Abstract
Metastasis remains the most common cause of death in cancer patients. Inhibition of metalloproteinases (MMPs) is an interesting approach to cancer therapy because of their role in the degradation of extracellular matrix (ECM), cell-cell, and cell-ECM interactions, modulating key events in cell migration and invasion. Herein, we show the cytotoxic and antimetastatic effects of the third fraction (FR3) from Bauhinia variegata candida (Bvc) stem on human cervical tumor cells (HeLa) and human peripheral blood mononuclear cells (PBMCs). FR3 inhibited MMP-2 and MMP-9 activity, indicated by zymogram. This fraction was cytotoxic to HeLa cells and noncytotoxic to PBMCs and decreased HeLa cell migration and invasion. FR3 is believed to stimulate extrinsic apoptosis together with necroptosis, assessed by western blotting. FR3 inhibited MMP-2 activity in the HeLa supernatant, differently from the control. The atomic mass spectrometry (ESI-MS) characterization suggested the presence of glucopyranosides, D-pinitol, fatty acids, and phenolic acid. These findings provide insight suggesting that FR3 contains components with potential tumor-selective cytotoxic action in addition to the action on the migration of tumor cells, which may be due to inhibition of MMPs.
1. Introduction
Cervical cancer (CC) is the fourth most frequent type of neoplasm among women in the worlds and the main cause of cancer-related death in low- and middle-income countries [1]. In 2012, more than 270,000 CC deaths occurred worldwide [2]. Human papilloma virus (HPV) is the main etiologic infectious agent associated with cervical cancer. 70% of cases of cervical cancer are induced by HPV types 16 and 18, but over 200 types of HPV have been identified [3]. HPV interferes with the cell cycle regulation because it infects the cervical mucosa and integrates its genome in cells. The viral E6 and E7 oncoproteins are critical for inducing malignant transformation because they lead to the suppression of p53 and pRB, fundamental tumor suppressor genes. Persistent infection leads to cervical intraepithelial neoplasia (CIN), which, if untreated, may progress to cancer cervical [4].
Metastasis is the main cause of cancer death [5]. One of the first steps in the metastatic cascade includes the intravasation of tumor cells into the circulation, and the processes correlated with this event, such as migration and cell invasion, are dependent on the tumor microenvironment [6]. Cytoskeletal rearrangements, combined with the degradation of cell-cell and cell-extracellular matrix (ECM) interactions, are the main mechanisms of premetastatic tissue remodeling [7]. ECM metalloproteinases (MMPs), especially MMP-2 and MMP-9, cleave ECM components, degrade the basement membrane, and allow tumor cells to penetrate the adjacent matrix stroma [8]. This type of remodeling has been associated with invasive CCs [9] and overexpression of MMP-2 and MMP-9 is also correlated with a worse prognosis in patients with this tumor type [10].
The lack of improvement in cervical cancer treatment is mainly due to resistance to cytotoxic agents [11]. Thus, the search for new therapies with low cost and minimal side effects has become paramount, with many researchers looking for compounds with anticancer activity from natural compounds [12]. Recent studies by our research group have shown that Bauhinia ungulata extract fractions have potential antimetastatic activity [13].
In the present work, we characterized phytochemically a B. variegata candida fraction with inhibitory activity against MMPs and established the mechanism of action of these compounds on cell viability in human cervical carcinoma (HeLa) and human peripheral blood mononuclear cells (PBMCs). Migration and invasion, MMP-2 activity, and the cell death pathways of HeLa cells treated with this fraction were also evaluated.
2. Materials and Methods
2.1. Sample and Preparation of Extracts
Bauhinia variegata candida (Bvc) stems were collected from an urban area of Divinópolis, Brazil, 20°08′20′′S; 44°53′02′′W; 712 m, and powdered. The powdered material (100 g) was extracted with 70% ethanol (600 mL, five times) for seven days at room temperature to obtain the crude extract. The crude extract was partitioned using hexane, ethyl acetate, and chloroform (160 mL, each). From this, the ethyl acetate partition (PA) was fractionated by open chromatographic column (silica gel 60 G). The column was eluted with hexane, dichloromethane, ethyl acetate, methanol, and water (50/50, v/v), obtaining seven fractions. All extracts were frozen (−80°C) and lyophilized.
2.2. Cancer Cell Line, Cell Culture Conditions, and Antibodies
Human cervical adenocarcinoma cells (HeLa) were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% (v/v) heat inactivated fetal bovine serum (SBF) and 1% streptomycin/penicillin (v/v) and maintained in a humidified atmosphere of 5% CO2 at 37°C [14]. The commercial cell line HeLa was authenticated by STR analysis and tested for mycoplasma contamination by PCR [15]. The DMEM culture medium, (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (MTT), Ficoll-Paque Plus, and MMP-2 and MMP-9 (M9445 and M8945, resp.) were purchased from Sigma Aldrich (St. Louis, Missouri, USA). Fetal bovine serum and Bioacoats were purchased from Becton Dickinson (BD) (Bedford, MA, USA). Antibodies were purchased from Cell Signaling Technology (Danvers, Massachusetts, USA).
2.3. Peripheral Blood Mononuclear Cells
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood (40 mL) obtained by venipuncture from two healthy volunteers. The obtained blood was centrifuged by density gradient using Ficoll-Paque Plus. The isolated cells were suspended in DMEM culture medium (10% FBS and 1% v/v). Adherent cells were considered mononuclear leukocytes [16]. This work was approved by the Ethics Committee in Research with Human Beings of the Federal University of São João del-Rei, under the number of opinions: 2.007.582.
2.4. Zymography
Each fraction of B. variegata candida was diluted in dimethyl sulfoxide (DMSO 1%) and applied 90 μg/mL in each well to 10% SDS-polyacrylamide gel containing 1 mg/ml gelatin, along with 1.8 μg/mL of MMP-2 and MMP-9 diluted in sample buffer (2.5% SDS and 1 g% sucrose). Electrophoresis was performed under reducing conditions according to [13]. Photographs of the gels were obtained and the MMPs activities, indicated by clear bands, were quantified by densitometry (ImageJ1.42q/Java1.6.0-10). The fraction capable of totally inhibiting the gelatinolytic activity of MMP-2 and MMP-9 was directed to subsequent tests.
For analysis of active MMP-2 in the cell culture supernatant, 5 × 105 HeLa cells/well were plated in a 24-well plate and incubated for 24 hours. The cells were treated with DMEM (2% FBS) with or without the selected fraction (IC50 concentration). After 24 hours, the cell culture medium was collected, lyophilized, and diluted in sample buffer (2 : 1, v/v). In each well, 20 μg/proteins were applied and electrophoresis conducted under the same conditions.
2.5. Cell Viability Assay
Cell viability was determined by MTT assay. Hela and PBMCs cells were plated in 96-well plates (2.5 × 105 cells/100 μl/well) and incubated in 5% CO2, at 37°C. After incubation for 24h the medium was removed, and cells were treated with 5, 10, 25, and 50 μg/ml of the fraction for 72 h. Then, 100 μL of DMEM with MTT (2.5 mg/mL) was added to each well and cells were incubated at 37°C for 3 h. After this time, the formazan crystals formed was dissolved by adding 100 μl/well of DMSO and the optical density was measured using microplate reader (Power Wave XS2 Biotek) at 570 nm.
2.6. Wound Healing Assay
The HeLa cells were seeded (5 × 105 cells/well) in 24-well plates and left for 24 h, at 37°C in CO2 (5%) until 90% confluency [17]. Then, the adherent cell layer was scratched with a sterile tip of pipette 200 μl. Cell debris was removed by washing with PBS and the cells were treated with the selected fraction (15 μg/mL). Photographs of the wounds were obtained at 0 and 72 hours of treatment using a microscope Axio Vert A1 FL (Carl Zeiss®), at 400x magnification, and the relative migration distance was measured by the following formula:
| (1) |
where A corresponds to the width of cell wounds before incubation and B the width of cell wounds after incubation [18].
2.7. Invasion and Migration Assay
Hela cells (2.5 × 104) were plated on the chambers Matrigel coated transwell (8 mm pore-size) in 24-well plates. Inside the chambers 1 mL of DMEM (serum free) containing 15 and 25 μg/mL of the treatment was added whereas DMEM containing 10% SFB was added outside of the chamber. Cisplatin (15 μg/mL) was used as control [19]. After 24 hours, cells that had degraded the Matrigel and invaded to the lower surface of the Matrigel coated membrane were fixed (methanol 70%) and stained with Hematoxylin/Eosin. The cells were photographed (50x) and counted. The same procedure was performed using the plates without Matrigel to evaluate the migratory capacity of the cells [15].
2.8. Western Blot Analysis
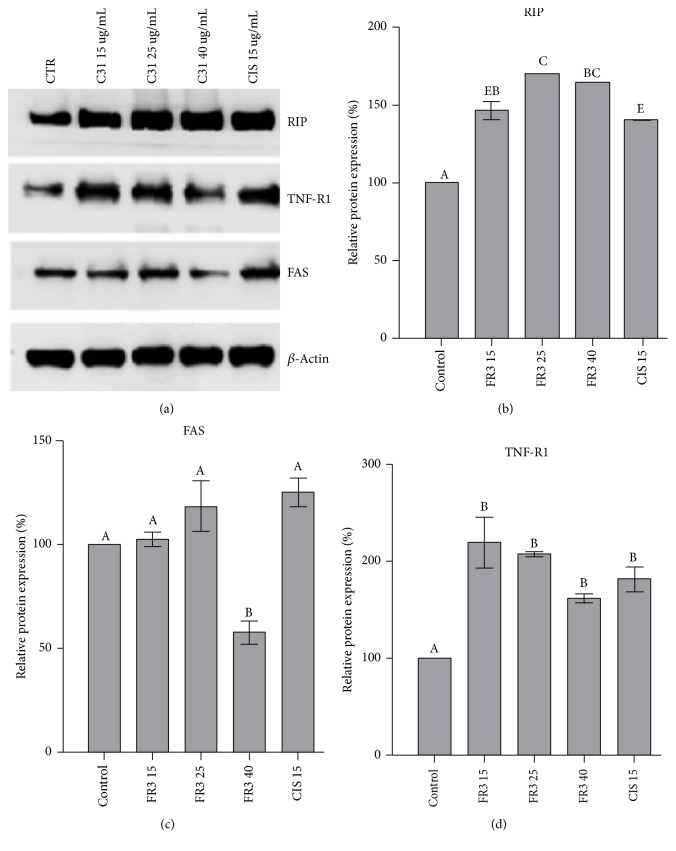
HeLa cells were treated for 24 hours at the concentrations of 15, 25, and 40 μg/ml of FR3. The dose choice was based on the IC50 value calculation. After that, the cells were lysed in a buffer containing 50 mM Tris pH 7, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 10 mM sodium pyrophosphate, 1% NP-40, and Protease Inhibitors containing 1 mM Na3VO4, 10 mM NaF, 10 mM sodium pyrophosphate, 1% NP-40, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM DTT, 0.01 M EDTA, and 1 mM PMSF. After electrophoresis, the proteins from the gel were transferred (TE 70 PWR Semi-Dry Transfer Unit, GE Healthcare) to a nitrocellulose membrane (Amersham Protram supported 0.45 μm NC, GE Healthcare) using a buffer with 25 mM Tris, 193 mM glycine, and 20% methanol. The expressions of the total and cleaved Parp (# 4967), total and cleaved caspase-8 (# 9746), total and cleaved caspase-3 (# 9662), FAS protein (# 4233), RIP protein (# 4926), and tumor necrosis receptor 1 (TNF-R1) (# 3736) were evaluated. All antibodies were diluted and incubated, according to the manufacturer's recommendations (1/1000 v/v). After washing with TBS-T, membranes were incubated with the secondary anti-rabbit antibody (# 7074) at 1 : 5000 (v/v) dilution. Then, the strip detection was performed by chemiluminescence (ECL, Western Blotting Detection Reagents, RPN2109; GE Healthcare, Piscataway, NJ) using a 1 : 1 (v/v) dilution and subsequently the membranes were photographed using ImageQuant LAS 4000 mini GE Healthcare). All experiments were performed in triplicate.
2.9. ESI(−)FT-ICR MS
Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) analysis was performed using a mass spectrometer (model 9.4 T Solarix, BrukerDaltonik GmbH, Bremen, Germany). The extract FR3 was analyzed by negative electrospray ionization, ESI(−), which was infused at a flow rate of 4 μL min−1 into the ESI source. The ESI(−) source conditions were set as follows: nebulizer gas pressure of 1.5 bar, capillary voltage of 3.9 kV, transfer capillary temperature of 200°C, and endplate offset of −500 V. The front and back trapping voltages in the ICR cell were +0.80 V and +0.85 V, respectively. The ion accumulation time in the hexapole was 0.010 s, followed by transport to the analyzer cell (ICR) through the multipole ion guide system (another hexapole). The mass spectra were acquired by accumulating 64 scans of time-domain transient signals in four mega-point time-domain data sets. The mass spectrum was processed using the Compass Data Analysis software package (BrukerDaltonics, Bremen, Germany). The proposed molecules were assigned using the Chemspider database (http://www.chemspider.com). The degree of unsaturation for each molecule was deduced directly from its DBE value according to equation: DBE = c − h/2 + n/2 + 1, where c, h, and n are the carbon, hydrogen, and nitrogen numbers, respectively, in the molecular formula [20].
2.10. Statistical Analysis
Statistical analyses for single comparisons were performed by Student's t-test, and the differences between the groups were tested using the One Way ANOVA multiple comparisons test using the GraphPad Prims program version 7 p < 0.05. All results underwent the Shapiro-Wilk normality test with p > 0.05.
3. Results
3.1. FR3 Inhibits the Gelatinolytic Activity of MMP-2 and MMP-9 In Vitro and Selectively Decreases the Viability of Tumor Cells
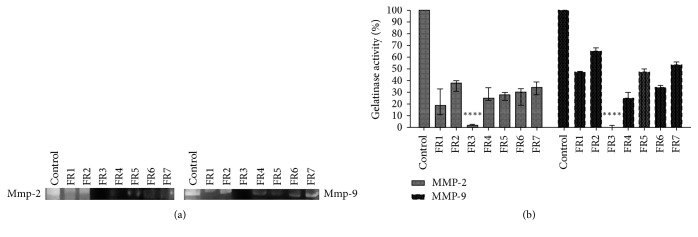
We observed that FR3 was the only fraction tested that completely inhibited MMP-2 and MMP-9 (Figure 1), standing out from the others. The FR3 reduced HeLa cell viability in a dose-dependent manner (Figure 2). Furthermore, at higher concentrations (25 and 50 ug/ml) FR3 decreased HeLa cell viability (50.66 ± 6.02% and 21% ± 3.60, resp.) (IC50: 25 μg/mL) and showed lower toxicity to nontumor PBMCs (87.33% ± 5.03 and 71% ± 10.81) at the same concentrations.
Figure 1.
The gelatinolytic activity of MMP-2 and MMP-9 treated with the B. variegata candida fractions was inhibited by FR3 (a) when treated for 3 hours of incubation, with a total decrease of the percentage of active gelatinases, significantly different from the other fractions (b). ∗∗∗∗ represents the significant difference with a value of p < 0.0001.
Figure 2.
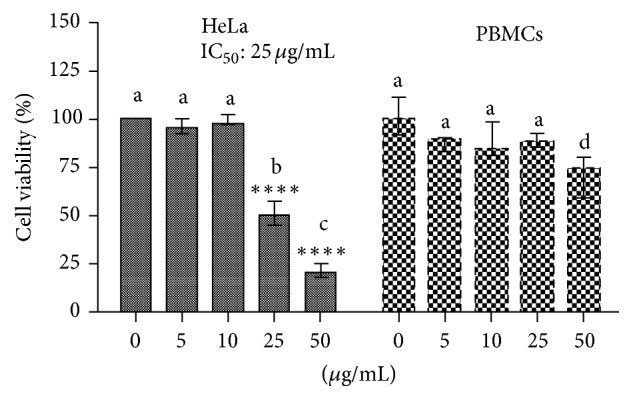
Viability analysis (MTT) of PBMCs and HeLa cell line exposed to the FR3 (0.5; 10; 25; and 50 μg/mL) for 72 hours. The results were expressed in relation to the DMSO control. ∗∗∗∗ indicates statistically significant differences between treatment and control in the same cell line. Different letters indicate statistically significant differences between each treatment between both cell lines (p < 0.0001).
3.2. FR3 Inhibits HeLa Cell Migration and Invasion and the Presence of Active MMP-2 in the Cell Supernatant
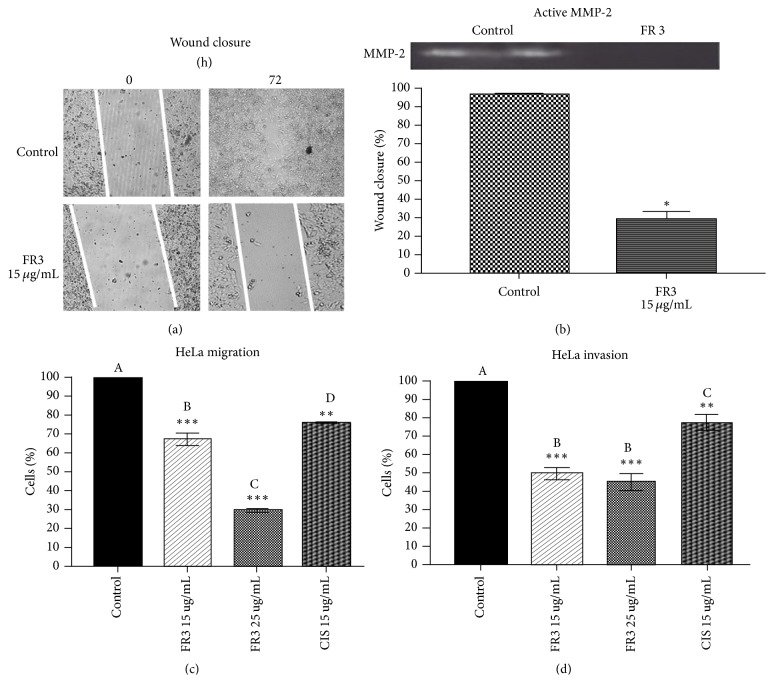
FR3 significantly inhibited HeLa cell migration (Figures 3(a) and 3(b)) in the wound closure assay. FR3 showed greater efficacy in HeLa cell transwell migration inhibition than Cisplatin (Figure 3(c)) according to the number of cells counted (Figure 4). Similarly, FR3 inhibited HeLa cell invasion significantly more than Cisplatin in the Matrigel assay (Figure 3(d)) according to the number of cells counted (Figure 4). Active MMP-2 was not observed without supernatant from the treated cells in which the control cell supernatant showed a clear band corresponding to the activity of this enzyme (Figure 3(b)).
Figure 3.
FR3 inhibited wound closure of the HeLa cell monolayer after 72 hours of treatment (a) in 70% (p ≤ 0.05) and decreased the expression of active MMP-2 in the supernatant of these cells (b). FR3 inhibited the migration of HeLa cells (15 ug/ml and 30 ug/ml) by the Boyden Chamber assay, as well as treatment with Cisplatin (15 ug/ml) for 24 hours (c) and inhibited the invasion of HeLa cells treated for 24 hours using Matrigel (d). The significant difference with p ≤ 0.05 values. ∗∗∗ indicates statistically significant differences between treatment and control with value of p ≤ 0.001. ∗∗ indicates statistically significant differences between treatment and control with value of p ≤ 0.005. ∗ represents the significant difference with a value of p ≤ 0.05. Different letters indicate statistically significant differences between each treatment with p ≤ 0.0001.
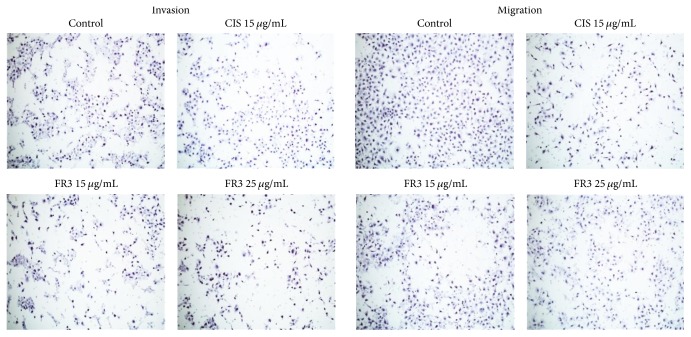
Figure 4.
FR3 decreased the number of cells that migrated through the Boyden Chamber in Migration and Invasion assay. 100x to magnification.
3.3. FR3 Induces Apoptosis by the Extrinsic Pathway in HeLa Cells
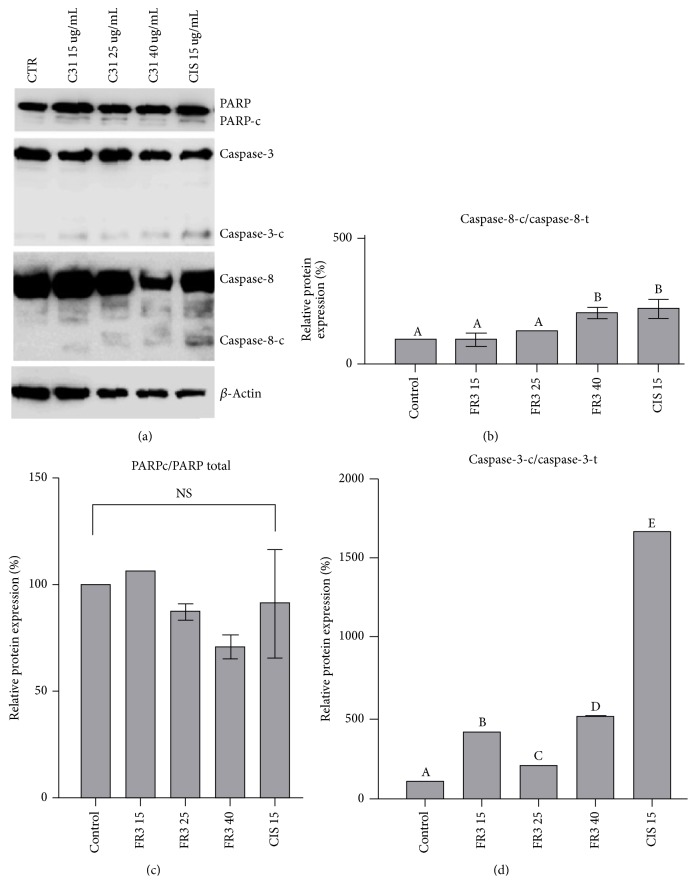
HeLa cell treatment with FR3 showed a significant increase (206.2% ± 20.98, 40 μg/mL) in cleaved caspase-8 expression (Figure 5(b)) and caspase-3c/caspase-3 (15, 25, and 40 μg/mL) (Figure 5(d)). The caspases are a group of cysteine proteases that regulate death mechanisms such as apoptosis.
Figure 5.
The FR3 acted on PARPc/PARP cell death marker proteins, caspase-3c/caspase-3, and caspase-8c/caspase-8 in 24-hour treated HeLa cells. The concentrations used were 15 μg/mL, 25 μg/mL, and 40 μg/mL FR3 and 15 μg/mL Cisplatin. (b)–(d) Densitometric analysis of the western blot transfer data of the proteins. The significant difference with values of p < 0.0001. Different letters indicate statistically significant differences between each treatment with p ≤ 0.0001.
The increase of expression of RIP (a receptor-interacting protein 1, the DD-containing serine/threonine kinase, plays a crucial role in switching between death and survival signaling) and TNF-R1 (canonical death receptor essential for TNF-induced apoptosis) (Figures 6(b) and 6(d)) and decreased FAS expression (40 ug/mL) (Figure 6(c)) are also observed, confirming that FR3 cytotoxicity against HeLa cells was due to induction of cell death by apoptosis. The ratio of PARPc/PARP expression showed no significant difference (Figure 6(c)).
Figure 6.
Effect of the FR3 on RIP, FAS, and TNF-R1 in 24-hour treated HeLa cells at concentrations of 15 μg/mL, 25 μg/mL, and 40 μg/mL and Cisplatin at 15 μg/mL concentrations. (b)–(d) Densitometric analysis of the western blot transfer data of the three proteins. The significant difference with values of p < 0.0001. Different letters indicate statistically significant differences between each treatment with p ≤ 0.0001.
3.4. ESI(−)FT-ICR MS Analysis
Among the assigned compounds in FR3 sample using ESI(−)FT-ICR MS, are the ethyl a-D-glucopyranoside, D-pinitol, and fatty acids such as palmitic acid, oleic acid, and phenolic acid. These compounds are present in plant extracts with proven antitumor activities (Table 1).
Table 1.
Molecular formulas and compounds estimated from components present in FR3 by ESI(-)M.
| Experimental m/z | Error (ppm) | Proposed compounds | Reference |
|---|---|---|---|
| 207.08744 | 0.11 | Ethyl a-D-glucopyranoside | Ndongo et al., 2015 |
| 229.04849 | 0.20 | D-Pinitol | Tien-Huang et al., 2013 |
| 243.06412 | 0.13 | Ethyl a-D-glucopyranoside | - |
| 255.23301 | 0.21 | Palmitic acid | Harada et al., 2002 |
| 281.24867 | 0.24 | Oleic acid | Harada et al., 2002 |
| 421.22677 | 0.5 | Phenolic acid | Chen et al., 2012. |
4. Discussion
In the present study, we showed that the Bauhinia variegata candida fraction (FR3) inhibits the MMP-2 and MMP-9 gelatinolytic activity. We also demonstrated that this fraction decreased HeLa cell viability and exhibited low toxicity in normal cells. The migration and invasion decreased and the amount of active MMP-2 in the HeLa cell supernatant decreased. Moreover, we showed that exposure to FR3 induced cell death via TNFR-1 and RIP1. Finally, mass spectrometry showed that Bauhinia variegata candida fraction FR3 contained glucopyranosides, D-pinitol, fatty acids, and phenolic acid.
It is reported that some plant-derived compounds can exhibit an inhibitory action against MMP-2 and MMP-9 gelatinolytic activity in vitro studies [21–23]. However, few studies have interrogated the action of Bauhinia extracts with inhibition of these enzymes. In a previous study conducted by our group, Bauhinia ungulate extracts also inhibited the activity of MMP-2 and MMP-9 [13]. Thus, it is believed that species of the genus Bauhinia may contain promising components with inhibitory action against MMPs. The FR3 presents as a promising compound for cancer treatment, as it was selectively cytotoxic to the HeLa line. It is thought that this activity is correlated with the presence of ethyl a-d-glucopyranoside, already described in Bauhinia petrandra extracts [24] and belongs to a class of molecules that has been highlighted by the antitumor activity of this cell line [25, 26]. Another component of FR3 that may be correlated with this activity is palmitic acid, which has selective cytotoxic action on the MOLT-4 leukemia cell line [27]. The FR3 is also considered promising because it inhibits MMP-2 and MMP-9, enzymes that are closely linked to dissemination [28] of a type of cancer considered highly invasive and highly expressed in cervical cancer [29].
Our results showed increased expression of TNFR-1 (tumor necrosis factor receptor), which is a membrane receptor that interacts with FADD proteins (FAS-associated death domain) and which, in turn, recruits caspase-8, which cleaves caspase-3, triggering the cell death process extrinsic pathway. Thus, since FR3 also provided for caspase-8 overexpression, it is possible that the cytotoxicity evidenced in the results is correlated with this cell death pathway [30]. However, intricately, death of HeLa cells may also be related to the newly discovered necroapoptosis pathway, as the results showed overexpression of RIPK1 (receptor-interacting serine/threonine protein kinase 1), which is a protein present in the necrosome, a characteristic structure of necroapoptosis formed from the binding of TNF-α to its TNFR-1 ligand on the cell surface [31]. Among the compounds found in FR3, oleic acid, linoleic acid, and glucopyranoside can be correlated with this activity, once they have already demonstrated the activation of the extrinsic pathway of the HeLa lineage [32].
Furthermore, it is believed that the decrease in MMP-2 activity in the cell supernatant is linked to decreased invasion and migration of HeLa cells, as several studies have established a relationship between the in vitro antimetastatic potential of plant extracts and the decrease in active MMPs [33–35]. This can be explained by the fact that MMPs act, via the degradation of the extracellular matrix, through mechanisms that allow tumor growth, angiogenesis, and metastasis [36]. D-pinitol, described as a component of FR3, is believed to present some function in this context, as it has already shown ability to inhibit cell migration, wound closure, and invasion of prostate cancer cells [37].
In conclusion, the present study suggests that the molecules present in the FR3 reduce cervical cancer cell viability. Moreover, we demonstrated a reduction on the migration and invasion activity. These finds can be correlated with MMPs inhibitory activity.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Fundação CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), and FINEP (MCTI/FINEP/MS/SCTIE/DECIT-01/2013-FPXII-BIOPLAT).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.GLOBOCAN. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. 2012. Available in: http://globocan.iarc.fr/Default.aspx.
- 2.Murillo R., Herrero R., Sierra M. S., Forman D. Cervical cancer in Central and South America: Burden of disease and status of disease control. Cancer Epidemiology. 2016;44:S121–S130. doi: 10.1016/j.canep.2016.07.015. http://dx.doi.org/10.1016/j.canep.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Barra F., Lorusso D., Leone Roberti Maggiore U., et al. Investigational drugs for the treatment of cervical cancer. Expert Opinion on Investigational Drugs. 2017;26(4):389–402. doi: 10.1080/13543784.2017.1302427. http://dx.doi.org/10.1080/13543784.2017.1302427. [DOI] [PubMed] [Google Scholar]
- 4.Conway M. J., Meyers C. Replication and assembly of human papillomaviruses. Journal of Dental Research. 2009;88(4):307–317. doi: 10.1177/0022034509333446. http://dx.doi.org/10.1177/0022034509333446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tansi F. L., Rüger R., Böhm C., et al. Potential of activatable FAP-targeting immunoliposomes in intraoperative imaging of spontaneous metastases. Biomaterials. 2016;88:70–82. doi: 10.1016/j.biomaterials.2016.02.028. http://dx.doi.org/10.1016/j.biomaterials.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Clark A. G., Vignjevic D. M. Modes of cancer cell invasion and the role of the microenvironment. Current Opinion in Cell Biology. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. http://dx.doi.org/10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Massagué J., Obenauf A. C. Metastatic colonization by circulating tumour cells. Nature. 2016;529(7586):298–306. doi: 10.1038/nature17038. http://dx.doi.org/10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gialeli C., Theocharis A. D., Karamanos N. K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS Journal. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. http://dx.doi.org/10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 9.Sheu B.-C., Chiou S.-H., Lin H.-H., et al. Up-regulation of inhibitory natural killer receptors CD94/NKG2A with suppressed intracellular perforin expression of tumor-infiltrating CD8+ T lymphocytes in human cervical carcinoma. Cancer Research. 2005;65(7):2921–2929. doi: 10.1158/0008-5472.CAN-04-2108. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D., Ye M., Zhang W. E6/E7 oncoproteins of high risk HPV-16 upregulate MT1-MMP, MMP-2 and MMP-9 and promote the migration of cervical cancer cells. International Journal of Clinical and Experimetal Pathology. 2015;8(5)C4503063 [PMC free article] [PubMed] [Google Scholar]
- 11.Roque D. R., Wysham W. Z., Soper J. T. The surgical management of cervical cancer: An overview and literature review. Obstetrical & Gynecological Survey. 2014;69(7):426–441. doi: 10.1097/OGX.0000000000000089. http://dx.doi.org/10.1097/OGX.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 12.Jaconodino C. B., Amestoy S. C., Thofehrn M. B. A utilização de terapias alternativas por pacientes em tratamento Quimioterápico. Cogitare Enfermagem. 2008;13(1) doi: 10.5380/ce.v13i1.11953. http://dx.doi.org/10.5380/ce.v13i1.11953. [DOI] [Google Scholar]
- 13.dos Santos K. M., de Fátima Nunes D. A., de Faria Gomes I. N., da Silva S. L., Ribeiro R. I. D. A. Inhibition of gelatinase activity of mmp-2 and mmp-9 by extracts of Bauhinia ungulata L. Bioscience Journal. 2015;31(2):584–590. doi: 10.14393/BJ-v31n2a2015-23477. http://dx.doi.org/10.14393/BJ-v31n2a2015-23477. [DOI] [Google Scholar]
- 14.Solmaz P., Mohammad S., Seyed H. Cytotoxic and apoptogenic effects of Bryonia aspera root extract against Hela and HN-5 cancer cell lines. Avicenna Journal of Phytomedicine. 2017;7(1)C5329178 [PMC free article] [PubMed] [Google Scholar]
- 15.Silva-Oliveira R. J., Silva V. A. O., Martinho O., et al. Cytotoxicity of allitinib, an irreversible anti-EGFR agent, in a large panel of human cancer-derived cell lines: KRAS mutation status as a predictive biomarker. Cellular Oncology. 2016;39(3):253–263. doi: 10.1007/s13402-016-0270-z. http://dx.doi.org/10.1007/s13402-016-0270-z. [DOI] [PubMed] [Google Scholar]
- 16.Bargalló M. E., Guardo A. C., Maleno M. J., et al. Utility of Systematic Isolation of immune cell subsets from HIV-infected individuals for miRNA profiling. Journal of Immunological Methods. 2017;442:12–19. doi: 10.1016/j.jim.2016.12.005. https://doi.org/10.1016/j.jim.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Martinho O., Silva-Oliveira R., Cury F. P., et al. HER family receptors are important theranostic biomarkers for cervical cancer: Blocking glucose metabolism enhances the therapeutic effect of HER inhibitors. Theranostics. 2017;7(3):717–732. doi: 10.7150/thno.17154. http://dx.doi.org/10.7150/thno.17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudsai T., Wattanapiromsakul C., Tewtrakul S. Wound healing property of isolated compounds from Boesenbergia kingii rhizomes. Journal of Ethnopharmacology. 2016;184:42–48. doi: 10.1016/j.jep.2016.03.001. http://dx.doi.org/10.1016/j.jep.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Moniz S., Martinho O., Pinto F., et al. Loss of WNK2 expression by promoter gene methylation occurs in adult gliomas and triggers Rac1-mediated tumour cell invasiveness. Human Molecular Genetics. 2013;22(1):84–95. doi: 10.1093/hmg/dds405. http://dx.doi.org/10.1093/hmg/dds405.dds405 [DOI] [PubMed] [Google Scholar]
- 20.Baliano A. P., Pimentel E. F., Buzin A. R., et al. Brown seaweed Padina gymnospora is a prominent natural wound-care product. Revista Brasileira de Farmacognosia. 2016;26(6):714–719. doi: 10.1016/j.bjp.2016.07.003. http://dx.doi.org/10.1016/j.bjp.2016.07.003. [DOI] [Google Scholar]
- 21.Lin H.-J., Kao S.-T., Siao Y.-M., Yeh C.-C. The Chinese medicine Sini-San inhibits HBx-induced migration and invasiveness of human hepatocellular carcinoma cells. BMC Complementary and Alternative Medicine. 2015;15(1, article no. 348) doi: 10.1186/s12906-015-0870-6. http://dx.doi.org/10.1186/s12906-015-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poachanukoon O., Meesuk L., Pattanacharoenchai N., Monthanapisut P., Ayudhya T. D. N., Koontongkaew S. Zingiber cassumunar ROXb. and its active constituent inhibit MMP-9 direct activation by house dust mite allergens and MMP-9 expression in PMA-stimulated human airway epithelial cells. Asian Pacific Journal of Allergy and Immunology. 2015;33(1):42–51. doi: 10.12932/AP0490.33.1.2015. http://dx.doi.org/10.12932/AP0490.33.1.2015. [DOI] [PubMed] [Google Scholar]
- 23.Jeong J.-W., Kim J. W., Ku S. K., et al. Essential oils purified from Schisandrae semen inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 activation and migration of human aortic smooth muscle cells. BMC Complementary and Alternative Medicine. 2015;15(1, article no. 7) doi: 10.1186/s12906-015-0523-9. http://dx.doi.org/10.1186/s12906-015-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida M. C. S. D. Contribuição ao conhecimento químico de Plantas Medicinais do Nordeste: Bauhinia pentandra (BONG.) D.DIETR e Bauhinia monandra KURZ. 2015. 257 f. Tese (Doutorado em Química) Universidade Federal do Ceará, Fortaleza, 2015.
- 25.Ndongo J. T., Issa M. E., Messi A. N., et al. Cytotoxic flavonoids and other constituents from the stem bark of Ochna schweinfurthiana. Natural Product Research. 2015;29(17):1684–1687. doi: 10.1080/14786419.2014.991321. http://dx.doi.org/10.1080/14786419.2014.99132. [DOI] [PubMed] [Google Scholar]
- 26.Li C. W., Dong H. J., Cui C. B. The synthesis and antitumor activity of twelve galloyl glucosides. Molecules. 2015;20(12):2034–2060. doi: 10.3390/molecules20022034. http://dx.doi.org/10.3390/molecules20022034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada H., Yamashita U., Kurihara H., Fukushi E., Kawabata J., Kamei Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Reseach. 2002;22(5) http://dx.doi.org/12529968. [PubMed] [Google Scholar]
- 28.Kato Y., Yamashita T., Ishikawa M. Relationship between expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 and invasion ability of cervical cancer cells. Oncology Reports. 2002 doi: 10.3892/or.9.3.565. http://dx.doi.org/11956628. [DOI] [PubMed] [Google Scholar]
- 29.Cardeal L. B. D. S., Brohem C. A., Corrêa T. C. S. Higher expression and activity of metalloproteinases in human cervical carcinoma cell lines is associated with HPV presence. The International Journal of Biochemistry & Cell Biology. 2006;84(5):713–719. doi: 10.1139/O06-084. http://dx.doi.org/10.1139/o06-084. [DOI] [PubMed] [Google Scholar]
- 30.Karmakar U. K., Ishikawa N., Arai M. A., et al. Boesenberols, Pimarane Diterpenes with TRAIL-Resistance-Overcoming Activity from Boesenbergia pandurata. Journal of Natural Products. 2016;79(8):2075–2082. doi: 10.1021/acs.jnatprod.6b00424. http://dx.doi.org/10.1021/acs.jnatprod.6b00424. [DOI] [PubMed] [Google Scholar]
- 31.Hannes S., Abhari B. A., Fulda S. Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked. Cancer Letters. 2016;380(1):31–38. doi: 10.1016/j.canlet.2016.05.036. http://dx.doi.org/10.1016/j.canlet.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Xiong Y., Wu X., Rao L. Tetrastigma hemsleyanum (Sanyeqing) root tuber extracts induces apoptosis in human cervical carcinoma HeLa cells. Journal of Ethnopharmacology. 2015;165:46–53. doi: 10.1016/j.jep.2015.02.030. http://dx.doi.org/10.1016/j.jep.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Chiu C.-T., Chen J. H., Chou F.-P., Lin H.-H. Hibiscus sabdariffa leaf extract inhibits human prostate cancer cell invasion via down-regulation of Akt/NF- κB/MMP-9 pathway. Nutrients. 2015;7(7):5065–5087. doi: 10.3390/nu7075065. http://dx.doi.org/10.3390/nu7075065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nho K. J., Chun J. M., Kim D.-S., Kim H. K. Ampelopsis japonica ethanol extract suppresses migration and invasion in human MDA-MB-231 breast cancer cells. Molecular Medicine Reports. 2015;11(5):3722–3728. doi: 10.3892/mmr.2015.3179. http://dx.doi.org/10.3390/nu7075065. [DOI] [PubMed] [Google Scholar]
- 35.Lin C.-Y., Chen P.-N., Hsu L.-S., Kuo D. Y., Chu S.-C., Hsieh Y.-S. Inhibition of the invasion and migration of renal carcinoma 786-o-si3 cells in vitro and in vivo by Koelreuteria formosana extract. Molecular Medicine Reports. 2014;10(6):3334–3342. doi: 10.3892/mmr.2014.2587. http://dx.doi.org/10.3892/mmr.2014.2587. [DOI] [PubMed] [Google Scholar]
- 36.Deryugina E. I., Quigley J. P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biology. 2015;44–46:94–112. doi: 10.1016/j.matbio.2015.04.004. http://dx.doi.org/10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin T.-H., Tan T.-W., Tsai T.-H., et al. D-pinitol inhibits prostate cancer metastasis through inhibition of αVβ3 integrin by modulating FAK, c-Src and NF-κB pathways. International Journal of Molecular Sciences. 2013;14(5):9790–9802. doi: 10.3390/ijms14059790. http://dx.doi.org/10.3390/ijms14059790. [DOI] [PMC free article] [PubMed] [Google Scholar]