Abstract
The neuropeptide calcitonin gene-related peptide (CGRP) is implicated in the underlying pathology of migraine by promoting the development of a sensitized state of primary and secondary nociceptive neurons. The ability of CGRP to initiate and maintain peripheral and central sensitization is mediated by modulation of neuronal, glial, and immune cells in the trigeminal nociceptive signaling pathway. There is accumulating evidence to support a key role of CGRP in promoting cross excitation within the trigeminal ganglion that may help to explain the high co-morbidity of migraine with rhinosinusitis and temporomandibular joint disorder. In addition, there is emerging evidence that CGRP facilitates and sustains a hyperresponsive neuronal state in migraineurs mediated by reported risk factors such as stress and anxiety. In this review, the significant role of CGRP as a modulator of the trigeminal system will be discussed to provide a better understanding of the underlying pathology associated with the migraine phenotype.
Keywords: Calcitonin gene-related peptide, Co-morbidity, Glia, Migraine, Sensitization, Trigeminal
Introduction
Migraine is a complex chronic, debilitating disease that affects 12 % of the general population and is characterized by a headache lasting from 4 h to 3 days [1, 2]. Associated symptoms include nausea; vomiting; and sensitivity to light, sound, and head movements. A key underlying feature of migraine pathology involves sensitization and excitation of trigeminal ganglion nerves [3, 4••]. Activation of trigeminovascular afferents in the meninges releases inflammatory neuropeptides and other small molecules that promote neurogenic inflammation and development of peripheral sensitization of the primary nociceptive neurons. In addition, activation stimulates efferent release of neuropeptides and other pro-inflammatory mediators that contribute to the development of central sensitization characterized by a lower activation threshold of second-order nociceptive neurons, hyperalgesia, and allodynia. While migraine triggers are thought to initiate an attack, the development and maintenance of a prolonged state of sensitization of trigeminal neurons, as seen in frequent and chronic migraine, is likely mediated by risk factors that sustain peripheral and central sensitization of trigeminal neurons [5, 6].
Based on the seminal studies in the early 1990s [7, 8], the neuropeptide calcitonin gene-related peptide (CGRP) has been strongly linked to migraine pathology and treatment of episodic migraine. Based on current migraine models, release of CGRP from trigeminal nerves is thought to play a central role in the painful phase of migraine due to its ability to mediate neurogenic inflammation and convey nociceptive information to the central nervous system [9, 10]. Lending support to this notion are findings that serum levels of CGRP obtained from the external jugular vein are elevated in patients during migraine (with and without aura) [7, 11, 12]. In addition, increased CGRP levels have been reported in saliva and cerebrospinal fluid during migraine attacks [13, 14]. Further evidence of the involvement of CGRP in migraine is provided by data from clinical trials, in which triptans, acute anti-migraine drugs that block CGRP release [15, 16], and molecules that block activity of CGRP or its receptor could effectively alleviate headache pain [17]. Support for a causative role of CGRP in episodic migraine was demonstrated when administration of CGRP to migraine patients caused a headache and associated symptoms that were indistinguishable from the patient’s typical migraine attack [18]. Taken together, these data provide strong evidence that CGRP is a key modulator of the trigeminal system and thus plays an important role in the pathophysiology of migraine.
Key to the underlying pathology of migraine is the concept of peripheral and central sensitization of trigeminal nociceptive neurons. Migraineurs are characterized by a hypervigilant nervous system that likely is the result of genetic predisposition, environmental factors, and lifestyle choices [6, 19]. An important question to address is what factors are increasing the risk of having an attack in migraine-susceptible individuals. Of the risk factors cited by migraineurs, stress, anxiety, and neck muscle tension are often most commonly reported to temporally correlate with their attacks. Interestingly, these factors are known to cause dysfunction of descending inhibition that can lead to the development and maintenance of central sensitization [20]. In addition, reported migraine risk factors include rhinosinusitis [21, 22] and temporomandibular joint disorders [23–26], which are prevalent orofacial pain conditions that also involve sensitization of primary and secondary trigeminal nociceptive neurons. In this review, evidence for a fundamental role of CGRP in migraine via its ability to promote and sustain peripheral and central sensitization of trigeminal nociceptive neurons will be discussed. Furthermore, we will provide evidence that these CGRP-mediated physiological events involve modulation of neurons, glial cells, and immune cells within the trigeminal system.
CGRP: Structure and Function
In humans, CGRP exists as two isoforms referred to originally as α-CGRP and β-CGRP but now referred to as CGRP1 and CGRP2 that are synthesized by two different genes yet perform similar biological functions [27–29]. Based on immunohistological studies with human tissues, we know that α-CGRP is preferentially expressed in sensory neurons when compared to levels of β-CGRP [30]. The role of α-CGRP, which will be referred to as simply CGRP, will be the primary focus of this review since this isoform is the predominant form expressed in trigeminal ganglial neurons [31].
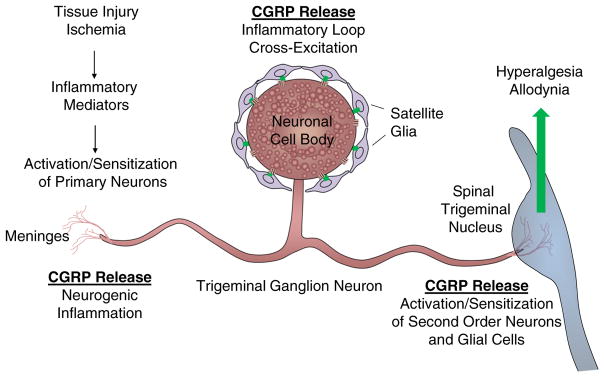
CGRP is one of the most abundant peptides in nerve tissue and is widely expressed in both peripheral and central neurons [32, 33]. While a broad range of biological functions have been attributed to CGRP, its role in promoting neurogenic inflammation and sensitization of trigeminal neurons are most relevant to migraine pathology [3]. CGRP’s role in migraine pathology likely involves its release from the following three distinct sites in trigeminal ganglion neurons: the afferent terminals that innervate the meninges, the cell body localized in the ganglion, and from the efferent fibers that terminate in the upper spinal cord (Fig. 1). The physiological effects of CGRP in promoting neurogenic inflammation and peripheral and central sensitization of nociceptive neurons are mediated via activation of the CGRP receptor. Functional human CGRP receptors are comprised of three subunits that include the G protein-coupled receptor referred to as calcitonin receptor-like receptor (CLR), the receptor activity-modifying protein (RAMP), and a receptor component protein (RCP) [34]. While the associated RAMP subunit is essential in delineating the relative potency of receptor ligands, the RCP determines the G protein that will couple to CLR activation [35]. Binding of CGRP to the CLR/RAMP1 receptor is known to cause activation of the enzyme adenylate cyclase that generates the secondary messenger cAMP leading to elevated intracellular levels of protein kinase A in many cell types including neuronal and glial cells [33, 34]. Within the trigeminal system, CGRP receptors are reported to be expressed by a diverse number of cell types including mast cells in the meninges, and second-order neurons, astrocytes, and microglia in the upper spinal cord [32, 36•, 37]. In addition, the existence of functional CGRP receptors on the cell body of trigeminal ganglial neurons as well as associated satellite glial cells has been reported [38–40].
Fig. 1.
A schematic illustration of potential sites of CGRP secretion from trigeminal ganglion neurons associated with migraine pathology. In response to tissue injury or ischemia, inflammatory mediators would cause activation of primary nociceptive neurons and subsequent CGRP release in the meninges to promote neurogenic inflammation, from the cell body in the ganglion to facilitate development of an inflammatory loop and cross excitation, and in the spinal trigeminal nucleus to cause activation of second-order neuronal and glial cells resulting in hyperalgesia and allodynia. While CGRP release in the meninges and the ganglion would initiate and sustain peripheral sensitization of primary trigeminal neurons, elevated CGRP levels in the upper spinal cord would promote development of central sensitization
CGRP and Migraine: Cellular Targets in Trigeminal Pathway
The diverse roles of CGRP implicated in migraine pathology have been delineated from results from human and animal studies investigating the effects of pharmaceutical and experimental compounds that target trigeminal neurons. Based on these findings, the therapeutic anti-migraine properties of current drugs is thought to involve inhibiting the activation and sensitization of primary nociceptive trigeminal neurons and the subsequent release of CGRP both peripherally in the meninges and ganglion and centrally in the upper spinal cord [15]. For example, the ability of the triptan class of serotonergic drugs to abort migraine attacks is believed to involve blocking CGRP secretion from the primary afferents that promote dilation of meningeal blood vessels and activation of resident mast cells [41]. Once activated, mast cells can release histamine and other pro-inflammatory mediators implicated in the development of neurogenic inflammation [42]. CGRP also functions to promote protein plasma extravasation by potentiating the physiological effects of the neuropeptide substance P and is known to play a role in the recruitment and activation of other immune cells including mast cells. Triptans also block secretion of CGRP from nerve fibers that project into the outer lamina of the upper spinal cord [43]. Importantly, central release of CGRP leading to elevated levels in the spinal cord is known to facilitate sensitization of second-order neurons by lowering the activation threshold to glutamate, which is co-released with CGRP [44]. Increased CGRP levels in the spinal cord can also stimulate astrocytes and microglial cells to release inflammatory cytokines and other pro-inflammatory mediators known to promote and maintain a state of central sensitization [45, 46]. Another site of CGRP release is from the cell body of trigeminal neurons localized in the ganglion that are known to express serotonergic receptors targeted by triptan drugs [47]. CGRP secretion within the ganglion can promote changes in trigeminal neurons and satellite glial cells that are found in close association with neuronal cell bodies and known to modulate the excitability state of primary nociceptive neurons [48]. Activation of CGRP receptors on trigeminal neurons is reported to work in an autocrine manner to increase its own expression by stimulating further synthesis and secretion from the neuronal cell body [38]. In addition, CGRP is known to function as a paracrine signal to stimulate secretion of pro-inflammatory cytokines and nitric oxide from satellite glial cells that would help to maintain a sensitized state of primary nociceptive neurons [39, 49, 50]. Taken together, CGRP functions at multiple sites within the trigeminal system to initiate and sustain peripheral and central sensitization via its ability to modulate the activity of immune cells, glial cells, and neuronal cells. Furthermore, CGRP’s role in promoting increased communication between neuronal and glial cells both in the ganglion and upper spinal cord has important implications, given that increased neuronglial signaling is associated with development of more chronic pain states [51, 52].
CGRP Release—Two Independent Pathways in Trigeminal Neurons
After being synthesized in the cell body of trigeminal ganglion neurons, CGRP is stored in dense core vesicles within the cell body and in peripheral and central nerve processes and terminals [47]. In response to activation of trigeminal neurons, CGRP can be released from the afferent and efferent fibers but also from the neuronal cell body. Stimulated CGRP secretion is thought to be primarily mediated via a calcium-dependent mechanism involving the vesicle-docking protein known as synaptosomal-associated protein 25 (SNAP-25), which is required for membrane fusion [32, 53–55]. Significantly, the inhibitory effects of triptans, drugs commonly used as an abortive migraine therapy [15], and onabotulinumtoxinA, which has been approved as a treatment for chronic migraine, are thought to involve blocking the calcium/SNAP-25-mediated release of CGRP from trigeminal neurons [56]. Although triptans and onabotulinumtoxinA are approved therapies for episodic and chronic migraine, respectively, there still remain a significant percentage of migraine sufferers that fail to respond to these treatment options [57, 58]. A plausible explanation for their lack of efficacy may be attributable to their ability to inhibit calcium/SNAP-25-dependent release of CGRP, but not calcium/SNAP-25-independent neurotransmitter secretion, which has been reported in sensory neurons including the trigeminal nerves [59].
CGRP can be secreted from cultured trigeminal neurons in response to a decrease in extracellular pH [60, 61], as proposed to occur within the meninges during a migraine attack [3]. The decrease in pH correlates with an increase in the concentration of extracellular protons, which mediates CGRP release by a novel calcium and SNAP-25-independent mechanism [59]. Protons induce CGRP secretion by a mechanism that involves increases in intracellular sodium ions and activation of the acid-sensitive ion channel ASIC3. The existence of two independent vesicle pools involved in CGRP release may allow trigeminal neurons to more finely regulate CGRP secretion in response to the type of inflammatory stimuli and severity of the pathology. In this way, the amount of CGRP released from trigeminal neurons would be greatest if both calcium-dependent as well as calcium-independent mechanisms were induced by an inflammatory soup as proposed to occur during a migraine attack [62, 63]. It is interesting to note that many of the pro-inflammatory molecules known to promote and sustain neurogenic inflammation within the dura have been shown to stimulate the expression and activity of ASIC3 [64, 65]. Thus, increased levels of pro-inflammatory molecules would promote elevated levels of ASIC3, which would facilitate an enhanced inflammatory response to protons during an attack by stimulating release of CGRP. In summary, the ability of trigeminal neurons to secrete CGRP via two distinct mechanisms may help to explain the significantly elevated levels of CGRP reported during a severe migraine attack.
Importantly, the proton-mediated release of CGRP from cultured trigeminal neurons was not blocked by the anti-migraine therapeutic agents rizatriptan and onabotulinumtoxinA [59]. However, KCl-induced stimulation of CGRP release, which involves a calcium/SNAP-25-dependent mechanism, was inhibited by rizatriptan and onabotulinumtoxinA. Thus, these findings support the notion that these therapies would not be effective in treating migraine headaches that primarily involve elevated extracellular proton levels (acidic pH). While there are currently no drugs approved for the treatment of migraine that selectively target ASICs, nonsteroidal anti-inflammatory drugs (NSAIDs), which are commonly used to effectively treat migraine, are known to inhibit ASIC channel activity at therapeutic doses that produce analgesic effects [66]. Furthermore, findings from clinical studies provide evidence that the combination of a triptan (sumatriptan) with an NSAID (naproxen) was more effective than treatment with either drug alone, possibly, because they inhibit both pathways involved in CGRP release [67]. Although not approved as a migraine therapeutic in the USA, the drug amiloride, which is known to inhibit ASICs, has been reported to be an effective therapy in some individuals with medically refractory migraine with prolonged aura [68].
Rhinosinusitis and Temporomandibular Joint Disorder as Migraine Risk Factors: Role of Cross Excitation in Trigeminal Ganglion
Rhinosinusitis and temporomandibular joint disorder (TMD), which involve tissue inflammation and sensitization and activation of trigeminal ganglion neurons, are considered risk factors for migraine [24, 69]. The phenomenon of cross excitation within the trigeminal ganglion may help to explain why activation of one branch of the trigeminal ganglion can promote cellular changes associated with peripheral sensitization within the entire ganglion. In an earlier study, injection of inflammatory molecules into the temporomandibular joint (TMJ), which was predicted to mediate activation of only a few sensory neurons in the V3 region of the trigeminal ganglion, resulted in intracellular changes in neighboring neuronal and glial cells within all regions of the ganglion including V1 and V2 [40]. This type of intraganglion communication involving cross depolarization or cross excitation has also been reported to occur between sensory neurons via release of diffusible molecules from the neuronal cell body in nodose ganglion [70] and dorsal root ganglion [71–73]. The release of CGRP from the neuronal cell body and processes is thought to facilitate this spreading of the inflammatory signal throughout the ganglion that likely promotes peripheral sensitization of trigeminal nociceptive neurons [74]. Based on results from several studies on the trigeminal ganglion [40, 73, 75], CGRP would be expected to cause excitation of other neuronal cells and satellite glial cells given that functional CGRP receptors are expressed on those cells [38, 39]. Thus, CGRP release from neuronal cell bodies or neuronal processes within the ganglion can function to cause sensitization of other neuronal and glial cells not initially involved in mediating the inflammatory response. The stimulatory effects of CGRP in the ganglion likely involve increased expression and release of other inflammatory molecules such as cytokines and ATP. Elevated levels of these molecules would establish an inflammatory loop between neurons and satellite glial cells that promote the spreading of the signal to other regions and maintain a sensitized or hyperexcitable state of the trigeminal neurons [48].
Further evidence of cross excitation in the trigeminal ganglion was demonstrated in a novel in vivo model of acute TMJ inflammation involving co-injection of nitric oxide (NO) and protons [49] into the TMJ capsule since elevated levels of these molecules in human TMJ capsules are implicated in joint inflammation and pain [76, 77]. Similar to the Thalakoti study, stimulation of V3 neurons that innervate the TMJ resulted in temporal and spatial changes in the expression of key inflammatory proteins in both neurons and satellite glial cells in all regions of the trigeminal ganglion. Results from another study designed to determine if tumor necrosis factor (TNF)-mediated sensitization of V2 neurons would lower the activation threshold of V1 neurons provided further evidence of cross excitation. The rationale for that study was based on the report that migraineurs often cite sinus pain and pressure as a risk factor of their headaches and the high co-morbidity reported between migraine and allergic rhinitis and acute sinusitis [69]. In the study by Damodaram et al. [78], injection of TNF-α, a cytokine whose levels are elevated in nasal secretions during allergic rhinitis and acute sinusitis, in a facial region (whisker pad) that is innervated by V2 neurons lowered the activation threshold to a subthreshold concentration of capsaicin in V1 neurons. However, TNF-α or capsaicin treatment alone was not sufficient to cause cellular changes in V1 neurons. Sensitization and activation of trigeminal neurons originating in the V1/V2 region of the ganglion that provide sensory innervation of the meninges are thought to be involved in the pathology of migraine [3]. Taken together, finding from these studies support a model by which CGRP promotes activation of neurons and satellite glial cell in one region of the ganglion that initiate an inflammatory cascade involving other neurons as well as satellite glial cells, leading to increased intraganglion and neuronal-glial communication and the development of peripheral sensitization throughout the ganglion. Furthermore, these data may provide a cellular basis for why TMD pathology and rhinosinusitis are considered risk factors for migraine by maintaining a sensitized state of the nociceptors within the trigeminal ganglion.
Migraine Risk Factors and CGRP: Role of Stress, Anxiety, and Neck Muscle Tension
Stress, neck muscle tenderness, and anxiety are some of the most commonly reported risk factors associated with episodic and chronic migraine [6]. There is emerging evidence that in many complex neurological diseases that environmental factors such as stress and anxiety greatly influence disease onset, progression, and maintenance of the clinical phenotype [79], the pathophysiological effects of unmanaged stress can include increased tension in the muscles of the neck and shoulders especially in females. Based on immunohistochemical studies, skeletal muscles are predominantly innervated by CGRP-containing neurons that facilitate pain signal transmission to the spinal cord [80–82]. Chronic muscle overload and tension in the neck and shoulders can lead to persistent fiber contraction, local ischemia, and the release of pro-inflammatory mediators, including bradykinin, glutamate, and CGRP, which can promote sensitization and activation of primary nociceptors [3]. Excitation of nociceptive neurons, which occurs in response to tonic muscle activity associated with myogenic trigger points, can lead to hypersensitization and lower-pain thresholds of second-order nociceptive neuron characteristic of central sensitization [83]. Convergence in the upper spinal cord of nerves providing sensory innervation of the neck and shoulder muscles and those emanating from the trigeminal ganglion may help to explain why neck and shoulder pathology is often associated with migraine. Elevated levels of CGRP in the spinal cord, as can occur in response to prolonged muscle tension in the neck, are implicated in the development of central sensitization and a prolonged state of neuronal sensitization [44]. The sensitizing effect of CGRP is thought to involve upregulation of protein kinase A and protein kinase C in second-order neurons, astrocytes, and microglia that are known to promote and sustain a highly sensitized state of nociceptive neurons indicative of a chronic pain state. In support of this notion, spinal application of CGRP facilitates nociceptive behavior and sensitizes the responses of dorsal horn neurons to innocuous and noxious peripheral stimulation [84, 85]. In contrast, blocking CGRP receptors with the peptide antagonist (CGRP8–37) [86] or anti-serum [87] has been reported to be anti-nociceptive in animal models of inflammatory or central neuropathic pain. In sum, these findings support a fundamental role of CGRP in promoting the development of a more persistent sensitized state of trigeminal primary and secondary nociceptive neuron characteristics of frequent and chronic migraine.
OnabotulinumtoxinA, which has been approved as a therapeutic option for chronic migraine, has traditionally been used clinically for the treatment of neuromuscular disorders including focal dystonias and relief of pain associated with cervical and oromandibular dystonias [88]. At the cellular level, onabotulinumtoxinA is known to inhibit the presynaptic release of the neurotransmitter acetylcholine from motor neurons at neuromuscular junctions and thus can suppress overactivity of muscle fibers [89]. Of clinical significance, muscle pain and tenderness, especially in the shoulders and neck, are physiological symptoms associated with migraine and are more commonly observed as migraine becomes more frequent [6]. Thus, sustained signaling from tonic contraction of craniofacial muscles is likely to provide a mechanism to induce prolonged sensitization of nociceptive neurons. This notion is possible because of the convergence of nociceptive signals in the upper spinal cord of cervical spinal cord neurons that innervate craniofacial muscles and trigeminal nociceptive neurons that provide sensory innervation of the dura [90]. With respect to the treatment of chronic migraine, it is interesting to note that the sites of onabotulinumtoxinA injections are topographically similar to the myogenic trigger points associated with referred pain locations in the head, neck, and shoulders [91]. Thus, onabotulinumtoxinA may function, at least in part, to suppress the activity of myogenic trigger points and decrease the persistent sensory barrage that promotes and helps maintain a state of central sensitization in the upper spinal cord. Insupport of this notion, injection of onabotulinumtoxinA into craniofacial muscles was shown to rapidly inhibit mechanical sensitivity of temporal muscle nociceptors by inhibiting the central release of glutamate and CGRP from muscle nociceptors [92]. Similarly, administration of botulinum toxin type A subcutaneously or injected intrathecally was found to diminish bilateral hyperalgesia in a model of sustained muscle pain caused by unilateral repeated injections of acidic saline [93]. Furthermore, data from a clinical study of abobotulinumtoxinA (Dysport) provided evidence of the anti-nociceptive effect of toxin injection in the ten most tender trigger points in patients with moderate to severe myofacial pain syndrome affecting their cervical and shoulder muscles [94]. Taken together, the therapeutic benefit of using botulinum toxin A is likely to involve its ability to inhibit nociceptive signaling from muscles to the upper spinal cord and thus suppress maintenance of central sensitization. As such, botulinum toxin type A inhibition of CGRP release centrally would be expected to minimize sensory input caused by inflammation and nociception in head, neck, and shoulder muscles, which is a commonly reported risk factor of migraine.
Similar to stress, anxiety is often cited as a risk factor for progression from frequent to chronic migraine. Based on findings from a recent study [95•], CGRP is likely to contribute to migraine pathology by facilitating anxiety-like states mediated via projections of the bed nucleus of the stria terminalis to autonomic and neuroendocrine regulatory structures of the brain. Injection of CGRP was shown to potentiate anxiety-like behaviors and increase neural activity in bed nucleus of the stria terminalis targets. The bed nucleus of the stria terminalis functions as a relay site within the hypothalamic-pituitary-adrenal axis to regulate its activity in response to acute stress caused by real or perceived threats [96]. Importantly, anxiety can lead to dysfunction of the descending inhibitory pathway, which is known to play a key role in modulation of the ascending spino-parabrachioamygdaloid tract [97] and is associated with the development of a more persistent pain state [98, 99]. In summary, elevated CGRP levels have been shown to alter the activity of neurons responsible for promoting anxiety-related behaviors, which are associated with progression to more frequent migraine attacks. Sustained CGRP levels are likely to contribute to migraine pathology by suppressing the descending inhibition pathway that functions to maintain neuronal homeostasis in the spinal cord in response to nociceptive signaling.
Summary and Conclusions
Our understanding of the diverse roles mediated by CGRP that contribute to migraine pathology has been greatly expanded since the early 1990s when CGRP’s primary role was thought to only involve vasodilation and promotion of neurogenic inflammation. We now know that CGRP functions to modulate the activity state of neurons, glial cells, and immune cells within the trigeminal system to promote peripheral and central sensitization and a persistent state of hypervigilance associated with the migraine phenotype. Importantly, CGRP release from primary trigeminal neurons can occur via two independent mechanisms, a finding that has important implications for understanding why triptans and onabotulinumtoxinA may not be an effective therapy of some migraine attacks. Given its ability to modulate neuronal and glial functions within the trigeminal ganglion, CGRP is now thought to play a key role in promoting cross excitation, which is involved in lowering the threshold of activation of nociceptive neurons in other branches of the ganglion. In this way, CGRP functions to mediate the development of a neuronal-glial inflammatory loop that initiates and sustains peripheral sensitization of all branches, which may help to explain the high co-morbidity of migraine with rhinosinusitis and TMD. In addition, elevated levels of CGRP within the central nervous system are implicated in the development of central sensitization by commonly reported migraine risk factors including neck and shoulder muscle tension/inflammation and anxiety. Based on the important involvement of CGRP in migraine pathology, it is not surprising that novel therapies utilizing humanized monoclonal antibodies that specifically target CGRP or its receptor are currently being tested in clinical trials. Although CGRP has had a long history with respect to migraine pathology, there is still much to learn about its complex role in mediating pathophysiological changes associated with episodic and chronic migraine.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Paul L. Durham declares no conflict of interest.
Human and Animal Rights and Informed Consent All studies by Paul Durham involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ferrari MD. Migraine. Lancet. 1998;351(9108):1043–51. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- 2.Lipton R, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–57. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 3.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–91. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 4••.Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015. This is an excellent comprehensive review of our current understanding of migraine pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DI, De ver Dye T. Migraine and the environment. Headache. 2009;49(6):941–52. doi: 10.1111/j.1526-4610.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27(5):394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 7.Goadsby P, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 8.Goadsby PJ, Edvinsson L Joint 1994 Wolff Award Presentation. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache. 1994;34(7):394–9. doi: 10.1111/j.1526-4610.1994.hed3407394.x. [DOI] [PubMed] [Google Scholar]
- 9.Benemei S, et al. Pain pharmacology in migraine: focus on CGRP and CGRP receptors. Neurol Sci. 2007;28(Suppl 2):S89–93. doi: 10.1007/s10072-007-0757-5. [DOI] [PubMed] [Google Scholar]
- 10.Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124(3):309–23. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Goadsby P, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117:427–34. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- 12.Fanciullacci M, et al. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60(2):119–23. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- 13.Durham P, Papapetropoulos S. Biomarkers associated with migraine and their potential role in migraine management. Headache. 2013;53(8):1262–77. doi: 10.1111/head.12174. [DOI] [PubMed] [Google Scholar]
- 14.van Dongen RM, et al. Migraine biomarkers in cerebrospinal fluid: a systematic review and meta-analysis. Cephalalgia. 2016 doi: 10.1177/0333102415625614. [DOI] [PubMed] [Google Scholar]
- 15.Bigal ME, Krymchantowski AV, Hargreaves R. The triptans. Expert Rev Neurother. 2009;9(5):649–59. doi: 10.1586/ern.09.15. [DOI] [PubMed] [Google Scholar]
- 16.Villalon CM, et al. Migraine: pathophysiology, pharmacology, treatment and future trends. Curr Vasc Pharmacol. 2003;1(1):71–84. doi: 10.2174/1570161033386826. [DOI] [PubMed] [Google Scholar]
- 17.Olesen J, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Eng J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 18.Lassen L, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 19.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 2006;5(2):148–57. doi: 10.1016/S1474-4422(06)70348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren K, Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain. 1999;13(3):155–63. discussion 164–71. [PubMed] [Google Scholar]
- 21.Bellamy J, et al. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23(8):2057–66. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cady RK, et al. Sinus headache: a neurology, otolaryngology, allergy, and primary care consensus on diagnosis and treatment. Mayo Clin Proc. 2005;80(7):908–16. doi: 10.4065/80.7.908. [DOI] [PubMed] [Google Scholar]
- 23.Ballegaard V, et al. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia. 2008;28(8):832–41. doi: 10.1111/j.1468-2982.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 24.Bevilaqua Grossi D, Lipton RB, Bigal ME. Temporomandibular disorders and migraine chronification. Curr Pain Headache Rep. 2009;13(4):314–8. doi: 10.1007/s11916-009-0050-9. [DOI] [PubMed] [Google Scholar]
- 25.Dahan H, et al. Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. J Headache Pain. 2015;16:528. doi: 10.1186/s10194-015-0528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graff-Radford SB. Temporomandibular disorders and headache. Dent Clin N Am. 2007;51(1):129–44. vi–vii. doi: 10.1016/j.cden.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Amara S, et al. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–4. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld M, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–35. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- 29.Fischer JA, Born W. Novel peptides from the calcitonin gene: expression, receptors and biological function. Peptides. 1985;6(Suppl 3):265–71. doi: 10.1016/0196-9781(85)90384-5. [DOI] [PubMed] [Google Scholar]
- 30.Mulderry PK, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25(1):195–205. doi: 10.1016/0306-4522(88)90018-8. [DOI] [PubMed] [Google Scholar]
- 31.Amara SG, et al. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229(4718):1094–7. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- 32.van Rossum D, Hanisch U, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21(5):649–78. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 33.Wimalawansa S. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev. 1996;17(5):533–85. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- 34.Poyner D, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54(2):233–46. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 35.Mallee J, et al. Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem. 2002;277(16):14294–8. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- 36•.Russell FA, et al. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–142. doi: 10.1152/physrev.00034.2013. This review provides a thorough summary of the diverse physiological and pathophysiological roles of CGRP following its release from sensory neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messlinger K, Fischer MJ, Lennerz JK. Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine. Keio J Med. 2011;60(3):82–9. doi: 10.2302/kjm.60.82. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, et al. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27(10):2693–703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Vause C, Durham P. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thalakoti S, et al. Neuron-Glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47(7):1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy D, et al. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(1–2):166–76. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peroutka SJ. Neurogenic inflammation and migraine: implications for the therapeutics. Mol Interv. 2005;5(5):304–11. doi: 10.1124/mi.5.5.10. [DOI] [PubMed] [Google Scholar]
- 43.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101(12):4274–9. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–91. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- 45.Xie YF. Glial involvement in trigeminal central sensitization. Acta Pharmacol Sin. 2008;29(6):641–5. doi: 10.1111/j.1745-7254.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 46.Old EA, Clark AK, Malcangio M. The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb Exp Pharmacol. 2015;227:145–70. doi: 10.1007/978-3-662-46450-2_8. [DOI] [PubMed] [Google Scholar]
- 47.Durham PL, Russo AF. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J Neurosci. 1999;19(9):3423–9. doi: 10.1523/JNEUROSCI.19-09-03423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durham PL, Garrett FG. Emerging importance of neuron-satellite glia interactions within trigeminal ganglia in craniofacial pain. Open Pain J. 2010;3:3–13. [Google Scholar]
- 49.Freeman S, Patil V, Durham P. Nitric oxide-proton stimulation of trigeminal ganglion neurons increases mitogen-activated protein kinase and phosphatase expression in neurons and satellite glial cells. Neuroscience. 2008;157:542–55. doi: 10.1016/j.neuroscience.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J Neurochem. 2009;110(3):811–21. doi: 10.1111/j.1471-4159.2009.06154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren K. Neuron, glia and reciprocal relationships in pain processing. Open Pain J. 2009;2:7–31. doi: 10.2174/1876386301003010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng J, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29(15):4981–92. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng J, et al. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci. 2007;120(Pt 16):2864–74. doi: 10.1242/jcs.012211. [DOI] [PubMed] [Google Scholar]
- 55.Durham PL, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache. 2004;44(1):35–42. doi: 10.1111/j.1526-4610.2004.04007.x. Discussion 42–3. [DOI] [PubMed] [Google Scholar]
- 56.Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. Eur J Neurol. 2006;13(Suppl 4):1–9. doi: 10.1111/j.1468-1331.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 57.Bigal M, et al. Satisfaction with current migraine therapy: experience from 3 centers in US and Sweden. Headache. 2007;47(4):475–9. doi: 10.1111/j.1526-4610.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 58.Diener HC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 59.Durham PL, Masterson CG. Two mechanisms involved in trigeminal CGRP release: implications for migraine treatment. Headache. 2013;53(1):67–80. doi: 10.1111/j.1526-4610.2012.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vause C, et al. Effect of carbon dioxide on calcitonin gene-related peptide secretion from trigeminal neurons. Headache. 2007;47(10):1385–97. doi: 10.1111/j.1526-4610.2007.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan J, et al. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152(1):106–13. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolay H, Moskowitz MA. Mechanisms of pain modulation in chronic syndromes. Neurology. 2002;59(5 Suppl 2):S2–7. doi: 10.1212/wnl.59.5_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 63.Hargreaves RJ, Shepheard SL. Pathophysiology of migraine—new insights. Can J Neurol Sci. 1999;26(Suppl 3):S12–9. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 64.Mamet J, et al. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22(24):10662–70. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282(24):17325–9. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- 66.Voilley N, et al. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21(20):8026–33. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandes JL, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443–54. doi: 10.1001/jama.297.13.1443. [DOI] [PubMed] [Google Scholar]
- 68.Holland PR, et al. Acid-sensing ion channel 1: a novel therapeutic target for migraine with aura. Ann Neurol. 2012;72(4):559–63. doi: 10.1002/ana.23653. [DOI] [PubMed] [Google Scholar]
- 69.Cady RK, Schreiber CP. Sinus problems as a cause of headache refractoriness and migraine chronification. Curr Pain Headache Rep. 2009;13(4):319–25. doi: 10.1007/s11916-009-0051-8. [DOI] [PubMed] [Google Scholar]
- 70.Oh EJ, Weinreich D. Chemical communication between vagal afferent somata in nodose ganglia of the rat and the guinea pig in vitro. J Neurophysiol. 2002;87:2801–7. doi: 10.1152/jn.2002.87.6.2801. [DOI] [PubMed] [Google Scholar]
- 71.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16(15):4733–41. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amir R, Devor M. Functional cross-excitation between afferent A-and C-neurons in dorsal root ganglia. Neuroscience. 2000;95(1):189–95. doi: 10.1016/s0306-4522(99)00388-7. [DOI] [PubMed] [Google Scholar]
- 73.Ulrich-Lai YM, et al. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from rat trigeminal ganglion: evidence for intraganglionic neurotransmission. Pain. 2001;91(3):219–26. doi: 10.1016/S0304-3959(00)00439-5. [DOI] [PubMed] [Google Scholar]
- 74.Cheng J, Ji R. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33(10):1970–8. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neubert JK, et al. Inflammation-induced changes in primary afferent-evoked release of substance P within trigeminal ganglia in vivo. Brain Res. 2000;871(2):181–91. doi: 10.1016/s0006-8993(00)02440-9. [DOI] [PubMed] [Google Scholar]
- 76.Arinci A, et al. Molecular correlates of temporomandibular joint disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:666–70. doi: 10.1016/j.tripleo.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi T, et al. Association between arthroscopic diagnosis of temporomandibular joint osteoarthritis and synovial fluid nitric oxide levels. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:129–36. doi: 10.1016/s1079-2104(99)70105-8. [DOI] [PubMed] [Google Scholar]
- 78.Damodaram S, et al. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache. 2009;49(1):5–20. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buse DC, et al. Psychiatric comorbidities of episodic and chronic migraine. J Neurol. 2013;260(8):1960–9. doi: 10.1007/s00415-012-6725-x. [DOI] [PubMed] [Google Scholar]
- 80.Barry CM, et al. Sensory nerve fibers containing calcitonin gene-related peptide in gastrocnemius, latissimus dorsi and erector spinae muscles and thoracolumbar fascia in mice. Neuroscience. 2015;291:106–17. doi: 10.1016/j.neuroscience.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 81.Dudek A, et al. Immunohistochemical properties of motoneurons supplying the trapezius muscle in the rat. Pol J Vet Sci. 2011;14(2):199–205. doi: 10.2478/v10181-011-0030-y. [DOI] [PubMed] [Google Scholar]
- 82.Tsukagoshi M, Goris RC, Funakoshi K. Differential distribution of vanilloid receptors in the primary sensory neurons projecting to the dorsal skin and muscles. Histochem Cell Biol. 2006;126(3):343–52. doi: 10.1007/s00418-006-0167-4. [DOI] [PubMed] [Google Scholar]
- 83.Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep. 2002;4(4):313–21. doi: 10.1007/s11926-002-0040-y. [DOI] [PubMed] [Google Scholar]
- 84.Neugebauer V, Rumenapp P, Schaible HG. Calcitonin gene-related peptide is involved in the spinal processing of mechanosensory input from the rat’s knee joint and in the generation and maintenance of hyperexcitability of dorsal horn-neurons during development of acute inflammation. Neuroscience. 1996;71(4):1095–109. doi: 10.1016/0306-4522(95)00473-4. [DOI] [PubMed] [Google Scholar]
- 85.Sun R, et al. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–66. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 86.Yu LC, Hansson P, Lundeberg T. The calcitonin gene-related peptide antagonist CGRP8-37 increases the latency to withdrawal responses in rats. Brain Res. 1994;653(1–2):223–30. doi: 10.1016/0006-8993(94)90393-x. [DOI] [PubMed] [Google Scholar]
- 87.Kawamura M, et al. Antinociceptive effect of intrathecally administered antiserum against calcitonin gene-related peptide on thermal and mechanical noxious stimuli in experimental hyperalgesic rats. Brain Res. 1989;497(1):199–203. doi: 10.1016/0006-8993(89)90990-6. [DOI] [PubMed] [Google Scholar]
- 88.Evidente VG, Adler CH. An update on the neurologic applications of botulinum toxins. Curr Neurol Neurosci Rep. 2010;10(5):338–44. doi: 10.1007/s11910-010-0129-z. [DOI] [PubMed] [Google Scholar]
- 89.Dolly O. Synaptic transmission: inhibition of neurotransmitter release by botulinum toxins. Headache. 2003;43(Suppl 1):S16–24. doi: 10.1046/j.1526-4610.43.7s.4.x. [DOI] [PubMed] [Google Scholar]
- 90.Morch CD, et al. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Eur J Neurosci. 2007;26(1):142–54. doi: 10.1111/j.1460-9568.2007.05608.x. [DOI] [PubMed] [Google Scholar]
- 91.Fernandez-de-las-Penas C, et al. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr Pain Headache Rep. 2007;11(5):365–72. doi: 10.1007/s11916-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 92.Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception II: activation and central sensitization in brainstem neurons with deep craniofacial afferent input. Brain Res. 2009;1253:48–59. doi: 10.1016/j.brainres.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 93.Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94(2):234–8. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 94.Gobel H, et al. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain. 2006;125(1–2):82–8. doi: 10.1016/j.pain.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 95•.Gungor NZ, Pare D. CGRP inhibits neurons of the bed nucleus of the stria terminalis: implications for the regulation of fear and anxiety. J Neurosci. 2014;34(1):60–5. doi: 10.1523/JNEUROSCI.3473-13.2014. This study provides evidence of the emerging role of CGRP in anxiety-related behaviors that is likely to have important implications for progression of migraine pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi DC, et al. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27(8):2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rouwette T, et al. The amygdala, a relay station for switching on and off pain. Eur J Pain. 2012;16(6):782–92. doi: 10.1002/j.1532-2149.2011.00071.x. [DOI] [PubMed] [Google Scholar]
- 98.Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry. 2013;1(1):9. doi: 10.1186/2049-9256-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–36. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]