To the Editor
The NF-κB family of transcription factors regulates thousands of genes controlling innate and adaptive immunity. The transcription factors comprise hetero- or homo-dimers of five subunits: NF-κB1/p50, NF-κB2/p52, RELA/p65, c-REL, and REL-B. Mutations in these subunits generally cause immunologic disorders with variable penetrance and expressivity.1 For example, NF-κB1 mutations causing haploinsufficiency can trigger common variable immunodeficiency (CVID), hyper-inflammatory/autoimmune syndromes, combined immunodeficiency, or no disease. The phenotype is influenced by the location and biochemical nature of the causal variant and other genetic and environmental factors.1 Recently, 4 related patients with RELA haploinsufficiency were described with TNF-dependent mucocutaneous ulcerations and inflammatory intestinal disease, both treatable with infliximab therapy.2
We studied a male born to healthy parents diagnosed at age 5 with Autoimmune Lymphoproliferative Syndrome (ALPS) with refractory immune thrombocytopenic purpura (ITP), anemia, neutropenia and splenomegaly (Figures 1A and 1B), with mild lymphadenopathy. The patient was splenectomized at age 7 and subsequently received corticosteroids, mycophenolate mofetil (MMF), intravenous immunoglobulin (IVIG), eltrombopag, and rituximab for refractory ITP. Platelet counts normalized after MMF and IVIG treatment, suggesting an autoimmune etiology, though the patient did not have elevated autoantibodies (ENA, DNA, RNP, ANA, anti-Cardiolipin, and anti-Thyroid). The patient had episodic aseptic meningitis, with lumbar punctures revealing reactive lymphocytosis in the cerebrospinal fluid consisting of 85% T cells in a 6:1 CD4+ to CD8+ ratio. Additionally, the patient had a systemic reaction to the pneumococcal 23-valent vaccine with leukocytosis and hypertension. Finally, the patient complained of severe recurrent headaches, abdominal pain, vomiting, diarrhea, and weight loss. These complaints were possibly related to MMF therapy and were reduced after switching to eltrombopag. ITP resolved following a third round of rituximab therapy though the patient continues to suffer from recurrent debilitating headaches.
Figure 1.
RELA haploinsufficiency leads to a T cell mediated autoimmune lymphoproliferative disease
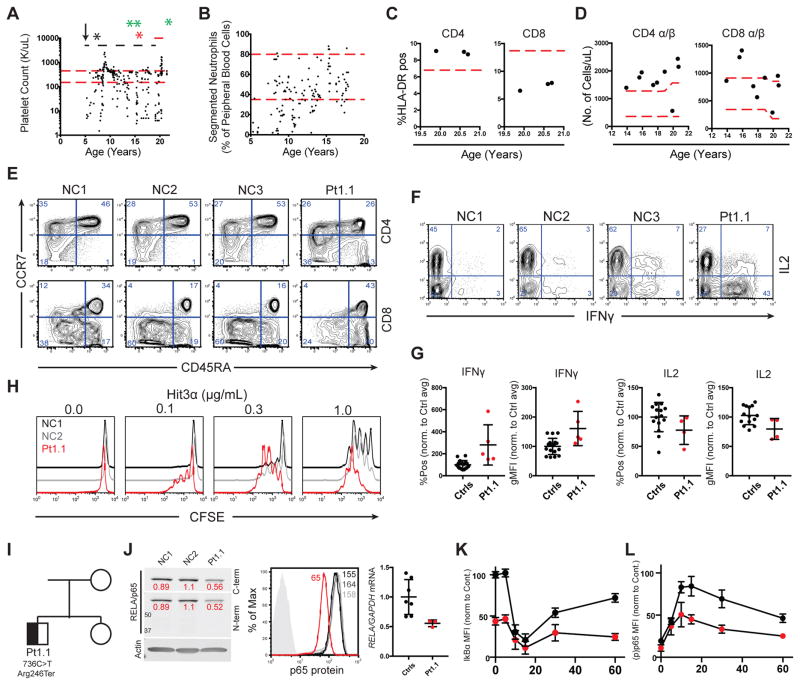
Patient platelet and neutrophil counts (red dashed lines, arrow, black, green, and red asterisks represent the normal range, age of onset, splenectomy, rituximab administration, and systemic immune response to pneumococcal vaccine, respectively. Black dashed and solid red lines represent MMF and eltrombopag administration, respectively (A/B). CD4+ and CD8+ cells expressing HLA-DR, red line indicates high end of normal range (C). CD4+ and CD8+ α/β T cells/uL of patient blood, red lines indicate normal range (D). CCR7 and CD45RA expression on CD3/CD4+/+ lymphocytes (E). Representative IFNγ and IL2 production in phorbol 12-myristate 13-acetate and ionomycin (PMA/I) restimulated CD4+ T cells (F). IFNγ and IL2 production in cells stimulated as in F, pooled from 4 separate experiments (G). Proliferation of CD4+ blasts following re-stimulation with 1 μg/mL anti-CD28 and the indicated dose of anti-CD3 (n=3) (H). Family pedigree and de-novo RELA variant (I). Protein expression in CD4+ T cell blasts by western blot (RELA/Actin ratios in red) (left), flow cytometry (center), and RELA mRNA levels determined by qRT-PCR (right) (J). IkBα degradation and p65 phosphorylation following PMA/I stimulation, pooled from 3 separate experiments (K and L, respectively).
Despite ALPS features, we found no defect in Fas, TCR, or cytokine withdrawal-mediated apoptosis (Figure E1 in the online repository). Immunophenotyping revealed slightly elevated CD3+ double negative T cells (2.4%), increased activated (HLA-DR+) CD4+ T cells, and increased peripheral blood T cells (Figure 1C and 1D). This was accompanied by a decrease in naïve (CCR7+/CD45RA+) CD4+ T cells and an increase in terminally differentiated (CCR7−/CD45RA+) effector T cells (Figure 1E). Furthermore, patient CD4+ T cells had an enhanced Th1-like phenotype (CXCR3+/CCR6−) (Figure E2A in the Online Repository). Patient naïve CD4+ T cells were intrinsically skewed towards a Th1-effector phenotype with increased production of the NF-κB target cytokine IFNγ (Figure 1F and 1G), though not all NF-κB targets showed increased production, as patient cells made less IL-2 upon re-stimulation. Patient CD4+ T cells were more sensitive to TCR stimulation than control cells (Figure 1H), suggesting that increased antigen sensitivity may account for the elevated effector and diminished naïve T cells. Patient T cells may be further activated due to decreased regulatory responses, as we found decreased iTreg differentiation despite normal levels of CD25+/CD127low nTreg cells (Figure E2B, E2C, and E2D in the Online Repository); this may be attributable to either increased IFNγ or decreased p65/c-Rel dependent FOXP3 transcription.3 Finally, B cell populations and immunoglobulins appeared normal (Figure E3 in the Online Repository). Thus, the disease was apparently largely driven by augmented T cell activation and effector function.
Whole genome sequencing revealed a de-novo heterozygous nonsense mutation (NM_021975.3:c.736C>T, p.Arg246*) in RELA (Figure 1I) leading to reduced p65 protein and mRNA, consistent with nonsense-mediated decay of the variant transcript (Figure 1J). By contrast, c-Rel, NF-κB1, and NF-κB2 protein levels were normal (Figure E4A and E4B in the online repository). Interestingly, while significantly less p65 was immunoprecipitated from patient cells, c-Rel, p50, and p52 were co-immunoprecipitated at higher relative levels in patient cells compared to controls, suggesting possible loss of the p65 homodimer (Figure E4A, E4C, and E4D in the online repository). While patient cells had lower baseline IκBα expression and maximum phospho-p65 after stimulation, NF-κB activation kinetics were comparable to healthy donor cells (Figure 1K and 1L). Hence, the patient’s disease likely results from insufficient p65 dimers and altered regulation of their specific targets.4
Given the differences between our case and those previously reported, we conclude that RELA haploinsufficiency, like NF-κB1 haploinsufficiency, can cause widely different clinical manifestations.2,5 While the variant type and overall genetic/environmental variability are key modifying factors, variants in other genes also determine the observed clinical phenotype in an apparently “monogenic” disease. To wit, our patient has multiple rare and potentially deleterious variants inherited from asymptomatic parents in genes affecting immune responses (Table E1 in the Online Repository). In particular, the variants in NF-κB regulators IKBIP and IKBKE may potentially alter the patient’s phenotype. IKBIP encodes IKK-β-interacting protein (IKIP) that is induced by p53 and whose overexpression causes apoptosis.6 The patient’s L363S IKBIP variant could possibly attenuate TNF-induced apoptosis via the TNF-p53 pathway. IKBKE encodes for IKK-E, a homologue of IKK-A and IKK-B, which was originally identified as an IKK-A/B independent IκBα kinase and can phosphorylate STAT1 during viral responses.7,8 The patient’s E50K IKBK variant eliminates a critical hydrogen bond with the catalytic lysine (K38 in IKK-E) that likely inactivates the kinase.9 This mutation may further depress NF-κB activation under certain circumstances, though it should be noted that overexpression of kinase dead IKK-E (K38A) attenuates PMA-induced NF-κB activation, a feature that we did not observe. Though further investigation is needed to determine whether these variants modify the patient’s condition, it is unlikely that they cause disease by themselves since they were inherited from the unaffected father.
In summary, we expand the known clinical phenotype of RELA haploinsufficiency by linking it to an ALPS-like disease associated with refractory autoimmune cytopenias, increased T cell activation and proliferation, and enhanced Th1 effector responses in the absence of mucocutaneous ulcerations and inflammatory intestinal disease.
Supplementary Material
Acknowledgments
FUNDING
We thank our clinical collaborators, patients and their families. We thank MERCK Pharmaceuticals and Joshua J. McElwee for providing funds for WGS of patient samples. This project was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, ClinicalTrials.gov number (NCT00246857), a fellowship Grant from the American Diabetes Association (Grant # 1-16-PDF-025), and a F12 Post-doctoral fellowship from NIGMS (1FI2GM119979-01) to W. A. C.
Footnotes
CONFLICT of INTEREST
The authors have no conflicts of interest to report.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-kappaB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badran YR, Dedeoglu F, Lewa Castillo JM, Bainter W, Ohsumi TK, Bousvaros A, et al. Human RELA haploinsufficiency results in autosomal-dominant chronic mucocutaneous ulceration. J Exp Med. 2017 doi: 10.1084/jem.20160724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q, Kameswaran V, Tone Y, Li L, Liuo HC, Green MI, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siggers T, Chang AB, Teixeira A, Wong D, Williams KJ, Ahmed B, et al. Principles of dimer-specific gene regulation revealed by a comprehensive characterization of NF-kappaB family DNA binding. Nat Immunol. 2011;13:95–102. doi: 10.1038/ni.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fliegauf M, Bryant VL, Frede N, Slade C, Woon ST, Lehnert K, et al. Haploinsufficiency of the NF-kappaB1 Subunit p50 in Common Variable Immunodeficiency. Am J Hum Genet. 2015;97:389–403. doi: 10.1016/j.ajhg.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofer-Warbinek R, Schmid JA, Mayer H, Winsauer G, Orel L, Mueller B, et al. A highly conserved proapoptotic gene, IKIP, located next to the APAF1 gene locus, is regulated by p53. Cell Death Differ. 2004;11:1317–1325. doi: 10.1038/sj.cdd.4401502. [DOI] [PubMed] [Google Scholar]
- 7.Peters RT, Liao SM, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- 8.Tenoever BR, Ng SL, Chua MA, McWhirter SM, García-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Misquitta YR, Olland A, Johnson MA, Kelleher KS, Kriz R, et al. Crystal structure of a human IkappaB kinase beta asymmetric dimer. J Biol Chem. 2013;288:22758–22767. doi: 10.1074/jbc.M113.482596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.