Abstract
Background and Objectives
β-arrestin2 (β-arr2) basically regulates multiple signaling pathways in mammalian cells by desensitization and internalization of G-protein coupled receptors (GPCRs). We investigated impacts of β-arr2 on survival, mobility, and tube formation of cardiac progenitor cells (CPCs) obtained from wild-type (WT) mouse (CPC-WT), and β-arr2 knock-out (KO) mouse (CPC-KO) cultured in presence or absence of serum and oxygen as non-canonical roles in GPCR system.
Methods
CPCs were cultured in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 -based media containing fetal bovine serum and growth factors. Survival of 2 types of CPCs in hypoxia and/or serum deprivation was measured by fluorescence-activated cell sorting. Wound healing ability, and tube formation ability on Matrigel of 2 kinds of CPCs were compared in normoxic and hypoxic cultures. Protein expression related to survival and mobility were measured with the Western blot for each culture conditions.
Results
CPC-KO showed significantly worse mobility in the wound healing assay and in tube formation on Matrigel especially in hypoxic culture than did the CPC-WT. Also, CPC-KO showed significantly higher apoptosis fraction in both normoxic and hypoxic cultures than did the CPC-WT. Expression of proteins associated with cell survival and mobility, e.g., protein kinase B (Akt), β-catenin, and glycogen synthase kinase-3β (GSK-3β) was significantly worse in CPC-KO.
Conclusions
The CPC-KO had significantly worse cell mobility, tube formation ability, and survival than the CPC-WT, especially in the hypoxic cultures. Apparently, β-arr2 is important on CPC survival by means of mobility and tube formation in myocardial ischemia.
Keywords: Beta-arrestins, Stem cells, Cell movement, Myocardial ischemia, Apoptosis
INTRODUCTION
Heart failure (HF) is one of major causes of death in developed countries, and ischemic heart disease, e.g., myocardial infarction (MI), is an important contributor to HF.1),2) Prolonged myocardial ischemia causes cardiomyocyte (CMC) necrosis and myocardial fibrosis.3) So, replacement of cells in the ischemic region can be an important therapeutic modality for medically intractable ischemic HF.3) The ultimate purpose of cell therapy is to repair cardiac function by replacing damaged CMCs.3) Resident cardiac progenitor cells (CPCs) can be used as an candidate source of stem cell therapy for ischemic HF, because CPCs can proliferate, differentiate, be mobile, and form tubes to make new vessels in either in vivo or in vitro.3) CPC was firstly reported in 2003 as being able to self-renew and proliferate.4) Also, CPCs can differentiate into certain cell types, such as CMC, endothelial cells (ECs), and vascular smooth muscle cells (VSMCs) in vivo.4) Consequently, CPCs have the potential to repair damaged myocardial tissue provoked by acute MI or chronic myocardial ischemia.3) Interestingly, previous studies reported that CPCs injected into myocardial tissue could differentiate into CMCs and ECs, thus improving myocardial systolic function.5) Basically, CPCs injected into ischemic jeopardized myocardium can migrate to the ischemic area and form new vessels to supply oxygen, nutrients, and various cytokines to promote healing of myocardium.5) So, the ability of CPCs to migrate and form tubes can be important characteristics to rescue ischemic myocardium.
The arrestin family consists of arrestin 1 to 4, which mainly reside in the retinal rods and cones, and arrestin 2, 3 (newly named β-arrestin 1, 2; β-arrs), which are ubiquitous in mammalian cells.6) β-arrestin1 (β-arr1) and β-arrestin2 (β-arr2) act as adaptor and scaffold proteins of 7 transmembrane domain receptors, or G-protein coupled receptors (GPCRs), and lead to desensitization and internalization of GPCRs.6) Aside from this canonical action of β-arrs on GPCR, these proteins play important roles in regulating cell proliferation, migration, and tube formation related to various signaling pathways in certain cell types.6) In addition, β-arrs regulate cytoskeleton and actin reorganization, and affect migration of ECs or malignant cells.7) β-arrs action for promoting cell migration requires extracellular signal-regulated kinase (ERK) pathway activation within the leading edge of migrating cells.7) β-arrs dependent ERK phosphorylation is important for inducing cell mobility and tube formation.7) Phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and Wnt/β-catenin signaling is crucial for cell mobility and tube formation of colon cancer cells, and β-arrs control this signaling that is related to cancer metastasis and progression.8)
Unvascularized MI, even in well-vascularized MI, would cause certain extent of myocardial damage, and secondary myocardial remodeling, it provokes ischemic HF. In this situation, GPCR and β-arr have important roles in HF pathogenesis because of their canonical β-adrenergic receptor signaling, and CPC starts proliferating and migrating to recover damaged myocardium. Non-canonical and GPCR-independent molecular mechanisms of β-arr may also be important for survival, mobility, and tube formation by CPCs.9) In these series of adaptation pathways in ischemic HF, we speculate that β-arr has a special role in the migration, proliferation, and tube formation of CPCs, especially in a hypoxic condition, which is similar to acute or chronic myocardial ischemia in vivo.
But, there is no specific study about roles and molecular mechanisms of β-arr in CPC under serum deprivation or prolonged hypoxia. With this background, this study was designed to find out the effects of β-arr2 in CPC survival, mobility, and tube formation, and the molecular mechanisms related to these actions, by using wild-type (WT) mouse CPC (CPC-WT) and β-arr2 knock-out (KO) mouse CPC (CPC-KO) in both normoxic and hypoxic cultures to simulate the normoxic and ischemic myocardium.
METHODS
CPC isolation and culture
β-arr2 KO mouse was generated by inactivation of the β-arr2 gene using the homogenous recombination in Professor Robert J. Lefkowitz Laboratory in Duke University (Durham, NC, USA), and the methodology was finely described in elsewhere.10) β-arr2 was knocked-out with conventional KO method. Silencing of β-arr2 gene was confirmed by the Southern blot, real-time polymerase chain reaction (RT-PCR) and the Western blot in DNA, mRNA and protein level of mouse tissue. Simultaneous KO of β-arr1 and β-arr2 made intrauterine fetal death related to vascular and lymphatic system development failure, but mouse with single KO of β-arr1 or β-arr2 did not show apparent abnormality of cardiovascular tissue development.11) Detailed methods of CPC isolation from mouse heart were described in elsewhere.12) Basically, CPCs were separated from non-CMC populations selected with collagenase-perfused WT and β-arr2 KO male C57BL/6 mice hearts, using a CD117 magnetic bead separation Kit (Miltenyl Biotec GmbH, Bergisch Gladbach, Germany). Those c-kit positive CPCs were cultured for 3 to 5 days in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12)-based culture media (Welgene, Gyeongsan, Korea) containing 10% fetal bovine serum (FBS; Biowest, Kansas, MO, USA), 20 ng/mL of recombinant human basic fibroblast growth factor (PeproTech, Oak Park, CA, USA), 40 ng/mL of epidermal growth factor (PeproTech), 100 µL/mL of Leukemia Inhibitory Factor (PeproTech), 2 mL/L of 500X Insulin-Transferrin-Selenium (Lonza, Basel, Switzerland), and 10 mL/L of 100X penicillin/streptomycin solution (Welgene) at 37°C. CPCs were washed with 1X Phosphate-buffered saline (PBS; Welgene) without Ca2+ and Mg2+. Culture plates were coated with 0.1% gelatin solution (Sigma-Aldrich, St. Louis, MO, USA) before all experiments. We used passage No. 5–7 CPCs for all experiments, and each type experiment condition was examined at least 3 times. c-kit, as a marker of CPC was checked with TSA PLUS Biotin Kit (Tyramide Signal Amplification, PerkinElmer, Waltham, MA, USA) using Passage 3 of CPC-WT and CPC KO attached on cell culturing chamber slides according to manufacturer's recommendation. DAPI was used to statin CPC nucleus. c-kit positivity has been checked in passage 3 to 7 CPCs.
Generation of hypoxia
CPC-WT and CPC-KO were cultured in normoxic (O2 20%) and hypoxic conditions (O2 1%) in a 37°C CO2 incubator (Thermo Fisher Scientific, Waltham, MA, USA) to perform experiments. The hypoxic condition was generated with BD GasPak™ EZ Anaerobe Gas Generating Pouch System (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Fluorescence-activated cell sorting (FACS)
The survival of CPC-WT and CPC-KO was measured by FACS.13) CPCs were transferred into 50 mL conical tube with cultured media. The adherent cells on the culture plate were detached with 0.025% of Trypsin-EDTA (Welgene). The whole-cell fluid was centrifuged at 1,200 rpm. The supernatant was removed from the tube, and the cells were dispersed with 1 mL PBS (Welgene) and counted. Cells were resuspended with 1X binding buffer at 5×106 cells/mL; 100 µL of the cell solution containing 5×105 cells was then transferred to an FACS tube. The cells were stained with FITC Annexin-V apoptosis kit (Becton, Dickinson and Company). CPC apoptosis and necrosis was detected with a FACSCalibur system (Becton, Dickinson and Company).
Wound healing assay
Cell migration ability was measured by the wound healing assay.14) CPC-WT and CPC-KO were seeded at 5×105 cells/well on 0.1% gelatin (Sigma-Aldrich) coated 6-well plates in serum containing media, and then culture media was changed to serum-free media to limit cell proliferation when sub-confluent state of CPC was achieved. After 24 hours of serum-free state, a cell monolayer was scratched with a 200 µL pipette tip to create a cell-free area. Cell plates were washed twice with 1X PBS (Welgene) to remove floating matter and cells, and replaced with serum-containing or serum-free medium as the experimental design in each well. Cell plates were maintained in the normoxic and hypoxic cultures. We carried out the wound healing assay in normoxia or hypoxia, and with or without FBS, so the assay was done for the 4 culture conditions of O2+/serum+, O2+/serum-, O2−/serum+, and O2−/serum−. 24 hours serum starvation has been applied to minimize the proliferation of CPC before starting experimental conditions including oxygen and serum to clarify the effect of CPC migration in the wound healing assay. The closure area made by cell migration was photographed and analyzed with a phase-contrast microscope (Olympus Corporation, Tokyo, Japan) and analyzed with the ImageJ software (ImageJ 1.43u; National Institute of Health, Bethesda, MD, USA).
Matrigel tube formation assay
Several 24-well culture plates (Sigma-Aldrich) were coated with 100 µL/well of growth factor reduced Matrigel (Becton, Dickinson and Company), and were solidified at 37°C for 1 hour. Before initiation of the tube formation assay, CPC-WT and CPC-KO were washed twice with PBS to remove floating matter and cells, and detached with 0.025% Trypsin-EDTA (Welgene). We have used CPC with or without pre-treatment of serum deprivation culture condition to see the effects of serum deprivation on CPC. Detached CPCs were resuspended with 1 mL serum-containing DMEM/F12 media (Welgene), and counted. 8×104 cells of CPC were plated on growth factor reduced Matrigel coated 24-well culture plates. CPCs were kept in normoxic and hypoxic cultures. We took images of tube formation on a phase-contrast microscope (Olympus Corporation) with a Flex camera (Nikon Corporation, Tokyo, Japan) at the baseline time and 24 hours later.
Western blotting
The Western blot analysis was performed as previously described.15) The membrane was incubated with primary antibody against total-extracellular signal-regulated kinase (t-ERK), phosphorylated-extracellular signal-regulated kinase (p-ERK), total-protein kinase-B (t-Akt), phosphorylated-protein kinase-B (p-Akt), β-catenin, glycogen synthase kinase-3β (GSK-3β) (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-arr1/2, β-arr2 (1:1,000; Cell Signaling Technology, Danvers, MA, USA), and β-actin (1:5,000; Santa Cruz Biotechnology) was used as a loading control. For chemiluminescence detection, we used the WEST-ZOL®Plus (iNtRON Biotechnology, Seongnam, Korea), and Las-3000 (Fujifilm Holding Corporation, Tokyo, Japan). The protein expression was analyzed using the Multi Gauge V3.1 program (Fujifilm Holding Corporation). We performed the Western blotting with FACS-derived cellular proteins to compare cell survival between CPC-WT and CPC-KO in routine culture condition to check the expression of survival proteins, e.g., ERK, Akt, GSK-3β, and β-catenin. Proteins were obtained from cells after the wound healing assay and tube formation assay to measure protein expression related to cell migration in both CPC-WT and CPC-KO.
Statistical analysis
Each experiment was performed independently a minimum of 3 times. Results are expressed as mean±standard deviation. Statistical analysis was performed using the two-tailed Student's t-test with a commercial statistics package (SPSS, Inc., Chicago, IL, USA). A p value<0.05 was considered statistically significant.
RESULTS
Basic morphologic difference of 2 types of CPC and higher apoptotic rate of CPC-KO than CPC-WT in the regular culture condition
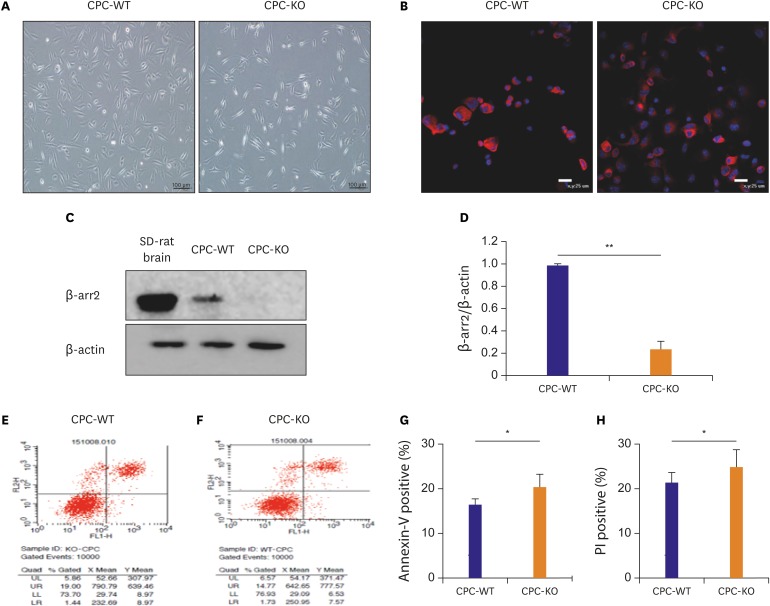
Basic morphologic differences exist between CPC-WT and CPC-KO in regular culture condition. CPC is like a long, shining lantern; CPC-KO is more slender and longer than CPC-WT, and CPC-WT is thicker and more triangular than CPC-KO (Figure 1A). Before the main experiment, we have checked c-kit positivity to confirm CPC in both WT and KO of β-arr2 in passage 3. More than 95% of cells showed c-kit positivity (red) on the DAPI stained nucleus (blue) in Figure 1B. This positivity continued at least in passage 7 cells which we have used in this experiment. And, we confirmed the silenced β-arr2 expression in CPC-KO with the Western blot analysis, using a Sprague Dawley (SD)-rat brain as a positive control for high expression of β-arr2 in Figure 1C. The β-arr2 expression was observed in CPC-WT, but rarely in CPC-KO with the optical density (OD) ratio on 1.00±0.01 vs. 0.23±0.06 (p<0.01; Figure 1C). The baseline survival rate of CPC-WT and CPC-KO in the routine culture condition was measured by FACS. Propodium iodide (PI), a marker of cellular necrosis, was shown on the y-axis, and the apoptosis marker, annexin V, was shown on the x-axis, in Figure 1D-G. Compared to CPC-WT, CPC-KO showed a higher rate of apoptosis, as measured by annexin V positivity (20.44±2.91% vs. 16.51±1.33%, p<0.05; Figure. 1E-G) and necrosis rate, as measured by PI positivity (24.86 ± 3.94% vs. 21.34 ± 2.31%, p<0.05; Figure 1E, F, and H). According to the FACS data, CPC-KO showed higher vulnerability to survival compared to CPC-WT, even in regular culture condition.
Figure 1.
Basic morphologic difference of 2 types of CPC and higher apoptotic rate of CPC-KO than CPC-WT in the regular culture condition. (A) Morphologic distinction of CPC-WT and CPC-KO in the routine culture. (B) c-kit positivity (red) was observed in more than 95% of cell in CPC-WT and CPC-KO in passage 3. Blue was nucleus of CPCs stained with DAPI. (C, D) Protein expression of β-arr2 was strong in CPC-WT, but was not observed in CPC-KO; brain tissue of SD-rat was used as positive control. (E-H) CPC-KO was more apoptotic and necrotic compared to CPC-WT in in the routine culture.
CPC = cardiac progenitor cell; CPC-KO = CPC obtained from β-arr2 knock-out mouse; CPC-WT = CPC obtained from wild-type mouse; DAPI = 4′,6-diamidino-2-phenylindole; PI = propodium iodide; SD = Sprague Dawley; β-arr2 = β-arrestin2.
*p<0.05, †p<0.01.
CPC-WT showed better expression profiles of proteins related with mobility especially in serum starvation compared to CPC-KO
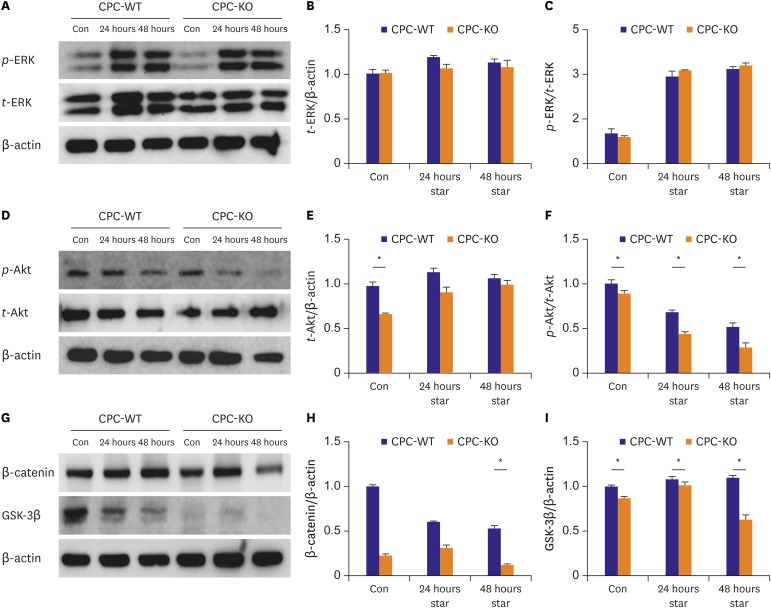
We performed the Western blot analysis to compare the difference of protein expression pattern related with CPC mobility and tube formation between CPC-WT and CPC-KO under serum starvation. Expression of t-ERK was similar in CPC-WT and CPC-KO in the all conditions. (Figure 2A and B). p-ERK/t-ERK expression showed steep increment after 24 and 48 hours serum starvation both in CPC-WT and CPC-KO compared to control condition. But, there was no significant difference of p-ERK/t-ERK expression between CPC-WT and CPC-KO in baseline and serum starvation culture condition. Expression of t-Akt was significantly higher in CPC-WT than in CPC-KO for the control condition (1.00±0.02 vs. 0.65±0.02, p<0.05), but turned out to similar expression on serum starvation for 24 hours (1.11±0.01 vs. 0.89±0.06, p>0.05) and 48 hours (1.04±0.01 vs. 0.97±0.06, p>0.05; Figure 2D and E). But, the p-Akt/t-Akt was significantly higher in CPC-WT than CPC-KO at both regular and serum starvation culture conditions (Figure 2D and F). β-catenin expression was similar in CPC-WT and CPC-KO in the regular culture and serum starvation for 24 hours. But it was significantly higher in CPC-WT than CPC-KO in prolonged serum starvation for 48 hours (1.10±0.03 vs. 0.63±0.05, p<0.05; Figure 2G and H). CPC-WT showed markedly higher GSK-3β expression than CPC-KO in all culture conditions (Figure 2G and I). CPC-WT generally showed higher expression pattern of proteins related with cell mobility and survival than CPC-KO in the regular culture and these patterns were exaggerated by serum starvation.
Figure 2.
CPC-WT showed better expression profiles of proteins related with mobility especially in serum starvation compared to CPC-KO. (A-C) t-ERK expression was similar in CPC-WT and CPC-KO in all culture conditions. p-ERK/t-ERK expression increased similarly both in CPC-WT and CPC-KO in serum-free culture for 24 and 48 hours. (D-F) Expression of t-Akt was higher in CPC-WT in control condition and p-Akt/t-Akt was significantly higher in CPC-WT at all culture condition compared to CPC-KO. (G-I) β-catenin expression was sharply decreased in CPC-KO at 48 hours serum starvation culture. GSK-3β expression was significantly higher in CPC-WT than in CPC-KO in all culture conditions.
Con = control; CPC-KO = cardiac progenitor cell obtained from β-arr2 knock-out mouse; CPC-WT = cardiac progenitor cell obtained from wild-type mouse; GSK-3β = glycogen synthase kinase-3β; p-Akt = phosphorylated-protein kinase-B; p-ERK = phosphorylated-extracellular signal-regulated kinase; star = serum starvation; t-Akt = total-protein kinase-B; t-ERK = total-extracellular signal-regulated kinase; β-arr2 = β-arrestin2.
*p<0.05.
CPC-KO showed worse mobility compared to CPC-WT in normoxic and hypoxic cultures
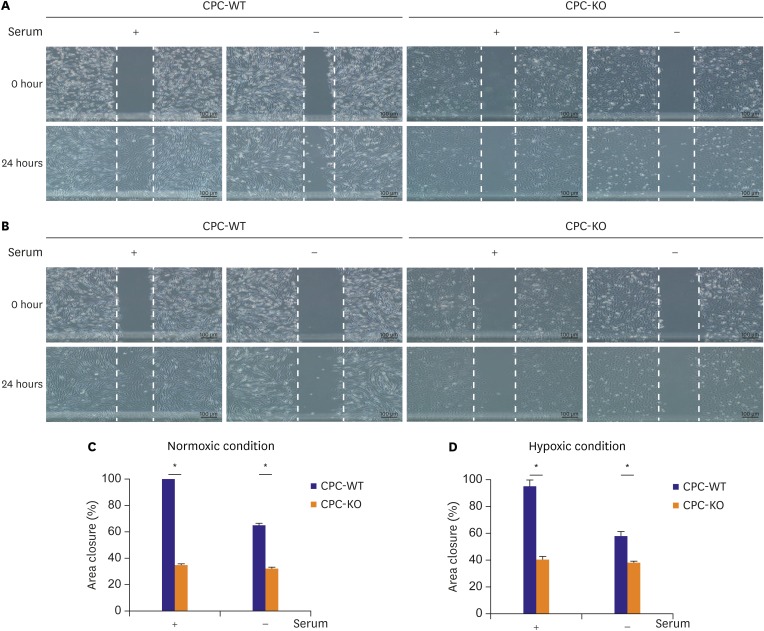
Before we started the wound healing assay, CPC-WT and CPC-KO had been pretreated with serum starvation for 24 hours to limit cell proliferation. It clarifies that wound closure might be induced by cell mobility, not by cell proliferation.14) In all combinations of oxygen and serum supply conditions, CPC-WT showed higher percentage of wound closure compared to CPC-KO. The percentage of wound area closure was significantly higher in CPC-WT than CPC-KO in O2+/serum+ (99.57±0.11% vs. 34.99±1.51%, p<0.05) and O2+/serum− (65.32±1.24% vs. 32.68±0.80%, p<0.05; Figure 3A-C), in O2−/serum+ (95.62±5.32% vs. 41.25±3.71%, p<0.05) and in O2−/serum− (58.21±2.25% vs. 38.41±1.46%, p<0.05; Figure 3A, B, and D). CPC-WT showed slower wound closure capability in the O2−/serum− than in the other culture conditions, but it was still faster than CPC-KO under the O2−/serum− condition. CPC-WT was able to heal wounds significantly faster compared to CPC-KO by faster cell mobility, not by cell proliferation activity which was hinder by 24 hours serum starvation pretreatment14) in all 4 cultures.
Figure 3.
CPC-KO showed worse mobility compared to CPC-WT in normoxic and hypoxic cultures. (A) Normoxic and (B) hypoxic culture. (C, D) Percentage of area scratched wound closure was higher in CPC-WT than in CPC-KO.
CPC-KO = cardiac progenitor cell obtained from β-arr2 knock-out mouse; CPC-WT = cardiac progenitor cell obtained from wild-type mouse; β-arr2 = β-arrestin2.
*p<0.05.
CPC-WT showed better expression profiles of proteins related cell mobility and tube formation in normoxic and hypoxic cultures compared to CPC-KO
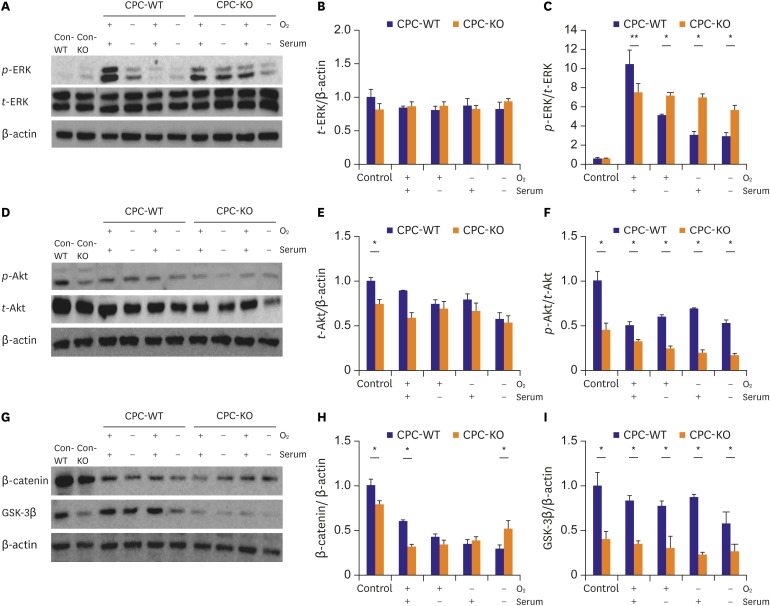
We measured expression patterns of various proteins related with cell mobility and tube formation, such as ERK, Akt, β-catenin, and GSK-3β. Those proteins were obtained from CPC-WT and CPC-KO in all four culture conditions (Figure 4). These cells were starved for 24 hours with serum-free medium before the start of wound healing assay under the each condition. t-ERK expression was similar in CPC-WT and CPC-KO in all cultures (Figure 4A and B). The p-ERK/t-ERK expression pattern was similar in CPC-KO and CPC-WT at baseline, but it was significantly higher in CPC-WT than in CPC-KO for the O2+/serum+ (10.47±0.18 vs. 7.54±0.14, p<0.01). The p-ERK/t-ERK expression of CPC-WT was lower than that of CPC-KO for the O2+/serum− (5.11±0.08 vs. 7.20±0.11, p<0.05), O2−/serum+ (3.02±0.05 vs. 6.97±0.08, p<0.05), and O2−/serum− (2.89±0.03 vs. 5.66±0.09, p<0.05; Figure 4A and C). The t-Akt expression was higher in CPC-WT than in CPC-KO at baseline (1.00±0.04 vs. 0.68±0.06, p<0.05), but there was no significant difference in the other cultures between the 2 cell types (Figure 4D and E). But, the p-Akt/t-Akt expression was significantly higher in CPC-WT than in CPC-KO at baseline (1.00±0.11 vs. 0.45±0.08, p<0.05), in O2+/serum+ (0.51±0.03 vs. 0.30±0.04, p<0.05), O2+/serum− (0.62±0.01 vs. 0.24±0.03, p<0.05), O2−/serum+ (0.69±0.01 vs. 0.19±0.03, p<0.05), and O2−/serum− (0.52±0.04 vs. 0.16±0.03, p<0.05; Figure 4D and F). The β-catenin expression was also higher in CPC-WT than in CPC-KO at baseline (1.00±0.07 vs. 0.78±0.05, p<0.05) and in O2+/serum+ (0.59±0.02 vs. 0.31±0.03, p<0.05), but it was higher in CPC-KO only for O2−/serum− (0.51±0.09 vs. 0.29±0.05, p<0.05; Figure 4G and H). The GSK-3β expression was also higher in CPC-WT and CPC-KO in all cultures (Figure 4G and I). CPC-WT showed a significantly higher expression of proteins associated with cell mobility and tube formation, for example, p-Akt, β-catenin, and GSK-3β, than did CPC-KO in all experimental cultures.
Figure 4.
CPC-WT showed better expression profiles of proteins related cell mobility and tube formation in normoxic and hypoxic cultures compared to CPC-KO. Proteins of Con-WT and Con-KO were obtained from the routine culture condition. And other cells were pre-treated with serum-free medium for 24 hours. And the experiment was carried out under each condition. (A-C) p-ERK/t-ERK expression level was higher in CPC-KO than CPC-WT in all culture conditions except the O2+/serum+ culture. (D-F) p-Akt/t-Akt was higher in CPC-WT than in CPC-KO in all cultures. (G-I) CPC-WT showed higher expression of β-catenin in control and O2+/serum+ culture. CPC-KO showed higher expression of β-catenin in O2−/serum− culture. GSK-3β was significantly higher in CPC-WT than in CPC-KO in all cultures.
Con-KO = β-arrestin2 knock-out cardiac progenitor cell in the routine culture condition; Con-WT = wild-type cardiac progenitor cell in the routine culture condition; CPC-WT = cardiac progenitor cell obtained from wild-type mouse; CPC-KO = cardiac progenitor cell obtained from β-arr2 knock-out mouse; GSK-3β = glycogen synthase kinase-3β; p-Akt = phosphorylated-protein kinase-B; p-ERK = phosphorylated-extracellular signal-regulated kinase; t-Akt = total-protein kinase-B; t-ERK = total-extracellular signal-regulated kinase; β-arr2 = β-arrestin2.
*p<0.05.
CPC-WT showed better tube formation ability compared to CPC-KO in normoxic and hypoxic cultures
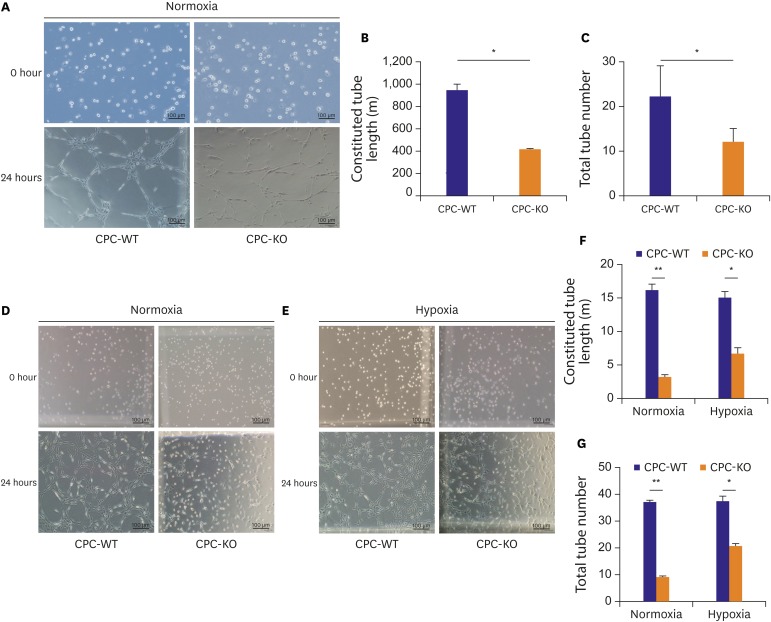
A Matrigel tube formation assay was carried out in 2 ways. For the experiment, CPCs were divided into 2 groups. One group received pretreatment of serum starvation for 24 hours, and then was transferred into the regular culture condition containing serum and oxygen for 24 hours to check the tube formation ability (Figure 5A) and the order group of cells did not pretreat with serum starvation and left in normoxic and hypoxic condition for 24 hours (Figure 5D-G). In the condition of serum starvation pretreatment, constituted tube length was significantly longer in CPC-WT than CPC-KO in the regular culture condition (936.81±59.91 vs. 411.64±15.59 µm, p<0.05; Figure 5A and B). And total complete tube number was also higher in CPC-WT than CPC-KO in the regular culture condition (22.00±7.50 vs. 12.00±3.21, p<0.05; Figure 5A and C). Without pretreatment of serum starvation, CPC-WT also had longer constituted tube lengths than CPC-KO in both normoxic (15.92±0.73 vs. 3.02±0.47 µm, p<0.01) and hypoxic culture (14.86±1.92 vs. 7.71±1.1 µm, p<0.05; Figure 5D-F). Total tube number was also higher for CPC-WT than for CPC-KO in the normoxic (37.25±1.62 vs. 9.25±1.36, p<0.01) and hypoxic cultures (37.50±4.59 vs. 20.75±3.13, p<0.05; Figure 5D, E, and G). So, fully constituted tube length and number was significantly higher in CPC-WT compared to CPC-KO both in normoxic and hypoxic condition for 24 hours after the serum starvation pretreatment or not.
Figure 5.
CPC-WT showed better tube formation ability compared to CPC-KO in normoxic and hypoxic cultures. (A-C) Constituted tube length and total tube number was higher in CPC-WT than in CPC-KO under normoxic condition after pretreatment of serum-starvation. (D-G) Constituted tube length and total tube number was higher in CPC-WT than in CPC-KO under normoxic and hypoxic culture without pretreatment of serum starvation.
CPC-KO = cardiac progenitor cell obtained from β-arr2 knock-out mouse; CPC-WT = cardiac progenitor cell obtained from wild-type mouse.
*p<0.05, †p<0.01.
DISCUSSION
The heart is composed of various types of cells, such as CMC, VSMC, EC, cardiac fibroblasts, and immune cells.16) However, there are other resident cells in the heart. CPCs comprise less than 1% of the non-CMC population in the heart, and have been sub-classified into c-kit, Sca-1, and Isl-1 positive cells, according to their patterns of cell-surface markers.4) Among them, c-kit positive CPCs can self-renew, be pluripotent, and differentiate into CMC, VSMCs, or ECs in suitable conditions.4) Therefore, CPCs can regenerate damaged myocardium by means of CMC replacement and new vessel constitution in MI or chronic myocardial ischemia.4) Mobilization and active proliferation of CPC in the model of mouse or rat MI have already been elucidated, and injection of ex vivo expansion CPC effectively repaired left ventricular systolic dysfunction in a rat MI model.4) These favorable effects of CPC in murine ischemic cardiomyopathy was consistently reproduced in feline and swine models of MI.17) A significant increase of c-kit positive cells was observed in a human failing heart also.10) It means that the failing heart can be a cue to start proliferation of nascent CPC to regenerate myocardium.10) CPCs defined with c-kit positive population from human myocardial tissue expands well in DMEM/F12-based culture medium, and a certain proportion of them proved to have ex vivo expansion capability and differentiation ability to CMC.11) With such promising data, as an alternative cell source for ischemic HF stem-cell therapy, clinical trials were conducted recently.4) Makkar et al.18) injected CPCs as a form of cardiosphere in a failing human heart by an intracoronary route; the whole injection procedure was safe, and the rescuing of HF in the CPC-injected group was evident. van Berlo et al.19) have shown that endogenous CPC produced new CMC within the heart although at a percentage of approximately 0.03 or less, and CPC amply generated cardiac EC effectively. Cell migration is a critical process for intrauterine fetal development and tissue recovery in various organs.5) It requires several spatially regulated events involving rearrangement of the actin cytoskeleton, formation of a leading edge, and contraction of the cell cortex.7)
β-arrs are versatile adaptor and scaffold proteins related to GPCRs.6) They play key roles in the various receptor functions by acting as either desensitizing and internalizing GPCRs or signal transducers by coupling activated GPCRs.6) β-arrs are ubiquitous in mammalian cells, but strong expression of β-arrs is observed especially in the brain and spleen.20) β-arrs are involved in cell proliferation, survival, and mobility, and they control numerous metabolic signaling pathways independently from the canonical GPCR action.21) β-arrs are important regulators of actin and cytoskeleton reorganization, and are closely related to cell migration.7) In addition, splenocytes derived from β-arr2 KO mice showed reduced CXCL12-induced migration.22) Those results indicate that β-arr2 affects cell migration through various molecular pathways.
In this study, we demonstrated that CPC-KO healed scratched wound significantly slower than did CPC-WT for all 4 combinations of oxygen concentration and serum supply in culture media (Figure 3). In the Western blot analysis, t-ERK showed similar expression in CPC-WT and CPC-KO for the all culture conditions (Figure 4A and B). p-ERK/t-ERK expression was also similar in both CPC-WT and CPC-KO in regular culture condition (Figure 4A and C). However, CPC-WT showed stronger expression of p-ERK/t-ERK than did CPC-KO for the O2+/serum+ culture condition, but CPC-KO showed stronger p-ERK/t-ERK expression in the other culture conditions (Figure 4A and C). In the wound healing assay, CPC-WT showed faster migration capability compared to CPC-KO in all conditions. Ge et al.23) have shown that phosphorylated ERK1/2 causes cell migration in NIH3T3 cells, one type of fibroblast. Our results are opposite to theirs; however, the role of interaction between ERK1/2 and β-arr2 in CPC migration has not been clearly demonstrated and it needs further research to discover about the direct association between β-arr2 and EKR1/2 in migration of CPC. We can neither explain the exact roles of ERK in CPC migration, nor the patterning of contradictory expression of p-ERK between O2+/serum+ condition and the other condition. It needs more detailed experiments and researches about the expression pattern and role of ERK in CPC.
Proteins in Wnt family bind to GPCRs known as frizzled receptors. β-arr2 is an important mediator in the Wnt5A-induced activation of the frizzled 4 receptor.24) Accumulation of β-catenin in cytosols is involved in cellular proliferation, survival and motility.24) We demonstrate that the expression of β-catenin was significantly lower in CPC-KO than CPC-WT in routine culture (Figure 4G and H). However, in the more harmful culture conditions, the β-catenin expression of CPC-KO gradually increased, while that of CPC-WT decreased (Figure 4G and H). Recent reports have mentioned that β-arr2 KO has been related to lower expression of β-catenin than in the presence of β-arr2.25) GSK-3β interacts with various pathways, such as Wnt/β-catenin, PI3-K/Akt/mTORC1, and Ras/Raf/MEK/ERK.24) The GSK-3 gene consists of GSK-3α and GSK-3β. GSK-3β is phosphorylated by Akt, and then GSK-3β is inactivated.24) Our results showed that CPC-WT expressed a significantly higher amount of GSK-3β in all cultures than CPC-KO did (Figures 2G, 2I, 4G, and 4I). Akt activation plays important roles in many cellular functions, such as survival, proliferation, migration, and tube formation. Deactivation of Akt stimulates GSK-3β and increases cell activity.24),26) These results showed lower expression of p-Akt in CPC-KO than CPC-WT in all cultures (Figures 2D, 2F, 4D, and 4F). Cardiac tube formation or angiogenesis is important for delivering oxygen, various growth factors, cytokines, and chemokines to damaged regions.27) Also, β-arrs are a main regulator of tumor angiogenesis,28) but the role of β-arrs in CPC is not clear. Our results showed that CPC-WT generated more complete tubes than CPC-KO in all cultures (Figure 5). Under pre-serum starvation, CPC-WT and CPC-KO both increased the tube length and number of complete tubes more than they did without pre-serum starvation (Figure 5A-C). Hypoxia generally provokes rapid tube formation in mesenchymal stem cell family compared to normoxic condition.29) CPC-KO showed more constitution of tube in hypoxia compared to normoxia, but this common phenomenon was not reproduced in CPC-WT in Figure 5. We surmise that CPC-WT showed relatively fast tube formation compared to previous data based on bone marrow derived mesenchymal stem cell or adipose tissue derived stem cell, so hypoxia did not have great affection to formation of tube in CPC-WT compared to CPC-KO.30) This point should be confirmed by comparative experiment using CPC, mesenchymal stem cell and adipose derived stem cell in same culture media under stimulating condition. Molecular pathway exploit about tube formation of CPC-WT in hypoxia is new research target in our laboratory. With above our data about β-arr2 actions in CPC, we surmise that β-arr2 will be very important scaffolding protein beside of canonical action in GPCR to make CPC migration, proliferation, and new vessel formation in MI inducing advanced HF. This hypothesis should be examined in MI model in β-arr2 KO mouse. There are some limitations in our data. First of all, we did not perform experiments with antagonist for effector molecules, e.g., Akt, GSK-3β, or β-catenin on CPC mobility, tube formation or survival on serum deprivation or hypoxemia. So, we could not make direct interaction between β-arr2 and tentative effector molecules in CPC performances. Second, the role of ERK in CPC was very ambiguous. According to previous data, ERK showed contradictory roles in cell mobility and survival in various cell types.7),8) p-ERK expression patterns in serum deprivation conditions were very ambiguous in CPC, and it was very difficult to estimate the role of ERK in CPC. We thought that it must be considered to do fine experiments to know the exact roles of effector molecules in CPC.
In summary, β-arr2 deficiency reduced mobility and tube formation ability of CPC in the presence or absence of serum and oxygen. It would be related to CPC survival given hypoxia or serum deprivation. Therefore, β-arr2 deficiency may be related to delayed myocardial regeneration via CPC mobility and tube formation disorder in ischemia.
Footnotes
Funding: The work was supported by research grant in 2015 from the Korean Society of Cardiology.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Bae JW, Seo SK, Koch WJ.
- Data curation: Seo SK, Kim N, Lee JH, Kim SM, Lee SY, Bae JW, Hwang KK, Kim DW, Koch WJ, Cho MC.
- Formal analysis: Seo SK.
- Funding acquisition: Bae JW, Hwang KK.
- Investigation: Seo SK, Kim N, Bae JW.
- Methodology: Seo SK.
- Writing - original draft: Seo SK.
- Writing - review & editing: Bae JW, Koch WJ.
The part of this study was presented as an abstract at the Annual Scientific meeting of the Korean Society of Cardiology in 2015.
References
- 1.Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. 2017;47:16–24. doi: 10.4070/kcj.2016.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016;46:658–664. doi: 10.4070/kcj.2016.46.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang SX, Phillips WD. Migration of resident cardiac stem cells in myocardial infarction. Anat Rec (Hoboken) 2013;296:184–191. doi: 10.1002/ar.22633. [DOI] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Kuang D, Zhao X, Xiao G, et al. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res Cardiol. 2008;103:265–273. doi: 10.1007/s00395-007-0690-z. [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for β-arrestin in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.DeFea KA. Arrestins in actin reorganization and cell migration. Prog Mol Biol Transl Sci. 2013;118:205–222. doi: 10.1016/B978-0-12-394440-5.00008-5. [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Li Z, Wang Y, Zhang L, Wu H, Li Z. Secreted pyruvate kinase M2 facilitates cell migration via PI3K/Akt and Wnt/β-catenin pathway in colon cancer cells. Biochem Biophys Res Commun. 2015;459:327–332. doi: 10.1016/j.bbrc.2015.02.112. [DOI] [PubMed] [Google Scholar]
- 9.Traynham CJ, Hullmann J, Koch WJ. Canonical and non-canonical actions of GRK5 in the heart. J Mol Cell Cardiol. 2016;92:196–202. doi: 10.1016/j.yjmcc.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 11.Attramadal H, Arriza JL, Aoki C, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 12.Fransioli J, Bailey B, Gude NA, et al. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun WJ, Nah DY, Bae JH, Chung JW, Lee H, Moon IS. Glucose-insulin-potassium solution protects ventricular myocytes of neonatal rat in an in vitro coverslip ischemia/reperfusion model. Korean Circ J. 2015;45:234–241. doi: 10.4070/kcj.2015.45.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naaldijk Y, Johnson AA, Ishak S, Meisel HJ, Hohaus C, Stolzing A. Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp Cell Res. 2015;338:97–104. doi: 10.1016/j.yexcr.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Park KJ, Kim YJ, Choi EJ, et al. Expression pattern of the thioredoxin system in human endothelial progenitor cells and endothelial cells under hypoxic injury. Korean Circ J. 2010;40:651–658. doi: 10.4070/kcj.2010.40.12.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda N, Manabe I. Cellular interplay between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J Inflamm. 2011;2011:535241. doi: 10.4061/2011/535241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan FG, DuBois RN. Emerging roles of beta-arrestins. Cell Cycle. 2006;5:2060–2063. doi: 10.4161/cc.5.18.3212. [DOI] [PubMed] [Google Scholar]
- 22.Walker JK, Fong AM, Lawson BL, et al. β-Arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 24.McCubrey JA, Steelman LS, Bertrand FE, et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia. 2014;28:15–33. doi: 10.1038/leu.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 26.Mannoury la Cour C, Salles MJ, Pasteau V, Millan MJ. Signaling pathways leading to phosphorylation of Akt and GSK-3β by activation of cloned human and rat cerebral D2 and D3 receptors. Mol Pharmacol. 2011;79:91–105. doi: 10.1124/mol.110.065409. [DOI] [PubMed] [Google Scholar]
- 27.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou L, Yang R, Chai J, Pei G. Rapid xenograft tumor progression in beta-arrestin1 transgenic mice due to enhanced tumor angiogenesis. FASEB J. 2008;22:355–364. doi: 10.1096/fj.07-9046com. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013;4:70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda K, Sowa Y, Nishino K, Itoh K, Fushiki S. Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann Plast Surg. 2015;74:728–736. doi: 10.1097/SAP.0000000000000084. [DOI] [PubMed] [Google Scholar]