Abstract
High-yielding varieties developed in the 1960s and 1970s at the International Rice Research Institute (IRRI) and elsewhere benefited farmers and the public, ultimately increasing yields and reducing the cost of rice to consumers. Most of these varieties, however, did not have the optimum cooking quality that was possessed by many of the traditional varieties they replaced. In 1985, the IRRI-developed indica variety IR64 was released in the Philippines. In addition to its high yield, early maturity and disease resistance, it had excellent cooking quality, matching that of the best varieties available. These merits resulted in its rapid spread and cultivation on over 10 million ha in the two decades after it was released. It has intermediate amylose content and gelatinization temperature, and good taste. It is resistant to blast and bacterial blight diseases, and to brown planthopper. Because of its success as a variety, it has been used extensively in scientific studies and has been well-characterized genetically. Many valuable genes have been introduced into IR64 through backcross breeding and it has been used in thousands of crosses. Its area of cultivation has declined in the past 10 years, but it has been replaced by a new generation of high-quality varieties that are mostly its progeny or relatives. Continued basic studies on IR64 and related varieties should help in unraveling the complex genetic control of yield and other desirable traits that are prized by rice farmers and consumers.
Background
In November 2016, the International Rice Research Institute (IRRI) and others commemorated the 50th anniversary of the release of IR8, its first developed variety and a beginning of the Green Revolution in rice (http://irri.org/ir8). This variety established the basic plant type of the high-yielding varieties (HYVs) that have now spread over most rice growing areas.
IR8 had a very high grain yield, but also a number of defects, most importantly, poor grain quality, lack of disease and insect resistance, and late maturity. The varieties subsequently developed and released over the next two decades improved greatly on these traits (Khush 1999). During the early 1980s, one of the most popular varieties grown was IR36. In addition to its disease and insect resistance, it achieved its high yield in a period of only 111 days from seed to seed, compared to 130 days for IR8 (Khush and Virk 2005). It spread rapidly and was estimated to be planted on more than 10 million ha during the 1980s.
While much improved over IR8, IR36 still lacked the quality of the best varieties grown in the Philippines and Indonesia before the Green Revolution. IR64, released in the Philippines in 1985, represented a breakthrough in combining excellent palatability of cooked rice with the other traits found in previous IRRI HYVs. IR64 replaced IR36 in most growing areas and spread rapidly in new areas. Because of its wide adaptation, early maturity, and improved quality, it became a standard for high-quality rice and was highly desired by the rice industry. Because of its popularity, it has been used widely as a representative indica variety in research studies. It has also been used extensively as a parent in breeding programs, and to develop populations for genetic analysis.
In this article, we describe the development of IR64 and its major characteristics. We also discuss the use of this variety in further breeding and rice research.
Review
Breeding history, including parentage and selection history, evaluation and release
The breeding history of IR64 is summarized in Khush and Virk (2005). The breeding program at IRRI focused on combining the different traits desired by farmers, including high yield, resistance to biotic and abiotic stresses, early maturity, and improved grain quality. The Genetic Evaluation and Utilization (GEU) program was formulated at IRRI to focus research efforts on combining these traits through interdisciplinary collaboration (Khush and Coffman 1977). In segregating generations and in evaluation of fixed lines, plants and breeding lines were evaluated for these traits either in the pedigree breeding nursery or special screening nurseries. This improved the chance of combining multiple traits into a single variety.
The cross of IR5657-33-2-1/IR2061-465-1-5-5, was made in early 1977 and was designated IR18348. The female parent was noted for its good cooking quality (intermediate amylose), as well as having some tolerance to salinity. The male parent was a high-yielding breeding line derived from a highly productive cross that resulted in a number of other varieties from sister lines, including IR28, IR29 and IR34. The full pedigree (Fig. 1) shows the derivation of IR64 from 19 traditional rice varieties.
Fig. 1.
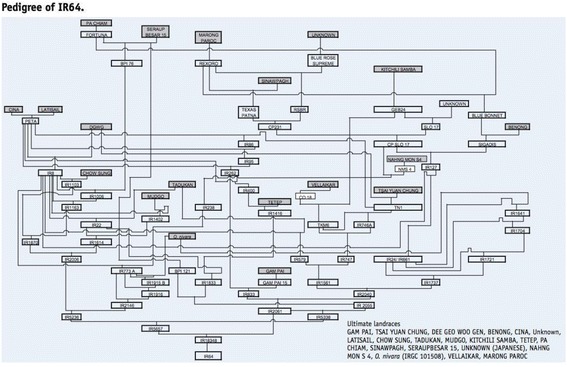
Pedigree of IR64 showing the ultimate landraces in its ancestry (Khush and Virk 2005)
The F2 population was evaluated in 1978, and the pedigree method of breeding was followed in the F3 and F4 populations, grown in 1979. The breeding line IR18348-36-3-3 resulted from the bulk harvest of a F5 family in 1980, and was subsequently evaluated in yield trials in 1981–83 at IRRI, as well as in the Philippine National Trials. It out-yielded IR36 by 21% in these trials (IRRI 1986). It was released by the Philippine Seed Board with the designation ‘IR64’ in 1985, and this designation has been subsequently used by IRRI (IRRI 1986).
The early varieties developed at IRRI were high yielding and had disease and insect resistance, but their grain quality was inferior to the best available varieties. At the time, the standard for grain quality in the Philippines was the variety BPI-76 and its sister line BPI-121, derived from the cross Fortuna/Seraup Besar 15 (Cada and Escuro 1972). The parent variety Fortuna is from the USA, and Seraup Besar 15 was originally introduced from Malaysia. BPI-76 was released in 1960 in the Philippines, and non-photoperiod sensitive selections, BPI-76 (n.s.) and BPI-76-1, were also released (Dalrymple 1978). Another popular variety noted for its quality was C4-63, and it was derived from the cross Peta/BPI-76. Before IR64, C4-63 was considered one of the main high-quality varieties in the Philippines. In 1970, a green-base C4-63 was released to replace the original seed stocks of C4-63. Because of its good eating quality (intermediate amylose content) it had spread in Indonesia, Malaysia, and Burma (Yoshida 1981). Rice was even widely marketed under the C4 name up to the 1980s; however, many of the market samples actually turned out to be other varieties or mixtures of varieties with inferior quality that were being grown by farmers (Juliano et al. 1989).
BPI-121-407 is considered the most likely contributor of superior quality in the pedigree of IR64, although BPI-76 is also in its pedigree. BPI-121-407 is a short-statured breeding line with superior quality, and was selected as an induced mutant of the original BPI-121 (Cada and Escuro 1972). In the advanced evaluation of the breeding line IR18348-36-3-3 at IRRI, taste panels were used to ensure that the quality of BPI-76 (or BPI-121) was captured. Taste panels were also applied in the government of the Philippines rice program to assist in providing the data for release of this variety.
Adoption and impact
The major breakthrough in the development of IR64 was the combination of the high yield and disease and insect resistance of earlier IRRI varieties with the superior grain quality associated with varieties like BPI-76 and C4-63. It’s first release was by the Philippine government in 1985. It was also released in the following countries: Bhutan, Burkina Faso (as FKR42), Cambodia, China, Ecuador (as NIAP11), Gambia, India, Indonesia, Mauritania, Mozambique, and Vietnam (as OM89) (Khush and Virk 2005). Its wide adaptation is notable, and it became widely grown in Southeast and South Asia. It is also noted to be well adapted to the Sahelian regions of West Africa (Devries et al. 2011; Julia and Dingkuhn 2013).
By 1995, IR64 was already estimated to be grown on 8 million ha (Khush 1995), and by the turn of the century this rose to over 10 million ha. The long persistence of IR64 in farmers’ fields after its release was attributed to its excellent eating quality (Champagne et al. 2010). The area of production gradually declined in the Philippines during the period 2000–2007, partly due to pressure from tungro disease (Laborte et al. 2015). Indonesia was a major producer of IR64, which was grown on more than 40% of its total area for around a decade (Fig. 2), and was still popular in 2009 (Brennan and Malabayabas 2011). It is also widely grown in India. During 1998–2006 IR64 accounted for over 10% of the breeder seed produced in India and was still above 3% in 2015, suggesting that it was grown on 2–3 million ha annually during the period (data provided by A. K. Singh).
Fig. 2.
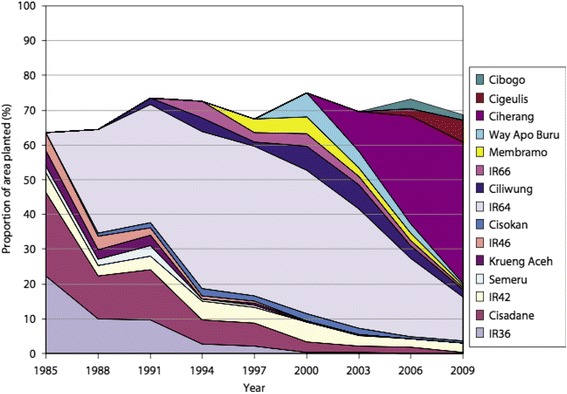
Share of leading varieties in Indonesia during 1985–2009 (Brennan and Malabayabas 2011)
A specific estimate of the impact of this variety has not been attempted, even though it is known as the most popular variety in terms of area, particularly in tropical Asia. IR64 has contributed greatly to farmer incomes not only through higher yields, but through improved quality that results in higher price and earlier maturity that allows higher cropping intensity.
As with other IRRI varieties, seed of IR64 was distributed freely to researchers and farmers and no intellectual property protection was sought on it or any progeny developed from it.
Major characteristics, including key traits
IR64 is a semidwarf indica rice variety, with average mature plant height of approximately 100 cm in the Philippines (Fig. 3). It is a relatively early duration variety, with total growth duration of about 117 days (Khush and Virk 2005). It inherits the same semidwarf sd1 allele as other IRRI semidwarf varieties, ultimately derived from Dee-geo-woo-gen. According to Wei et al. (2016) it has the loss of function alleles for Hd1 and Ehd1, which confer earlier duration and insensitivity to photoperiod. At the time of its release, IRRI (1986) listed the valuable traits as resistance to brown planthopper (BPH) biotypes 1 and 3, green leafhopper (GLH), white backed planthopper (WBPH), bacterial blight, grassy stunt virus; and moderate resistance to blast, BPH biotype 2, and stem borer. It was the first IRRI variety to combine intermediate amylose content and intermediate gelatinization temperature (GT).
Fig. 3.

Plot of IR64 growing in the field at IRRI, Los Baños, Philippines (photo from IRRI)
IR64 has high yield, especially compared to earlier-released IRRI varieties, but not as high as some of the subsequently released varieties like IR72. Peng et al. (2000) list its grain yield as 8.76 and 8.28 t ha− 1 compared to 9.50 and 9.06 t ha− 1 for IR72 in the dry season at IRRI in 1996 and 1998, respectively. It has high grain filling percentage and grain weight, but a relatively low spikelet number per m2 (Peng et al. 2000). According to Ujiie et al. (2016), IR64 possesses a gene GS3 for grain size and the narrow-leaf gene (NAL1) that improve grain yields. IR64 is considered a typical high-tillering indica type cultivar, in contrast to the “new plant type” NPT varieties that have lower tillering but large tiller and panicle size (Okami et al. 2015).
IR64 has relatively durable resistance to BPH, and it is known to carry the major gene Bph1. However, it is reported to have better resistance than other varieties carrying Bph1 and has good field resistance to the pest, exhibiting antiobiosis, antixenosis and tolerance (Cohen et al. 1997). This is partly attributed to its possessing additional QTLs controlling BPH resistance which confer greater durability of the resistance (Alam and Cohen 1998).
IR64 has very good levels of resistance to blast disease, including major-gene resistance and partial resistance (Bastiaans and Roumen 1993; Grand et al. 2012; Roumen 1992). Sallaud et al. (2003) reported six resistance genes, designated Pi25(t), Pi-27(t), Pi29(t), Pi30(t), Pi31(t), and Pi32(t). It also has resistance genes Pita (Lee et al. 2011) and Pi20 (Khush and Virk 2005), as well as Pi33 on chromosome 8 which was derived from O. rufipogon (accession IRGC101508, sometimes referred to as O. nivara) in its pedigree (Ballini et al. 2007; Berruyer et al. 2003). Sreewongchai et al. (2010) found that IR64 has broad resistance to blast disease in Thailand and this was conferred mainly by QTLs on chromosomes 2 and 12. It was also resistant in a blast hotspot in India (Thakur et al. 2013). Kongprakhon et al. (2010) identified QTLs that may correspond to Pi25 (chromosome 2), Pi29 (chromosome 8), and Pi28 (chromosome 10).
IR64 is resistant to Bacterial Blight (BB) disease (caused by Xanthomonas oryzae pv. oryzae) and possesses the major gene Xa4 for resistance (Adhikari et al. 1994; Khush and Virk 2005). The gene is thought to confer additional agronomic benefits in addition to BB resistance (Hu et al. 2017). It is also resistant against African strains of X. oryzae, and several QTLs for resistance were identified (Djedatin et al. 2016).
IR64 is susceptible to tungro disease, including Rice Tungro Spherical Virus (RTSV) (Lee et al. 2010) and Rice Tungro Bacilliform Virus (RTBV) (Zenna et al. 2006).
IR64 was developed primarily for irrigated rice production, and abiotic stress resistance was not an objective. While it is generally considered susceptible to abiotic stresses, it has been widely grown in more favorable rainfed situations and under mildly unfavorable soils.
IR64 is considered susceptible to drought stress and yield reductions can be considerable (Anantha et al. 2016). Yields under aerobic conditions (favorable upland) are also relatively low (Zhao et al. 2010). Vikram et al. (2015) attributed drought susceptibility of many modern high-yielding varieties to linkage between a drought susceptibility QTL and the semidwarf gene sd1. IR64 has a relatively shallow root system and low root length density (Henry et al. 2011; Shrestha et al. 2014). Under water stress, IR64 has relatively low water uptake rate (Gowda et al. 2012).
IR64 is considered sensitive to heat (Coast et al. 2016; Gonzalez-Schain et al. 2016; Shanmugavadivel et al. 2017; Ye et al. 2015), although this has not been reported as a problem with previous or current production environments. However, Jagadish et al. (2008) indicated that IR64 is actually moderately tolerant of high temperature at flowering. It is also susceptible to Fe toxicity (Wu et al. 1997), anaerobic germination (Miro and Ismail 2013), and low temperature at the vegetative stage (Chawade et al. 2013). IR64 lacks the P deficiency gene PSTOL1 like a number of other high-yielding varieties (Gamuyao et al. 2012). IR64 was reported to be sensitive to low P conditions (Mori et al. 2016; Vejchasarn et al. 2016). It is also relatively sensitive to Zn deficiency (Impa et al. 2013). While IR64 does not possess the submergence tolerance allele of SUB1, it has some moderate tolerance to submergence during the vegetative stage (Singh et al. 2010; Singh et al. 2013).
Grain and market characteristics
IR64 grain has good physical appearance and is a typical long-grain variety with high head rice yield (IRRI 1986). As mentioned above, IR64 was the first IRRI variety to have both intermediate amylose content and intermediate GT. These traits are considered important for the ideal texture of cooked rice, especially for many rice consumers in South and Southeast Asia. However, these two traits alone do not account for the superior cooking quality of the variety, and methods to evaluate cooking quality are still inadequate aside from laborious sensory methods (Concepcion et al. 2015). This is why the use of taste panels was essential to identify the superior quality of IR64.
Rice texture is mostly controlled by the allele at the waxy locus (Wx) on chromosome 6. Among the major alleles at this locus, IR64 carries the Wxin allele at the waxy locus signifying intermediate amylose content (Zhang et al. 2012). However, various sequence features of the Wx gene are associated with grain quality of rice, including the number of CT repeats in the 5′ untranslated part of the gene and the SNPs at specific sites in intron 1, exon 6, and exon 10 (Bligh et al. 1998; Bligh et al. 1995; Larkin and Park 2003). Based on the DNA sequence, IR64 has the (CT)17 allele of Wx (Roferos et al. 2008; Teng et al. 2012). (CT)17 and (CT)20 are associated with intermediate amylose and soft to medium hardness (Roferos et al. 2008). The SNPs at the Wx locus are G-C-C for intron 1, exon 6 and exon 10, a common haplotype for intermediate amylose varieties (Chen et al. 2010).
Azucena and IR64 both have intermediate amylose and intermediate GT, and the doubled haploid population of the cross between the two was used to identify QTLs for several grain quality related traits, including starch properties measured by Rapid Visco Analyzer (RVA) (Bao et al. 2002). The study showed that there are genetic differences for these traits despite both varieties having similar amylose content and GT.
Part of the improved quality superiority is from improved flavor, including sweet and corn notes (Calingacion et al. 2015; Champagne et al. 2010). Reduced yellow color, improved texture and mouthfeel, and superior “sweet” taste were noted in IR64 vs. the lower-quality IRRI-132 (Champagne et al. 2010) (Table 1). Metabolomics analysis showed very different profiles for IR64 vs. the lower quality variety Apo (Calingacion et al. 2015).
Table 1.
Comparison of flavor attributes of high quality IR64 and low-quality Apo (Calingacion et al. 2015)
| Flavor | Apoa | IR64a | Apo | IR64 | ||
|---|---|---|---|---|---|---|
| Irrigated | Drought | Irrigated | Drought | |||
| Sweet taste | + | ++ | + | + | ++ | + |
| Corn | + | + | + | |||
| Sweet aromatic | + | + | + | |||
| Astringent | ++ | + | + | + | + | |
| Water like metallic | ++ | + | ++ | ++ | + | |
| Sewer/animal | ++ | ++ | ++ | |||
| Sour/silage | ++ | + | + | + | ||
| Hay-like musty | ++ | ++ | ++ | + | ||
a reported from a previous study (Champagne et al. 2010)
Calingacion et al. (2014) surveyed grain quality preferences in different countries and regions based on the most popular varieties in each area. However, two countries where IR64 dominated have different preferences (Indonesia and Philippines). These preferences can change over time.
Genetic and genomic characteristics
IR64 has been used extensively in genetic studies of rice, mainly because it represents a high-yielding and high-quality indica variety that is widely adapted to tropical lowland growing conditions. The most well-known mapping population was a doubled haploid population of about 146 lines derived from the cross IR64/Azucena (Guiderdoni et al. 1992), and first used by Huang et al. (1997) to map important agronomic traits and by Wu et al. (1997) to map tolerance to Fe toxicity. Other mapping populations with IR64 as a parent have been developed using both indica and japonica parents. A recombinant inbred line (RIL) population of 171 families was developed in a cross between IR64 and the wild relative O. rufipogon and tolerance to Al toxicity was mapped (Nguyen et al. 2003). Reciprocal chromosome segment substitution lines (CSSL) were also developed in IR64 and Koshihikari backgrounds to study the inheritance of grain shape (Nagata et al. 2015).
The original genome sequence of rice used the japonica variety Nipponbare (IRGSP 2005). In the first report of resequencing multiple varieties, IR64 was used among 20 diverse varieties, although this only included 100 Mb of the unique fraction of the genome (McNally et al. 2009). Schatz et al. (2014) reported whole genome de novo assembly for IR64 as well as the japonica variety Nipponbare and the aus variety DJ123. The genome coverage was 88.5% and included 37,758 genes. IR64 was estimated to have 381 genes not present in either of the other two varieties, and DJ123 and Nipponbare had 297 and 786 genes not found in the other varieties. Jain et al. (2014) also reported whole genome sequencing of IR64 along with Pokkali and N22, achieving 84.5% coverage. Methylation pattern has also been studied in IR64 and compared with a japonica variety Dianjingyou1 and two wild ancestors (Li et al. 2012). Gene expression and identification of functional roles of genes have been carried out with this model variety, for example drought responsive genes (Ray et al. 2011), gene expression changes during different stages of development (Sharma et al. 2012), and salinity tolerance (Wang et al. 2016).
Important progeny
The excellent grain quality of IR64 has become the standard for rice quality requirements in a number of countries. Because of its popularity with farmers, IR64 has been used widely as a parent in rice breeding, as a recipient of new genes through marker-assisted backcrossing and genetic transformation, and as a standard check for basic studies by many rice researchers. IR64 figured prominently in the mapping of many QTLs when genome-wide markers became available.
In the Philippines, IR64 was replaced by newer varieties in the early 2000s mainly due to its susceptibility to tungro disease. However, the breeders have attempted to retain the quality traits of IR64. PSB Rc82 is an example of a variety that became very popular, and IR64 is one of its grandparents. In India as well, the variety MTU 1010 became very popular, and it is a cross of Krishnaveni/IR64.
IR64 was a dominant variety in Indonesia for over two decades. In the last 10 years, it has been replaced by the variety Ciherang, which has very similar grain quality and improved yields. This variety is from the cross IR18349-53-1-3-1-3/IR19661-131-3-1//IR19661-131-3-1/IR64/IR64, and has high genetic similarity with IR64 (IRRI 2015; Septiningsih et al. 2014). It has very similar grain quality to IR64 and is also morphologically and genetically similar (Muhamad et al. 2017).
The availability of genome-wide molecular markers for marker assisted selection enabled the transfer of important traits into popular varieties like IR64 through marker-assisted backcrossing (MABC) (Collard and Mackill 2008). Early examples of this included varieties developed by pyramiding BB resistance genes into IR64, including Angke and Conde in Indonesia, and NSIC Rc142 in the Philippines (Verdier et al. 2012).
The rice submergence tolerance gene SUB1 was introduced by marker assisted backcrossing into IR64 and several other popular varieties (Septiningsih et al. 2009) (Fig. 4). Because IR64 had moderate tolerance to submergence, IR64-Sub1 tended to perform better under submergence than some of the other Sub1 lines, and its relatively early maturity allowed it to recover and produce yields after prolonged flooding and before the onset of low temperatures (Singh et al. 2010; Singh et al. 2013). IR64-Sub1 was released as Submarino 1 in the Philippines in 2009. The genetic similarity of Ciherang with IR64 was exploited to develop a submergence-tolerant version of Ciherang with only one backcross (Septiningsih et al. 2014). IR64-Sub1 was also used to develop submergence tolerant varieties in Vietnam (Lang et al. 2015).
Fig. 4.
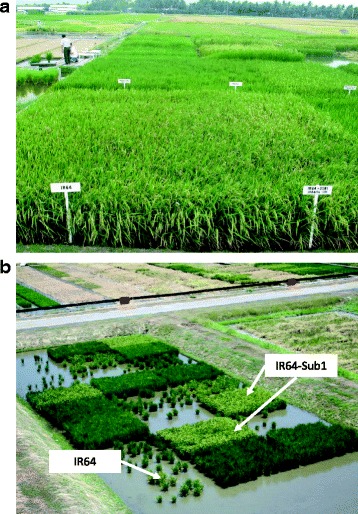
a IR64 and IR64-Sub1 under non-flooded conditions at IRRI. b Trials after submergence showing the survival of IR64-Sub1 compared to IR64. Fields were submerged for 17 days at 28 days after seeding. (Photos from IRRI)
Many interesting genes and QTLs have been backcrossed into IR64 and these progeny are being evaluated for useful traits (Table 2). One of the most important is drought tolerance. This trait was introduced by backcrossing from drought tolerance donor Aday Sel into IR64 (Venuprasad et al. 2011), and some of the derived lines showed yield increases of 528 to 1875 kg ha-1 over IR64 under severe drought conditions (Swamy et al. 2013). Breeding lines with two drought-tolerance QTLs (qDTY2.2 + qDTY4.1) introgressed into IR64 showed improved performance under drought stress. These lines had improved hydraulic conductivity and higher root length density (Henry et al. 2015). The gene DRO1, conferring deeper rooting and drought tolerance, was also transferred into IR64 by backcrossing, and resulted in NILs with higher drought tolerance (Uga et al. 2013). According to the authors, a single bp deletion in DRO1 resulted in a stop codon in IR64 and caused shallow rooting and drought intolerance, and this deletion was observed in IR64 and some of its progeny but not in its ancestors. The IR64 Dro1-NIL had a 14% higher yield than IR64 (Deshmukh et al. 2017). IR64-Sub1 is being used as a recipient for drought tolerance QTLs to develop a version of IR64 tolerant of both stresses (Singh et al. 2016).
Table 2.
Near Isogenic Lines (NILs) developed in IR64 genetic background with genes conferring novel and improved traits
| Trait/QTL | Comments | References |
|---|---|---|
| Submergence tolerance (SUB1) | The SUB1 major gene was introduced by Marker Assisted Backcrossing (MABC) into IR64 and released in several countries. | (Septiningsih et al. 2009) |
| Drought tolerance (DRO1) | Breeding line developed by MABC showed improved drought tolerance through deeper root system. | (Uga et al. 2013) |
| Drought tolerance (qDTY2.2 + qDTY4.1) | Lines derived by MABC showed improved yield under severe drought stress. | (Swamy et al. 2013) |
| SPIKE gene (NARROW LEAF1) | NIL with this gene showed 15–36% higher yield when introgressed into IR64. The gene increases spikelet number. | (Fujita et al. 2013). |
| Improved agronomic traits | 334 introgression lines developed in IR64 background using tropical japonica donors | (Farooq et al. 2010; Fujita et al. 2009; Kato et al. 2010; Tagle et al. 2016) |
| Anaerobic germination (AG1) | IR64-AG1 was developed by introgressing the AG1 QTL into IR64. | (Toledo et al. 2015) |
| Yield QTL identified from O. rufipogon | Some QTLs from low yielding wild rice O. rufipogon can increase yield in IR64 background. | (Cheema et al. 2008; Septiningsih et al. 2003) |
| Drought tolerance from O. glaberrima | A population of alien introgression lines using an accession of African rice O. glaberrima backcrossed to IR64 (BC2), and identified QTLs associated with drought-related traits. | (Bimpong et al. 2011) |
| Early-morning flowering (qEMF3) | NIL IR64 + qEMF3 with early morning flowering was developed using three backcrosses by marker assisted backcrossing and it flowered 1.5–2.0 h earlier in the day than IR64. In this case the donor was wild rice O. officinalis. This trait can confer tolerance to high temperature at anthesis. | (Hirabayashi et al. 2015) |
| Tolerance to P deficiency (Pup1) | Tolerance of P deficiency was introduced into IR64-Pup1, with the Pup1 gene for more efficient P uptake. | (Chin et al. 2011; Wissuwa et al. 2016) |
| Resistance to rice yellow mottle virus (RYMV) | Resistance to RYMV was introduced into IR64 background by marker assisted backcrossing. | (Ahmadi et al. 2001) |
IR64 is generally considered a restorer line for hybrid rice, particularly in the WA CMS system widely used in indica “three-line” hybrid breeding (Xie et al. 2014). However, Toriyama and Kazama (2016) developed a new IR64 cms termed CW type (Chinese wild rice), restored by the Rf17 gene.
At IRRI, induced mutation was used to generate a large collection of mutants in IR64. This collection has been used to discover many genes controlling important traits in rice (Wu et al. 2005). Some of these mutants show favorable phenotypes that could be used directly in rice breeding. Examples include tolerance to salinity (Nakhoda et al. 2012), resistance to blast disease (Madamba et al. 2009), resistance to tungro disease (Zenna et al. 2008), and drought tolerance (Cairns et al. 2009).
Transgenic applications have been limited for rice and are currently not in commercial cultivation. However, Agrobacterium mediated transformation protocols are well developed and used for IR64 (Ignacimuthu and Raveendar 2011). For example, it has been transformed with genes conferring higher Fe and Zn content in the endosperm (Oliva et al. 2014; Trijatmiko et al. 2016) and herbicide tolerance (Chhapekar et al. 2015).
Conclusions
This brief review attempts to document the value of the variety IR64 for rice breeding and genetics but cannot contain all the information available. At one time, this variety was estimated to be grown on 9–10 million ha annually (Laird and Kate 1999). Considering the many years it has been in production, over approximately two decades, it has been providing hundreds of millions of consumers with high-quality rice. In this sense it resembles some of the other mega varieties like Swarna and Samba Mahsuri, grown in India. However, breeders have been quick to take advantage of this variety to make further advances. As an example, IR64 has been replaced in most of the Philippines and Indonesia by new varieties with similar quality attributes but improved agronomic traits like disease resistance and higher grain yield.
Rice breeding is a continual process and all varieties are expected to be replaced by improved varieties over time. IR64 is still a popular variety in some areas, particularly in India where it is a popular variety in the north. Its area is gradually declining, due to release of improved varieties. However, as outlined here, it lives on through its progeny that are cultivated or under evaluation throughout the region. Some of the most important factors that enabled the development of such a superior variety include: a large breeding program where many crosses were made annually and large segregating populations were grown, well-defined objectives with focus on the most necessary traits including preferred quality attributes, systematic screening by skilled researchers for required traits, sensory data to confirm quality of the cooked rice, a suitable evaluation program to measure yield as early in the breeding program as possible, and an effective outreach effort to evaluate advanced selections under farmers’ conditions and ensure that seed was widely available.
The development of high-quality mega varieties like IR64 has provided a challenge to rice breeders to make further improvements to varieties that are widely-accepted by farmers. This challenge has been met well in the Philippines and Indonesia where new varieties have replaced IR64, but less well in India where the variety is still popular. In general, new varieties that aim to replace the mega varieties must offer a clear advantage, such as improved stress tolerance or higher yield. Additionally, the rice processing industry must be supportive of the efforts to replace existing varieties with new ones, so that farmers will have a suitable market for their crop produced from these new varieties.
Acknowledgments
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Abbreviations
- BB
Bacterial blight
- BPH
Brown Planthopper
- CSSL
Chromosome segment substitution library
- GEU
Genetic Evaluation and Utilization
- GLH
Green leafhopper
- GT
Gelatinization temperature
- HYV
High-yielding variety
- IRRI
International Rice Research Institute
- MABC
Marker assisted backcrossing
- RIL
Recombinant inbred population
- RTBV
Rice tungro bacilliform virus
- RTSV
Rice tungro spherical virus
- RVA
Rapid visco analyzer
- WBPH
White backed planthopper
Authors’ contributions
DJM and GSK conceived of the manuscript. DJM served as lead author and wrote most of the manuscript. GSK reviewed the entire manuscript and provided corrections and additional information. Both authors approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adhikari TB, Mew TW, Teng PS. Progress of bacterial blight on rice cultivars carrying different Xa genes for resistance in the field. Plant Dis. 1994;78:73–77. doi: 10.1094/PD-78-0073. [DOI] [Google Scholar]
- Ahmadi N, Albar L, Pressoir G, Pinel A, Fargette D, Ghesquiere A. Genetic basis and mapping of the resistance to Rice yellow mottle virus. III. Analysis of QTL efficiency in introgressed progenies confirmed the hypothesis of complementary epistasis between two resistance QTLs. Theor Appl Genet. 2001;103:1084–1092. doi: 10.1007/s001220100642. [DOI] [Google Scholar]
- Alam SN, Cohen MB. Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a doubled-haploid rice population. Theor Appl Genet. 1998;97:1370–1379. doi: 10.1007/s001220051031. [DOI] [Google Scholar]
- Anantha MS, Patel D, Quintana M, Swain P, Dwivedi JL, Torres RO, Verulkar SB, Variar M, Mandal NP, Kumar A, Henry A. Trait combinations that improve rice yield under drought: Sahbhagi Dhan and new drought-tolerant varieties in South Asia. Crop Sci. 2016;56:408–421. doi: 10.2135/cropsci2015.06.0344. [DOI] [Google Scholar]
- Ballini E, Berruyer R, Morel JB, Lebrun MH, Notteghem JL, Tharreau D. Modern elite rice varieties of the 'Green Revolution' have retained a large introgression from wild rice around the Pi33 rice blast resistance locus. New Phytol. 2007;175:340–350. doi: 10.1111/j.1469-8137.2007.02105.x. [DOI] [PubMed] [Google Scholar]
- Bao JS, Wu YR, Hu B, Wu P, Cui HR, Shu QY. QTL for rice grain quality based on a DH population derived from parents with similar apparent amylose content. Euphytica. 2002;128:317–324. doi: 10.1023/A:1021262926145. [DOI] [Google Scholar]
- Bastiaans L, Roumen EC. Effect on leaf photosynthetic rate by leaf blast for rice cultivars with different types and levels of resistance. Euphytica. 1993;66:81–87. doi: 10.1007/BF00023511. [DOI] [Google Scholar]
- Berruyer R, Adreit H, Milazzo J, Gaillard S, Berger A, Dioh W, Lebrun MH, Tharreau D (2003) Identification and fine mapping of Pi33, the rice resistance gene corresponding to the Magnaporthe grisea avirulence gene ACE1. Theor Appl Genet 107:1139–1147 [DOI] [PubMed]
- Bimpong IK, Serraj R, Chin JH, Ramos J, Mendoza EMT, Hernandez JE, Mendioro MS, Brar DS (2011) Identification of QTLs for drought-related traits in alien introgression lines derived from crosses of rice (Oryza sativa cv. IR64) x O. glaberrima under lowland moisture stress. J Plant Biol 54:237–250
- Bligh HFJ, Larkin PD, Roach PS, Jones CA, Fu HY, Park WD. Use of alternate splice sites in granule-bound starch synthase mRNA from low-amylose rice varieties. Plant Mol Biol. 1998;38:407–415. doi: 10.1023/A:1006021807799. [DOI] [PubMed] [Google Scholar]
- Bligh HFJ, Till RI, Jones CA. A microsatellite sequence closely linked to the waxy gene of Oryza sativa. Euphytica. 1995;86:83–85. doi: 10.1007/BF00022012. [DOI] [Google Scholar]
- Brennan JP, Malabayabas A (2011) International Rice Research Institute’s contribution to rice varietal yield improvement in South-East Asia. ACIAR impact assessment No. 74. Australian Centre for International Agricultural Research, Canberra
- Cada EC, Escuro PB. Rice varietal improvement in the Philippines. Los Baños: Rice breeding. International Rice Research Institute; 1972. pp. 161–166. [Google Scholar]
- Cairns JE, Acuna TLB, Simborio FA, Dimayuga G, Praba ML, Leung H, Torres R, Lafitte HR. Identification of deletion mutants with improved performance under water-limited environments in rice (Oryza sativa L.) Field Crop Res. 2009;114:159–168. doi: 10.1016/j.fcr.2009.07.019. [DOI] [Google Scholar]
- Calingacion M, Fang L, Quiatchon-Baeza L, Mumm R, Riedel A, Hall RD, Fitzgerald M. Delving deeper into technological innovations to understand differences in rice quality. Rice. 2015;8:1–10. doi: 10.1186/s12284-015-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calingacion M, Laborte A, Nelson A, Resurreccion A, Concepcion JC, Daygon VD, Mumm R, Reinke R, Dipti S, Bassinello PZ, Manful J, Sophany S, Lara KC, Bao JS, Xie LH, Loaiza K, El-hissewy A, Gayin J, Sharma N, Rajeswari S, Manonmani S, Rani NS, Kota S, Indrasari SD, Habibi F, Hosseini M, Tavasoli F, Suzuki K, Umemoto T, Boualaphanh C, Lee HH, Hung YP, Ramli A, Aung PP, Ahmad R, Wattoo JI, Bandonill E, Romero M, Brites CM, Hafeel R, Lur HS, Cheaupun K, Jongdee S, Blanco P, Bryant R, Lang NT, Hall RD, Fitzgerald M (2014) Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLOS ONE 9(1): e85106. 10.1371/journal.pone.0085106 [DOI] [PMC free article] [PubMed]
- Champagne ET, Bett-Garber KL, Fitzgerald MA, Grimm CC, Lea J, Ohtsubo K, Jongdee S, Xie LH, Bassinello PZ, Resurreccion A, Ahmad R, Habibi F, Reinke R (2010) Important sensory properties differentiating premium rice varieties. Rice 3:270–281
- Chawade A, Lindlof A, Olsson B, Olsson O (2013) Global expression profiling of low temperature induced genes in the chilling tolerant japonica rice Jumli Marshi. PLOS ONE 8(12): e81729. 10.1371/journal.pone.0081729 [DOI] [PMC free article] [PubMed]
- Cheema KK, Bains NS, Mangat GS, Das A, Vikal Y, Brar DS, Khush GS, Singh K (2008) Development of high yielding IR64 x Oryza rufipogon (Griff.) introgression lines and identification of introgressed alien chromosome segments using SSR markers. Euphytica 160:401–409
- Chen MH, Fjellstrom RG, Christensen EF, Bergman CJ (2010) Development of three allele-specific codominant rice Waxy gene PCR markers suitable for marker-assisted selection of amylose content and paste viscosity. Mol Breed 26:513–523
- Chhapekar S, Raghavendrarao S, Pavan G, Ramakrishna C, Singh VK, Phanindra MLV, Dhandapani G, Sreevathsa R, Kumar PA. Transgenic rice expressing a codon-modified synthetic CP4-EPSPS confers tolerance to broad-spectrum herbicide, glyphosate. Plant Cell Rep. 2015;34:721–731. doi: 10.1007/s00299-014-1732-2. [DOI] [PubMed] [Google Scholar]
- Chin JH, Gamuyao R, Dalid C, Bustamam M, Prasetiyono J, Moeljopawiro S, Wissuwa M, Heuer S (2011) Developing rice with high yield under phosphorus deficiency: Pup1 sequence to application. Plant Physiol 156:1202–1216 [DOI] [PMC free article] [PubMed]
- Coast O, Murdoch AJ, Ellis RH, Hay FR, Jagadish KSV. Resilience of rice (Oryza spp.) pollen germination and tube growth to temperature stress. Plant Cell Environ. 2016;39:26–37. doi: 10.1111/pce.12475. [DOI] [PubMed] [Google Scholar]
- Cohen MB, Alam SN, Medina EB, Bernal CC. Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: mechanism and role in successful N. lugens management in central Luzon, Philippines. Entomol Exp Appl. 1997;85:221–229. doi: 10.1046/j.1570-7458.1997.00252.x. [DOI] [Google Scholar]
- Collard BCY, Mackill DJ. Marker-assisted selection: an approach for precision plant breeding in the 21st century. Phil Trans Royal Soc B Rev. 2008;363:557–572. doi: 10.1098/rstb.2007.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion JCT, Ouk M, Zhao D, Fitzgerald MA. The need for new tools and investment to improve the accuracy of selecting for grain quality in rice. Field Crop Res. 2015;182:60–67. doi: 10.1016/j.fcr.2015.05.003. [DOI] [Google Scholar]
- Dalrymple DG (1978) Development and spread of high yielding varieties of wheat and rice in the less developed nations. USDA Foreign Agricultural Economic Report No. 95. U. S. Department of Agriculture, Washington DC.
- Deshmukh V, Kamoshita A, Norisada M, Uga Y. Near-isogenic lines of IR64 (Oryza sativa subsp indica cv.) introgressed with DEEPER ROOTING 1 and STELE TRANSVERSAL AREA 1 improve rice yield formation over the background parent across three water management regimes. Plant Prod Sci. 2017;20:249–261. doi: 10.1080/1343943X.2017.1305868. [DOI] [Google Scholar]
- Devries ME, Leffelaar PA, Sakane N, Bado BV, Giller KE (2011) Adaptability of irrigated rice to temperature change in Sahelian environments. Exp Agric 47:69–87
- Djedatin G, Ndjiondjop MN, Sanni A, Lorieux M, Verdier V, Ghesquiere A. Identification of novel major and minor QTLs associated with Xanthomonas oryzae pv. oryzae (African strains) resistance in rice (Oryza sativa L.) Rice. 2016;9:18. doi: 10.1186/s12284-016-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Tagle AG, Santos RE, Ebron LA, Fujita D, Kobayashi N. Quantitative trait loci mapping for leaf length and leaf width in rice cv. IR64 derived lines. J Integr Plant Biol. 2010;52:578–584. doi: 10.1111/j.1744-7909.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- Fujita D, Santos RE, Ebron LA, Telebanco-Yanoria MJ, Kato H, Kobayashi S, Uga Y, Araki E, Takai T, Tsunematsu H, Imbe T, Khush GS, Brar DS, Fukuta Y, Kobayashi N. Development of introgression lines of an Indica-type rice variety, IR64, for unique agronomic traits and detection of the responsible chromosomal regions. Field Crop Res. 2009;114:244–254. doi: 10.1016/j.fcr.2009.08.004. [DOI] [Google Scholar]
- Fujita D, Trijatmiko KR, Tagle AG, Sapasap MV, Koide Y, Sasaki K, Tsakirpaloglou N, Gannaban RB, Nishimura T, Yanagihara S, Fukuta Y, Koshiba T, Slamet-Loedin IH, Ishimaru T, Kobayashi N (2013) NAL1 allele from a rice landrace greatly increases yield in modern indica cultivars. Proc Natl Acad Sci U S A 110:20431-20436 [DOI] [PMC free article] [PubMed]
- Gamuyao R, Chin JH, Pariasca-Tanaka J, Pesaresi P, Catausan S, Dalid C, Slamet-Loedin I, Tecson-Mendoza EM, Wissuwa M, Heuer S. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature. 2012;488:535–539. doi: 10.1038/nature11346. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Schain N, Dreni L, Lawas LMF, Galbiati M, Colombo L, Heuer S, Jagadish KSV, Kater MM. Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol. 2016;57:57–68. doi: 10.1093/pcp/pcv174. [DOI] [PubMed] [Google Scholar]
- Gowda VRP, Henry A, Vadez V, Shashidhar HE, Serraj R (2012) Water uptake dynamics under progressive drought stress in diverse accessions of the OryzaSNP panel of rice (Oryza sativa). Funct Plant Biol 39:402–411 [DOI] [PubMed]
- Grand X, Espinoza R, Michel C, Cros S, Chalvon V, Jacobs J, Morel JB. Identification of positive and negative regulators of disease resistance to rice blast fungus using constitutive gene expression patterns. Plant Biotechnol J. 2012;10:840–850. doi: 10.1111/j.1467-7652.2012.00703.x. [DOI] [PubMed] [Google Scholar]
- Guiderdoni E, Galinato E, Luistro J, Vergara G (1992) Anther culture of tropical japonica x indica hybrids of rice (Oryza sativa L.). Euphytica 62:219–224
- Henry A, Gowda VRP, Torres RO, McNally KL, Serraj R (2011) Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crop Res 120:205–214
- Henry A, Swamy BPM, Dixit S, Torres RD, Batoto TC, Manalili M, Anantha MS, Mandal NP, Kumar A. Physiological mechanisms contributing to the QTL-combination effects on improved performance of IR64 rice NILs under drought. J Exp Bot. 2015;66:1787–1799. doi: 10.1093/jxb/eru506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi H, Sasaki K, Kambe T, Gannaban RB, Miras MA, Mendioro MS, Simon EV, Lumanglas PD, Fujita D, Takemoto-Kuno Y, Takeuchi Y, Kaji R, Kondo M, Kobayashi N, Ogawa T, Ando I, Jagadish KSV, Ishimaru T. qEMF3, a novel QTL for the early-morning flowering trait from wild rice, Oryza officinalis, to mitigate heat stress damage at flowering in rice, O. sativa. J Exp Bot. 2015;66:1227–1236. doi: 10.1093/jxb/eru474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Cao J, Zhang J, Xia F, Ke Y, Zhang H, Xie W, Liu H, Cui Y, Cao Y, Sun X, Xiao J, Li X, Zhang Q, Wang S (2017) Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nature Plants 3. 10.1038/nplants.2017.1039 [DOI] [PubMed]
- Huang N, Parco A, Mew T, Magpantay G, Mccouch S, Guiderdoni E, Xu JC, Subudhi P, Angeles ER, Khush GS. RFLP mapping of isozymes, RAPD and QTLs for grain shape, brown planthopper resistance in a doubled haploid rice population. Mol Breeding. 1997;3:105–113. doi: 10.1023/A:1009683603862. [DOI] [Google Scholar]
- Ignacimuthu S, Raveendar S (2011) Agrobacterium mediated transformation of indica rice (Oryza sativa L.) for insect resistance. Euphytica 179:277–286
- Impa SM, Morete MJ, Ismail AM, Schulin R, Johnson-Beebout SE (2013) Zn uptake, translocation and grain Zn loading in rice (Oryza sativa L.) genotypes selected for Zn deficiency tolerance and high grain Zn. J Exp Bot 64:2739–2751 [DOI] [PMC free article] [PubMed]
- IRGSP The map-based sequence of the rice genome. Nature. 2005;436:793–800. doi: 10.1038/nature03895. [DOI] [PubMed] [Google Scholar]
- IRRI . Annual report for 1985. Los Baños: International Rice Research Institute; 1986. [Google Scholar]
- IRRI . Growing rice, cultivating partnerships: 40 years of Indonesia-IRRI collaboration. Los Baños: Int. Rice Res. Inst; 2015. [Google Scholar]
- Jagadish SVK, Craufurd PQ, Wheeler TR. Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci. 2008;48:1140–1146. doi: 10.2135/cropsci2007.10.0559. [DOI] [Google Scholar]
- Jain M, Moharana KC, Shankar R, Kumari R, Garg R. Genomewide discovery of DNA polymorphisms in rice cultivars with contrasting drought and salinity stress response and their functional relevance. Plant Biotechnol J. 2014;12:253–264. doi: 10.1111/pbi.12133. [DOI] [PubMed] [Google Scholar]
- Julia C, Dingkuhn M. Predicting temperature induced sterility of rice spikelets requires simulation of crop-generated microclimate. Eur J Agron. 2013;49:50–60. doi: 10.1016/j.eja.2013.03.006. [DOI] [Google Scholar]
- Juliano BO, Perez CM, Maranan CL, Abansi CL, Duff B. Grain quality characteristics of rice in Philippine retail markets. Philipp Agriculturalist. 1989;72:113–122. [Google Scholar]
- Kato Y, Okami M, Tajima R, Fujita D, Kobayashi N. Root response to aerobic conditions in rice, estimated by Comair root length scanner and scanner-based image analysis. Field Crop Res. 2010;118:194–198. doi: 10.1016/j.fcr.2010.04.013. [DOI] [Google Scholar]
- Khush GS. Modern varieties - their real contribution to food supply and equity. GeoJournal. 1995;35:275–284. doi: 10.1007/BF00989135. [DOI] [Google Scholar]
- Khush GS. Green revolution: preparing for the 21st century. Genome. 1999;42:646–655. doi: 10.1139/g99-044. [DOI] [PubMed] [Google Scholar]
- Khush GS, Coffman WR. Genetic evaluation and utilization (GEU) program: the rice improvement program of the international rice research institute. Theor Appl Genet. 1977;51:97–110. doi: 10.1007/BF00273821. [DOI] [PubMed] [Google Scholar]
- Khush GS, Virk PS. IR varieties and their impact. Los Baños: Int. Rice Res. Inst; 2005. [Google Scholar]
- Kongprakhon P, Cuesta-Marcos A, Hayes PM, Hongtrakul V, Sirithunya P, Toojinda T, Sangduen N (2010) Four QTL in rice associated with broad spectrum resistance to blast isolates from rice and barley. J Phytopathol 158:125–131
- Laborte AG, Paguirigan NC, Moya PF, Nelson A, Sparks AH, Gregorio GB (2015) Farmers’ preference for rice traits: insights from farm surveys in central Luzon, Philippines, 1966-2012. PLOS ONE 10(8): e0136562. 10.1371/journal.pone.0136562 [DOI] [PMC free article] [PubMed]
- Laird SA, Kate K (1999) The commercial use of biodiversity : access to genetic resources and benefit-sharing. Earthscan, London.
- Lang NT, Phuoc NT, Ha PTT, Toan TB, Buu BC, Reinke R, Ismail AM, Wassmann R. Development of submergence tolerant breeding lines for Vietnam. SABRAO. J Genet Breed. 2015;47:448–459. [Google Scholar]
- Larkin PD, Park WD (2003) Association of waxy gene single nucleotide polymorphisms with starch characteristics in rice (Oryza sativa L.) Mol Breed 12:335–339
- Lee JH, Muhsin M, Atienza GA, Kwak DY, Kim SM, De Leon TB, Angeles ER, Coloquio E, Kondoh H, Satoh K, Cabunagan RC, Cabauatan PQ, Kikuchi S, Leung H, Choi IR (2010) Single nucleotide polymorphisms in a gene for translation initiation factor (eIF4G) of rice (Oryza sativa) associated with resistance to Rice tungro spherical virus. Mol Plant-Microbe Interact 23:29–38 [DOI] [PubMed]
- Lee S, Jia YL, Jia M, Gealy DR, Olsen KM, Caicedo AL (2011) Molecular evolution of the rice blast resistance gene Pi-ta in invasive weedy rice in the USA. PLOS ONE 6(10): e26260. 10.1371/journal.pone.0026260 [DOI] [PMC free article] [PubMed]
- Li X, Zhu JD, Hu FY, Ge S, Ye MZ, Xiang H, Zhang GJ, Zheng XM, Zhang HY, Zhang SL, Li Q, Luo RB, Yu C, Yu J, Sun JF, Zou XY, Cao XF, Xie XF, Wang J, Wang W (2012) Single-base resolution maps of cultivated and wild rice methylomes and regulatory roles of DNA methylation in plant gene expression. BMC Genomics 13:300. 10.1186/1471-2164-13-300 [DOI] [PMC free article] [PubMed]
- Madamba MRS, Sugiyama N, Bordeos A, Mauleon R, Satoh K, Baraoidan M, Kikuchi S, Shimamoto K, Leung H (2009) A recessive mutation in rice conferring non-race-specific resistance to bacterial blight and blast. Rice 2:104–114
- McNally KL, Childs KL, Bohnert R, Davidson RM, Zhao K, Ulat VJ, Zeller G, Clark RM, Hoen DR, Bureau TE, Stokowski R, Ballinger DG, Frazer KA, Cox DR, Padhukasahasram B, Bustamante CD, Weigel D, Mackill DJ, Bruskiewich RM, Ratsch G, Buell CR, Leung H, Leach JE. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc Natl Acad Sci U S A. 2009;106:12273–12278. doi: 10.1073/pnas.0900992106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro B, Ismail AM (2013) Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.) Front Plant Sci 4:269. 10.3389/fpls.2013.00269. [DOI] [PMC free article] [PubMed]
- Mori A, Fukuda T, Vejchasarn P, Nestler J, Pariasca-Tanaka J, Wissuwa M. The role of root size versus root efficiency in phosphorus acquisition in rice. J Exp Bot. 2016;67:1179–1189. doi: 10.1093/jxb/erv557. [DOI] [PubMed] [Google Scholar]
- Muhamad K, Ebana K, Fukuoka S, Okuno K. Genetic relationships among improved varieties of rice (Oryza sativa L.) in Indonesia over the last 60 years as revealed by morphological traits and DNA markers. Genet Resour Crop Evol. 2017;64:701–715. doi: 10.1007/s10722-016-0392-1. [DOI] [Google Scholar]
- Nagata K, Ando T, Nonoue Y, Mizubayashi T, Kitazawa N, Shomura A, Matsubara K, Ono N, Mizobuchi R, Shibaya T, Ogiso-Tanaka E, Hori K, Yano M, Fukuoka S. Advanced backcross QTL analysis reveals complicated genetic control of rice grain shape in a japonica x indica cross. Breed Sci. 2015;65:308–318. doi: 10.1270/jsbbs.65.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoda B, Leung H, Mendioro MS, Mohammadi-nejad G, Ismail AM (2012) Isolation, characterization, and field evaluation of rice (Oryza sativa L., Var. IR64) mutants with altered responses to salt stress. Field Crop Res 127:191–202
- Nguyen BD, Brar DS, Bui BC, Nguyen TV, Pham LN, Nguyen HT. Identification and mapping of the QTL for aluminum tolerance introgressed from the new source, Oryza rufipogon Griff., into indica rice (Oryza sativa L.) Theor Appl Genet. 2003;106:583–593. doi: 10.1007/s00122-002-1072-4. [DOI] [PubMed] [Google Scholar]
- Okami M, Kato Y, Kobayashi N, Yamagishi J (2015) Morphological traits associated with vegetative growth of rice (Oryza sativa L.) during the recovery phase after early-season drought. Eur J Agron 64:58–66
- Oliva N, Chadha-Mohanty P, Poletti S, Abrigo E, Atienza G, Torrizo L, Garcia R, Duenas C, Poncio MA, Balindong J, Manzanilla M, Montecillo F, Zaidem M, Barry G, Herve P, Shou HX, Slamet-Loedin IH. Large-scale production and evaluation of marker-free indica rice IR64 expressing phytoferritin genes. Mol Breed. 2014;33:23–37. doi: 10.1007/s11032-013-9931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Laza RC, Visperas RM, Sanico AL, Cassman KG, Khush GS. Grain yield of rice cultivars and lines developed in the Philippines since 1966. Crop Sci. 2000;40:307–314. doi: 10.2135/cropsci2000.402307x. [DOI] [Google Scholar]
- Ray S, Dansana PK, Giri J, Deveshwar P, Arora R, Agarwal P, Khurana JP, Kapoor S, Tyagi AK. Modulation of transcription factor and metabolic pathway genes in response to water-deficit stress in rice. Funct Integr Genomics. 2011;11:157–178. doi: 10.1007/s10142-010-0187-y. [DOI] [PubMed] [Google Scholar]
- Roferos LT, Butardo VM, Fitzgerald MA, Juliano BO (2008) Association between alleles of the waxy gene and traits of grain quality in Philippine Seed Board rice varieties. Philipp Agric Sci 91:334–337.
- Roumen EC. Effect of leaf age on components of partial resistance in rice to leaf blast. Euphytica. 1992;63:271–279. [Google Scholar]
- Sallaud C, Lorieux M, Roumen E, Tharreau D, Berruyer R, Svestasrani P, Garsmeur O, Ghesquiere A, Notteghem JL. Identification of five new blast resistance genes in the highly blast-resistant rice variety IR64 using a QTL mapping strategy. Theor Appl Genet. 2003;106:794–803. doi: 10.1007/s00122-002-1088-9. [DOI] [PubMed] [Google Scholar]
- Schatz MC, Maron LG, Stein JC, Wences AH, Gurtowski J, Biggers E, Lee H, Kramer M, Antoniou E, Ghiban E, Wright MH, Chia JM, Ware D, McCouch SR, McCombie WR (2014) Whole genome de novo assemblies of three divergent strains of rice, Oryza sativa, document novel gene space of aus and indica. Genome Biol 15:506. 10.1186/s13059-014-0506-z [DOI] [PMC free article] [PubMed]
- Septiningsih EM, Hidayatun N, Sanchez DL, Nugraha Y, Carandang J, Pamplona AM, Collard BCY, Ismail AM, Mackill DJ (2014) Accelerating the development of new submergence tolerant rice varieties: the case of Ciherang-Sub1 and PSB Rc18-Sub1. Euphytica 202:259–268
- Septiningsih EM, Pamplona AM, Sanchez DL, Neeraja CN, Vergara GV, Heuer S, Ismail AM, Mackill DJ. Development of submergence tolerant rice cultivars: the Sub1 locus and beyond. Ann Bot. 2009;103:151–160. doi: 10.1093/aob/mcn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Septiningsih EM, Prasetiyono J, Lubis E, Tai TH, Tjubaryat T, Moeljopawiro S, McCouch SR. Identification of quantitative trait loci for yield and yield components in an advanced backcross population derived from the Oryza sativa variety IR64 and the wild relative O. rufipogon. Theor Appl Genet. 2003;107:1419–1432. doi: 10.1007/s00122-003-1373-2. [DOI] [PubMed] [Google Scholar]
- Shanmugavadivel PS, Mithra SVA, Prakash C, Ramkumar MK, Tiwari R, Mohapatra T, Singh NK. High resolution mapping of QTLs for heat tolerance in rice using a 5K SNP array. Rice. 2017;10:28. doi: 10.1186/s12284-017-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Agarwal P, Ray S, Deveshwar P, Sharma P, Sharma N, Nijhawan A, Jain M, Singh AK, Singh VP, Khurana JP, Tyagi AK, Kapoor S (2012) Expression dynamics of metabolic and regulatory components across stages of panicle and seed development in indica rice. Funct Integr Genomics 12:229–248 [DOI] [PubMed]
- Shrestha R, Al-Shugeairy Z, Al-Ogaidi F, Munasinghe M, Radermacher M, Vandenhirtz J, Price AH. Comparing simple root phenotyping methods on a core set of rice genotypes. Plant Biol. 2014;16:632–642. doi: 10.1111/plb.12096. [DOI] [PubMed] [Google Scholar]
- Singh N, Dang TTM, Vergara GV, Pandey DM, Sanchez D, Neeraja CN, Septiningsih EM, Mendioro M, Tecson-Mendoza EM, Ismail AM, Mackill DJ, Heuer S. Molecular marker survey and expression analyses of the rice submergence-tolerance gene SUB1A. Theor Appl Genet. 2010;121:1441–1453. doi: 10.1007/s00122-010-1400-z. [DOI] [PubMed] [Google Scholar]
- Singh R, Singh Y, Xalaxo S, Verulkar S, Yadav N, Singh S, Singh N, Prasad KSN, Kondayya K, Rao PVR, Rani MG, Anuradha T, Suraynarayana Y, Sharma PC, Krishnamurthy SL, Sharma SK, Dwivedi JL, Singh AK, Singh PK, Nilanjay, Singh NK, Kumar R, Chetia SK, Ahmad T, Rai M, Perraju P, Pande A, Singh DN, Mandal NP, Reddy JN, Singh ON, Katara JL, Marandi B, Swain P, Sarkar RK, Singh DP, Mohapatra T, Padmawathi G, Ram T, Kathiresan RM, Paramsivam K, Nadarajan S, Thirumeni S, Nagarajan M, Singh AK, Vikram P, Kumar A, Septiningshih E, Singh US, Ismail AM, Mackill D, Singh NK. From QTL to variety-harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Sci. 2016;242:278–287. doi: 10.1016/j.plantsci.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Singh US, Dar MH, Singh S, Zaidi NW, Bari MA, Mackill DJ, Collard BCY, Singh VN, Singh JP, Reddy JN, Singh RK, Ismail AM. Field performance, dissemination, impact and tracking of submergence tolerant (Sub1) rice varieties in South Aisa. SABRAO J Breed Genet. 2013;45:112–131. [Google Scholar]
- Sreewongchai T, Toojinda T, Thanintorn N, Kosawang C, Vanavichit A, Tharreau D, Sirithunya P. Development of elite indica rice lines with wide spectrum of resistance to Thai blast isolates by pyramiding multiple resistance QTLs. Plant Breed. 2010;129:176–180. doi: 10.1111/j.1439-0523.2009.01669.x. [DOI] [Google Scholar]
- Swamy BPM, Ahmed HU, Henry A, Mauleon R, Dixit S, Vikram P, Tilatto R, Verulkar SB, Perraju P, Mandal NP, Variar M, Robin S, Chandrababu R, Singh ON, Dwivedi JL, Das SP, Mishra KK, Yadaw RB, Aditya TL, Karmakar B, Satoh K, Moumeni A, Kikuchi S, Leung H, Kumar A (2013) Genetic, physiological, and gene expression analyses reveal that multiple QTL enhance yield of rice mega-variety IR64 under drought. PLOS ONE 8(5): e62795. 10.1371/journal.pone.0062795 [DOI] [PMC free article] [PubMed]
- Tagle AG, Fujita D, Ebron LA, Telebanco-Yanoria MJ, Sasaki K, Ishimaru T, Fukuta Y, Kobayashi N. Characterization of QTL for unique agronomic traits of new-plant-type rice varieties using introgression lines of IR64. Crop J. 2016;4:12–20. doi: 10.1016/j.cj.2015.10.001. [DOI] [Google Scholar]
- Teng B, Zeng RZ, Wang YC, Liu ZQ, Zhang ZM, Zhu HT, Ding XH, Li WT, Zhang GQ (2012) Detection of allelic variation at the Wx locus with single-segment substitution lines in rice (Oryza sativa L.) Mol Breed 30:583–595
- Thakur S, Singh PK, Rathour R, Variar M, Prashanthi SK, Singh AK, Singh UD, Chand D, Singh NK, Sharma TR (2013) Positive selection pressure on rice blast resistance allele Piz-t makes it divergent in Indian land races. J Plant Interact 8:34–44
- Toledo AMU, Ignacio JCI, Casal Jr C, Gonzaga ZJ, Mendioro MS, Septiningsih EM. Development of improved Ciherang-Sub1 having tolerance to anaerobic germination conditions. Plant Breed Biotech. 2015;3:77–87. doi: 10.9787/PBB.2015.3.2.077. [DOI] [Google Scholar]
- Toriyama K, Kazama T. Development of cytoplasmic male sterile IR24 and IR64 using CW-CMS/Rf17 system. Rice. 2016;9:22. doi: 10.1186/s12284-016-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trijatmiko KR, Dueñas C, Tsakirpaloglou N, Torrizo L, Arines FM, Adeva C, Balindong J, Oliva N, Sapasap MV, Borrero J, Rey J, Francisco P, Nelson A, Nakanishi H, Lombi E, Tako E, Glahn RP, Stangoulis J, Chadha-Mohanty P, Johnson AAT, Tohme J, Barry G, Slamet-Loedin IH. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci Rep. 2016;6:19792. doi: 10.1038/srep19792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H, Takehisa H, Motoyama R, Nagamura Y, Wu J, Matsumoto T, Takai T, Okuno K, Yano M (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45:1097–1102 [DOI] [PubMed]
- Ujiie K, Yamamoto T, Yano M, Ishimaru K. Genetic factors determining varietal differences in characters affecting yield between two rice (Oryza sativa L.) varieties, Koshihikari and IR64. Genet Resour Crop Evol. 2016;63:97–123. doi: 10.1007/s10722-015-0237-3. [DOI] [Google Scholar]
- Vejchasarn P, Lynch JP, Brown KM. Genetic variability in phosphorus responses of rice root phenotypes. Rice. 2016;9:29. doi: 10.1186/s12284-016-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuprasad R, Impa SM, Gowda RPV, Atlin GN, Serraj R. Rice near-isogenic-lines (NILs) contrasting for grain yield under lowland drought stress. Field Crop Res. 2011;123:38–46. doi: 10.1016/j.fcr.2011.04.009. [DOI] [Google Scholar]
- Verdier V, Cruz CV, Leach JE. Controlling rice bacterial blight in Africa: needs and prospects. J Biotechnol. 2012;159:320–328. doi: 10.1016/j.jbiotec.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Vikram P, Swamy BP, Dixit S, Singh R, Singh BP, Miro B, Kohli A, Henry A, Singh NK, Kumar A. Drought susceptibility of modern rice varieties: an effect of linkage of drought tolerance with undesirable traits. Sci Rep. 2015;5:14799. doi: 10.1038/srep14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W-S, Zhao X-Q, Li M, Huang L-Y, Xu J-L, Zhang F, Cui Y-R, Fu B-Y, Li Z-K. Complex molecular mechanisms underlying seedling salt tolerance in rice revealed by comparative transcriptome and metabolomic profiling. J Exp Bot. 2016;67:405–419. doi: 10.1093/jxb/erv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei FJ, Tsai YC, Wu HP, Huang LT, Chen YC, Chen YF, Wu CC, Tseng YT, Hsing YIC. Both Hd1 and Ehd1 are important for artificial selection of flowering time in cultivated rice. Plant Sci. 2016;242:187–194. doi: 10.1016/j.plantsci.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Wissuwa M, Kretzschmar T, Rose TJ. From promise to application: root traits for enhanced nutrient capture in rice breeding. J Exp Bot. 2016;67:3605–3615. doi: 10.1093/jxb/erw061. [DOI] [PubMed] [Google Scholar]
- Wu JL, Wu C, Lei C, Baraoidan M, Bordeos A, Madamba MR, Ramos-Pamplona M, Mauleon R, Portugal A, Ulat VJ, Bruskiewich R, Wang G, Leach J, Khush G, Leung H. Chemical- and irradiation-induced mutants of indica rice IR64 for forward and reverse genetics. Plant Mol Biol. 2005;59:85–97. doi: 10.1007/s11103-004-5112-0. [DOI] [PubMed] [Google Scholar]
- Wu P, Luo A, Zhu J, Yang J, Huang N, Senadhira D. Molecular markers linked to genes underlying seedling tolerance for ferrous iron toxicity. Plant Soil. 1997;196:317–320. doi: 10.1023/A:1004288427140. [DOI] [Google Scholar]
- Xie F, He Z, Esguerra M, Qiu F, Ramanathan V (2014) Determination of heterotic groups for tropical Indica hybrid rice germplasm. Theor Appl Genet 127:407–417 [DOI] [PubMed]
- Ye C, Tenorio F, Redoña E, Morales-Cortezano P, Cabrega G, Jagadish KV, Gregorio G. Fine-mapping and validating qHTSF4.1 to increase spikelet fertility under heat stress at flowering in rice. Theor Appl Genet. 2015;128:1507–1517. doi: 10.1007/s00122-015-2526-9. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Rice crop science. Los Baños, Philippines: Int. Rice Res. Inst; 1981. [Google Scholar]
- Zenna NS, Cabauatan PQ, Baraoidan M, Leung H, Choi IR. Characterization of a putative rice mutant for reaction to rice tungro disease. Crop Sci. 2008;48:480–486. doi: 10.2135/cropsci2007.03.0127. [DOI] [Google Scholar]
- Zenna NS, Cruz FCS, Javier EL, Duka IA, Barrion AA, Azzam O (2006) Genetic analysis of tolerance to rice tungro bacilliform virus in rice (Oryza sativa L.) through agroinoculation. J Phytopathol 154:197–203
- Zhang ZJ, Li M, Fang YW, Liu FC, Lu Y, Meng QC, Jun CC, Yi XH, Gu MH, Yan CJ (2012) Diversification of the waxy gene is closely related to variations in rice eating and cooking quality. Plant Mol Biol Rep 30:462–469
- Zhao DL, Atlin GN, Amante M, Cruz MTS, Kumar A (2010) Developing aerobic rice cultivars for water-short irrigated and drought-prone rainfed areas in the tropics. Crop Sci 50:2268–2276
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


