Heart failure (HF) is a multi-factorial disease characterized by the inability of the heart to pump sufficient blood throughout the surrounding tissues. Scientific advances have recently aided in decreasing deaths attributed to HF; however, prevalence continues to increase as it threatens at least 26 million people globally.1) HF progression depends on several pre-existing conditions such as ischemic diseases like myocardial infarction (MI), eventually resulting in the death of cardiac cells. However, regeneration of cardiac cells in the adult heart is limited after injury and is instead replaced with a fibrotic scar. Therefore, researchers have resorted to stem cell therapy to restore these lost cells, and have demonstrated in numerous occasions the regenerative capabilities of cardiac progenitor cells (CPCs).2) CPCs can differentiate into various cell types including cardiomyocytes, and are capable of migration and tube formation, an important characteristic for developing therapies for ischemic myocardium.
Current HF treatments include targeting G-protein-coupled receptors (GPCRs) such as β-adrenergic receptors (βARs). GPCRs are responsible for mediating neurotransmitter or hormone signals to physiological responses; its dysregulation contributing to pathogenesis GPCR ligands activates G-protein independent pathways involving receptor phosphorylation by G protein-coupled receptor kinases (GRKs), eventually resulting in the recruitment of β-arrestin (β-arr). β-arr is a desensitizer (negative regulator) of GPCRs which have 2 isoforms, β-arrestin1 and β-arrestin2 (β-arr1 and β-arr2). Aside from its role in GPCR signaling, it links GPCRs with various downstream effectors such as the mitogen-activated protein kinase (MAPK) cascade, Src, and Mdm2.3) β-arr is known to play signaling roles in cancer, cardiac cells, stem cells, and even is pivotal in the progression of pathological states of the heart (Figure 1).4),5) It was previously demonstrated that β-arr1 is responsible for βAR desensitization and downregulation in vivo in the heart. Cardiac β-arr1 also counters the anti-apoptosis and inhibition of inflammation caused by β-arr2 in the myocardium.6) Interestingly, a new study titled “β-arrestin2 Affects Cardiac Progenitor Cell Survival through Cell Mobility and Tube Formation in Severe Hypoxia” published in the current issue of Korean Circulation Journal investigated the regulatory role of β-arr2 in myocardial regeneration with the use of CPCs. The study has observed that removal of β-arr2 resulted in decreased mobility tube formation and significantly lower expression of proteins related to survival and mobility, in addition to higher apoptotic rates in both normoxic and hypoxic conditions.7)
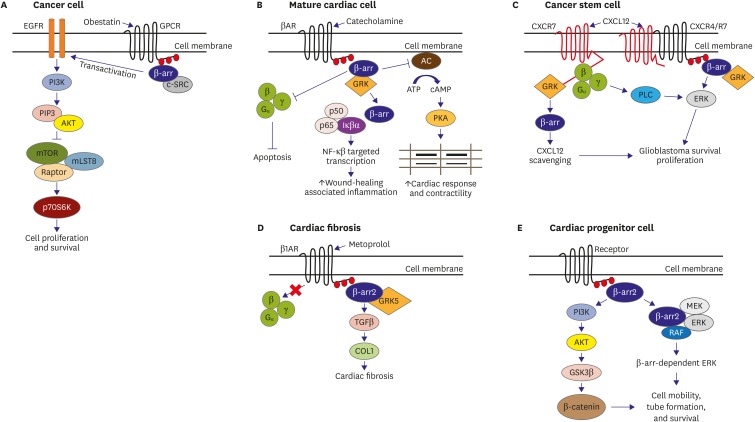
Figure 1.
β-arr is a potential therapeutic target for various diseases. (A) β-arr signaling in cancer cells. When a peptide such a obestatin binds to a receptor, phosphorylation of GPCR occurs leading to the attraction of β-arr. β-arr is then translocated which allows for its association with Src. The β-arr/Src interaction results in EGFR transactivation and Akt signaling. (B) β-arr signaling in mature cardiac cells. Catecholamines (e.g., epinephrine) can elicit a reaction through G-protein signaling or through β-arr2-mediated signaling which leads to improved cardiac response and contractility. Wound-healing associated inflammation also occurs via NF-κB pathway as activated by β-arr2, which are essential to preservation of cardiac function post-MI. (C) β-arr signaling in cancer stem cells. When CXCL12 binds to CXCR7, β-arr pathway is activated through GRK to internalize CXCR7 resulting in CXCL12 scavenging. It can also signal PLC to activate ERK 1/2 which leads to cell survival in certain cancer cells (for example, glioblastoma). (D) β-arr signaling in metoprolol-mediated cardiac fibrosis. Cardiac fibrosis induced by metoprolol via GRK5/β-arr2 is fairly miniscule compared to the fibrosis caused by heart failure. In addition, β-blocking capacity by metoprolol induces a positive action that can overshadow the fibrosis induced. (E) Possible β-arr signaling in cardiac progenitor cells. β-arr2 can affect expression of β-catenin via PI3K/GSK-3β signaling, resulting in cell survival brought about by regulation of cell mobility and tube formation. An alternative pathway can trigger β-arr-dependent ERK signaling via ERK 1/2.
AC = adenylyl cyclase; Akt = protein kinase B; ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate; COL1 = collagen 1; c-Src = proto-oncogene tyrosine-protein kinase Src; CXCL12 = C-X-C motif chemokine ligand 12; CXCR4 = C-X-C chemokine receptor type 4; CXCR7 = C-X-C chemokine receptor type 7; EGFR = epithelial growth factor receptor; ERK = extracellular signal-regulated kinase; GPCR = G-protein-coupled receptor; GRK = G protein-coupled receptor kinase; GSK-3β = glycogen synthase kinase-3β; Iκβα = nuclear factor κB inhibitor of nuclear factor κB; MEK = mitogen-activated protein kinase kinase; MI = myocardial infarction; mLST8 = mammalian lethal with SEC13 protein-8; mTOR = mechanistic target of rapamycin; NF-κB = nuclear factor-κB; p70s6k = 70 kDa ribosomal S6 kinase; PI3K = phosphoinositide 3-kinase; PKA = protein kinase A; PLC = phospholipase C; RAF = rapidly accelerated fibrosarcoma; Raptor = regulatory associated protein of mTOR; TGF = transforming growth factor; βAR = β-adrenergic receptor; β-arr = β-arrestin; β-catenin = β-catenin.
Although the study has demonstrated the novel importance of β-arr2 in CPC survival, there are still numerous issues that need to be addressed before we can consider β-arr2 as a viable target to treat diseases. For example, while the current study showed the role of β-arr2 by using a knockout (KO) model, it is also important to investigate how overexpression (OE) of β-arr2 in CPCs can be useful for regenerative purposes. A previous work by McCrink et al.8) using transgenic mouse hearts observed that cardiac β-arr2 OE increases cardiac function while reducing remodeling in post-MI. It will be very interesting to see the results of β-arr2 OE when employed in CPCs. In addition, we still have to uncover how β-arr2 fits into the whole timeline of HF. Future studies should consider investigating for the effect of β-arr1 and β-arr2 at different stages of HF progression. And even before we can ask this, we still have to have a better understanding of β-arr2 and the mechanisms related to it. What the authors failed to include in the current study is how β-arr2 KO affects βAR signaling in CPC survival, and how β-arr2 KO in CPCs directly interacts with known regulatory pathways by providing a direct evidence. Take for example, there were no clear links showing how protein kinase B (Akt), glycogen synthase kinase-3β (GSK-3β), and β-catenin is linked to β-arr2, and whether an alternative pathway via extracellular signal-regulated kinase (ERK) 1/2 can result in the same fate of cell survival. Furthermore, scientists are concerned regarding the effectivity of stem cell therapy. For instance, a clinical trial termed Stem Cell Infusion in Patients with Ischemic Cardiomyopathy (SCIPIO) involving the use of CPCs showed encouraging results in the use of these cells in patients with HF,9) but was later questioned by the journal, The Lancet, with Harvard University conducting its own investigations with regards to data integrity.
The recent findings from the studies previously discussed demonstrate that the role β-arr is much more important than what we originally imagined. Targeting β-arr might not only be useful in HF-related diseases, but also in other metabolic diseases such as cancer, which was found to be related to tumor initiation time and cancer malignancy outcomes.10) But as previously mentioned, there is still a lot to unravel. Researchers and clinicians should investigate how β-arr signal transduction can trigger a cascade of mechanisms that lead to the inhibition or progression of disease. Only after painting a clearer picture on its importance can we proceed with developing rational methods on how to harness β-arr for therapeutics. Nonetheless, the future of β-arr is bright and can be considered as a potential target for HF and metabolic disease management.
Footnotes
Funding: This work was supported by the Priority Research Centers Program (2010-0020224) and the Basic Science Research Program (2015R1A2A1A13001900) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Marquez J, Han J.
- Funding acquisition: Han J.
- Resources: Han J.
- Supervision: Han J.
- Writing - original draft: Marquez J.
- Writing - review & editing: Marquez J, Han J.
The contents of the report are the author's own views and do not necessarily reflect the views of the Korean Circulation Journal.
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le T, Chong J. Cardiac progenitor cells for heart repair. Cell Death Dis. 2016;2:16052. doi: 10.1038/cddiscovery.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noor N, Patel CB, Rockman HA. β-arrestin: a signaling molecule and potential therapeutic target for heart failure. J Mol Cell Cardiol. 2011;51:534–541. doi: 10.1016/j.yjmcc.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Würth R, Bajetto A, Harrison JK, Barbieri F, Florio T. CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front Cell Neurosci. 2014;8:144. doi: 10.3389/fncel.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakaya M, Chikura S, Watari K, et al. Induction of cardiac fibrosis by β-blocker in G protein-independent and G protein-coupled receptor kinase 5/β-arrestin2-dependent signaling pathways. J Biol Chem. 2012;287:35669–35677. doi: 10.1074/jbc.M112.357871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bathgate-Siryk A, Dabul S, Pandya K, et al. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension. 2014;63:404–412. doi: 10.1161/HYPERTENSIONAHA.113.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo SK, Kim N, Lee JH, et al. β-arrestin2 affects cardiac progenitor cell survival through cell mobility and tube formation in severe hypoxia. Korean Circ J. 2018;48:296–309. doi: 10.4070/kcj.2017.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrink KA, Maning J, Vu A, et al. β-arrestin2 improves post-myocardial infarction heart failure via sarco(endo)plasmic reticulum Ca(2+)-ATPase-dependent positive inotropy in cardiomyocytes. Hypertension. 2017;70:972–981. doi: 10.1161/HYPERTENSIONAHA.117.09817. [DOI] [PubMed] [Google Scholar]
- 9.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Hu S, Wang D, Wu J, et al. Involvement of β-arrestins in cancer progression. Mol Biol Rep. 2013;40:1065–1071. doi: 10.1007/s11033-012-2148-0. [DOI] [PubMed] [Google Scholar]