgery (Table 6). The results of the OS curve are presented in Figure 3. Finally, a ROC curve was generated to assess the prognostic accuracy of the combination of plasma fibrinogen and CA199. The results showed that for OS, the AUC of the combination of plasma fibrinogen and CA199 was 0.765 (95%CI: 0.688-0.841) (Figure 4), which was higher than that of plasma fibrinogen (0.735, 95%CI: 0.654-0.816) (Figure 1A) and that of CA199 (0.729, 95%CI: 0.650-0.808) (Figure 1B). These results indicated that the combination of plasma fibrinogen and CA199 may serve as a significant prognostic biomarker that is superior to either plasma fibrinogen or CA199 alone.
Table 6.
Univariate and multivariate analysis of overall survival in gallbladder cancer patients according to the combination of fibrinogen and CA199
| Characteristics | HR (95%CI) | Wald | P value |
| Univariate analysis | |||
| Combined fibrinogen and CA199 | - | < 0.001 | |
| 0 | |||
| 1 | |||
| 1.1 | |||
| 2.1 | |||
| Multivariate analysis | |||
| Resection margin status | 1.973 (1.289-3.020) | 0.002 | |
| Negative | |||
| Positive | |||
| TNM stage | 11.299 | 0.011 | |
| IIA-IIB stage/0-1 stage | 1.342 (0.318-5.659) | 0.160 | 0.689 |
| IIIA-IIIB stage/0-1 stage | 3.812 (1.158-12.545) | 4.848 | 0.028 |
| IVA-IVB stage/0-1 stage | 5.189 (1.491-18.055) | 6.699 | 0.010 |
| Combined fibrinogen and CA199 | 14.218 | 0.003 | |
| 1/0 | 1.784 (0.775-4.104) | 1.854 | 0.173 |
| 1.1/0 | 1.895 (0.943-3.806) | 3.222 | 0.073 |
| 2.1/0 | 3.195 (1.676-6.090) | 12.454 | 0.000 |
The number 0 for fibrinogen concentration > 3.47g/L with CA199 level > 25.45 U/mL group, number 1 for fibrinogen concentration > 3.47 g/L with CA199 level ≤ 25.45 U/mL group, number 1.1 for fibrinogen concentration ≤ 3.47g/L with CA199 level > 25.45 U/mL group, and number 2.1 for fibrinogen concentration ≤ 3.47 g/L with CA199 level ≤ 25.45 U/mL group.
Figure 3.
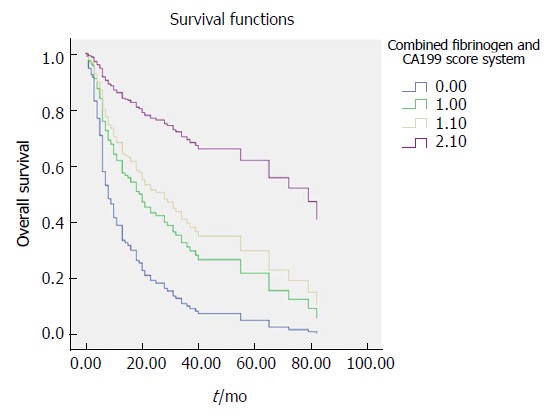
Survival curve according to the combined fibrinogen and CA199 scoring system. Data compares the fibrinogen concentration > 3.47 g/L with CA199 level > 25.45 U/mA group, fibrinogen concentration > 3.47 g/L with CA199 level ≤ 25.45 U/mL group, fibrinogen concentration ≤ 3.47 g/L with CA199 level > 25.45 U/mL group and fibrinogen concentration ≤ 3.47 g/L with CA199 level ≤ 25.45 U/mL group. The number 0 for fibrinogen concentration > 3.47 g/L with CA199 level > 25.45 U/mL group, number 1 for fibrinogen concentration > 3.47g/L with CA199 level ≤ 25.45 U/mL group, number 1.1 for fibrinogen concentration ≤ 3.47 g/L with CA199 level > 25.45 U/mL group, number 2.1 for fibrinogen concentration ≤ 3.47 g/L with CA199 level ≤ 25.45 U/mL group.
Figure 4.
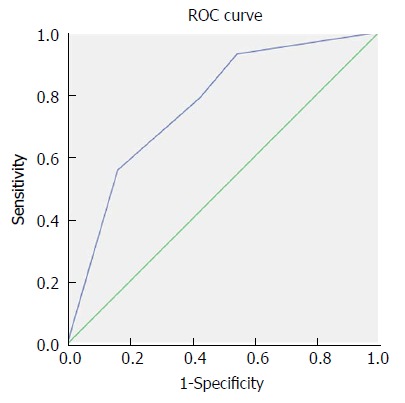
Receiver operating characteristic curve analysis based on the combination of fibrinogen and CA199 for overall survival. AUC indicates the diagnostic power of the combination of fibrinogen and CA199. In this model, AUC was 0.765 (95%CI: 0.688-0.841). AUC: Area under curve.
DISCUSSION
The incidence of GBC appears to be increasing worldwide, creating an enormous public health and economic burden. Due to a lack of effective prognostic biomarkers, the prognosis of GBC is typically poor. In the present study, we investigated the correlations between biomarkers, clinicopathological characteristics, and survival in patients with GBC undergoing surgical resection. Our results showed that plasma fibrinogen, CA199, resection margin and TNM stage were independent prognostic factors associated with OS in patients with GBC. Elevated plasma fibrinogen and CA199 levels were significantly correlated with worse OS. Moreover, to the best of our knowledge, the current study indicated for the first time that the combination of plasma fibrinogen and CA199 was more efficient than plasma fibrinogen or CA199 alone in predicting the prognosis of GBC patients who have undergone surgical resection.
Due to the low incidence of GBC, few studies have examined the correlations between inflammation-related factors and GBC prognosis. The inflammation-related factors explored in previous studies include platelet count (PLT)[35], platelet to lymphocyte ratio (PLR)[32,33], neutrophil to lymphocyte ratio (NLR)[36,37] and plasma fibrinogen level[30].
Wang et al[35] showed that a PLT > 178 × 109/L was significantly correlated with worse prognosis of GBC (HR = 1.541, 95%CI: 1.038-2.287, P = 0.032) and identified 178 × 109/L as the optimal cut-off value (AUC = 0.798, 95%CI: 0.737-0.858, sensitivity: 0.746, specificity: 0.722). The prognostic accuracy of PLT for GBC was higher than that of plasma fibrinogen in our study (AUC = 0.735, 95%CI: 0.654-0.816, sensitivity: 0.709, specificity: 0.721). However, Pang et al[32] and Zhang et al[33] indicated that PLT was not associated with the prognosis of GBC (HR = 1.013, 95%CI: 0.647-1.594, P = 0.956, and HR = 1.172, 95%CI: 0.794-1.731, P = 0.423). These results may be related to the different cut-off values chosen (300 × 109/L and 200 × 109/L) and different sample sizes (316 patients and 145 patients). Therefore, the prognostic significance of PLT in GBC patients requires further validation.
Zhang et al[33] and Zhang et al[36] demonstrated that NLR was significantly associated with an unfavorable prognosis of GBC (HR = 2.059, 95%CI: 1.253-3.384, P = 0.004, and HR = 1.65, P < 0.001), but the prognostic accuracy of NLR for GBC according to Lingqiang Zhang et al[36] (AUC = 0.637, 95%CI: 0.556-0.718, sensitivity: 0.713, specificity: 0.565) was not higher than that of plasma fibrinogen in our study (AUC = 0.735, 95%CI: 0.654-0.816, sensitivity: 0.709, specificity: 0.721).
Pang et al[32] showed that PLR was a negative prognostic factor for GBC patients (HR = 2.02, 95%CI: 1.24-3.28, P < 0.001), although the prognostic accuracy of PLR for GBC (AUC = 0.620, 95%CI: 0.542-0.698, P = 0.040, sensitivity: 0.736, specificity: 0.532) was not superior to that of plasma fibrinogen in our study (AUC = 0.735, 95%CI: 0.654-0.816, sensitivity: 0.709, specificity: 0.721). However, Zhang et al[33] showed that PLR was not associated with the prognosis of patients with GBC. Therefore, the prognostic significance of PLR in GBC patients requires further validation.
To date, the only study to explore the prognostic significance of plasma fibrinogen in GBC patients was conducted by Shu et al[30]. Consistent with the results of our study, their results indicated that an elevated preoperative plasma fibrinogen level, poorer margin status and higher TNM stage were independently associated with worse OS. They also showed that lymphatic metastasis was a negative prognostic factor for GBC, but in our study, lymphatic metastasis was not identified as a prognostic factor. This difference may be related to the TNM stage classification standard used in the present study. The newly published AJCC-8 indicated that the number of positive lymph nodes rather than the location of lymph node metastasis is closely related to the prognosis of GBC. The new N stage defined 1-3 positive lymph nodes as N1, 4 or more positive lymph nodes as N2, and no positive lymph nodes as N0.
The prognostic significance of plasma fibrinogen in the study conducted by Shu et al[30] (AUR = 0.751, 95%CI: 0.653-0.848) was slightly superior to that observed in our study (AUC = 0.735, 95%CI: 0.654-0.816, sensitivity: 0.709, specificity: 0.721) and had a positive predictive value of 92.73%. This difference may be related to the different optimal cut-off values between the two studies (4.02 g/L and 3.47 g/L) and the different methods used to determine the optimal cut-off values. In their study, the dichotomous variable that was used to determine the optimal cut-off value on the ROC curve was TNM stage, whereas the dichotomous variable used in our study was OS. Our method is accepted by most researchers. Nonetheless, the results of their study support the conclusion reached in our study that the prognostic accuracy of the combination of plasma fibrinogen and CA199 (AUC = 0.765, 95%CI: 0.688-0.841) is higher than that of plasma fibrinogen alone (AUR = 0.751, 95%CI: 0.653-0.848).
Many recent studies have demonstrated that an elevated plasma fibrinogen level, as a marker of coagulation and fibrinolytic activation, is a strong predictor of poor prognosis for various malignant tumors[13,15,19,23,25]. Additionally, it was found that fibrinogen synthesis is significantly upregulated by inflammation[38]. However, the molecular mechanisms underlying the association between plasma fibrinogen and cancer prognosis is still uncertain. In an in vitro study, Shu et al[30] found that plasma fibrinogen at a high concentration induced epithelial-mesenchymal transition (EMT), thus increasing the migration, invasion, and metastatic capacity of co-cultured GBC cells by increasing the expression of vimentin (a mesenchymal marker) and reducing the expression of E-cadherin (an epithelial marker). EMT is known to confer migration, invasion, and metastatic capacity and multidrug resistance to cells[38,39]. However, this explanation needs to be verified through basic research in the future.
One of the methodological innovations of our study is that we assigned different scores to patients according to the HR for elevated plasma fibrinogen levels and elevated CA199 levels, instead of assigning all patients a score of 1 as in the previous scoring system. This new scoring method further distinguished the difference in prognostic efficiency between plasma fibrinogen and CA199, rather than simply assigning a score of 1 to each parameter, considering that the HRs for the two parameters are not the same (CA199: 1.842, plasma fibrinogen: 1.711). From the OS curve (Figure 3) and ROC curve (Figure 4), we observed that the new scoring system effectively improved the prognostic accuracy of the biomarkers.
This study is the first to investigate the prognostic significance of the combination of plasma fibrinogen and CA199 in GBC patients. Inevitably, our study has some limitations that should be acknowledged. First, this study was performed using a retrospective design. Second, although this new scoring method distinguished the prognostic value of different biomarkers and improved prognostic accuracy, the validity and predictive value of this scoring method still require further verification. Third, the data were obtained from a single institution and the sample size was relatively small, which may have influenced the final conclusions. Fourth, to obtain more data, we did not distinguish between patients who underwent radical surgery and those who received palliative cholecystectomy or extended resection. Therefore, our results should be validated by prospective, multicenter studies with a large sample size.
In conclusion, elevated preoperative levels of both plasma fibrinogen and CA199 are independent prognostic factors for GBC. Additionally, the combination of plasma fibrinogen and CA199 showed superior prognostic accuracy compared with either parameter alone. Therefore, the combination of plasma fibrinogen and CA199 can facilitate the identification of GBC patients with poorer survival prognosis before surgery. We hypothesize that the combination of plasma fibrinogen and CA199 could be used as an inexpensive, simple, reliable and reproducible method to determine GBC prognosis in clinical practice.
ARTICLE HIGHLIGHTS
Research background
Gallbladder cancer is a rare hepatobiliary tumor with a relatively low incidence. Due to the lack of significant specific symptoms at the early stage, the prognosis of gallbladder cancer is poor and can be fatal. Therefore, determining a convenient and cost-effective prognostic biomarker is urgently required for patients with gallbladder cancer. Elevated fibrinogen has been demonstrated to be associated with poor prognosis in multiple malignancies, while CA199 is considered a widely accepted diagnostic and prognostic marker of gallbladder cancer. There have been very few studies on the role of fibrinogen in the prognosis of patients with gallbladder cancer. To date, studies on the combined use of fibrinogen and CA199 to predict the prognosis of patients with gallbladder cancer have not been conducted. The combined use of fibrinogen and CA199 avoids inconsistencies caused by the use of a single indicator and enhances predictive efficacy.
Research motivation
The main aim of this study was to validate and identify a convenient and inexpensive combination of biomarkers with a higher prognostic value for patients with gallbladder cancer. From our research, we found that the combination of preoperative plasma fibrinogen and CA199 was a more efficient prognostic factor than either parameter alone in patients with gallbladder cancer. Due to this finding, we can screen potential high-risk candidates for gallbladder cancer and provide prognostic guidance for surgical patients with gallbladder cancer. In addition, this study also provides clinical evidence and preconditions for the future study of how fibrinogen promotes the proliferation and metastasis of malignant tumor cells.
Research objectives
The main objective of this study was to identify a convenient and more efficient prognostic biomarker for gallbladder cancer patients. From this study, we found that both elevated fibrinogen and elevated CA199 were independent risk factors for gallbladder cancer patients. Furthermore, the combination of preoperative plasma fibrinogen and CA199 was a more efficient prognostic factor than either parameter alone in patients with gallbladder cancer. These findings not only provide a further example which proves the relationship between hemostasis and tumor, but also provide powerful clinical evidence for related basic research in the future.
Research methods
We used an Excel table to organize research-related clinical data, and imported these variables into SPSS 24.0 statistical software. We then assigned the different types of variables appropriately. We determined the optimal cut-off values for fibrinogen and CA199 by plotting ROC curves, and then determined the association of fibrinogen and CA199 with other clinicopathological variables using the R × C table. Finally, univariate and multivariate analyses were performed to determine the independent prognostic factors in patients with gallbladder cancer.
Given the different HRs of the two parameters (CA199: 1.842, plasma fibrinogen: 1.711), one methodological innovation of this study was that we assigned different scores to elevated plasma fibrinogen levels and elevated CA199 levels, instead of assigning the same score as in the previous scoring system. This scoring approach further differentiates the difference in the prognostic efficiency between plasma fibrinogen and CA199, rather than simply assigning each parameter with 1 point. Based on the overall survival curve (Figure 3) and the ROC curve (Figure 4) in the text, we observed that the new scoring system effectively improved the prognostic accuracy of the biomarkers.
Research results
Our study demonstrated that the best cut-off values for pretreatment fibrinogen and CA199 were 3.47 g/L and 25.45 U/mL, respectively, in patients with gallbladder cancer. After single factor and multivariate analysis, it was shown that elevated pretreatment fibrinogen, elevated pretreatment CA199, resection margin and TNM stage were independent risk factors for gallbladder cancer patients. When the elevated pretreatment fibrinogen and elevated pretreatment CA199 were combined with different assigned scores according to their different HRs, the prognostic accuracy and power was significantly improved (the AUROC increased to 0.765, a relatively high value). These research findings confirm the relationship between hemostatic factors and cancer, in this case gallbladder cancer. How does the hemostatic factor fibrinogen influence the development, growth, and metastasis of gallbladder cancer cells? The underlying mechanism is still unknown, and further studies are required to identify and confirm the mechanism involved.
Research conclusions
In the present study, we found that the combination of preoperative plasma fibrinogen and CA199 is a more efficient prognostic factor than either parameter alone in patients with gallbladder cancer. We proposed that the combination of hemostatic factor and specific oncology markers can better predict the prognosis of gallbladder cancer, as hemostatic and oncology markers can compensate for each other’s inconsistency in predicting tumor prognosis and thus enhance overall prognostic efficacy. Fibrinogen is associated with the development, growth, and metastasis of cancer cells, and CA199 is a product of tumor cell growth and metabolism. However, from our study findings, we observed that they had different prognostic efficacy (as they had different HRs) in gallbladder cancer patients; therefore, we assigned different scores to them which differed from the previous traditional scoring system. Using this new method, the prognostic efficacy of these two prognostic biomarkers combined was significantly improved, and this combination was used to screen potential high-risk gallbladder cancer candidates, identify appropriate surgical patients, and adopt the best follow-up strategy.
Research perspectives
In this study, we found that the combination of a hemostatic factor and oncology factor could compensate for each other’s inconsistency in predicting tumor prognosis and improve the overall prognostic efficacy. The combination of these factors was more efficient than either parameter alone in predicting the prognosis of gallbladder cancer patients. These factors are inexpensive, easy-to-use and highly accurate for determining the prognosis of gallbladder cancer patients. Further large-scale, well-designed and prospective studies to verify the findings and conclusions of this investigation are required.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Supported by National Key Project Research and Development Projects, No. S2016G9012; International Science and Technology Cooperation Projects, No. 2015DFA30650; and The Capital Special Research Project for Clinical Application, No. Z151100004015170.
Institutional review board statement: The publication of this manuscript has been reviewed and approved by the PUMCH institutional review board.
Informed consent statement: All patients and their families signed informed consent statements before surgery, and the type of surgical procedure was performed according to the approved guidelines.
Conflict-of-interest statement: We declare that the authors have no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: January 26, 2018
First decision: February 10, 2018
Article in press: March 10, 2018
P- Reviewer: Armellini E, Osuga T, Tokunaga Y S- Editor: Gong ZM L- Editor: Webster JR E- Editor: Huang Y
Contributor Information
Wei-Yu Xu, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Hao-Hai Zhang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Xiao-Bo Yang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Yi Bai, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Jian-Zhen Lin, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Jun-Yu Long, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Jian-Ping Xiong, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Jun-Wei Zhang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Xin-Ting Sang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China.
Hai-Tao Zhao, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China. zhaoht@pumch.cn.
References
- 1.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu XS, Shi LB, Li ML, Ding Q, Weng H, Wu WG, Cao Y, Bao RF, Shu YJ, Ding QC, et al. Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma. Ann Surg Oncol. 2014;21:449–457. doi: 10.1245/s10434-013-3292-z. [DOI] [PubMed] [Google Scholar]
- 3.Liu YB, He XW, Wang JW, Li JT, Li KQ, Liu FB, Xue JF, Zhu JH, Li B, Peng SY. [Establishment of liver metastasis model of human gallbladder cancer and isolation of the subpopulation with high metastatic potential] Zhonghua Yi Xue Za Zhi. 2006;86:2117–2121. [PubMed] [Google Scholar]
- 4.Boutros C, Gary M, Baldwin K, Somasundar P. Gallbladder cancer: past, present and an uncertain future. Surg Oncol. 2012;21:e183–e191. doi: 10.1016/j.suronc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava K, Srivastava A, Mittal B. Potential biomarkers in gallbladder cancer: present status and future directions. Biomarkers. 2013;18:1–9. doi: 10.3109/1354750X.2012.717105. [DOI] [PubMed] [Google Scholar]
- 6.Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. Fourteen year surgical experience of gallbladder cancer: validity of curative resection affecting survival. Hepatogastroenterology. 2012;59:36–41. doi: 10.5754/hge10297. [DOI] [PubMed] [Google Scholar]
- 7.Wang RT, Xu XS, Liu J, Liu C. Gallbladder carcinoma: analysis of prognostic factors in 132 cases. Asian Pac J Cancer Prev. 2012;13:2511–2514. doi: 10.7314/apjcp.2012.13.6.2511. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005;3:25. doi: 10.1186/1479-5876-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence SO, Simpson-Haidaris PJ. Regulated de novo biosynthesis of fibrinogen in extrahepatic epithelial cells in response to inflammation. Thromb Haemost. 2004;92:234–243. doi: 10.1160/TH04-01-0024. [DOI] [PubMed] [Google Scholar]
- 11.Collen D, Tytgat GN, Claeys H, Piessens R. Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. Br J Haematol. 1972;22:681–700. doi: 10.1111/j.1365-2141.1972.tb05715.x. [DOI] [PubMed] [Google Scholar]
- 12.Koenig W. Fibrin (ogen) in cardiovascular disease: an update. Thromb Haemost. 2003;89:601–609. [PubMed] [Google Scholar]
- 13.Kim KH, Park TY, Lee JY, Lee SM, Yim JJ, Yoo CG, Kim YW, Han SK, Yang SC. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci. 2014;29:507–511. doi: 10.3346/jkms.2014.29.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanim B, Hoda MA, Klikovits T, Winter MP, Alimohammadi A, Grusch M, Dome B, Arns M, Schenk P, Jakopovic M, et al. Circulating fibrinogen is a prognostic and predictive biomarker in malignant pleural mesothelioma. Br J Cancer. 2014;110:984–990. doi: 10.1038/bjc.2013.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang SS, Lei YY, Cai XL, Yang H, Xia X, Luo KJ, Su CH, Zou JY, Zeng B, Hu Y, et al. Preoperative serum fibrinogen is an independent prognostic factor in operable esophageal cancer. Oncotarget. 2016;7:25461–25469. doi: 10.18632/oncotarget.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang GY, Jiang N, Yi HM, Wang GS, Zhang JW, Li H, Zhang J, Zhang Q, Yang Y, Chen GH. Pretransplant Elevated Plasma Fibrinogen Level is a Novel Prognostic Predictor for Hepatocellular Carcinoma Recurrence and Patient Survival Following Liver Transplantation. Ann Transplant. 2016;21:125–130. doi: 10.12659/aot.895416. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Kurokawa Y, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. Usefulness of Preoperative Plasma Fibrinogen Versus Other Prognostic Markers for Predicting Gastric Cancer Recurrence. World J Surg. 2016;40:1904–1909. doi: 10.1007/s00268-016-3474-5. [DOI] [PubMed] [Google Scholar]
- 18.Hong T, Shen D, Chen X, Wu X, Hua D. Preoperative plasma fibrinogen, but not D-dimer might represent a prognostic factor in non-metastatic colorectal cancer: A prospective cohort study. Cancer Biomark. 2017;19:103–111. doi: 10.3233/CBM-160510. [DOI] [PubMed] [Google Scholar]
- 19.Zhao K, Deng H, Qin Y, Liao W, Liang W. Prognostic significance of pretreatment plasma fibrinogen and platelet levels in patients with early-stage cervical cancer. Gynecol Obstet Invest. 2015;79:25–33. doi: 10.1159/000365477. [DOI] [PubMed] [Google Scholar]
- 20.Seebacher V, Polterauer S, Grimm C, Husslein H, Leipold H, Hefler-Frischmuth K, Tempfer C, Reinthaller A, Hefler L. The prognostic value of plasma fibrinogen levels in patients with endometrial cancer: a multi-centre trial. Br J Cancer. 2010;102:952–956. doi: 10.1038/sj.bjc.6605547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekos C, Grimm C, Brodowicz T, Petru E, Hefler L, Reimer D, Koch H, Reinthaller A, Polterauer S, Polterauer M. Prognostic role of plasma fibrinogen in patients with uterine leiomyosarcoma - a multicenter study. Sci Rep. 2017;7:14474. doi: 10.1038/s41598-017-13934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei Y, Zhao S, Lu X, Liu H, Li X, Ma R. Clinical and Prognostic Significance of Preoperative Plasma Fibrinogen Levels in Patients with Operable Breast Cancer. PLoS One. 2016;11:e0146233. doi: 10.1371/journal.pone.0146233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selzer E, Grah A, Heiduschka G, Kornek G, Thurnher D. Pre-therapeutic fibrinogen levels are of prognostic significance in locally advanced head and neck cancer. Wien Klin Wochenschr. 2016;128:320–328. doi: 10.1007/s00508-016-0963-3. [DOI] [PubMed] [Google Scholar]
- 24.Holzinger D, Danilovic I, Seemann R, Kornek G, Engelmann J, Pillerstorff R, Holawe S, Psyrri A, Erovic BM, Farwell G, et al. Prognostic Impact of Pretreatment Plasma Fibrinogen in Patients with Locally Advanced Oral and Oropharyngeal Cancer. PLoS One. 2016;11:e0158697. doi: 10.1371/journal.pone.0158697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Song Y, Jin J, Zhou LQ, He ZS, Shen C, He Q, Li J, Liu LB, Wang C, et al. Preoperative Plasma Fibrinogen Level Represents an Independent Prognostic Factor in a Chinese Cohort of Patients with Upper Tract Urothelial Carcinoma. PLoS One. 2016;11:e0150193. doi: 10.1371/journal.pone.0150193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C, Zhou Y, Zhou S, Zhao K, Lu B, Sun E. Preoperative peripheral plasma fibrinogen level is an independent prognostic marker in penile cancer. Oncotarget. 2017;8:12355–12363. doi: 10.18632/oncotarget.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troppan KT, Melchardt T, Wenzl K, Schlick K, Deutsch A, Bullock MD, Reitz D, Beham-Schmid C, Weiss L, Neureiter D, et al. The clinical significance of fibrinogen plasma levels in patients with diffuse large B cell lymphoma. J Clin Pathol. 2016;69:326–330. doi: 10.1136/jclinpath-2015-203356. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Zhou X, Bao W, Chen Y, Cheng L, Qiu G, Sheng L, Ji Y, Du X. Plasma fibrinogen levels are correlated with postoperative distant metastasis and prognosis in esophageal squamous cell carcinoma. Oncotarget. 2015;6:38410–38420. doi: 10.18632/oncotarget.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu LR, Li J, Chen P, Jiang Q, Tang XP. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol. 2016;18:178–188. doi: 10.1007/s12094-015-1350-7. [DOI] [PubMed] [Google Scholar]
- 30.Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao Y, Wang XA, Zhang F, Xiang SS, Li HF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. doi: 10.1186/1471-2407-14-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, Jiang XQ, Peng ZH. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085–4092. doi: 10.3748/wjg.v20.i14.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang Q, Zhang LQ, Wang RT, Bi JB, Zhang JY, Qu K, Liu SS, Song SD, Xu XS, Wang ZX, et al. Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma. World J Gastroenterol. 2015;21:6675–6683. doi: 10.3748/wjg.v21.i21.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Jiang C, Li J, Sun J, Qu X. Prognostic significance of preoperative neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with gallbladder carcinoma. Clin Transl Oncol. 2015;17:810–818. doi: 10.1007/s12094-015-1310-2. [DOI] [PubMed] [Google Scholar]
- 34.Cl A. [Rapid physiological coagulation method in determination of fibrinogen] Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 35.Wang RT, Zhang LQ, Mu YP, Li JB, Xu XS, Pang Q, Sun LK, Zhang X, Dong SB, Wang L, et al. Prognostic significance of preoperative platelet count in patients with gallbladder cancer. World J Gastroenterol. 2015;21:5303–5310. doi: 10.3748/wjg.v21.i17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Wang R, Chen W, Xu X, Dong S, Fan H, Liu C. Prognostic significance of neutrophil to lymphocyte ratio in patients with gallbladder carcinoma. HPB (Oxford) 2016;18:600–607. doi: 10.1016/j.hpb.2016.03.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whelton SP, Narla V, Blaha MJ, Nasir K, Blumenthal RS, Jenny NS, Al-Mallah MH, Michos ED. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2014;113:644–649. doi: 10.1016/j.amjcard.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romano S, Staibano S, Greco A, Brunetti A, Nappo G, Ilardi G, Martinelli R, Sorrentino A, Di Pace A, Mascolo M, et al. FK506 binding protein 51 positively regulates melanoma stemness and metastatic potential. Cell Death Dis. 2013;4:e578. doi: 10.1038/cddis.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He L, Zhou X, Qu C, Hu L, Tang Y, Zhang Q, Liang M, Hong J. Musashi2 predicts poor prognosis and invasion in hepatocellular carcinoma by driving epithelial-mesenchymal transition. J Cell Mol Med. 2014;18:49–58. doi: 10.1111/jcmm.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]


