Abstract
Lung cancer is a common disease and the leading cause of cancer-related mortality, with non-small cell lung cancer (NSCLC) accounting for the majority of cases. Following diagnosis of lung cancer, accurate staging is essential to guide clinical management and inform prognosis. Positron emission tomography (PET) in conjunction with computed tomography (CT)—as PET-CT has developed as an important tool in the multi-disciplinary management of lung cancer. This article will review the current evidence for the role of 18F-fluorodeoxyglucose (FDG) PET-CT in NSCLC diagnosis, staging, response assessment and follow up.
Keywords: Positron emission tomography (PET), non-small cell lung cancer (NSCLC), diagnosis, staging, response assessment, follow up
Introduction
Lung cancer remains the leading cause of cancer-related mortality in both men and women. Non-small cell lung cancer (NSCLC) accounts for 75–80% of cases (1). In the United Kingdom, 5-year survival has been reported to be just 8% (2). Patients suspected or diagnosed with lung cancer are managed by a multidisciplinary team whose role is to accurately diagnose, stage and then treat patients. Traditional radiological imaging techniques such as chest radiography, ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI), play an essential role in staging, which ultimately determines the management options available to a patient. However, the development of positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG), a radiolabelled glucose analogue, has significantly impacted on the contribution of radiology to the management of this disease. This is routinely performed as a hybrid technique with PET-CT, which combines anatomical localisation and morphological information from CT with functional data provided by PET. Malignant tumours are often highly metabolically active, with increased glucose metabolism and consequently FDG uptake, and can be detected as ‘hot spots’ with higher standardised uptake values (SUV). This is not always the case and often small tumours or those of the ‘bronchoalveolar’ type have lower SUV. A meta-analysis concluded that FDG-PET can diagnose malignant pulmonary lesions with an estimated sensitivity of 94.2% and specificity of 83.3% (3). Combining the FDG-PET scan with simultaneous CT scan further increases its accuracy by avoiding the technical challenges of interpreting the two scans independently. Subsequently PET-CT has become a standard of care (Figure 1) (4-6). The success of PET-CT has resulted in a search for similar radiolabelled ligands to be utilised in conjunction with other radiological modalities such as MRI, and this is an important area of research.
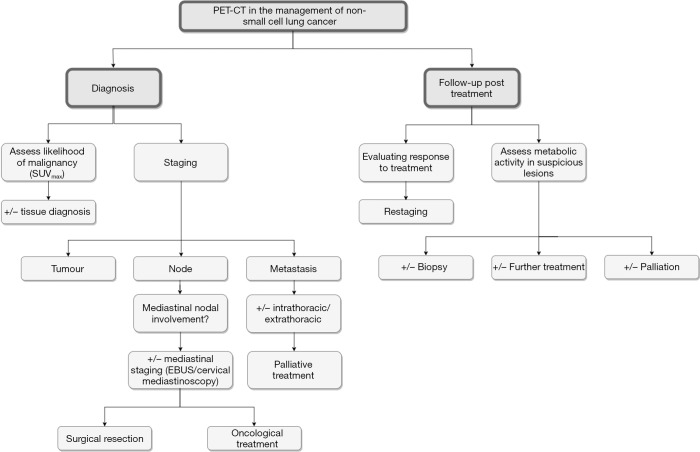
Figure 1.
The roles of PET-CT in the management of non-small cell lung carcinoma. PET-CT, positron emission tomography-computed tomography.
Role of PET-CT in the diagnosis and evaluation of lung lesions
Small pulmonary nodules are frequently identified as an incidental finding on imaging performed for other reasons. It has been reported that they are identified on up to 0.2% of chest radiographs (7) and around 1% of thoracic CT scans (8). Although the majority of solitary pulmonary nodules are benign lesions such as a granuloma or hamartoma, in up to 20% of cases they represent a malignant tumour, especially in older patients and smokers (8). The incidence of malignancy in these higher risk groups can approach 70% (9).
After a nodule has been identified, patients are referred and managed by a multidisciplinary team to investigate the nature of the nodule. For suspicious lesions, PET-CT has an important role in aiding differentiation between benign and malignant lesions, with metabolically active lesions more likely to represent malignancy. PET-CT is relied upon to guide decision making in regard to proceeding to obtain a tissue diagnosis. A large recent retrospective analysis reported PET-CT to have a diagnostic accuracy of 93.5% in diagnosing malignant pulmonary nodules, and a false positive rate of 6.5% (10). Interestingly, there is some data suggesting that the degree of FDG uptake on PET-CT can differentiate between different tumour histology. Data has been presented, for example, that demonstrated that squamous cell carcinomas tend to have significantly higher FDG uptake than bronchioloalveolar carcinoma, adenocarcinoma in situ and carcinoid tumours (11,12). In these tumour types false-negative PET-CT studies can occur. This is also the case for sub-centimeter pulmonary nodules (13). On the other hand, false-positive results can be observed in metabolically active, often inflammatory, benign lesions such as bacterial pneumonia, pyogenic abscesses, tuberculosis, sarcoidosis, infective granulomas and fibrosing mediastinitis (10)
Subsequently, if suggestive of malignancy on PET-CT, a tissue diagnosis is sought in order to appropriately manage the patient. A tissue diagnosis can be obtained through multiple routes depending on the location of the tumour. Peripheral lesions are routinely sampled with CT-guided biopsy which is now well established as a diagnostic technique for pulmonary nodules, with a diagnostic accuracy of up to 98% (14,15), although occasionally can be complicated by pneumothorax. Central lesions may be more amenable to bronchoscopic techniques including endobronchial ultrasound (EBUS) guided biopsy. The detection of metastases, mediastinal or more distally, on the PET-CT can direct an alternative route to obtaining tissue for pathological diagnosis. For example, an accessible mediastinal or supraclavicular lymph node may be deemed a more appropriate biopsy target, with lower risk, than CT-guided biopsy of the main lesion—particularly for patients with more advanced tumours in which a tissue diagnosis is required to determine the appropriate oncological therapy.
Role of PET-CT in the staging of lung carcinoma
Following diagnosis of a lung cancer, the disease must be staged which is an assessment of tumour size, tumour location, involvement of mediastinal lymph nodes and the presence of metastases. Since the patients prognosis and therefore management is determined by disease staging, it is essential that this can be performed reliably and accurately (16). Staging requires the combination of imaging modalities to identify the extent of tumour progression and the ability to obtain biopsies of suspicious pulmonary nodules, lymph nodes and/or potential metastases.
The TNM staging system is the standard staging system used worldwide for lung carcinoma (17). Recently the 8th edition was published although it is not yet in routine use (18). The system assesses the tumour (T), lymph nodes (N) and metastases (M). The tumour is assessed based for its size, location and spread beyond the visceral pleura; the presence of enlarged or abnormal lymph nodes within the lung, hilum and mediastinum is quantified; and the presence of intrathoracic or extrathoracic metastatic involvement is assessed. The stage of disease determines the optimum management (19-21). For early, stage I, disease surgical resection by lobectomy or pneumonectomy with curative intent is the gold standard currently. Patients with stage II disease with a good performance status will usually be offered surgical resection, followed by adjuvant chemotherapy to reduce the risk of tumour recurrence (22). Stage IIIA disease can be treated with tri-modality therapy: combined chemotherapy and radiotherapy, followed by surgical resection for patients who are ‘down-staged’ following the oncological therapy and have a good performance status. Patients with stage IIIB disease, are usually treated with a combination of chemotherapy and radiotherapy, with surgery having little role. Patients with more advanced, stage IV disease, will be offered a combination of systemic chemotherapy and/or radiotherapy depending on their performance status, and palliative radiotherapy for symptom relief (23). PET-CT is now a standard staging investigation in patients with lung carcinoma (19-21) and can aid detection of nodal and distant metastases.
Nodal staging
Lymph node staging is an important step in determining whether the patient is offered surgical resection and therefore, considerable effort is made to evaluate mediastinal lymph nodes (24,25). PET-CT has a significantly greater accuracy than conventional CT imaging which relies on lymph node size alone (26). PET-CT has been reported to have an accuracy of 90% for correctly diagnosing the presence or absence of mediastinal lymph node involvement, with a sensitivity of 79–85% and specificity of 87–92%. This compares very favourably to the values reported for CT alone—an accuracy of 75%, sensitivity of 57–68% and a specificity of 76–82% (Figure 2) (27-30). However, it is important to be aware that there is reduced accuracy of PET-CT detecting malignancy in small nodes <10–15 mm diameter (31). As such, occult nodal metastases are often detected by post-operative histopathology. This is termed stage migration (32). One recent study reported a 25.9% rate of occult nodal metastases (30). The authors identified that combining tumour size with SUVmax offered some predictive ability—for tumours >2.5 cm with SUVmax >4.35, there was an 88.9% chance of detecting occult lymph node metastases.
Figure 2.
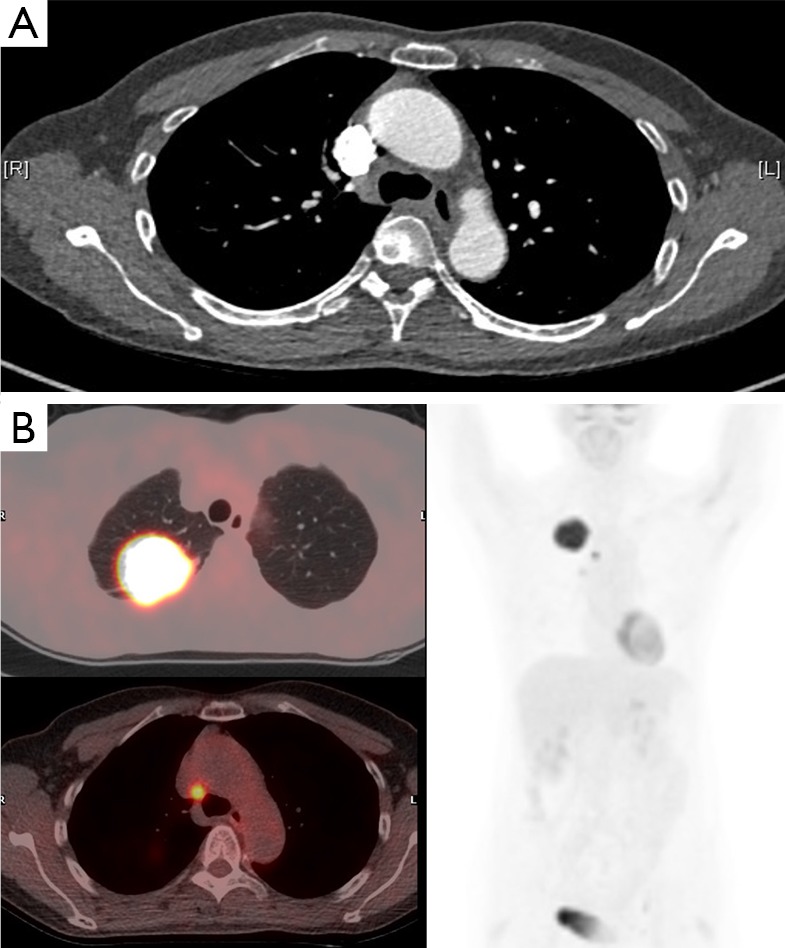
The value of PET-CT to staging. Patient with non-small cell lung carcinoma. The tumour in the right upper lobe shows avid tracer uptake (SUVmax =19.4). There is a 5 mm station 4R node adjacent to the azygos vein and superior vena cava on the staging CT scan. This shows tracer uptake on the PET-CT (SUVmax =9.7) and was shown to contain tumour cells on endo-bronchial ultrasound guided sampling. If PET-CT had not been performed this N2 node would have been missed. PET-CT, positron emission tomography-computed tomography.
In view of the diagnostic accuracy recorded with PET-CT, when there is suggestion of malignant involvement of mediastinal lymph nodes, further assessment is necessary (33). If the PET-CT scan is positive for mediastinal or hilar nodes, the lymph node status needs histological confirmation in order to accurately stage the patient (34), whereas patients with negative mediastinal nodes on PET-CT examination can generally proceed to surgical resection without the need for invasive mediastinal staging (35). Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) are becoming the investigations of choice for obtaining tissue for mediastinal staging and have been demonstrated to be both clinically and cost effective when compared to surgical staging in a randomised controlled trial (36-38). It is becoming increasingly appreciated that combining PET-CT and EBUS-TBNA can significantly increase the diagnostic accuracy of nodal staging (39).
For mediastinal lymph nodes that are suspicious based on size-criteria on the CT, but negative on PET-CT, further staging with histological examination should be sought by the multidisciplinary team to ensure surgical resection is appropriate. Conversely, patients with mediastinal nodes positive on PET should not be denied potentially curative surgery without histological confirmation, with knowledge that positivity can be due to other factors such as infection or inflammation.
Distant metastases
Forty percent of patients with NSCLC are reported to present with metastatic disease. The commonest sites of NSCLC metastasis include the adrenal glands, bones, liver, or brain (40). PET-CT is more accurate than conventional CT at diagnosing the presence of metastases. It has been reported that up to 10% of patients are found to have metastases on PET-CT that were not detected on prior CT, and therefore offers an important additional benefit to patient staging (26,41).
In up to 20% of patients with NSCLC, adrenal tumours are identified on CT. Incidental adrenal adenomas are not uncommon in the population though (25). The ability of PET-CT to differentiate metabolically active, potentially metastatic, lesions from metabolically quiescent lesions can be very useful, although caution should be exercised when interpreting PET-CT for small adrenal nodules, as the false-positive and false-negative rates will be higher (42). One study reported accuracy of 95% in diagnosing adrenal metastasis with PET-CT. They reported a positive predictive value of 95% and a negative predictive value of 94% (43).
FDG-PET can also aid the diagnosis of bone metastases which are seen as ‘hot spots’ within the bone.
The more traditional approach was with bone scintigraphy. Reports describe sensitivity in the range 90%, but specificity as low as 60%. This is due to uptake of the radio-nucleotide tracer in areas of inflammation and degeneration that may be associated with arthritic changes or post-trauma (44). PET-CT is found to be superior to bone scintigraphy for detection of bone metastases with accuracy quoted to be as high as 96%, with sensitivity (90%) and a specificity (98%) (44).
FDG-PET offers superiority in the diagnosis of liver metastases when compared with conventional imaging modalities. Uptake of FGD is highly suspicious of malignancy (45). In contrast, FDG-PET detects brain metastases with a sensitivity of lower than 46% due to the high levels of glucose uptake within normal brain tissue (46). Conventional CT or MRI is considered the investigation of choice for accurately diagnosing the presence of brain metastases.
The added benefit of FDG-PET can be appreciated by comparing the staging pre and post PET-CT. It has been observed that in up to 62% of cases the TNM stage is modified following a PET-CT scan (Table 1) (42). This is obviously of crucial importance as management decisions are determined by the final stage of disease and as such, a PET-CT can prevent patients from being subjected to unnecessary further investigations and surgical procedures that will offer them no prognostic benefit (54,55).
Table1. Impact of PET-CT on patient staging and management in non-small cell lung carcinoma (NSCLC).
| Study | N of patients | Change of stage (%) | Impact on management (%) |
|---|---|---|---|
| Lewis et al. (47) | 34 | – | 41 |
| Bury et al. (48) | 109 | 34 | 25 |
| Saunders et al. (49) | 97 | 27 | 37 |
| Pieterman et al. (50) | 102 | 62 | – |
| Hicks et al. (51) | 153 | 43 | 35 |
| Hoekstra et al. (52) | 57 | 30 | 19 |
| Schmucking et al. (53) | 63 | – | 52 |
| Zhou et al. (30) | 54 | 25.9 | – |
| Takeuchi et al. (54) | 592 | 28.7 | 37.2 |
| Taus et al. (55) | 246 | 35 | – |
| Zheng et al. (56) | 23 | 35 | – |
| Kirmani et al. (32) | 312 | 25.3 | – |
PET-CT, positron emission tomography-computed tomography.
The value of PET-CT in the staging of lung cancer has been reinforced in international guidelines—all of which emphasise the importance of having rapid access to PET-CT (19-21,57) due to its ability to facilitate accurate disease staging which is important to guide management decisions and allow prognosis to be predicted.
Role of PET-CT in evaluating response to treatment
PET-CT scans are not necessarily just performed as a one-off test and can be used to track disease over time—particularly to assess the impact of oncological therapies—i.e., chemotherapy and radiotherapy (56,58). FDG-PET offers benefit over conventional CT where although tumour shrinkage may be observed, radiation-induced inflammation and fibrosis after neoadjuvant chemotherapy or radiation therapy can make assessment difficult (11). On conventional CT imaging identifying a response to treatment is simply based on a reduction in size and volume of the tumour—however, this does not necessarily correlate with clinical outcomes. PET-CT offers the opportunity to assess the level of FDG uptake and therefore tumour activity which may be a better marker. Decreased FDG uptake as detected on PET-CT is found to correlate with improved outcomes and is a marker of effective responsiveness to the chemotherapy (59). Conversely therefore, if there is no change in activity level, this could direct a change in chemotherapeutic approach. It has been reported that evidence of high FDG uptake following the first chemotherapy cycle correlates with a poorer prognosis than patients with low FDG uptake, with median survival of 12 months compared with 34 months (41,60).
Role of PET-CT in disease surveillance
In addition to measuring the impact of therapy, PET-CT can also play an important role in the diagnosis of disease recurrence following an abnormality being detected on conventional imaging (61). The role of PET-CT in long-term follow up is not well described, and there are many studies which do not recommend PET-CT for the routine surveillance of patients following treatment for NSCLC (62). However, on conventional imaging, pathology such as atelectasis, consolidation and radiation fibrosis can easily be confused with disease recurrence, whilst different FDG uptake on PET-CT can facilitate this differentiation (11). Consequently, a PET-CT scan can be useful for identifying disease recurrence in patients with suspicious lesions on conventional imaging with a sensitivity reported to be 98%, specificity of 82% and overall accuracy of 93%. Moreover, a negative PET-CT at follow up is highly predictive of improved survival (49,60), even after adjustment for the therapy given, whereas conventional staging offers only modest prognostic stratification (42). While these benefits are recognised, PET-CT is not utilised as a routine first-line follow-up surveillance investigation, in current guidelines, but can be utilised if there is suspicion of tumour recurrence or metastatic disease detected on standard CT (19-21,57,63). The high cost is likely an important factor in this decision. There is some evidence correlating pre-operative tumour SUVmax with risk of recurrence and death in patients with NSCLC (41) and it may be that with greater understanding, PET-CT will play a greater role in follow-up of higher risk patients following curative therapy.
Limitations of PET-CT
Although the evidence reported above demonstrates PET-CT to be an important and accurate tool in the management of NSCLC, it is important to acknowledge the limitations of this imaging modality. One of them is the misregistration between the PET and CT components due to changes in the patient’s position during the imaging. It is commonly related to recording in different phase of the patient’s respiratory cycle, and some works are examining the potential benefit of respiratory gating of scans (64). Another point to consider is that several benign lesions, such as infection, inflammation and infarction, also have increased glucose metabolism and can therefore show as a hotspot on the PET-CT image and be potentially misinterpreted (17). In addition, the brain, heart, gastrointestinal tract, genitourinary tract, and skeletal muscles can all show increased FDG uptake on PET imaging, and may mask a malignant lesion or give false positive results, resulting in additional unnecessary investigation and patient stress. Finally, there are some technological limitations of PET-CT: small metastatic lesions (<1 cm) may appear ‘cold’ on the PET-CT image due to the resolution of the scanner. Lesions containing only micro metastases, low metabolic neoplasms, and highly differentiated tumours can all be misinterpreted as being benign (16). Furthermore, PET-CT is also more costly than alternative scanning modalities and this must be considered in the current financial climate (65,66).
Future development and prospects
PET-CT is now an established radiological modality widely used in the management of NSCLC. Novel radio ligands are being developed in an attempt to broaden the utility of PET imaging by improving the diagnostic accuracy for malignancy and predicting response to specific cancer therapies (67). For example, 11C-methionine has been demonstrated to be more specific and sensitive than 18F-FDG in differentiating benign and malignant thoracic nodules. Another ligand, 18F-fluorothymidine, which is a marker of cellular proliferation, is thought to be even more sensitive than 18F-FDG in evaluating treatment response. Other ligands are being developed to examine other specific aspects of tumour biology—for example the analysis of epidermal growth factor receptor (EGFR) expression, which it is hoped will provide an insight into tumour behaviour and sensitivity to specific chemotherapeutic agents, thereby facilitating individualised treatment regimens (68-70). Its use with other imaging such as MRI is also under development.
Conclusions
FDG-PET-CT currently plays an important and central role in the multidisciplinary management of lung cancer. PET-CT combines metabolic and morphologic data allowing it to offer increased ability to diagnose malignant lung nodules when compared to conventional CT imaging. Integrated PET-CT combines the benefit of PET and CT, whilst minimizing their limitations in the diagnosis, staging and treatment of NSCLC. PET-CT offers a superior assessment for lymph nodal involvement and for the presence of local or distant metastatic disease than can be achieved on conventional imaging alone, and is often used to interpret equivocal lesions identified on such imaging modalities. PET-CT scans can also offer predictive and prognostic information after both neoadjuvant and definitive therapy. Increasingly, the value of PET-CT in disease surveillance following treatment is being recognised and its role may increase in the future. An understanding of the limitations of FDG-PET will also provide a more accurate interpretation of the PET-CT findings.
In conclusion, the increased use of PET-CT scans in the investigation of patients with NSCLC allows for more accurate staging and therefore more appropriate management decisions. It is hoped that this translates to improved patient outcomes and increased cost-effectiveness, by avoiding inappropriate treatments.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. 10.3322/caac.20138 [DOI] [PubMed] [Google Scholar]
- 2.Tyczynski JE, Bray F, Parkin DM. Lung cancer in Europe in 2000: epidemiology, prevention, and early detection. Lancet Oncol 2003;4:45-55. 10.1016/S1470-2045(03)00960-4 [DOI] [PubMed] [Google Scholar]
- 3.Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24. 10.1001/jama.285.7.914 [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Singh H, Basu S, et al. Positron emission tomography-computed tomography in the management of lung cancer: An update. South Asian J Cancer 2013;2:171-8. 10.4103/2278-330X.114148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosini V, Nicolini S, Caroli P, et al. PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol 2012;81:988-1001. 10.1016/j.ejrad.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 6.Sahiner I, Vural GU. Positron emission tomography/computerized tomography in lung cancer. Quant Imaging Med Surg 2014;4:195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. 10.1056/NEJMcp012290 [DOI] [PubMed] [Google Scholar]
- 8.Alzahouri K, Velten M, Arveux P, et al. Management of SPN in France. Pathways for definitive diagnosis of solitary pulmonary nodule: a multicentre study in 18 French districts. BMC Cancer 2008;8:93. 10.1186/1471-2407-8-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murrmann GB, van Vollenhoven FH, Moodley L. Approach to a solid solitary pulmonary nodule in two different settings—“Common is common, rare is rare.” J Thorac Dis 2014;6:237-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng M, Yang X, Ma Q, et al. Retrospective analysis for the false positive diagnosis of PET-CT scan in lung cancer patients. Medicine (Baltimore) 2017;96:e7415. 10.1097/MD.0000000000007415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuaron J, Dunphy M, Rimner A. Role of FDG-PET scans in staging, response assessment, and follow-up care for non-small cell lung cancer. Front Oncol 2013;2:208. 10.3389/fonc.2012.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derlin T, Clauditz TS, Quaas A, et al. Synchronous bronchioloalveolar and squamous cell lung cancer with different 18F-FDG avidity on PET/CT. Clin Nucl Med 2012;37:e255-6. 10.1097/RLU.0b013e31824440f9 [DOI] [PubMed] [Google Scholar]
- 13.Liu Y. Lung Neoplasms with Low F18-Fluorodeoxyglucose Avidity. PET Clin 2018;13:11-8. 10.1016/j.cpet.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Zhan P, Zhu QQ, Miu YY, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2017;6:23-34. 10.21037/tlcr.2017.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeshita J, Masago K, Kato R, et al. CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. AJR Am J Roentgenol 2015;204:29-34. 10.2214/AJR.14.13151 [DOI] [PubMed] [Google Scholar]
- 16.Chao F, Zhang H. PET/CT in the Staging of the Non-Small-Cell Lung Cancer. J Biomed Biotechnol 2012;2012:783739. 10.1155/2012/783739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsim S, O’Dowd CA, Milroy R, et al. Staging of non-small cell lung cancer (NSCLC): A review. Respir Med 2010;104:1767-74. 10.1016/j.rmed.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 18.TNM classification of malignant tumours. Available online: https://www.ncbi.nlm.nih.gov/nlmcatalog/101691444
- 19.De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8. 10.1016/j.ejcts.2007.01.075 [DOI] [PubMed] [Google Scholar]
- 20.Lung cancer: diagnosis and management. Available online: https://www.nice.org.uk/guidance/CG121
- 21.Early-Stage and Locally Advanced (non-metastatic) Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines. Available online: http://www.esmo.org/Guidelines/Lung-and-Chest-Tumours/Early-Stage-and-Locally-Advanced-non-metastatic-Non-Small-Cell-Lung-Cancer [DOI] [PubMed]
- 22.Pisters KM, Le Chevalier T. Adjuvant Chemotherapy in Completely Resected Non–Small-Cell Lung Cancer. J Clin Oncol 2005;23:3270-8. 10.1200/JCO.2005.11.478 [DOI] [PubMed] [Google Scholar]
- 23.Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006;4:548-82. 10.6004/jnccn.2006.0046 [DOI] [PubMed] [Google Scholar]
- 24.Broderick SR, Patterson GA. Performance of integrated positron emission tomography/computed tomography for mediastinal nodal staging in non-small cell lung carcinoma. Thorac Surg Clin 2013;23:193-8. 10.1016/j.thorsurg.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 25.Vansteenkiste JF. Imaging in lung cancer: positron emission tomography scan. Eur Respir J Suppl 2002;35:49s-60s. 10.1183/09031936.02.00252402 [DOI] [PubMed] [Google Scholar]
- 26.McLoud TC. The role of PET scanning in the evaluation of lung carcinoma. Cancer Imaging 2003;3:83-4. [Google Scholar]
- 27.Vansteenkiste JF, Stroobants SG. The role of positron emission tomography with 18F-fluoro-2-deoxy-D-glucose in respiratory oncology. Eur Respir J 2001;17:802-20. 10.1183/09031936.01.17408020 [DOI] [PubMed] [Google Scholar]
- 28.Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005;79:375-82. 10.1016/j.athoracsur.2004.06.041 [DOI] [PubMed] [Google Scholar]
- 29.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:137S-146S. 10.1378/chest.123.1_suppl.137S [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Chen R, Huang G, et al. Potential clinical value of PET/CT in predicting occult nodal metastasis in T1-T2N0M0 lung cancer patients staged by PET/CT. Oncotarget 2017;8:82437-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu P, Sun Y, Sun Y, et al. The role of (18)F-FDG PET/CT for evaluation of metastatic mediastinal lymph nodes in patients with lung squamous-cell carcinoma or adenocarcinoma. Lung Cancer 2014;85:53-8. 10.1016/j.lungcan.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Kirmani BH, Rintoul RC, Win T, et al. Stage migration: results of lymph node dissection in the era of modern imaging and invasive staging for lung cancer. Eur J Cardio 2013;43:104-9; discussion 109-10. [DOI] [PubMed]
- 33.van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002;359:1388-93. 10.1016/S0140-6736(02)08352-6 [DOI] [PubMed] [Google Scholar]
- 34.Yousefi-Koma A, Panah-Moghaddam M, Kalff V. The Utility of Metabolic Imaging by 18F-FDG PET/CT in Lung Cancer: Impact on Diagnosis and Staging. Tanaffos 2013;12:16. [PMC free article] [PubMed] [Google Scholar]
- 35.Devaraj A, Cook GJR, Hansell DM. PET/CT in non-small cell lung cancer staging—promises and problems. Clin Radiol 2007;62:97-108. 10.1016/j.crad.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 36.Sharples LD, Jackson C, Wheaton E, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess 2012;16:1-75, iii-iv. 10.3310/hta16180 [DOI] [PubMed] [Google Scholar]
- 37.Rintoul RC, Glover MJ, Jackson C, et al. Cost effectiveness of endosonography versus surgical staging in potentially resectable lung cancer: a health economics analysis of the ASTER trial from a European perspective. Thorax 2014;69:679-81. 10.1136/thoraxjnl-2013-204374 [DOI] [PubMed] [Google Scholar]
- 38.Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. 10.1055/s-0034-1392040 [DOI] [PubMed] [Google Scholar]
- 39.Vial MR, O’Connell OJ, Grosu HB, et al. Diagnostic performance of endobronchial ultrasound-guided mediastinal lymph node sampling in early stage non-small cell lung cancer: A prospective study. Respirology 2018;23:76-81. 10.1111/resp.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quint LE, Tummala S, Brisson LJ, et al. Distribution of distant metastases from newly diagnosed non-small cell lung cancer. Ann Thorac Surg 1996;62:246-50. 10.1016/0003-4975(96)00220-2 [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Dong M, Sun X, et al. Prognostic Value of 18F-FDG PET/CT in Surgical Non-Small Cell Lung Cancer: A Meta-Analysis. PloS One 2016;11:e0146195. 10.1371/journal.pone.0146195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrevens L, Lorent N, Dooms C, et al. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist 2004;9:633-43. 10.1634/theoncologist.9-6-633 [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Xie D, Huang W, et al. 18F-FDG PET/CT in the evaluation of adrenal masses in lung cancer patients. Neoplasma 2010;57:129-34. 10.4149/neo_2010_02_129 [DOI] [PubMed] [Google Scholar]
- 44.Bury T, Barreto A, Daenen F, et al. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med 1998;25:1244-7. 10.1007/s002590050291 [DOI] [PubMed] [Google Scholar]
- 45.Hustinx R, Paulus P, Jacquet N, et al. Clinical evaluation of whole-body 18F-fluorodeoxyglucose positron emission tomography in the detection of liver metastases. Ann Oncol 1998;9:397-401. 10.1023/A:1008290027419 [DOI] [PubMed] [Google Scholar]
- 46.Brink I, Schumacher T, Mix M, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging 2004;31:1614-20. 10.1007/s00259-004-1606-x [DOI] [PubMed] [Google Scholar]
- 47.Lewis P, Griffin S, Marsden P, et al. Whole-body 18F-fluorodeoxyglucose positron emission tomography in preoperative evaluation of lung cancer. Lancet 1994;344:1265-6. 10.1016/S0140-6736(94)90753-6 [DOI] [PubMed] [Google Scholar]
- 48.Bury T, Dowlati A, Paulus P, et al. Whole-body 18FDG positron emission tomography in the staging of non-small cell lung cancer. Eur Respir J 1997;10:2529-34. 10.1183/09031936.97.10112529 [DOI] [PubMed] [Google Scholar]
- 49.Saunders CA, Dussek JE, O’Doherty MJ, et al. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg 1999;67:790-7. 10.1016/S0003-4975(98)01257-0 [DOI] [PubMed] [Google Scholar]
- 50.Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000;343:254-61. 10.1056/NEJM200007273430404 [DOI] [PubMed] [Google Scholar]
- 51.Hicks RJ, Kalff V, MacManus MP, et al. (18)F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med 2001;42:1596-604. [PubMed] [Google Scholar]
- 52.Hoekstra CJ, Stroobants SG, Hoekstra OS, et al. The value of [18F]fluoro-2-deoxy-D-glucose positron emission tomography in the selection of patients with stage IIIA-N2 non-small cell lung cancer for combined modality treatment. Lung Cancer 2003;39:151-7. 10.1016/S0169-5002(02)00446-4 [DOI] [PubMed] [Google Scholar]
- 53.Schmücking M, Baum RP, Griesinger F, et al. Molecular whole-body cancer staging using positron emission tomography: consequences for therapeutic management and metabolic radiation treatment planning. Recent Results Cancer Res 2003;162:195-202. 10.1007/978-3-642-59349-9_19 [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi S, Khiewvan B, Fox PS, et al. Impact of initial PET/CT staging in terms of clinical stage, management plan, and prognosis in 592 patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:906-14. 10.1007/s00259-013-2672-8 [DOI] [PubMed] [Google Scholar]
- 55.Taus Á, Aguiló R, Curull V, et al. Impact of 18F-FDG PET/CT in the treatment of patients with non-small cell lung cancer. Arch Bronconeumol 2014;50:99-104. [DOI] [PubMed] [Google Scholar]
- 56.Zheng Y, Sun X, Wang J, et al. FDG-PET/CT imaging for tumor staging and definition of tumor volumes in radiation treatment planning in non-small cell lung cancer. Oncol Lett 2014;7:1015-20. 10.3892/ol.2014.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-e92S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Ruysscher D, Nestle U, Jeraj R, et al. PET scans in radiotherapy planning of lung cancer. Lung Cancer 2012;75:141-5. 10.1016/j.lungcan.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 59.Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol 2003;21:2651-7. 10.1200/JCO.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 60.Patz EF, Connolly J, Herndon J. Prognostic Value of Thoracic FDG PET Imaging After Treatment for Non-Small Cell Lung Cancer. AJR Am J Roentgenol 2000;174:769-74. 10.2214/ajr.174.3.1740769 [DOI] [PubMed] [Google Scholar]
- 61.Duhaylongsod FG, Lowe VJ, Patz EF, Jr, et al. Detection of primary and recurrent lung cancer by means of F-18 fluorodeoxyglucose positron emission tomography (FDG PET). J Thorac Cardiovasc Surg 1995;110:130-9; discussion 139-40. 10.1016/S0022-5223(05)80018-2 [DOI] [PubMed] [Google Scholar]
- 62.Ettinger DS, Akerley W, Bepler G, et al. Non–Small Cell Lung Cancer. J Natl Compr Canc Netw 2010;8:740-801. 10.6004/jnccn.2010.0056 [DOI] [PubMed] [Google Scholar]
- 63.Beslic N, Sadija A, Ceric T, et al. Value of Positron Emission Tomography/Computed Tomography (PET-CT) in Suspected Non-small Cell Lung Cancer Recurrence and Impact on Patient Management. Acta Inform Med 2016;24:296-8. 10.5455/aim.2016.24.296-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuplov V, Holman BF, McClelland JR, et al. Issues in quantification of registered respiratory gated PET/CT in the lung. Phys Med Biol 2017;63:015007. 10.1088/1361-6560/aa950b [DOI] [PubMed] [Google Scholar]
- 65.Rankin S. PET/CT for staging and monitoring non small cell lung cancer. Cancer Imaging 2008;8:S27-31. 10.1102/1470-7330.2008.9006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao JQ, Rodrigues GB, Louie AV, et al. Systematic Review of the Cost-Effectiveness of Positron-Emission Tomography in Staging of Non–Small-Cell Lung Cancer and Management of Solitary Pulmonary Nodules. Clin Lung Cancer 2012;13:161-70. 10.1016/j.cllc.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 67.Szyszko TA, Yip C, Szlosarek P, et al. The role of new PET tracers for lung cancer. Lung Cancer 2016;94:7-14. 10.1016/j.lungcan.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 68.Langer A. A systematic review of PET and PET/CT in oncology: A way to personalize cancer treatment in a cost-effective manner? BMC Health Serv Res 2010;10:283. 10.1186/1472-6963-10-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hochhegger B, Alves GRT, Irion KL, et al. PET/CT imaging in lung cancer: indications and findings. J Bras Pneumol 2015;41:264-74. 10.1590/S1806-37132015000004479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Revel MP, Carette MF, Torrent M, et al. Diagnosis and standardized report for non-small cell lung cancer. Diagn Interv Imaging 2014;95:727-38. 10.1016/j.diii.2014.06.007 [DOI] [PubMed] [Google Scholar]