Abstract
Physiological need states and associated motivational drives can bias visual processing of cues that help meet these needs. Human neuroimaging studies consistently show a hunger-dependent, selective enhancement of responses to images of food in association cortex and amygdala. More recently, cellular-resolution imaging combined with circuit mapping experiments in behaving mice have revealed underlying neuronal population dynamics and enabled tracing of pathways by which hunger circuits influence the assignment of value to visual objects in association cortex, insular cortex, and amygdala. These experiments begin to provide a mechanistic understanding of motivation-specific neural processing of need-relevant cues in healthy humans and in disease states such as obesity and other eating disorders.
Introduction
A key behavioral goal for the health and survival of an animal is the maintenance of bodily homeostasis. Brain networks have evolved to promote specific actions that will restore and maintain homeostasis. A foundational example involves the hunger-related hypothalamic circuitry that promotes food seeking and feeding behaviors during states of acute or upcoming energy deficit. Hunger can bias neural processing in higher-order brain regions such as cortex and amygdala. These regions serve many purposes, and are unlikely to contain dedicated ‘hunger circuits’ per se. However, they can be recruited during states of energy deficit to optimize the search for sources of food, by biasing attention towards food-associated stimuli in the environment (Figure 1A). Indeed, attentional capture by cues predicting energy-dense foods is a notion familiar to anyone who has walked through a grocery store when hungry [1,2]. This once-useful adaptation can be counterproductive in modern Western society, where high-fat and high-sugar foods are more readily available, and food advertisements are inescapable. Emerging basic and clinical research in humans and rodents is beginning to elucidate the brain regions, circuits, and mechanisms that drive this hunger-dependent biasing of responses to visual food cues.
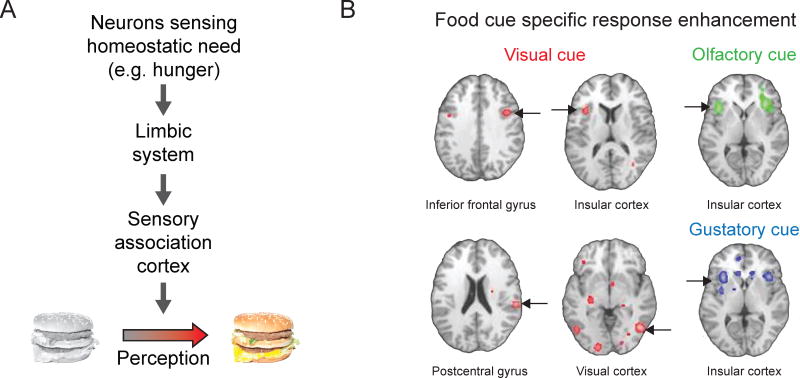
Figure 1. Physiological needs bias processing of salient visual cues.
A. To help meet the changing needs of our bodies, we direct our attention and neural processing towards relevant sensory cues in the environment. A specific physiological or homeostatic need is sensed by specialized neurons in the brain that coordinate complex search and consummatory behaviors to satisfy the need. This need state is relayed through limbic structures to cortical areas that process environmental stimuli, enhancing representations of salient objects (i.e., those relevant to the current physiological need). For example, hunger is a powerful homeostatic drive that biases visual processing towards food-associated sensory cues, such as images of cheeseburgers. B. A meta-analysis across human neuroimaging studies shows hunger-dependent enhancement of responses to visual food cues (red regions, left and middle columns) in many brain areas, including in association cortex (fusiform gyrus and parahippocampal gyrus) and insular cortex. Responses to other stimuli predicting food, including odors (top right) and tastes (bottom right), are also affected by hunger state in insular cortex. Pseudocolored pixels indicate brain regions with significant response enhancement in states of hunger vs. satiety. Modified from Obesity, 22, C. Huerta, P. Sarkar, T. Duong, A. Laird, and P. Fox, Neural bases of food perception: Coordinate-based meta-analyses of neuroimaging studies in multiple modalities, 1439–1446, 2014, with permission from John Wiley and Sons.
Hunger-dependent neural responses to visual food cues in humans
Humans have highly-evolved visual systems that can identify motivationally-relevant stimuli in cluttered environments. For example, attention to, and perception of food-associated visual cues is enhanced during states of hunger [3]. Using fMRI in hungry subjects, many studies have demonstrated stronger neural responses to food-related images vs. other images containing similar low-level visual features. Such effects are pronounced in visual association areas including inferotemporal cortex, parahippocampal gyrus, and fusiform gyrus (involved in complex object recognition), with lesser effects observed in primary visual cortex (Figure 1B) [4–8]. Critically, the enhanced responses to food-associated images are no longer present when the same subjects are scanned again following meal consumption. Moreover, enhancement of food cue responses can scale with level of hunger and with the caloric content of the associated food [7,9].
Brain areas not directly involved in visual processing, per se, also exhibit visual food cue response biases in hungry human subjects. These structures include limbic areas (amygdala and nucleus accumbens), gustatory and visceral areas (insular cortex), areas involved in executive function (prefrontal cortex and orbitofrontal cortex), and areas controlling bodily homeostasis (hypothalamus) [4–6,9–11]. Many of these areas activated by visual food cues are similarly activated by the taste or smell of food [8]. Food cue response enhancement in these areas is also reduced following meal consumption.
Human neuroimaging studies of food cue responses have also provided useful clinical insights, as patients with eating disorders demonstrate abnormal neural responses to food cues in both hungry and sated states. For example, anorexia patients, even when hungry, demonstrate reduced food cue responses in visual areas as compared to healthy controls [12]. In contrast, subjects suffering from obesity or other eating disorders can show enhanced responses to food cues in visual areas and in insular cortex that persists even following a meal [4,13,14]. One study even found that amygdala responses to high-calorie food during satiety could predict future weight gain [11].
Human neuroimaging studies involve indirect estimates of activity using measurements pooled across tens of thousands of neurons of varying functional properties, morphology, chemical makeup, and projection identity. Thus, while these studies uncover important information regarding the brain regions involved, they provide limited information about circuit mechanism and local population dynamics. A better understanding of the cellular and circuit mechanisms underlying these hunger-dependent responses to food cues may facilitate interpretation of human neuroimaging studies and enable targeting of specific cell types and pathways for treatment of obesity and eating disorders. Studies addressing this phenomenon using extracellular recordings from single neurons in non-human primates [15], while insightful, are limited by recording yield and duration. New tools and approaches for recording large numbers of single neurons across extended periods of time in mice are beginning to enable more detailed investigation of visual processing in the context of different homeostatic need states.
Neural mechanisms underlying hunger-dependent visual responses to food cues
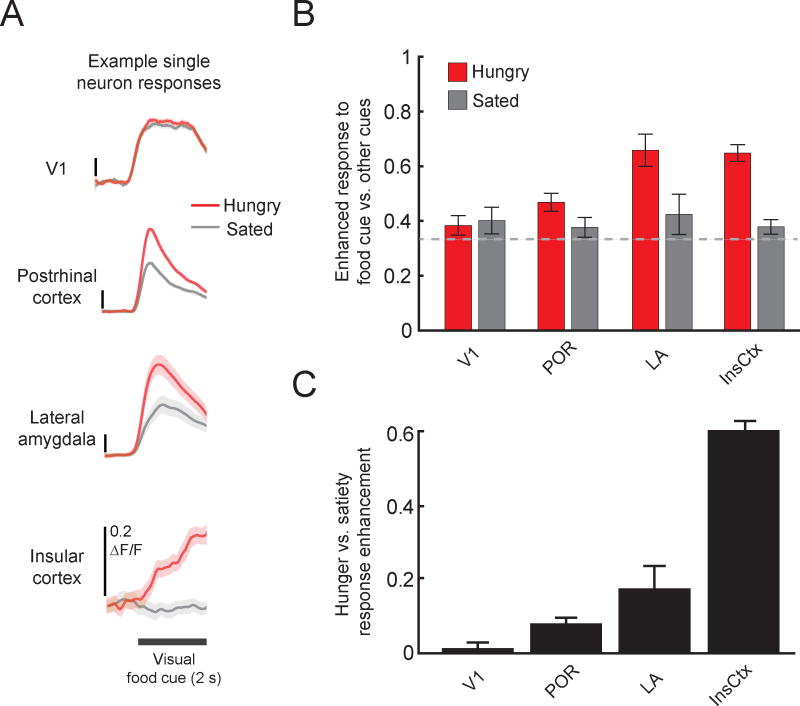
To understand the effects of hunger and satiety on association cortical responses to visual food cues at cellular resolution, we performed two-photon calcium imaging of the activity of hundreds of neurons simultaneously in postrhinal cortex (POR, defined here as a specific retinotopically-organized area in mouse lateral association cortex [16]). These recordings were conducted in head-fixed mice performing a Go-NoGo task. Following training, mice can discriminate between arbitrary visual cues (drifting visual gratings) associated with food (liquid Ensure, a high calorie liquid meal replacement) or with other outcomes [17]. We found that, in hungry mice, the average response across neurons to learned food cues was stronger than to neutral cues in postrhinal cortex, but not in primary visual cortex. This enhancement was due to an increased magnitude of responses to food cues in individual neurons, and to an increase in the number of neurons responsive to the food cue. Importantly, this food cue response bias was abolished following feeding to satiety (Figure 2). These cellular recordings support a role for hunger state in modifying or gating the flow of specific sensory information through the visual system. We observed even stronger effects of hunger on visual food cue responses in insular cortex (InsCtx [18]). Here, both the hunger-dependent food cue bias and visual cue-evoked responses in many cells were entirely suppressed following satiation (Figure 2). In addition, previous work has shown that ongoing InsCtx activity is also modulated by hunger state [19]. The hunger-dependent enhancement of food cue responses in mouse visual association cortex and InsCtx recapitulates key findings from the human neuroimaging studies discussed above.
Figure 2. Cellular imaging of enhanced responses to food cues in food-restricted mice.
A. Average food cue-evoked calcium response timecourses using two-photon imaging in mouse primary visual cortex (V1), postrhinal cortex (POR), lateral amygdala (LA; recorded from lateral amygdala axons in postrhinal cortex), and insular cortex (InsCtx). Neurons in POR, LA, and InsCtx tended to show increased activity in response to the same visual food-predicting cue when mice were hungry vs. after a period of feeding to satiety. ΔF/F: fractional change in fluorescence of the GCaMP6 calcium indicator. Mean +/− s.e.m. across trials. Reprinted from Neuron 91, C. Burgess, R. Ramesh, A. Sugden, K. Levandowski, M. Minnig, H. Fenselau, B. Lowell, and M. Andermann, Hunger-Dependent Enhancement of Food Cue Responses in Mouse Postrhinal Cortex and Lateral Amygdala, 1154–1169, 2016 with permission from Elsevier. B. At the population level, V1 did not show a bias towards food cues vs. neutral or aversive cues (food cue bias = (food cue response)/(sum of responses to all 3 visual cues); no bias = 0.33), regardless of hunger state. However, POR, LA, and InsCtx all showed a bias towards the food cue when the mice were hungry. Similar to findings from human neuroimaging studies, this bias was not present when mice were sated. C. Food cue response enhancement between sated and hungry states increases from V1 to POR, LA, and InsCtx. Hunger-satiety modulation index = (ResponseHungry − ResponseSated)/(ResponseHungry + ResponseSated).
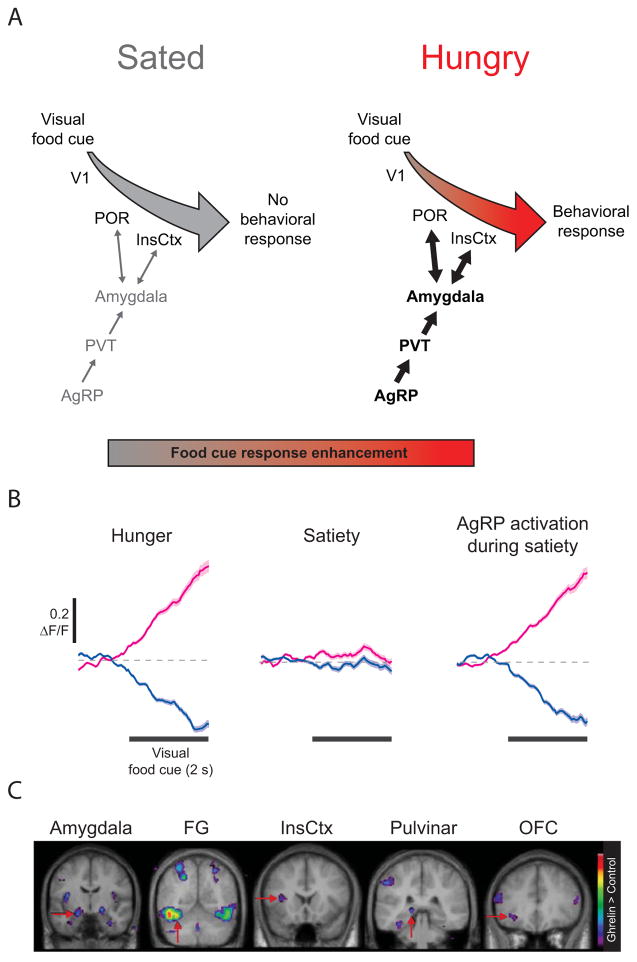
How does hunger modulate cortical areas such as POR and InsCtx? Genetic mouse models can provide new insights into how homeostatic signals bias cortical responses toward need-relevant stimuli, by enabling the use of cell type-specific circuit mapping and manipulation. Agouti-related peptide (AgRP) neurons constitute a powerful genetic entry point for evaluating putative pathways from hunger circuitry to cortex [20] (Figure 3A). Located in the arcuate nucleus of the hypothalamus, AgRP neurons are sensitive to hormonal signals of negative energy balance, and demonstrate high levels of activity during food restriction and lower levels following meal consumption [21–23]. Consistent with these findings, direct activation of AgRP neurons drives food seeking and feeding behaviors [24,25], and inhibition or ablation of AgRP neurons results in hypophagia [23,25,26]. We found that activation of AgRP neurons in sated mice restored engagement and accurate performance in a visual discrimination task for food rewards and, furthermore, restored food cue-evoked activity patterns in InsCtx (Figure 3B) [18]. This finding could shed light on previous studies in humans in which ghrelin administration restores enhanced food cue responses in cortex and amygdala [27], as ghrelin potently activates AgRP neurons (Figure 3C, [22,28,29]; for further discussion of the hypothalamic circuitry that drives feeding behaviors, see Andermann and Lowell, 2017).
Figure 3. Circuits regulating hunger-dependent enhancement of responses to visual food cues.
A. Circuit mapping experiments in mice have begun to trace the network of brain areas responsible for hunger-dependent visual processing of food-associated cues. Agouti-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus respond to caloric deficiency and drive feeding-related behaviors. This hunger drive is transmitted via the paraventricular thalamus (PVT) to the basolateral amygdala, which in turn exhibits reciprocal connections with postrhinal cortex (POR) and insular cortex (InsCtx). This connectivity integrates information about hunger state with the learned value of food-predicting cues, thereby enhancing responses to motivationally-salient visual stimuli and guiding behavioral choices upon presentation of food cues. Similar circuitry may be recruited by other need states (e.g. thirst, salt appetite) and may be hijacked by maladaptive drives, such as during drug seeking. B. Pharmacogenetic activation of AgRP neurons in sated mice was sufficient to restore InsCtx neuronal responses to visual food cues to levels observed in hungry mice. Subsets of InsCtx neurons responded to a visual food cue with either an increase (magenta) or decrease (blue) in activity when the mice were hungry. These responses were largely abolished when the mice were sated, but could be restored by activation of AgRP neurons. Reprinted with permission from Nature Publishing Group. C. Ghrelin administration in humans, which induces hunger, is also sufficient to induce enhanced responses to food cues vs. neutral cues, likely through actions on AgRP neurons. Colored pixels indicate the t-statistic for regions with significantly stronger responses to food images vs. scenery. Modified from Cell Metabolism, 7, S. Malik, F. McGlone, D Bedrossian and A. Dagher, Ghrelin modulates brain activity in areas that control appetitive behavior, 400–408, 2008, with permission from Elsevier. V1: primary visual cortex, FG: fusiform gyrus, OFC: orbitofrontal cortex.
We recently combined cellular imaging and circuit mapping to uncover a specific pathway from hypothalamic AgRP neurons to InsCtx via paraventricular thalamus (PVT; involved in feeding and other reward-related behaviors [30–32]) and basolateral amygdala (BLA; involved in encoding the value of learned cues [18,33–37]; Figure 3A). Specific manipulations revealed that components of this pathway were important for food cue responses in InsCtx, and for behavioral responses to food cues. The above pathway is, to our knowledge, the first to causally link hypothalamic interoceptive neurons to cortical circuits, and thus provides a starting point for further elucidation of the circuit mechanisms underlying need state-dependent enhancement of visual processing. In future, it will be important to define the roles of additional pathways relaying signals to cortex and amygdala from AgRP neurons and from other sources (e.g., visceral inputs, satiety centers [20,38]).
One particularly interesting relay, or gate, in the pathway described above is the basolateral amygdala complex (BLA). BLA responses to food cues are enhanced by hunger [6,10,11,39], and can encode the value of salient visual stimuli [33,34,37]. Moreover, reinforcer devaluation tasks demonstrate that the BLA plays a role in updating the value of stimuli based on selective satiation on a specific reinforcer [33,40]. These studies suggest that the amygdala may be involved in reducing the motivational value of visual food cues in cortex following restoration of energy balance via feeding to satiety. In particular, reciprocal loops between the lateral and basolateral amygdala and cortex may serve to bias sensory processing of motivationally-salient stimuli [17,41–43]. We recently found that hunger-dependent enhancement of cortical responses to food cues may be mediated in part by direct amygdalo-cortical projections: 1) amygdala feedback to POR showed a marked hunger-dependent enhancement of responses to food-associated cues [17], and 2) chemogenetic silencing of amygdalar inputs to InsCtx selectively blunted responses to learned visual food cues in InsCtx [18].
Surprisingly, need state-dependent processing of salient sensory cues has also been observed at the level of hypothalamic neurons that regulate energy balance and hunger drive. For example, three different groups recently demonstrated that AgRP neuron activity is rapidly reduced within seconds by sensory cues predicting food availability [21,29,44]. This drop in AgRP neuron activity in response to food-predicting cues was attenuated when mice were sated [29]. Similarly, rapid sensitivity to food cues was reported in arcuate nucleus proopiomelanocortin (POMC) neurons [21,29] and in leptin receptor-expressing GABAergic neurons in the dorsomedial hypothalamus [45]. However, the predictive cues in these studies were not strictly visual, because food presentation likely engages multiple sensory modalities. Using a visual discrimination task, we have shown that AgRP population activity is suppressed by an initially arbitrary visual stimulus that becomes associated with food availability [18]. The rapid drop in AgRP neuron activity in response to cues predicting food availability likely allows the animal to rapidly anticipate the eventual state of increased satiety following consumption of the associated food and absorption of nutrients, thereby preventing overconsumption [20]. However, at the level of understanding homeostatic biasing of cognitive circuits, these findings add complexity, as they reject the strict view of slow and steady homeostatic inputs to cognitive circuitry. Nevertheless, during receipt of very small rewards, as occurs during the operant visual discrimination task described above, transient changes in AgRP neuron activity are quite small relative to the overall difference in tonic activity levels between hungry and sated states [18].
Perspectives and Future Directions
Many of the same limbic and cortical circuits recruited during states of energy deficit to enhance representations of visual food cues also likely enhance non-visual sensory cues associated with food. For example, human subjects show enhanced neural responses to olfactory food cues in many of the same areas as visual food cues (Figure 1B) [8]. Odor processing in the olfactory bulb of rodents is also modulated by hunger state [46]. Moreover, ghrelin injection can reduce odor detection thresholds, while leptin, a satiety-related hormone released by adipose tissue, can decrease neural activity in the olfactory bulb in response to food odors [47,48]. Future studies could examine whether the circuitry described in Figure 3A also plays a role in hunger-dependent modulation of responses to cues of other sensory modalities.
Learned cues that predict reward (e.g. cues related to drugs of abuse) can also drive enhanced responses across many of the same brain areas, perhaps by hijacking the circuits normally used to drive attention towards physiologically-relevant cues [49]. For example, human neuroimaging results contrasting cocaine- and food-predicting cues show similar, but not identical, brain-wide response patterns [50]. Further, other need states may similarly shape visual processing of need-relevant cues. For example, studies in water-restricted humans find enhanced neural responses to water-predicting cues in association cortex and insular cortex [51,52]. Future studies could assess whether amygdalo-cortical reciprocal loops and/or other brain regions play similar roles in recruitment of cognitive resources in the context of different homeostatic and hedonic drives [44,53].
Other natural, non-homeostatic drives may also employ similar mechanisms to drive attention towards salient environmental cues. A recent study demonstrated that oxytocin, a neuropeptide implicated in social interaction, can directly modulate neural activity in the olfactory bulb of mice, enhancing signal-to-noise during odor coding [54]. Oxytocin also enhances representations of pup calls in auditory cortex of mothers [55]. We speculate that, for a given sensory modality, modulation of activity occurs at those stages in the sensory processing hierarchy which contain unambiguous representations of need-relevant objects in the environment. As such, modulation may occur at earlier stages for olfactory, gustatory, and auditory processing than for visual processing (hence the increased hunger-dependent modulation in POR vs. V1). While we have focused on mammalian brain areas involved in food cue processing, impressive efforts to elucidate circuitry linking need states and sensory processing in animal models with somewhat simpler circuitry promise to yield important insights [56,57].
We have illustrated how basic physiological need states may provide important and tractable entry points into understanding the roles of cortex in cognition. We suggest that an evolutionarily-conserved role of cortex is to help meet essential homeostatic needs of the body, by adding flexibility and context-dependence to sensory perception, memory, and decision-making in a dynamic world.
Highlights.
Hunger drives attention towards visual food-associated cues
In humans, neural response biases to food cues emerge in higher-order cortex and amygdala
In mice, cellular imaging reveals similar population biases, but local response diversity
Specific pathways between hypothalamus, amygdala, and cortex underlie these biases
Acknowledgments
Funding: This writing was supported by a National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NRSA F31 (grant number DK105678) (R.N.R.), an NIH New Innovator Award (grant number DP2 DK105570) R01 (grant number DK109930), a McKnight Scholar Award, and a Pew Scholar Award (M.L.A.).
Footnotes
Conflicts of interest: none
The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
** Of outstanding interest
* Of special interest
- 1.Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- 2.Loeber S, Grosshans M, Herpertz S, Kiefer F, Herpertz SC. Hunger modulates behavioral disinhibition and attention allocation to food-associated cues in normal-weight controls. Appetite. 2013;71:32–39. doi: 10.1016/j.appet.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Piech RM, Pastorino MT, Zald DH. All I saw was the cake. Hunger effects on attentional capture by visual food cues. Appetite. 2010;54:579–582. doi: 10.1016/j.appet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Cornier MA. The effects of overfeeding and propensity to weight gain on the neuronal responses to visual food cues. Physiol Behav. 2009;97:525–530. doi: 10.1016/j.physbeh.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 6.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 7.Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, Fritsche A, Preissl H. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 8**.Huerta CI, Sarkar PR, Duong TQ, Laird AR, Fox PT. Neural bases of food perception: coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity (Silver Spring) 2014;22:1439–1446. doi: 10.1002/oby.20659. The authors perform a meta-analysis of human fMRI studies of neural responses to food- and non-food images, odors, and tastes across hunger states. Food cues across sensory modalities displayed differing patterns of activation across cortical and subcortical areas. Presentation of images of food induced the most consistent pattern of responses. In hungry subjects, insular cortex was consistently activated by food-related cues across sensory modalities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, Schur EA. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96:989–999. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, Sinha R, Small DM. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci. 2015;35:7964–7976. doi: 10.1523/JNEUROSCI.3884-14.2015. The authors use fMRI in healthy human subjects to show that amygdala responses to palatable food when sated predict susceptibility to future weight gain. Their findings suggest that the amygdala relays information about the food to the hypothalamus when sated, perhaps engaging a homeostatic drive to eat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santel S, Baving L, Krauel K, Munte TF, Rotte M. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res. 2006;1114:138–148. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 13.Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Gao E, Burkhalter A. In vivo transcranial imaging of connections in mouse visual cortex. J Neurosci Methods. 2007;159:268–276. doi: 10.1016/j.jneumeth.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 17**.Burgess CR, Ramesh RN, Sugden AU, Levandowski KM, Minnig MA, Fenselau H, Lowell BB, Andermann ML. Hunger-Dependent Enhancement of Food Cue Responses in Mouse Postrhinal Cortex and Lateral Amygdala. Neuron. 2016;91:1154–1169. doi: 10.1016/j.neuron.2016.07.032. This paper uses in vivo two-photon calcium imaging of neurons in primary visual cortex (V1), in postrhinal cortex (POR), and in feedback axons from the lateral amygdala to postrhinal cortex to investigate the emergence of hunger-dependent enhancement of food cue responses in mice. Neurons in POR and lateral amygdala feedback axons to POR (but not neurons in V1) showed stronger responses to the food cues during hungry, but not sated, states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017 doi: 10.1038/nature22375. This paper uses a microprism-based approach to image activity of neurons in insular cortex in mice performing a visual discrimination task for food rewards. InsCtx neurons showed enhanced responses to learned food-predicting visual cues. These responses were strongly attenuated by satiety but were restored by chemogenetic activation of hunger-promoting hypothalamic AgRP neurons. A functionally important pathway is identified from AgRP neurons to InsCtx, via paraventricular thalamus and basolateral amygdala. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, Simon SA. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Andermann ML, Lowell BB. Toward a Wiring Diagram Understanding of Appetite Control. Neuron. 2017;95:757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015:4. doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 23.Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol Metab. 2014;3:694–704. doi: 10.1016/j.molmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 27.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Cone JJ, Roitman JD, Roitman MF. Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015;133:844–856. doi: 10.1111/jnc.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. This study uses in vivo fiber photometry to record bulk calcium activity of AgRP and POMC neurons in the arcuate nucleus of the hypothalamus. These neurons are known to promote hunger and satiety, respectively. The authors show that there is a rapid reduction in AgRP activity, and a rapid increase in POMC neuron activity, in response to presentation of food, even prior to the onset of consumption. These rapid changes in activity in response to food were attenuated when the mouse was sated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015;56:315–329. doi: 10.1016/j.neubiorev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stratford TR, Wirtshafter D. Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res. 2013;1490:128–133. doi: 10.1016/j.brainres.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 34.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, Tye KM. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron. 2016;90:348–361. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sternson SM, Eiselt AK. Three Pillars for the Neural Control of Appetite. Annu Rev Physiol. 2017;79:401–423. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- 39.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klavir O, Genud-Gabai R, Paz R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron. 2013;80:1290–1300. doi: 10.1016/j.neuron.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 42.Pitkanen A, Kelly JL, Amaral DG. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus. 2002;12:186–205. doi: 10.1002/hipo.1099. [DOI] [PubMed] [Google Scholar]
- 43.Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 1996;375:552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 44*.Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. This study uses a combination of optogenetic and chemogenetic manipulation, calcium imaging, and behavioral experiments to demonstrate that hunger and thirst sensations are mildly aversive. Stimulation of hunger-promoting AgRP neurons in the arcuate nucleus, or of thirst-promoting Nos1 neurons in the subfornical organ, can gradually lead to conditioned place avoidance. In vivo micro-endoscopic calcium imaging also demonstrates rapid suppression of AgRP neuron activity upon food presentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19:1628–1635. doi: 10.1038/nn.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pager J. Ascending olfactory information and centrifugal influxes contributing to a nutritional modulation of the rat mitral cell responses. Brain Res. 1978;140:251–269. doi: 10.1016/0006-8993(78)90459-6. [DOI] [PubMed] [Google Scholar]
- 47.Prud’homme MJ, Lacroix MC, Badonnel K, Gougis S, Baly C, Salesse R, Caillol M. Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience. 2009;162:1287–1298. doi: 10.1016/j.neuroscience.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 48.Tong J, Mannea E, Aime P, Pfluger PT, Yi CX, Castaneda TR, Davis HW, Ren X, Pixley S, Benoit S, et al. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci. 2011;31:5841–5846. doi: 10.1523/JNEUROSCI.5680-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ. Neuroimaging for drug addiction and related behaviors. Rev Neurosci. 2011;22:609–624. doi: 10.1515/RNS.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum Brain Mapp. 2015;36:120–136. doi: 10.1002/hbm.22617. The authors use fMRI imaging in human subjects to contrast neural responses to food- and cocaine-associated cues. Cocaine-associated cues and food-associated cues activated similar, but not identical, cortical networks. Activation of insular cortex by cocaine-associated cues was weaker than by food-associated cues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- 52.Saker P, Farrell MJ, Adib FR, Egan GF, McKinley MJ, Denton DA. Regional brain responses associated with drinking water during thirst and after its satiation. Proc Natl Acad Sci U S A. 2014;111:5379–5384. doi: 10.1073/pnas.1403382111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cone JJ, Fortin SM, McHenry JA, Stuber GD, McCutcheon JE, Roitman MF. Physiological state gates acquisition and expression of mesolimbic reward prediction signals. Proc Natl Acad Sci U S A. 2016;113:1943–1948. doi: 10.1073/pnas.1519643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, et al. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron. 2016;90:609–621. doi: 10.1016/j.neuron.2016.03.033. This study shows that oxytocin, which has been implicated in social interaction, exerts top-down effects on the olfactory bulb to enhance sensory coding of specific odors. This input is essential for social recognition in mice. These data demonstrate a role for direct hypothalamic input to olfactory bulb, and provide an example highlighting early modification of sensory processing in mammals by a non-homeostatic need state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. This study uses pup retrieval behavior in female mice to investigate the role of oxytocin in social cognition. Pup calls were preferentially expressed in left auditory cortex, and pairing of calls with oxytocin enhanced pup call auditory representations. These data suggest that oxytocin can influence sensory representations of pup calls, and that oxytocin is important for transforming auditory responses in experienced vs. pup-naïve mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jourjine N. Hunger and thirst interact to regulate ingestive behavior in flies and mammals. Bioessays. 2017:39. doi: 10.1002/bies.201600261. [DOI] [PubMed] [Google Scholar]
- 57.Zhang SX, Rogulja D, Crickmore MA. Dopaminergic Circuitry Underlying Mating Drive. Neuron. 2016;91:168–181. doi: 10.1016/j.neuron.2016.05.020. [DOI] [PubMed] [Google Scholar]