Abstract
To investigate whether the use of IL-6 receptor antagonist (tocilizumab) might be associated with hepatitis B virus (HBV) reactivation in rheumatoid arthritis (RA) patients, particularly in those with resolved HBV infection [HBV surface antigen (HBsAg) negative and antibody to HBV core antigen (anti-HBc) positive, serologically]. HBsAg, anti-HBc, antibody to HBsAg (anti-HBs), and HBV DNA titers were measured in RA patients who had continuously received tocilizumab for more than 3 months. Patients were divided into two groups according to the presence of anti-HBc. Clinical and laboratory data, in addition to medications administered along with tocilizumab during the treatment duration with tocilizumab, were compared between the two groups. HBV reactivation was defined as the presence of HBV DNA in sera, and alterations in HBsAg, anti-HBc, and anti-HBs titers according to the use of tocilizumab were also evaluated. Fifteen of 39 patients (38.5%) had anti-HBc positivity, while 24 patients (61.5%) did not. There were no differences in demographic data, serologic classification, and variables related to tocilizumab between the anti-HBc-positive and -negative groups. Comparison of the medications administered along with tocilizumab treatment revealed no meaningful differences. None of the patients experienced reactivation of HBV. In addition, in 15 patients with resolved HBV infection, no alterations in HBsAg, anti-HBc, and anti-HBs titers were observed with the use of tocilizumab. Tocilizumab may be applied to RA patients safely with few concerns for HBV reactivation, particularly in those with resolved HBV infection.
Keywords: Tocilizumab, hepatitis B, reactivation, rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory disease primarily affecting joints and their adjacent structures that leads to joint damage and deformity.1 Treatment of RA seeks to control both inflammation and pain and to reduce disability related to RA by the proper use of disease modifying antirheumatic drugs (DMARDs). Even though methotrexate remains a cornerstone in the treatment of RA, remarkable advances in the therapeutic regimens for RA have been made in the past decades. Particularly, biologics targeting inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), that play a crucial role in RA pathogenesis2 have ushered in a new era of RA management. Treatment guidelines currently recommend the application of biologics to patients who fail to respond to conventional DMARDs,3 and the number of patients receiving biologics have substantially increased in recent years.4
Hepatitis B virus (HBV) infection is one of the most common worldwide viral infections. Because chronic inflammation by HBV can provoke cirrhosis, liver failure, and even hepatocellular carcinoma, HBV still remains as a major public health concern. Global epidemiologic studies now show that more than 2 billion individuals have been infected by HBV and more than 350 million people have chronic HBV infection.5 Recently, there have been increasing demands for screening for HBV infection in the field of rheumatology, as patients receiving biologics are at risk of HBV reactivation.6,7 Among viral factors associated with HBV reactivation, chronic HBV infection and high baseline HBV DNA are likely to be meaningful risk factors.6 In addition, HBV reactivation can occur in patients with resolved HBV infection, which is defined as HBV surface antigen (HBsAg) negative and antibody to HBV core antigen (anti-HBc) positive, regardless of antibody to HBsAg (anti-HBs).7,8 So far, HBV reactivation in RA patients has been mainly assessed in patients receiving TNF-α blockade, but rarely in those receiving IL-6 receptor antagonist (tocilizumab). Furthermore, the safety of tocilizumab in regards to HBV reactivation in resolved HBV infection remains unclear. Hence, in this study, we investigated whether the use of tocilizumab might be associated with HBV reactivation in RA patients, particularly in those with resolved HBV infection.
We retrospectively reviewed the medical records of patients with RA receiving tocilizumab according to the following inclusion criteria: 1) patients diagnosed with RA according to the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA9,10 at the Division of Rheumatology, Yonsei University College of Medicine, Severance Hospital; 2) patients who had information on HBsAg, anti-HBc, and anti-HBs at the initiation of tocilizumab and who did not have HBsAg; 3) patients who were continuously receiving tocilizumab for more than 3 months; and 4) patients who provided blood samples for the cross-sectional analysis of HBsAg, anti-HBc, anti-HBs, and HBV DNA with informed consent forms at the time of study enrollment. This study was approved by the Institutional Review Board of Severance Hospital, and was conducted in accordance with the principles set forth in the Declaration of Helsinki (IRB approval number 4-2017-0761).
We collected information on age, gender, and disease duration as demographic data. Patients were classified as seropositive and seronegative RA according to the presence of rheumatoid factor and anti-citrullinated protein antibodies. Regarding tocilizumab usage, information on DAS28-ESR (disease activity score 28-erythrocyte sedimentation rate) on the initiation of tocilizumab, treatment duration, and the route of administration were also obtained. We also reviewed laboratory data for aspartate aminotransferase (AST), alanine aminotransferase (ALT), ESR, and C-reactive protein (CRP), both at initiation of tocilizumab and at study enrollment.
We measured HBsAg, anti-HBc, and anti-HBs both at initiation of tocilizumab and at study enrollment. Serum HBV DNA titers were measured only at study enrollment. Reactivation of HBV was defined as a serum HBV DNA titer above the lower detection limit.11 Serum levels of HBsAg, anti-HBc, and anti-HBs were detected and interpreted using the Abbott Architect i4000 immunoassay system (Abbott Diagnostics, Chicago, IL, USA). Serum HBV DNA was measured by real-time PCR assay (cobas CAP/CTM System, Roche Molecular Diagnostics, Indianapolis, IN, USA) with a lower detection limit of 15 IU/mL.
We reviewed medications that were administered along with tocilizumab throughout the duration of tocilizumab treatment. We particularly calculated the cumulative dosages of glucocorticoid and methotrexate administered during the treatment duration of tocilizumab. In addition, as it is known that HBV reactivation is more common within the first 6 months after the start of immunosuppressive therapies, we also calculated the cumulative dosage of both drugs for the initial 6 months after tocilizumab initiation.12
Continuous variables are presented as medians with interquartile ranges, and categorical variables are expressed as frequencies and percentages. Continuous variables were compared using the Mann-Whitney U test, and categorical data were compared using the Chi-squared test or Fisher's exact test, as appropriate. Comparison of serum titers of HBsAg, anti-HBc, and anti-HBs at initiation of tocilizumab and those at study enrollment was performed using the Wilcoxon signed rank test. All statistical analyses were conducted using the SPSS package for Windows, version 21 (IBM Corp., Armonk, NY, USA). p-values <0.05 were considered statistically significant.
The baseline characteristics of patients are described in Table 1. The median age was 55.0, and 28 (71.8%) patients were female. The median disease duration before the initiation of tocilizumab was 61.7 months. Thirty-five (89.7%) patients were classified as seropositive RA, while only 4 patients were as seronegative RA. Median DAS-28 ESR score at initiation of tocilizumab was 7.0. The median treatment duration with tocilizumab was 10.8 months.
Table 1. Characteristics of Patients with RA and Comparison of Variables between Patients with and without Anti-HBc.
| Total patients (n=39) | Patients with anti-HBc (n=15) | Patients without anti-HBc (n=24) | p value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (yr) | 55.0 (46.3–62.0) | 57.0 (48.5–68.5) | 50.0 (43.0–61.5) | 0.141 |
| Female gender | 28 (71.8) | 10 (66.7) | 19 (79.2) | 0.391 |
| Disease duration (month) | 61.7 (21.4–152.3) | 52.7 (20.9–121.5) | 67.7 (22.2–170.2) | 0.729 |
| Serologic classification | 0.999 | |||
| Seropositive RA | 35 (89.7) | 14 (93.3) | 21 (87.5) | |
| Seronegative RA | 4 (10.3) | 1 (6.7) | 3 (12.5) | |
| Tocilizumab | ||||
| DAS-28 ESR at initiation | 7.0 (6.4–7.4) | 7.2 (6.5–7.4) | 6.9 (6.3–7.5) | 0.581 |
| Treatment duration (month) | 10.8 (6.2–23.7) | 9.4 (5.7–25.8) | 11.0 (6.3–23.5) | 0.436 |
| Route of tocilizumab | 0.658 | |||
| Intravenous | 33 (84.6) | 12 (80.0) | 21 (87.5) | |
| Subcutaneous | 6 (15.4) | 3 (20.0) | 3 (12.5) | |
| Laboratory data at initiation of tocilizumab | ||||
| Anti-HBs positive | 24 (61.5) | 12 (80.0) | 12 (50.0) | 0.093 |
| AST (IU/L) | 16.0 (13.0–20.0) | 16.0 (13.0–21.5) | 17.0 (14.0–20.0) | 0.795 |
| ALT (IU/L) | 15.0 (9.3–25.8) | 14.0 (9.3–19.8) | 16.0 (9.0–27.0) | 0.654 |
| ESR (mm/hr) | 61.0 (40.5–77.8) | 62.0 (48.3–76.3) | 54.0 (39.0–78.0) | 0.603 |
| CRP (g/mL) | 13.6 (4.9–28.3) | 16.5 (8.0–53.0) | 13.0 (3.7–27.1) | 0.319 |
| Laboratory data at study enrollment | ||||
| AST (IU/L) | 20.0 (17.0–27.0) | 20.0 (17.0–25.3) | 20.5 (16.5–27.5) | 0.977 |
| ALT (IU/L) | 23.0 (14.0–29.8) | 22.0 (15.5–29.5) | 24.0 (13.0–29.5) | 0.840 |
| ESR (mm/hr) | 9.0 (3.0–21.5) | 12.0 (4.5–21.0) | 7.5 (2.5–21.0) | 0.347 |
| CRP (g/mL) | 0.2 (0.1–0.8) | 0.3 (0.2–0.9) | 0.2 (0.1–0.7) | 0.178 |
Anti-HBc, positive antibody to HBV core antigen; RA, rheumatoid arthritis; DAS, disease activity score; ESR, erythrocyte sedimentation rate; Anti-HBs, antibody to HBsAg; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CRP, C-reactive protein.
Values are expressed as the median (interquartile range) or number (percentage).
Among the patients enrolled, 15 of 39 patients (38.5%) had anti-HBc. Since no patients had a history of chronic HBV infection, none received antiviral therapy for hepatitis B prophylaxis. There were no differences in demographic data and serologic classification between patients with and without anti-HBc. Furthermore, variables related to tocilizumab did not differ between the two groups. In addition, patients with anti-HBc exhibited anti-HBs positivity more frequently than those without, although the difference was not significant (80.0% vs. 50.0%, p=0.093). Alterations in AST, ALT, ESR, and CRP over the duration of treatment with tocilizumab were not remarkable (Table 1).
To minimize the influence of other medications administered along with tocilizumab on HBV reactivation, we compared the frequencies of glucocorticoid and DMARDs and the cumulative dosages of glucocorticoid and methotrexate during the treatment duration of tocilizumab. Among the medications administered, glucocorticoid was the most commonly used medication, followed by methotrexate and sulfasalazine in the both groups. We found no meaningful differences in the frequencies of all medications between patients with and without anti-HBc. Furthermore, the cumulative dosages of both glucocorticoid and methotrexate and the cumulative dosages of both drugs within the first 6 months after tocilizumab initiation also did not differ (Table 2).
Table 2. Comparison of Medication Administered along with Tocilizumab between Patients with and without Anti-HBc.
| Medications | Patients with anti-HBc (n=15) | Patients without anti-HBc (n=24) | p value |
|---|---|---|---|
| Glucocorticoid | 14 (93.3) | 21 (87.5) | 0.999 |
| Cumulative glucocorticoid dosage (mg)* | 1015.0 (506.3–1760.0) | 1295.0 (519.1–3073.8) | 0.419 |
| Cumulative glucocorticoid dosage within the first 6 months (mg)* | 775.0 (340.0–905.0) | 800.0 (452.5–1061.3) | 0.523 |
| Methotrexate | 11 (73.3) | 17 (70.8) | 0.999 |
| Cumulative methotrexate dosage (mg)† | 557.1 (294.7–940.7) | 689.6 (444.6–1156.8) | 0.458 |
| Cumulative methotrexate dosage within the first 6 months (mg)† | 338.6 (266.1–490.0) | 316.4 (240.0–379.8) | 0.312 |
| Leflunomide | 0 (0.0) | 2 (8.3) | 0.514 |
| Hydroxychloroquine | 1 (6.7) | 6 (25.0) | 0.216 |
| Sulfasalazine | 3 (20.0) | 7 (29.2) | 0.711 |
| Tacrolimus | 1 (6.7) | 2 (8.3) | 0.999 |
Anti-HBc, positive antibody to HBV core antigen.
Values are expressed as the median (interquartile range) or number (percentage).
*The cumulative glucocorticoid dosage was represented in prednisolone equivalent dosage and was calculated in those treated with glucocorticoids, †The cumulative methotrexate dosage was calculated in those treated with methotrexate.
In this study, reactivation of HBV was defined as the presence of HBV DNA in sera. We measured HBV DNA at study enrollment in all 39 patients regardless of anti-HBc; however, we could find no patient in whom serum HBV DNA was detected. Therefore, we concluded that the use of tocilizumab more than 3 months was not associated with HBV reactivation.
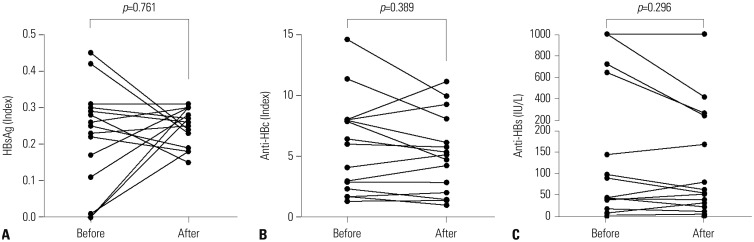
We also evaluated whether tocilizumab is associated with alterations in the titers of HBsAg, anti-HBc, and anti-HBs in 15 patients with resolved HBV infection. When we compared HBsAg, anti-HBc, and anti-HBs titers between at initiation of tocilizumab and at study enrollment, we could find no significant differences between the two (Fig. 1).
Fig. 1. Alterations in the titers of HBsAg (A), anti-HBc (B), and anti-HBs (C) in patients with resolved HBV infection. There were no meaningful differences in the titers of HBsAg, anti-HBc, and anti-HBs in 15 patients with resolved HBV infection at initiation of tocilizumab and at study enrollment. HBsAg, HBV surface antigen; Anti-HBc, positive antibody to HBV core antigen; Anti-HBs, antibody to HBsAg; HBV, hepatitis B virus.
In this study, we investigated whether the use of tocilizumab might be associated with HBV reactivation in RA patients, particularly in those with resolved HBV infection. We found that tocilizumab treatment with the median treatment duration of 9.4 months was not associated with HBV reactivation or the presence of HBV DNA in sera. Moreover, the use of tocilizumab did not contribute to alterations in HBsAg, anti-HBc, and anti-HBs titers in patients with resolved HBV infection. There is discordance between our study and previous studies. Previous studies reported that patients with autoimmune diseases accompanied by lower anti-HBs titer exhibit a higher rate of HBV reactivation13 and that TNF-α inhibitor treatment is associated with decreases in anti-HBs titers in patients with inflammatory arthritis.14 However, in the present study, no patients with resolved HBV infection experienced HBV reactivation, and furthermore, alterations in the titers of variables related to HBV did not depend on the use of tocilizumab, unlike TNF-α inhibitor.
Worldwide, HBV infection is the most common cause of chronic liver disease. South Korea is known as an endemic area of chronic HBV infection.15 Although successful implementation of national vaccination programs to eliminate HBV infection has shown progress in decreasing the prevalence of HBsAg positivity in the general population, South Korea is still classified as an intermediate HBV endemic area.16 In regions with intermediate to high risk of HBV infection, the assessment of HBV reactivation following the use of biologics stands to be a clinically important issue, as RA patients often experience elevated liver enzymes during treatment. Because a majority of RA patients are treated with a combination of medications, including glucocorticoids, DMARDs, and biologics, it is crucial to discriminate between viral reactivation and drug induced liver injury. The risk of HBV reactivation does not seem to be high in patients with resolved HBV infection,6 although evidence further indicates that biologics therapy could be associated with HBV reactivation. In a recent systematic review, the estimated proportion of patients experiencing HBV reactivation in those with resolved HBV infection during TNF-α treatment was 1.7%.17 However, the risk of HBV reactivation in resolved HBV infection due to the use of tocilizumab remains a debate: a literature review suggested that 8.6% of patients treated with tocilizumab suffered from HBV reactivation,18 while in a recent study, HBV reactivation was not reported in patients treated with tocilizumab.19,20 We assume that this discrepancy might be attributable to differences in both the definition of HBV reactivation and geographic and ethnic features. In this regard, data regarding the safety of tocilizumab in Korean patients with RA regardless of resolved HBV infection would be needed; however, there are no data thereon. For these reasons, we deduced that tocilizumab may not participate in provoking HBV reactivation in Korean patients with RA regardless of anti-HBc positivity and that, furthermore, it may not contribute to changes in the titers of variables related to HBV in patients with resolved HBV infection.
The strength of this study is that it is the first to demonstrate the safety of tocilizumab in RA patients, particularly in those with resolved HBV infection, in Korea. Because anti-viral agents should be applied to all patients with chronic HBV infection before the use of biologics, in this study, we focused on HBV reactivation in patients with resolved HBV infection. However, our study has several limitations. First, we had no information on the presence of HBV DNA at initiation of tocilizumab due to the absence of HBsAg in all patients. Second, the number of patients treated with tocilizumab was relatively small with a short follow-up duration. This might have affected the negative results that were observed in this study. In addition, it is unclear whether tocilizumab is more advantageous to TNF-α inhibitors in terms of HBV reactivation, especially in those with resolved HBV infection. Future studies with a larger number of patients treated with tocilizumab will clarify the link between the use of tocilizumab and HBV reactivation in RA patients with resolved HBV infection.
In conclusion, the use of tocilizumab was not associated with HBV reactivation in RA patients, particularly in those with resolved HBV infection, in Korea. We suggest that tocilizumab may be safely applied in terms of HBV reactivation to RA patients with HBsAg negativity and anti-HBc positivity, regardless of anti-HBs.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2016-0145) to Sang-Won Lee.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03029050).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 4.Harrold LR, Peterson D, Beard AJ, Gurwitz JH, Briesacher BA. Time trends in medication use and expenditures in older patients with rheumatoid arthritis. Am J Med. 2012;125:937.e9–937.e15. doi: 10.1016/j.amjmed.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 6.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5 Suppl):S156–S165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 8.Lee JI. Reactivation of hepatitis B virus in patients with rheumatologic disease treated with biologic disease modifying anti-rheumatic drugs: screening and treatment. J Rheum Dis. 2015;22:282–287. [Google Scholar]
- 9.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 10.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura J, Nagashima T, Nagatani K, Yoshio T, Iwamoto M, Minota S. Reactivation of hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int J Rheum Dis. 2016;19:470–475. doi: 10.1111/1756-185X.12359. [DOI] [PubMed] [Google Scholar]
- 12.Mochida S, Nakao M, Nakayama N, Uchida Y, Nagoshi S, Ido A, et al. Nationwide prospective and retrospective surveys for hepatitis B virus reactivation during immunosuppressive therapies. J Gastroenterol. 2016;51:999–1010. doi: 10.1007/s00535-016-1168-2. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Atsumi T, Kurita T, Odani T, Fujieda Y, Otomo K, et al. Hepatitis B virus reactivation by immunosuppressive therapy in patients with autoimmune diseases: risk analysis in Hepatitis B surface antigen-negative cases. J Rheumatol. 2011;38:2209–2214. doi: 10.3899/jrheum.110289. [DOI] [PubMed] [Google Scholar]
- 14.Charpin C, Guis S, Colson P, Borentain P, Mattéi JP, Alcaraz P, et al. Safety of TNF-blocking agents in rheumatic patients with serology suggesting past hepatitis B state: results from a cohort of 21 patients. Arthritis Res Ther. 2009;11:R179. doi: 10.1186/ar2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang BM, Kim CH, Kim JY. Cost of chronic hepatitis B infection in South Korea. J Clin Gastroenterol. 2004;38(10 Suppl 3):S153–S157. doi: 10.1097/00004836-200411003-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Shin AR, Chung HH, Kim MK, Lee JS, Shim JJ, et al. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med. 2013;28:413–419. doi: 10.3904/kjim.2013.28.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YH, Bae SC, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol. 2013;31:118–121. [PubMed] [Google Scholar]
- 18.Mori S, Fujiyama S. Hepatitis B virus reactivation associated with antirheumatic therapy: risk and prophylaxis recommendations. World J Gastroenterol. 2015;21:10274–10289. doi: 10.3748/wjg.v21.i36.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CT, Huang WN, Hsieh CW, Chen YM, Chen DY, Hsieh TY, et al. Safety and effectiveness of tocilizumab in treating patients with rheumatoid arthritis - A three-year study in Taiwan. J Microbiol Immunol Infect. 2017 Jul 01; doi: 10.1016/j.jmii.2017.04.002. [Epub] [DOI] [PubMed] [Google Scholar]
- 20.Papalopoulos I, Fanouriakis A, Kougkas N, Flouri I, Sourvinos G, Bertsias G, et al. Liver safety of non-tumour necrosis factor inhibitors in rheumatic patients with past hepatitis B virus infection: an observational, controlled, long-term study. Clin Exp Rheumatol. 2018;36:102–109. [PubMed] [Google Scholar]