Abstract
Immunotherapy has evolved at a phenomenal pace in cancer therapeutics. This has primarily been fueled by the much perceived necessity to procure an alternative to current standard of care chemotherapy agents, owing to several concerns such as treatment-related toxicity and poor long-term survival associated with the same. The knowledge of various mechanisms involved in regulation of immune response to cancer cells has served a fundamental role in identifying key molecules through which immune cell activity may be modulated. This in-turn led to the development of immune-checkpoint inhibitors. Presently, lung cancer is among the most enthusiastically investigated targets for treatment with immune-checkpoint inhibitors. Encouraging results with initial trials have now translated to attempts directed at further enhancement of outcomes through various strategies. Herein, we shall present a critical assessment of data from pivotal trials that led to the Food and Drug Administration (FDA) approval of various immune-checkpoint inhibitors and also discuss novel strategies that may potentially yield outcomes superior to standard of care chemotherapy in patients diagnosed with advanced non-small cell lung cancer (NSCLC).
Keywords: Immunotherapy, monoclonal antibodies, non-small cell lung cancer (NSCLC), programmed cell death receptor-1 (PD-1), t-lymphocytes
Introduction
Lung cancer accounts for almost a quarter of all cancer-related deaths in the United States (1). The standard of care treatment with platinum based chemotherapy has benefitted patients for almost two decades (2,3). A multidisciplinary approach combining the application of chemotherapy with alternate treatment modalities such as surgery and radiation therapy have aided in improving survival outcomes (2,4-9). Likewise, targeted therapy is another approach that has considerably enhanced survival indices in a select group of lung cancer patients (10,11). However, success with the above treatment modalities has continually been constrained by various limitations.
Historic data on standard of care chemotherapy has identified several issues associated with their clinical application. The incidence of treatment-related adverse events associated with platinum based chemotherapy has been a key concern since their inception in clinical practice. Retrospective analysis of data from 37 clinical trials attributed an increase in the incidence of nephrotoxicity, hematological toxicity and gastrointestinal adverse events to treatment with platinum based chemotherapy (12). Similarly, chemoradiation therapy indicated for stage III disease in lung cancer has been found to result in significant morbidity (13). Surgical resection, though being an effective modality to achieve cure in local disease, is precluded as a therapeutic option for many patients due to their poor pre-operative performance status (14). On the other hand, the application of targeted therapy is limited to the tumors that are positive for specific oncogenic mutations (3). Thus, it was prudent to explore alternative treatment strategies to overcome the challenges associated with standard of care therapy for lung cancer.
Immunotherapy is an upcoming treatment strategy in cancer therapeutics. It involves modulation of immune regulatory mechanisms to boost the immune response against cancer cells (15). A number of key target molecules that act as checkpoints in regulation of immune cell activity have been identified (Figure 1). One of the earliest to be discovered was the cytotoxic T lymphocyte associated antigen-4 (CTLA-4). The expression of CTLA-4 molecule is upregulated after the activation of T-cells (16). It acts to downregulate T-cell activity by inducing T-cell cycle arrest (17). In addition, it also inhibits CD28 mediated stimulation of T-cell activity by competitively binding with the B7 molecule on the antigen presenting cell (APC) (18-21). Akin to CTLA-4, programmed cell death receptor-1 (PD-1) is another molecule that is expressed by activated T-cells. It induces downregulation of T-cell activity and proliferation upon binding with programmed cell death receptor-ligand 1 (PD-L1) (22). Furthermore, recent studies have also uncovered certain molecules expressed by T-cells that deliver a positive costimulatory signal upon binding with the corresponding ligand. These include the 4-1BB receptor (CD137) and OX40 molecule, in addition to others (23-25). The binding of 4-1BB receptor with its corresponding ligand induces T-cell activation and proliferation whereas the engagement of OX40 receptor with its ligand primarily leads to increased T-cell survival and proliferation (26,27). A comprehensive understanding of the above and other mechanisms involved in regulation of immune response to cancer cells made way for the development of immune-checkpoint inhibitors (16,23,28,29).
Figure 1.
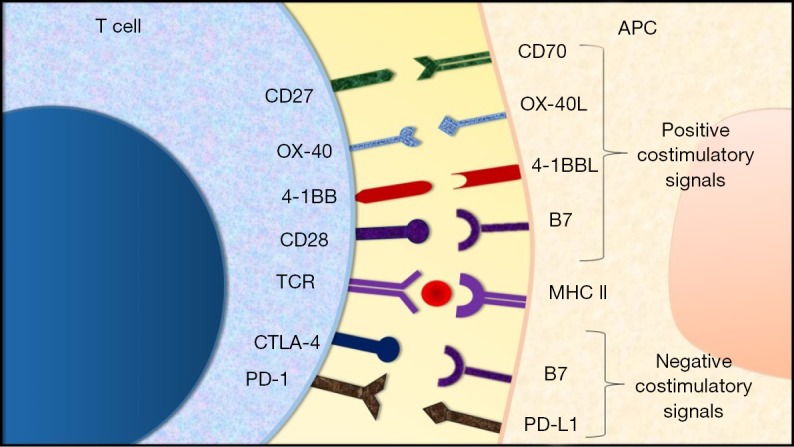
Costimulatory pathways for T cell activation. The activation of T cells is regulated through the interaction of various costimulatory molecules. Positive costimulatory signals are generated by the engagement of CD27-CD70, OX40-OX40L, 4-1BB-4-1BBL and CD28-B7 whereas PD-1-PD-L1 and CTLA-4-B7 interaction generates negative costimulatory signals. APC, antigen presenting cell; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death ligand-1; CTLA-4, cytotoxic T-lymphocyte associated antigen-4; MHC, major histocompatibility complex; TCR, T-cell receptor.
Immune-checkpoint inhibitors are a type of drug that block specific proteins involved in downregulation of immune response to cancer cells (15,30-32). Clinical studies evaluating response with immune-checkpoint inhibitors in various tumor histologies have demonstrated superior survival indices as well as toxicity profile in comparison to standard of care chemotherapy regimens (33-37). Clinical benefit observed with immunotherapy in pivotal trials has led to the approval of a select number of immune-checkpoint inhibitors for use in specific tumors by the Food and Drug Administration (FDA) (29). The first among them to be approved was ipilimumab for patients with late-stage melanoma (38). This was followed by the approval of nivolumab, pembrolizumab and nivolumab plus ipilimumab combination soon after (29). In addition to melanoma, immune-checkpoint inhibitors are also approved for application in several other tumor histologies. These include non-small cell lung cancer (NSCLC) (nivolumab, atezolizumab and pembrolizumab), renal cell carcinoma (nivolumab), urothelial carcinoma (nivolumab, atezolizumab and durvalumab), squamous cell carcinoma of head and neck (nivolumab and pembrolizumab), classical Hodgkin’s lymphoma (nivolumab and pembrolizumab) and Merkel cell carcinoma (avelumab) (34,35,39-50). Of note, anti-PD-1 therapy (pembrolizumab) has recently replaced chemotherapy in first-line setting for NSCLC with high PD-L1 expression (51). Ongoing efforts to optimize the dosing and schedule of currently approved immune-checkpoint inhibitors, together with various trials investigating new immunotherapy agents are expected to further diversify the indication for immunotherapy.
Presently, NSCLC is among the most thoroughly investigated cancer histologies for potential application of immunotherapy with immune-checkpoint inhibitors. Success with initial trials and an increasing list of FDA approvals have encouraged efforts to further improve outcomes through evaluation and implementation of novel treatment strategies. In this review, we will critically assess the pivotal trials that led to the approval of various immunotherapy agents for NSCLC. Further, we shall summarize and discuss the ongoing trials investigating new immunotherapy treatment protocols and novel agents for patients diagnosed with NSCLC. Building upon our experience thus far with immunotherapy, we will explore future directions for the advancement and successful application of immune-checkpoint inhibitors in the treatment of NSCLC.
FDA approved immune-checkpoint inhibitors for NSCLC- pivotal trials
Nivolumab
Nivolumab, an anti-PD-1 human monoclonal antibody (MoAb), became the first immune-checkpoint inhibitor to be approved by the FDA for patients diagnosed with NSCLC (30). It was approved to be administered as nivolumab 3 mg/kg once every 2 weeks for patients diagnosed with squamous NSCLC exhibiting progression of disease during or after prior treatment with platinum-based chemotherapy. The approval was based upon the observations made in the CheckMate 017 study, a phase 3 randomized control trial (RCT) (NCT01642004, Table 1). Squamous NSCLC patients that exhibited progression of disease with platinum-based chemotherapy were recruited for the study. Treatment with docetaxel 75 mg/m2 once every 3 weeks (n=137) was compared against nivolumab 3 mg/kg once every 2 weeks (n=135) (34). Patients receiving nivolumab exhibited a median overall survival (OS) of 9.2 months [95% confidence interval (CI): 7.3 to 13.3] as compared to 6.0 months (95% CI: 5.1 to 7.3) with docetaxel therapy. Similarly, median progression free survival (PFS) with nivolumab was recorded as 3.5 months (95% CI: 2.1 to 4.9) vs. 2.8 months (95% CI: 2.1 to 3.5) for patients treated with docetaxel. Furthermore, the 1 year-OS rate with nivolumab was almost twice as high as what was observed with docetaxel (42% vs. 24%). Nivolumab therapy outperformed treatment with docetaxel in toxicity profile as well, with only 7% (9 of 131 patients) patients experiencing grade 3–4 treatment related adverse events (TRAEs) in comparison to 55% (71 of 129 patients) participants that were treated with docetaxel (34).
Table 1. Pivotal trials for application of immunotherapy agents in NSCLC.
| Immunotherapy agent | Mechanism of action | Date of FDA approval | FDA approved regimen | Trial description | Outcomes | NCT number/reference |
|---|---|---|---|---|---|---|
| Nivolumab | Anti-PD-1 human MoAb | March 4, 2015 | Nivolumab 3 mg/kg IV over 60 min Q2W | Phase: phase 3 RCT; indication: metastatic/advanced squamous NSCLC presenting with disease progression after or during treatment with platinum-based doublet chemotherapy; design: patients randomized to receive docetaxel 75 mg/m2 Q3W or nivolumab 3 mg/kg Q2W | Median OS: nivolumab arm: 9.2 months, 95% CI: 7.3 to 13.3; docetaxel arm: 6.0 months, 95% CI: 5.1 to 7.3; median PFS: nivolumab arm: 3.5 months, 95% CI: 2.1 to 4.9; docetaxel arm: 2.8 months, 95% CI: 2.1 to 3.5; 1-year OS: nivolumab arm: 42%, 95% CI: 34 to 50; docetaxel arm: 24%, 95% CI: 17 to 31; grade 3–4 TRAE: nivolumab arm: 7% (9 of 131 patients); docetaxel arm: 55% (71 of 129 patients) |
NCT01642004; CheckMate 017 (34) |
| Pembrolizumab (MK-3475) | Anti-PD-1 human MoAb | October 2, 2015 | Pembrolizumab 2 mg/kg IV over 30 minutes Q3W | Phase: phase 1; indication: metastatic/advanced NSCLC presenting with disease progression after or during treatment with targeted therapy for EGFR/ALK mutations or platinum-based doublet chemotherapy; design: patients in arms C, F1 and F2 received pembrolizumab 2 mg/kg Q3W or 10 mg/kg Q2W/Q3W; patients in arm F3 received pembrolizumab 2 mg/kg Q3W | Confirmed ORR for tumors strongly positive for PD-L1: arms C, F1 and F2 (n=61): 41%, 95% CI: 28.6% to 58.3%; arm F3 (n=25): 28%, 95% CI: 12.1% to 49.4%; serious AE: 37.8% (208 of 550 patients); immune-related adverse events: 13% (71 of 550 patients) | NCT01295827; KEYNOTE 001 (39) |
| Nivolumab (BMS-936558) | Anti-PD-1 human MoAb | October 9, 2015 | Nivolumab 3 mg/kg IV Q2W | Phase: phase 3 RCT; indication: metastatic/advanced non-squamous NSCLC presenting with disease progression after or during treatment with platinum-based doublet chemotherapy or FDA-approved targeted therapy for EGFR/ALK mutations; design: patients randomized to receive nivolumab 3 mg/kg IV Q2W or docetaxel 75 mg/m2 IV Q3W | Median OS: nivolumab arm: 12.2 months, 95% CI: 9.7 to 15.0; docetaxel arm: 9.4 months, 95% CI: 8.1 to 10.7; HR: 0.73, 95% CI: 0.59 to 0.89, P=0.002; objective response: nivolumab arm: 19%, 95% CI: 15 to 24; docetaxel arm: 12%, 95% CI: 9 to 17; median PFS: nivolumab arm: 2.3 months, 95% CI: 2.2 to 3.3; docetaxel arm: 4.2 months, 95% CI: 3.5 to 4.9; HR: 0.92, 95% CI: 0.77 to 1.1, P=0.39; grade 3–4 TRAE: nivolumab arm: 10% (30 of 287 patients); docetaxel arm: 54% (144 of 268 patients) |
NCT01673867; CheckMate 057 (52) |
| Atezolizumab (MPDL3280A) | Anti-PD-L1 MoAb | October 18, 2016 | Atezolizumab 1,200 mg IV over 60 minutes Q3W | Phase: phase 3 RCT; indication: stage IIIB or IV NSCLC patients presenting with disease progression after or during prior treatment with platinum-based chemotherapy or FDA-approved targeted therapy for EGFR/ALK mutations; design: patients randomized to receive atezolizumab 1,200 mg/kg Q3W or docetaxel 75 mg/m2 Q3W | Median OS: atezolizumab arm: 13.8 months, 95% CI: 11.8 to 15.7; docetaxel arm: 9.6 months, 95% CI: 8.6 to 11.2; HR: 0.74, 95% CI: 0.63 to 0.87, P=0.0004; grade 3–4 TRAE: atezolizumab arm: 15% (90 of 609 patients); docetaxel arm: 43% (247 of 578 patients) |
NCT02008227; OAK trial (35) |
| Atezolizumab (MPDL3280A) | Anti-PD-L1 MoAb | October 18, 2016 | Atezolizumab 1,200 mg IV over 60 min Q3W | Phase: phase 2 RCT; indication: metastatic/locally advanced NSCLC presenting with disease progression after or during prior treatment with platinum-based chemotherapy; design: patients randomized to receive atezolizumab 1,200 mg/kg Q3W or docetaxel 75 mg/m2 Q3W | Median OS: atezolizumab arm: 12.6 months, 95% CI: 9.7 to 16.4; docetaxel arm: 9.7 months, 95% CI: 8.6 to 12.0; HR: 0.73, 95% CI: 0.53 to 0.99, P=0.04; grade 3–4 TRAE: atezolizumab arm: 11% (16 of 142 patients); docetaxel arm: 39% (52 of 135 patients) |
NCT01903993; POPLAR trial (53) |
| Pembrolizumab (MK-3475) | Anti-PD-1 human MoAb | October 24, 2016 | Pembrolizumab 200 mg IV Q3W | Phase: phase 3 RCT; indication: treatment naïve PD-L1 positive (TPS score ≥50%) EGFR wild-type advanced NSCLC without ALK translocation; design: patients randomized to receive investigator’s choice of platinum-based chemotherapy or pembrolizumab 200 mg once every 3 weeks | Median PFS: chemotherapy arm: 6.0 months (95% CI: 4.2 to 6.2); pembrolizumab arm: 10.3 months (95% CI: 6.7 to “not reached”); HR: 0.50, 95% CI: 0.37 to 0.68, P<0.001; response rate: chemotherapy arm: 27.8%; pembrolizumab arm: 44.8%; OS at 6 months: chemotherapy arm: 72.4%; pembrolizumab arm: 80.2%; grade 3–5 TRAE: chemotherapy arm: 53.3%; pembrolizumab arm: 26.6% |
NCT02142738; KEYNOTE 024 (51) |
| Pembrolizumab (MK-3475) | Anti-PD-1 human MoAb | October 24, 2016 | Pembrolizumab 2 mg/kg IV over 30 minutes Q3W | Phase: phase 2/phase 3 RCT; indication: PD-L1 positive (TPS score ≥1%) metastatic NSCLC presenting with progression of disease after prior treatment with platinum based chemotherapy or FDA-approved targeted therapy for EGFR/ALK mutations; design: patients randomized in 1:1:1 fashion to receive pembrolizumab 2 mg/kg Q3W, pembrolizumab 10 mg/kg Q3W or docetaxel 75 mg/m2 Q3W | Median OS for patients with TPS score ≥1%: pembrolizumab 2 mg/kg: 10.4 months; pembrolizumab 10 mg/kg: 12.7 months; docetaxel 75 mg/m2: 8.5 months; median PFS for patients with TPS score ≥1%: pembrolizumab 2 mg/kg: 3.9 months; pembrolizumab 10 mg/kg: 4.0 months; docetaxel 75 mg/m2: 4.0 months; median OS for patients with TPS score ≥50%: pembrolizumab 2 mg/kg: 14.9 months; pembrolizumab 10 mg/kg: 17.3 months; docetaxel 75 mg/m2: 8.2 months; median PFS for patients with TPS score ≥50%: pembrolizumab 2 mg/kg: 5.0 months; pembrolizumab 10 mg/kg: 5.2 months; docetaxel 75 mg/m2: 4.1 months; grade 3–5 TRAEs: pembrolizumab 2 mg/kg: 13% (43 of 339 patients); pembrolizumab 10 mg/kg: 16% (55 of 343 patients); docetaxel 75 mg/m2: 35% (109 of 309 patients) |
NCT01905657; KEYNOTE 010 (54) |
| Pembrolizumab (MK-3475) | Anti-PD-1 human MoAb | May 10, 2017 | Pembrolizumab 200 mg IV Q3W plus carboplatin AUC 5 Q3W and pemetrexed 500 mg/m2 Q3W | Phase: phase 2 RCT; indication: treatment naïve EGFR wild-type metastatic non-squamous NSCLC without ALK translocation, independent of PD-L1 status; design: (I) cohort G1: pembrolizumab 200 mg IV Q3W plus carboplatin AUC 5 Q3W and pemetrexed 500 mg/m2 Q3W; (II) cohort G2: carboplatin AUC 5 Q3W and pemetrexed 500 mg/m2 Q3W; (III) cohort H: pembrolizumab plus ipilimumab | Median PFS: cohort G1: 13.0 months (95% CI: 8.3 to “not estimated”); cohort G2: 8.9 months (95% CI: 4.4 to 10.3); HR: 0.53, 95% CI: 0.31 to 0.91, P=0.0205; ORR: cohort G1: 55% (95% CI: 42 to 68); cohort G2: 29% (95% CI: 18 to 41) |
NCT02039674; KEYNOTE 021 |
NSCLC, non-small cell lung cancer; MoAb, monoclonal antibody; PFS, progression free survival; OS, overall survival; ORR, objective response rate; NSCLC, non-small cell lung cancer; RCT, randomized control trial; EGFR, epithelial growth factor receptor; ALK, anaplastic lymphoma kinase; Q(x)W, every (x) weeks; AE, adverse events; TRAE, treatment-related adverse events; CI, confidence interval; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death ligand-1; HR, hazard ratio; TPS, tumor proportion score; AUC, area under curve.
The approval of nivolumab for squamous NSCLC was soon followed by expansion of indication to non-squamous NSCLC based upon the findings made in the CheckMate 057 study (NCT01673867). This was a phase 3 RCT that compared safety and efficacy with docetaxel 75 mg/m2 once every 3 weeks vs. nivolumab 3 mg/kg once every 2 weeks in patients with advanced/metastatic PD-1 positive non-squamous NSCLC, presenting with disease progression after or during treatment with platinum-based chemotherapy or FDA-approved targeted therapy for epithelial growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) mutations (52). Objective response was observed in 12% (95% CI: 9 to 17) patients receiving docetaxel, as opposed to 19% (95% CI: 15 to 24) of those treated with nivolumab. The median OS in docetaxel arm was reported as 9.4 months (95% CI: 8.1 to 10.7) vs. 12.2 months (95% CI: 9.7 to 15.0) in the nivolumab arm. The median PFS in docetaxel arm was found to be 4.2 months (95% CI: 3.5 to 4.9) as compared to 2.3 months (95% CI: 2.2 to 3.3) in nivolumab arm. Patients receiving nivolumab exhibited significantly lower grade 3–4 TRAEs (10%, 30 of 287 patients) than those treated with docetaxel (54%, 144 of 268 patients) (52). In view of the above, the FDA approved the use of nivolumab 3 mg/kg once every 2 weeks for the treatment of advanced/metastatic PD-1 positive non-squamous NSCLC that presented with disease progression after or during treatment with platinum-based chemotherapy or FDA-approved targeted therapy for EGFR/ALK mutations.
Pembrolizumab
Pembrolizumab or MK-3475, is an anti-PD-1 human MoAb akin to nivolumab that targets PD-1/PD-L1 interaction between T cells and APCs to enhance anti-cancer immune activity (30). The findings made in KEYNOTE 001 trial (phase 1 trial) laid ground for FDA approval of pembrolizumab in NSCLC (NCT01295827, Table 1). The C and F arms of this study investigated pembrolizumab for the treatment of advanced NSCLC patients presenting with disease progression after prior treatment with FDA approved targeted therapy for EGFR/ALK or platinum-based chemotherapy (39). Patients in arms C, F1 and F2 received pembrolizumab 10 mg/kg every 2 weeks (n=202), 10 mg/kg every 3 weeks (n=287) or 2 mg/kg every 3 weeks (n=6), whereas participants enrolled in an expansion cohort F3 received pembrolizumab 2 mg/kg every 3 weeks (n=55) only. Patients with tumors exhibiting strong PD-L1 positivity tumor proportion score (TPS) of ≥50% tumor cells) in arms C, F1 and F2 exhibited a confirmed objective response rate (ORR) of 41% (95% CI: 28.6–58.3%). The ORR for arm F3 was reported as 28% (95% CI: 12.1–49.4%). In regard to the toxicity profile, pooled data analysis revealed that serious adverse events and immune-related adverse events were reported in 37.8% and 13% of all patients that received pembrolizumab, respectively (39). Considering the above, the FDA approved pembrolizumab 2 mg/kg once every 3 weeks for advanced NSCLC patients with PD-L1 TPS of ≥50% tumor cells presenting with progression after prior treatment with platinum-based chemotherapy or FDA approved targeted therapy for EGFR/ALK mutations. However, the indication and dosage of pembrolizumab for lung cancer were soon revised in light of new evidence from KEYNOTE 010 and KEYNOTE 024 trials.
The revision of initial indication for pembrolizumab in lung cancer stemmed from the data published from two trials. The KEYNOTE 010 was a phase 2/3 RCT that compared therapy with pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg and docetaxel 75 mg/m2 for previously treated PD-L1 positive metastatic NSCLC patients (NCT01905657) (54). The PD-L1 positivity criteria in this study was defined as TPS score of ≥1%. The study reported a median OS and median PFS for patients receiving pembrolizumab 2 mg/kg as 10.4 months and 3.9 months, while that for pembrolizumab 10 mg/kg arm were recorded as 12.7 months and 4.0 months, respectively. Docetaxel treatment arm was observed to have a median OS of 8.5 months and a median PFS of 4.0 months. In addition to the above, this study also analyzed outcomes in patients with PD-L1 TPS score of ≥50%. The study reported a median OS and median PFS for pembrolizumab 2 mg/kg arm as 14.9 months and 5.0 months, pembrolizumab 10 mg/kg arm as 17.3 months and 5.2 months, and lastly for docetaxel arm as 8.2 months and 4.1 months, respectively (54). Grade 3, 4 or 5 TRAE were observed in 13% patients that received pembrolizumab 2 mg/kg, 16% of those that received pembrolizumab 10 mg/kg and 35% patients treated with docetaxel (54). The data from KEYNOTE 010 led to the approval of pembrolizumab for PD-L1 positive (TPS score ≥1%) metastatic NSCLC presenting with progression of disease after prior treatment with platinum based chemotherapy or FDA-approved targeted therapy for EGFR/ALK mutations.
Another trial, the KEYNOTE 024 was a phase 3 RCT that compared chemotherapy vs. fixed-dose pembrolizumab (200 mg once every 3 weeks) for treatment naïve PD-L1 positive EGFR wild-type advanced NSCLC without ALK translocation (NCT02142738) (51). In this study, the PD-L1 positivity criteria were defined as ≥50% staining. The median PFS of patients receiving chemotherapy was recorded as 6.0 months (95% CI: 4.2 to 6.2), while patients receiving pembrolizumab exhibited a median PFS of 10.3 months (95% CI: 6.7–not reached). Pembrolizumab outperformed chemotherapy in response rates (44.8% vs. 27.8%), OS at 6 months (80.2% vs. 72.4%) and grade 3–5 TRAEs (26.6% vs. 53.3%) as well (51). The above data prompted approval of pembrolizumab 200 mg IV once every 3 weeks for patients presenting with treatment naïve EGFR wild-type PD-L1 positive (TPS ≥50%) advanced/metastatic NSCLC without ALK translocation. Of note, this became the first FDA approval for the application of an immune-checkpoint inhibitor in first-line setting for advanced NSCLC.
Most recently, the FDA approved pembrolizumab 200 mg once every 3 weeks in combination with pemetrexed 500 mg/m2 plus carboplatin area under curve (AUC) 5 once every 3 weeks for treatment naïve EGFR wild-type metastatic non-squamous NSCLC without ALK translocation, independent of PD-L1 status. The approval was based on the KEYNOTE 021 study, a multi-arm phase 2 RCT (NCT02039674). The study noted a significant advantage in PFS with pembrolizumab plus carboplatin and pemetrexed combination as compared to chemotherapy alone [hazard ratio (HR): 0.53, 95% CI: 0.31–0.91, P=0.0205). The ORR for the combination therapy was reported to be 55% (95% CI: 42–68) as compared to 29% (95% CI: 18–41) with chemotherapy. Further data on toxicity profile and other survival parameters is awaited.
Atezolizumab
Atezolizumab, also referred to as MPDL3280A, is an anti PD-L1 human MoAb (30). It was approved for use as atezolizumab 1,200 mg to be administered IV once every 3 weeks for patients diagnosed with advanced stage IIIB/IV NSCLC presenting with disease progression after or during prior treatment with FDA approved targeted therapy for EGFR/ALK mutations or platinum-based chemotherapy. The FDA approval for the use of atezolizumab for NSCLC was based-off the clinical findings in the OAK (phase 3 RCT) and POPLAR (phase 2 RCT) studies (NCT02008227, NCT01903993; Table 1).
The OAK study enrolled patients with advanced stage IIIB/IV NSCLC presenting with disease progression after or during prior treatment with FDA approved targeted therapy for EGFR/ALK mutations or platinum-based chemotherapy (35). The study participants were randomized to receive atezolizumab 1,200 mg once every 3 weeks (n=425) or docetaxel 75 mg/m2 once every 3 weeks (n=425). The median OS with docetaxel and atezolizumab was found to be 9.6 months (95% CI: 8.6–11.2) and 13.8 months (95% CI: 11.8–15.7), respectively. Further, grade 3–4 TRAE we observed in only 15% of the patients receiving atezolizumab as compared to 43% of those who received docetaxel (35).
The POPLAR study enrolled patients with metastatic/locally advanced NSCLC presenting with disease progression after or during prior treatment with platinum-based chemotherapy (53). The study participants were randomized in 1:1 fashion to receive either atezolizumab 1,200 mg once every 3 weeks (n=143) or docetaxel 75 mg/m2 once every 3 weeks (n=135). The median OS with atezolizumab and docetaxel was found to be 12.6 months (95% CI: 9.7–16.4) and 9.7 months (95% CI: 8.6–12.0), respectively. Grade 3–4 TRAEs were reported in 11% patients that were treated with atezolizumab, as opposed to 39% patients that received therapy with docetaxel (53).
Immune-checkpoint inhibitors for NSCLC-ongoing registration trials
Nivolumab
Nivolumab has been evaluated as a first-line therapy for advanced NSCLC in two trials. The CheckMate 026, a phase 3 RCT, compared treatment with nivolumab 3 mg/kg once every 2 weeks vs. investigator’s choice chemotherapy (squamous NSCLC: gemcitabine/cisplatin, gemcitabine/carboplatin, paclitaxel/carboplatin; non-squamous NSCLC: pemetrexed/cisplatin, pemetrexed/carboplatin) for recurrent/stage IV PD-L1 positive NSCLC patients that were ineligible for EGFR/ALK targeted therapy (NCT02041533) (55). The PD-L1 positivity criteria were defined as ≥5% staining in this study. The median PFS in nivolumab arm was noted to be 4.2 months, as opposed to 5.9 months in chemotherapy arm (HR: 1.15; 95% CI: 0.91–1.45; P=0.25). The safety profile of nivolumab, however, was superior to that of chemotherapy. Grade 3–4 TRAEs with nivolumab were observed in only 18% patients as compared to 51% patients that received chemotherapy (55). The application of nivolumab as a first-line therapy for NSCLC has been attempted again through the Checkmate 227 trial.
The CheckMate 227 study, a phase 3 RCT, is presently recruiting chemotherapy naïve recurrent/stage IV NSCLC patients (NCT02477826). This is a multi-arm study that will be investigating monotherapy with nivolumab as a first-line option for advanced NSCLC, in addition to other regimens. Patients in comparator arm will receive platinum doublet chemotherapy only. The study participants will be stratified according to tumor PD-L1 status, with separate groups for patients with PD-L1 expression <1% and ≥1%. The primary objective of this study is to assess PFS and OS. The secondary objectives are assessment of ORR and changes in patient-reported cancer symptoms from baseline (evaluated using Lung Cancer Symptom Score).
Pembrolizumab
Presently, pembrolizumab is being investigated in phase 3 RCTs for application in NSCLC. This includes KEYNOTE 042, KEYNOTE 189 and KEYNOTE 407 (Table 2). Although these trials are evaluating the same fixed-dose of pembrolizumab (200 mg IV every 3 weeks), they vary significantly in other aspects, most important of them being the experimental regimen and indication.
Table 2. Registration trials for single-agent immunotherapy in NSCLC.
| Immunotherapy agent | Mechanism of action | Treatment regimen | Trial description | Enrollment number | Trial status | NCT number/reference |
|---|---|---|---|---|---|---|
| Nivolumab | Anti-PD-1 human MoAb | Nivolumab 3 mg/kg IV Q2W | Phase: phase 3 RCT; indication: treatment naïve PD-L1 positive (≥5% expression) EGFR wild-type stage IV/recurrent NSCLC without ALK translocation; treatment arms: (I) experimental arm: nivolumab 3 mg/kg IV Q2W; (II) active comparator: 6 cycles of investigators’ choice of platinum-based chemotherapy; results: median PFS: (I) nivolumab arm: 4.2 months; (II) chemotherapy arm: 5.9 months; grade 3–4 TRAE: (I) nivolumab arm: 18%; (II) chemotherapy arm: 51% | 541 | Closed; follow-up for survival data | NCT02041533; CheckMate 026 (55) |
| Pembrolizumab (MK-3475) | Anti-PD-1 human MoAb | Pembrolizumab 200 mg IV Q3W | Phase: phase 3 RCT; indication: patients with PD-L1 positive, ALK negative, EGFR negative NSCLC; treatment arms: patients randomized to receive platinum doublet chemotherapy for 6 cycles or pembrolizumab for a maximum of 35 cycles as first-line therapy; primary endpoint: OS of participants with strong PD-L1 positivity; secondary endpoints: PFS of participants with strong PD-L1 positivity; PFS and OS of all study participants | 1,240 | Active, not recruiting | NCT02220894; KEYNOTE 042 (56) |
| Pembrolizumab (MK-3475) | Anti-PD-1 human MoAb | Pembrolizumab 200 mg IV Q3W | Phase: phase 3 RCT; indication: Patients with non-squamous NSCLC that are not eligible for anti-EGFR/ALK mutation targeted therapy; treatment arms: (I) experimental arm: 4 cycles of pembrolizumab 200 mg Q3W plus chemotherapy (pemetrexed 500 mg/m2 and carboplatin AUC 5 or cisplatin 75 mg/m2 Q3W) followed by pemetrexed 500 mg/m2 Q3W plus pembrolizumab 200 mg Q3W; (II) control arm: placebo plus chemotherapy Q3W followed by pemetrexed 500 mg/m2 plus placebo Q3W; primary endpoint: PFS; secondary endpoints: safety, duration of response, OS, ORR, PFS for study participants with a PD-L1 expression TPS score ≥1% | 570 | Active, not recruiting | NCT02578680; KEYNOTE 189 (57) |
| Pembrolizumab (MK-3475) | Anti-PD-1 human MoAb | Pembrolizumab 200 mg IV Q3W | Phase: phase 3 RCT; indication: treatment naïve stage IV squamous NSCLC; treatment arms: (I) experimental arm: pembrolizumab 200 mg Q3W for a maximum of 35 cycles plus chemotherapy (4 cycles of carboplatin AUC 6 Q3W plus paclitaxel 200 mg/m2 or nab-paclitaxel 100 mg/m2 on days 1, 8, 15); (II) control arm: placebo (normal saline) Q3W for a maximum of 35 cycles plus chemotherapy; primary endpoint: PFS, OS (assessed up to 2 years); secondary endpoints: ORR (assessed up to 2 years) | 560 | Recruiting | NCT02775435; KEYNOTE 407 |
| Atezolizumab (MPDL3280A) | Anti-PD-L1 MoAb | Atezolizumab 1,200 mg IV Q3W | Phase: phase 3 RCT; indication: chemotherapy naïve stage IV non-squamous NSCLC; treatment arms: (I) experimental arm 1: Atezolizumab 1,200 mg IV Q3W plus chemotherapy (4 or 6 cycles of nab-paclitaxel 100 mg/m2 on day 1, 8 and 15 plus carboplatin AUC 6 Q3W); (II) control arm: chemotherapy only; primary endpoint: investigator assessed PFS using RECIST v1.1 (assessed up to 2.5 years); secondary endpoints: duration of response, objective response, PFS (evaluated by an independent review facility), OS (assessed up to 3.5 years), safety, changes in patient-reported cancer symptoms from baseline and TTD | 650 | Recruiting | NCT02366143; IMpower 130 (58) |
| Atezolizumab (MPDL3280A) | Anti-PD-L1 MoAb | Atezolizumab 1,200 mg IV Q3W | Phase: phase 3 RCT; indication: treatment naïve stage IV squamous NSCLC; treatment arms: (I) experimental arm 1: Atezolizumab 1,200 mg IV Q3W plus chemotherapy (4 or 6 cycles of nab-paclitaxel 100 mg/m2 on day 1, 8 and 15 plus carboplatin AUC 6 Q3W); (II) experimental arm 2: Atezolizumab plus chemotherapy (4 or 6 cycles of paclitaxel 200 mg/m2 plus carboplatin AUC 6 Q3W); (III) comparator arm: chemotherapy only (carboplatin plus nab-paclitaxel); primary endpoint: Investigator assessed PFS using RECIST v1.1 (evaluated up to 28 months), OS (evaluated up to 44 months); secondary endpoints: duration of response, objective response, PFS (evaluated by an independent review facility), TTR, TIR, incidence of AE, percent patients with anti-therapeutic antibody response to atezolizumab, changes in patient-reported cancer symptoms from baseline and TTD | 1,025 | Recruiting | NCT02367794; IMpower 131 (58) |
| Atezolizumab (MPDL3280A) | Anti-PD-L1 MoAb | Atezolizumab 1,200 mg IV Q3W | Phase: phase 3 RCT; indication: treatment naïve stage IV non-squamous NSCLC; treatment arms: (I) experimental arm 1: Atezolizumab 1,200 mg IV Q3W plus chemotherapy (4 or 6 cycles of paclitaxel 200 mg/m2 plus carboplatin AUC 6 Q3W); (II) experimental arm 2: Atezolizumab 1,200 mg IV Q3W plus chemotherapy and bevacizumab 15 mg/kg IV Q3W; (III) control arm: bevacizumab plus chemotherapy; primary endpoint: investigator assessed PFS using RECIST v1.1 (assessed up to 2 years); secondary endpoints: objective response, duration of response, PFS (evaluated by an independent review facility), OS (assessed up to 7 years), safety, changes in patient-reported cancer symptoms from baseline and TTD | 1,200 | Recruiting | NCT02366143; IMpower 150 (58) |
| Avelumab (MSB0010718C) | Anti-PD-L1 MoAb | Avelumab 10 mg/kg IV over 60 minutes Q2W | Phase: phase 3 RCT; indication: treatment naïve stage IV/recurrent PD-L1 positive NSCLC; treatment arms: (I) experimental arm 1: avelumab 10 mg/kg IV Q2W; (II) experimental arm 2: avelumab 10 mg/kg once a week for 12 weeks, later continued as Q2W till unacceptable toxicity or progression of disease; (III) active comparator arm: Platinum base chemotherapy for a maximum of 6 cycles; non-squamous NSCLC: pemetrexed 500 mg/m2 Q3W plus cisplatin 75 mg/m2 Q3W or pemetrexed 500 mg/m2 Q3W plus carboplatin AUC 6 mg/mL/min Q3W; squamous NSCLC: paclitaxel 200 mg/m2 Q3W plus carboplatin AUC 6 mg/mL/min Q3W, gemcitabine 1,000 mg/m2 on day 1, 8 of 3 weeks cycle plus carboplatin AUC 5 mg/mL/min Q3W or gemcitabine 1,250 mg/m2 on day 1, 8 of 3 weeks cycle plus cisplatin 75 mg/m2 Q3W; primary endpoint: PFS and OS for high PD-L1 expression tumors; secondary endpoint: PFS and OS for patients with high, moderate or any PD-L1 expression, duration of response, best overall response, change from baseline in EORTC QLQ-LC13, EORTC QLQ-C30 global health status and EQ-5D-5L health outcome questionnaire, number of patients with abnormal 12-lead ECG, ECOG performance status, treatment-emergent adverse events, vital signs, physical examination and safety laboratory tests graded by NCI-CTCAE | 1,095 | Recruiting | NCT02576574; JAVELIN lung 100 study |
| Avelumab (MSB0010718C) | Anti-PD-L1 MoAb | Avelumab 10 mg/kg IV over 60 minutes Q2W | Phase: phase 3 RCT; indication: PD-L1 positive NSCLC presenting with progression of disease after or during prior treatment with platinum doublet chemotherapy; treatment arms: (I) experimental arm: Avelumab 10 mg/kg IV Q2W; (II) comparator arm: Docetaxel 75 mg/m2 Q3W; (III) primary endpoint: OS, evaluated for a maximum of 7.6 years; secondary endpoint: PFS, best overall response, change from baseline in EORTC QLQ-LC13, EORTC QLQ-C30 global health status and EQ-5D-5L health outcome questionnaire, number of patients with treatment-emergent adverse events as graded by NCI-CTCAE |
792 | Active, not recruiting | NCT02395172; JAVELIN lung 200 study |
| Durvalumab MEDI4736 | anti-PD-L1 MoAb | NA | Phase: phase 3 RCT; indication: treatment naïve advanced NSCLC with high PD-L1 expression (≥25% staining) treatment arms: (I) experimental arm: durvalumab monotherapy; (II) active comparator: standard of care platinum-based chemotherapy (pemetrexed/cisplatin, pemetrexed/carboplatin, gemcitabine/cisplatin, gemcitabine/carboplatin); primary endpoint: PFS and OS; secondary endpoint: duration of response, ORR, PFS at 12 months, health-related quality of life as per EORTC QLQ-C30 questionnaire, cancer-related symptoms as per EORTC QLQ-LC13 questionnaire, determine immunogenicity of durvalumab by measurement of anti-drug antibodies, incidence of TRAEs | 440 | Recruiting | NCT03003962; PEARL trial |
MoAb, monoclonal antibody; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death ligand-1; AUC, area under concentration curve; RCT, randomized control trial; NSCLC, non-small cell lung cancer; PFS, progression free survival; OS, overall survival; ORR, objective response rate; TPS, tumor proportion score; TTD, time to deterioration; TTR, time to response; TIR, time in response; AE, adverse events; TRAE, treatment-related adverse events; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality of Life; EQ-5D-5L, European Quality of Life 5-dimensions; EORTC QLQ-LC13, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Lung Cancer 13; ECG, electrocardiogram; ECOG, Eastern Cooperative Oncology Group; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
The KEYNOTE 042 trial will analyze whether monotherapy with pembrolizumab is superior to standard of care chemotherapy in first-line setting for advanced NSCLC. This trial is currently underway to investigate treatment with pembrolizumab 200 mg IV every 3 weeks for 35 cycles as a first-line option for patients with PD-L1 positive, EGFR wild-type NSCLC without ALK translocation (NCT02220894) (56). The outcomes in the experimental arm will be compared against to those observed with standard of care platinum-based doublet chemotherapy (carboplatin/pemetrexed/paclitaxel), being administered in a parallel comparator arm. The randomization in the treatment arms will be stratified according to PD-L1 status, wherein outcomes in patients exhibiting 1–49% staining will be compared to those with ≥50% staining [as observed with immunohistochemistry (IHC) using 22C3 antibody]. Other stratification parameters include East Asian vs. non-East Asian region, non-squamous vs. squamous histology and performance status of 1 vs. 0. The primary objective of this study is to evaluate the OS of participants with strong PD-L1 positivity. Additionally, PFS and OS of all study participants and the PFS of participants with strong PD-L1 positivity will be also assessed as a secondary objective.
Next, the KEYNOTE 189 trial, is assessing combined application of pembrolizumab with chemotherapy in patients with non-squamous NSCLC that are not eligible for anti-EGFR/ALK mutation targeted therapy (NCT02578680). The experimental arm involves treatment with 4 cycles of pembrolizumab 200 mg once every 3 weeks plus pemetrexed 500 mg/m2 and carboplatin AUC 5 or cisplatin 75 mg/m2 once every 3 weeks, followed by pemetrexed 500 mg/m2 plus pembrolizumab 200 mg once every 3 weeks. The control arm has been designated to administer chemotherapy same as above but substitutes pembrolizumab with placebo. Assessment of PFS has been defined as the primary objective of this study. The duration of response, safety of experimental regimen vs. that of control arm, OS, ORR and PFS for study participants with a PD-L1 expression TPS score of ≥1% will be evaluated as a secondary endpoint during this trial.
Lastly, the KEYNOTE 407 trial is still in the recruitment phase. The aim of this study is to determine if the combined use of pembrolizumab with chemotherapy in treatment naïve stage IV squamous NSCLC would yield outcomes superior to standard of care chemotherapy (NCT02775435). The treatment regimen for the experimental arm has been defined as pembrolizumab 200 mg once every 3 weeks for 35 cycles plus 4 cycles of carboplatin AUC 6 once every 3 weeks plus paclitaxel 200 mg/m2 or nab-paclitaxel 100 mg/m2 on days 1, 8 and 15. The control arm will have the same regimen but will involve substitution of pembrolizumab with placebo. The primary endpoint of this trial would be to evaluate the PFS and OS over the course of 2 years, whereas the ORR will be assessed as a secondary objective.
Atezolizumab
Atezolizumab is currently being evaluated in three clinical trials, the IMpower 130, IMpower 131 and IMpower 150, to potentially expand its application in NSCLC (Table 2). Though the indication and treatment regimens are quite distinct among these trials, all the three trials have nominated the same FDA approved dose of atezolizumab (1,200 mg administered every 3 weeks) for investigation purposes.
The IMpower 130 study, a phase 3 RCT, intends to assess combined application of chemotherapy and atezolizumab in chemotherapy naïve stage IV non-squamous NSCLC patients (NCT02366143). This trial will compare experimental therapy consisting of atezolizumab 1,200 mg once every 3 weeks plus chemotherapy (4 or 6 cycles of nab-paclitaxel 100 mg/m2 on day 1, 8 and 15 plus carboplatin AUC 6 once every 3 weeks) vs. treatment with chemotherapy only. The primary endpoint for this trial has been defined as the investigator assessed PFS, which will be evaluated for 2.5 years. Assessment of the duration of response, objective response, PFS (evaluated by an independent review facility), OS, safety, changes in patient-reported cancer symptoms from baseline and time to deterioration (TTD) have been defined as the secondary endpoint for this study.
Next, the IMpower 131 study, a phase 3 RCT, will be investigating two different chemotherapy regimens in combination with atezolizumab for treatment naïve stage IV squamous NSCLC patients (NCT02367794). One of the experimental arms will administer atezolizumab 1,200 mg once every 3 weeks plus chemotherapy with 4 or 6 cycles of nab-paclitaxel 100 mg/m2 on day 1, 8 and 15 and carboplatin AUC 6 once every 3 weeks. The treatment regimen for the other experimental arm will consist of atezolizumab plus 4 or 6 cycles of paclitaxel 200 mg/m2 and carboplatin AUC 6 once every 3 weeks. Patients in the active comparator arm will be treated with carboplatin plus nab-paclitaxel chemotherapy only. The investigator-assessed PFS and OS have been defined as the primary objective for this trial. The secondary endpoints for this trial are objective response, assessment of the duration of response, PFS (evaluated by an independent review facility), time to response (TTR), time in response (TIR), incidence of adverse events, percent patients with anti-therapeutic antibody response to atezolizumab and changes in patient-reported cancer symptoms from baseline and TTD.
Lastly, the IMpower 150 study, a phase 3 RCT, aims to evaluate atezolizumab in combination with chemotherapy and bevacizumab for treating stage IV non-squamous NSCLC patients that have not received any prior therapy (NCT02366143). The first experimental regimen involved atezolizumab 1,200 mg once every 3 weeks plus 4 or 6 cycles of paclitaxel 200 mg/m2 and carboplatin AUC 6 once every 3 weeks. The second experimental regimen consists of atezolizumab 1,200 mg once every 3 weeks plus chemotherapy (as above) and bevacizumab 15 mg/kg IV once every three weeks. Study participants enrolled in the control arm will receive bevacizumab plus chemotherapy only. The primary objective of this study is to determine the investigator assessed PFS. The secondary endpoints include objective response, duration of response, changes in patient-reported cancer symptoms from baseline, PFS (evaluated by an independent review facility), OS, safety and TTD.
Avelumab
Avelumab is an anti-PD-L1 MoAb (30). It is presently being investigated for application in NSCLC in two trials, the JAVELIN lung 100 and the JAVELIN lung 200.
The JAVELIN lung 100 study, a phase 3 RCT, will investigate therapy with avelumab vs. chemotherapy for treatment naïve stage IV/recurrent PD-L1 positive NSCLC patients (NCT02576574). This study will consist of two experimental arms, each evaluating a unique avelumab monotherapy regimen. The first will be avelumab 10 mg/kg once every 2 weeks. The other experimental regimen will be avelumab 10 mg/kg once a week for 12 weeks, later continued as 10 mg/kg once every 2 weeks till unacceptable toxicity or progression of disease. Patients randomized to the comparator arm will be receiving platinum-based chemotherapy for a maximum of 6 cycles. This would consist of pemetrexed 500 mg/m2 plus cisplatin 75 mg/m2 once every 3 weeks or pemetrexed 500 mg/m2 plus carboplatin AUC 6 mg/mL/min once every 3 weeks for patients with non-squamous NSCLC. Patients with squamous NSCLC will be treated with either paclitaxel 200 mg/m2 plus carboplatin AUC 6 mg/mL/min once every 3 weeks, gemcitabine 1,000 mg/m2 on day 1, 8 of 3 weeks cycle plus carboplatin AUC 5 mg/mL/min once every 3 weeks or gemcitabine 1,250 mg/m2 on day 1, 8 of 3 weeks cycle plus cisplatin 75 mg/m2 once every 3 weeks. The primary objective of this study would be to determine the OS and the PFS for all treatment arms. As a secondary objective, this study will also assess PFS and OS for patients with high, moderate or any PD-L1 expression, duration of response and best overall response among other parameters.
The JAVELIN lung 200 study, a phase 3 RCT, will be comparing treatment with avelumab 10 mg/kg once every 2 weeks vs. docetaxel 75 mg/m2 once every 3 weeks for patients with PD-L1 positive NSCLC presenting with progression of disease after or during prior treatment with platinum doublet chemotherapy (NCT02395172). The primary objective of this study will be to evaluate and compare the OS recorded in avelumab and docetaxel treatment arms. As a secondary objective, the study will also assess the best overall response, PFS and number of patients with treatment-emergent adverse events as graded by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) among other parameters.
Durvalumab
Durvalumab, also known as MEDI4736, is an anti-PD-L1 MoAb (30). Presently, three trials are investigating potential application of monotherapy with durvalumab for NSCLC in different settings. These include the ARCTIC trial, MYSTIC trial and NCT03003962.
The ARCTIC trial, a phase 3 RCT, is a multi-arm study for patients with PD-L1 positive EGFR wild-type advanced/metastatic NSCLC without ALK translocation presenting after treatment failure on at-least 2 prior systemic therapies, with one being platinum-based chemotherapy (NCT02352948). In this study, the PD-L1 positivity criteria have been defined as ≥25% staining using VENTANA PD-L1 SP263 CDx assay. Herein, the efficacy of treatment with durvalumab monotherapy will be assessed for patients with both PD-L1 positive as well as PD-L1 negative tumors, in addition to other experimental regimens. Patients enrolled in comparator arm will be receiving chemotherapy with either vinorelbine 30 mg/m2 on days 1, 8, 15 and 22 of 28 days cycle, erlotinib 150 mg OD or gemcitabine 1,000 mg/m2 on days 1, 8, 15 of 28 days cycle. The primary objective of this study is to determine the OS and PFS for patients in each treatment arm, whereas the duration of response, ORR and OS at 12 months will be assessed as secondary endpoints.
The MYSTIC phase 3 RCT is yet another multi-arm study that will be evaluating monotherapy with durvalumab 20 mg/kg IV once every 4 weeks for treatment naïve EGFR wild-type metastatic/advanced NSCLC without ALK translocation (NCT02453282). Patients randomized to the comparator arm will receive treatment with standard of care platinum-based chemotherapy (carboplatin + pemetrexed/cisplatin + pemetrexed/carboplatin + gemcitabine/cisplatin + gemcitabine). The primary objective will be to determine and compare OS with durvalumab monotherapy vs. standard of care chemotherapy, whereas PFS, safety and ORR will be assessed as secondary endpoints.
The approval of durvalumab monotherapy for application in treatment naïve advanced NSCLC with high PD-L1 expression in Asia will be attempted through the phase 3 trial PEARL trial (NCT03003962). This trial defines the PD-L1 positivity criteria as ≥25% staining. Patients randomized to the experimental arm will receive durvalumab monotherapy whereas those enrolled in the comparator arm will be treated with standard of care platinum-based chemotherapy (pemetrexed/cisplatin, gemcitabine/carboplatin, pemetrexed/carboplatin or gemcitabine/cisplatin). The primary objective of this study will be to assess PFS and OS. The secondary objectives will include evaluation of ORR, PFS at 12 months, duration of response and incidence of TRAEs among other outcomes.
Dual immune-checkpoint inhibition therapy for NSCLC—ongoing registration trials
Currently, there are no approved dual immune-checkpoint inhibition regimens for NSCLC. Two regimens, nivolumab plus ipilimumab (anti-CTLA-4 MoAb) and tremelimumab (anti-CTLA-4 human MoAb) plus durvalumab (anti-PD-L1 MoAb), are presently being investigated in various clinical trials (Table 3) (30).
Table 3. Ongoing registration trials for combination immunotherapy in NSCLC.
| Immunotherapy agent | Mechanism of action | Treatment regimen | Trial description | Enrollment number | Trial status | NCT number/reference |
|---|---|---|---|---|---|---|
| Nivolumab, ipilimumab | Nivolumab: anti-PD-1 human MoAb; ipilimumab: anti-CTLA-4 MoAb | Nivolumab 3 mg/kg IV Q2W; ipilimumab 1 mg/kg IV Q6W | Phase: phase 3 RCT; indication: chemotherapy naïve recurrent/stage IV NSCLC; treatment arms: (I) experimental arm 1: nivolumab 3 mg/kg IV Q2W plus ipilimumab 1 mg/kg IV Q6W; (II) experimental arm 2: nivolumab monotherapy; (III) experimental arm 3: platinum doublet chemotherapy (carboplatin/cisplatin/paclitaxel/pemetrexed/gemcitabine) plus nivolumab; (IV) comparator arm: platinum doublet chemotherapy only; primary endpoint: PFS (assessed up to 40 months), OS (assessed up to 48 months); secondary endpoints: ORR and changes in patient-reported cancer symptoms from baseline (evaluated using Lung Cancer Symptom Score) | 2,220 | Recruiting |
NCT02477826; CheckMate 227 |
| Tremelimumab, durvalumab (MEDI4736) | Tremelimumab: anti-CTLA-4 human MoAb; durvalumab: anti-PD-L1 MoAb | Tremelimumab 1 mg/kg IV Q4W, durvalumab 20 mg/kg IV Q4W | Phase: phase 3 RCT; indication: chemotherapy naïve EGFR wild-type stage IV NSCLC without ALK translocation; treatment arms: (I) experimental arm: 4 doses of tremelimumab 1 mg/kg IV Q4W and 12 doses of durvalumab 20 mg/kg IV Q4W; (II) comparator arm: platinum doublet chemotherapy (carboplatin + pemetrexed/cisplatin + pemetrexed/carboplatin + gemcitabine/cisplatin + gemcitabine); primary endpoint: OS (assessed up to 4 years); secondary endpoints: ORR, PFS, duration of response, TRAE, assess and compare OS for PD-L1 negative patients | 960 | Recruiting |
NCT02542293; NEPTUNE trial (59) |
| Tremelimumab, durvalumab (MEDI4736) | Tremelimumab: anti-CTLA-4 human MoAb; durvalumab: anti-PD-L1 MoAb | Tremelimumab 1 mg/kg IV Q4W, durvalumab 20 mg/kg IV Q4W | Phase: phase 3 RCT; indication: treatment naïve EGFR wild-type metastatic/advanced NSCLC without ALK translocation; treatment arms: (I) experimental arm 1: tremelimumab 1 mg/kg IV Q4W plus durvalumab 20 mg/kg IV Q4W; (II) experimental arm 2: durvalumab monotherapy; (III) comparator arm: platinum doublet chemotherapy (carboplatin + pemetrexed/cisplatin + pemetrexed/carboplatin + gemcitabine/cisplatin + gemcitabine); primary endpoint: OS and PFS of combination therapy and OS of durvalumab monotherapy versus standard of care chemotherapy (assessed up to 3 years); secondary endpoints: ORR, PFS and safety | 1,092 | Active, not recruiting |
NCT02453282; MYSTIC trial |
| Tremelimumab, durvalumab (MEDI4736) | Tremelimumab: anti-CTLA-4 human MoAb; durvalumab: anti-PD-L1 MoAb | NA | Phase: phase 3 RCT; indication: PD-L1 positive EGFR wild-type advanced/metastatic NSCLC without ALK translocation presenting after treatment failure on at least 2 prior systemic therapies, with one being platinum-based chemotherapy; treatment arms for sub-study A (PD-L1 positive patients, defined as ≥PD-L1 positive patients, defined as ingCDx assay): (I) experimental arm 1: durvalumab monotherapy; (II) active comparator: investigator’s choice of chemotherapy (vinorelbine 30 mg/m2 on days 1, 8, 15 and 22 of 28 days cycle/erlotinib 150 mg OD/gemcitabine 1,000 mg/m2 on days 1, 8, 15 of 28 days cycle); treatment arms for sub-study B (PD-L1 negative patients): (I) experimental arm 1: durvalumab plus tremelimumab; (II) experimental arm 2: durvalumab monotherapy; (III) experimental arm 3: tremelimumab monotherapy; (IV) active comparator: investigator’s choice of chemotherapy (vinorelbine/gemcitabine/erlotinib); primary outcome: OS and PFS evaluated for up to 3 years; secondary outcomes: duration of response, ORR, OS at 12 months | 597 | Active, not recruiting |
NCT02352948; ARCTIC study (60) |
MoAb, monoclonal antibody; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death ligand-1; AUC, area under concentration curve; RCT, randomized control trial; NSCLC, non-small cell lung cancer; PFS, progression free survival; OS, overall survival; ORR, objective response rate; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; TRAE, treatment related adverse events; NA, not available.
The CheckMate 227 study, a phase 3 RCT, is assessing combination immunotherapy with nivolumab and ipilimumab in chemotherapy naïve recurrent/stage IV NSCLC patients (NCT02477826). The experimental regimen for dual-immune checkpoint inhibition consists of nivolumab 3 mg/kg once every 2 weeks plus ipilimumab 1 mg/kg once every 6 weeks. Participants randomized to the active comparator arm will receive treatment with platinum doublet chemotherapy only. The primary objective here is to assess PFS and OS whereas the secondary objectives are to assess the ORR and changes in patient-reported cancer symptoms from baseline (evaluated using Lung Cancer Symptom Score).
The ARCTIC trial, a phase 3 RCT, is a multi-arm study evaluating treatment with immune-checkpoint inhibitors in patients with PD-L1 positive EGFR wild-type advanced/metastatic NSCLC without ALK translocation presenting after treatment failure on at-least two prior systemic therapies, with one being platinum-based chemotherapy (NCT02352948). As mentioned earlier, the PD-L1 positivity criteria in this study has been defined as ≥25% staining using VENTANA PD-L1 SP263 CDx assay. In addition to durvalumab monotherapy treatment arms, this study will also be investigating treatment with tremelimumab monotherapy and combination immunotherapy with tremelimumab plus durvalumab for patients with PD-L1 negative tumors. Patients randomized to comparator arm will be receiving chemotherapy with either vinorelbine, erlotinib or gemcitabine. This study will determine the OS and PFS for patients for each treatment arm as a primary objective. The ORR, duration of response and OS at 12 months will be evaluated as secondary objectives.
The MYSTIC trial, a phase 3 RCT, is a multi-arm study recruiting patients with treatment naïve EGFR wild-type metastatic/advanced NSCLC without ALK translocation (NCT02453282). This study will evaluate combination immunotherapy with tremelimumab 1 mg/kg IV plus durvalumab 20 mg/kg IV once every 4 weeks. As stated previously, patients randomized to the comparator arm will receive standard of care platinum-based chemotherapy. The OS and PFS for combination with durvalumab and tremelimumab will be assessed as a primary endpoint whereas PFS, safety and ORR will be assessed as secondary objectives.
Lastly, the NEPTUNE trial is a phase 3 RCT evaluating combination therapy with tremelimumab and durvalumab for treatment naïve EGFR wild-type metastatic/advanced NSCLC without ALK translocation (NCT02542293). The patients randomized to receive investigational regimen will be treated with 4 doses of tremelimumab 1 mg/kg IV and 12 doses of durvalumab 20 mg/kg IV, once every 4 weeks. Patients in control arm will receive standard of care platinum doublet chemotherapy. The primary objective this trial will be to evaluate OS for up to 4 years. The OS for patients with PD-L1 negative tumors will be evaluated as a secondary endpoint.
Discussion
The success with immunotherapy in cancer therapeutics is exemplary in terms of the pace with which it is taking over the field of oncology. Immune-checkpoint inhibitors have demonstrated considerable advantage in both clinical efficacy and toxicity profile over present standard of care chemotherapy agents. The string of FDA approvals in a relatively short period of time has invigorated a surge in investigative efforts to further expand the indication and fast-track the development of various immunotherapy agents.
NSCLC has been inquisitively investigated as a target for immunotherapy agents. So far, nivolumab, pembrolizumab and atezolizumab have received approval by the FDA for use in advanced NSCLC. Remarkably, the process of approval for pembrolizumab followed an unprecedented course. Data from KEYNOTE 001 and KEYNOTE 021, a multi-arm phase 1/2 trial with unique lung cancer treatment arms, was used as the basis for approval. This allowed for an early introduction of drug, in-turn benefitting the patients in a time-efficient manner. The timeline for initiation of additional trials has also been impressive. Novel strategies that may potentially enhance outcomes with immune-checkpoint inhibitors are being actively explored in several ongoing trials (Tables 2,3). This includes both single-agent immunotherapy in combination with platinum based chemotherapy and dual-immune checkpoint inhibition regimens. In addition, a number of new immune-checkpoint inhibitors are presently being evaluated for potential application in both first-line and second-line settings.
The ideal PD-L1 positivity criteria for selection of patients that would benefit from anti-PD-1/PD-L1 immune-checkpoint inhibitors remains to be an enigma. In the KEYNOTE 024 trial, pembrolizumab outperformed chemotherapy in first-line settings for advanced NSCLC with a TPS score of ≥50% set as the criteria for PD-L1 positivity (51). On the other hand, the CheckMate 026 trial, wherein PD-L1 positivity was defined as ≥5% staining, nivolumab did not show any advantage over chemotherapy in survival outcomes when used in first-line settings for advanced NSCLC (55). In order to optimize case-selection for immunotherapy with anti-PD-1/PD-L1 immune-checkpoint inhibitors, upcoming trials have resorted to a more open approach. The CheckMate 227 trial, evaluating monotherapy with nivolumab in first-line setting for advanced NSCLC, will stratify outcomes on the basis of tumor PD-L1 expression ≥1% and <1%. Similarly, the KEYNOTE 042 trial that is presenting evaluating monotherapy with pembrolizumab in first-line setting for advanced NSCLC, will present stratified data for two groups, tumors with ≥50% PD-L1 expression and 1–49% PD-L1 expression. It is expected that these studies and others alike would provide crucial data to define the most appropriate PD-L1 positivity criteria and make way for expansion of indication for immune-checkpoint inhibitors.
The toxicity profile with immune-checkpoint inhibitors has been identified as a subject of concern. The FDA approved combination immunotherapy with nivolumab and ipilimumab for melanoma was noted to have a poor toxicity profile (61). A higher incidence of TRAEs may put therapy with immune-checkpoint inhibitors at a disadvantage to alternative regimens, especially when considering treatment options for patients with poor performance status. In lung cancer, however, attempts to meet this challenge have been made by introduction and evaluation of low-dose regimens that are less toxic and exhibit statistically significant clinical efficacy (62). Dual-immune-checkpoint inhibition with other immunotherapy agents such as tremelimumab and durvalumab presently being investigated may serve as an alternative strategy to achieve superior outcomes as compared to currently approved combination regimen. Furthermore, newly identified immune-checkpoint interactions are being explored for possible use as targets for novel immunotherapy agents. This includes OX-40/OX-40L, CD27/CD70 and 4-1BB/4-1BBL among others (23-26,63-66). Ongoing trials evaluating immunotherapy agents in variant settings are expected to yield critical data that would shape an ideal immunotherapy regimen.
Conclusions
Immunotherapy is an upcoming treatment strategy with the potential to change the landscape of cancer therapeutics. Several clinical studies have demonstrated outcomes with immune-checkpoint inhibitors to be superior in comparison with chemotherapy in advanced NSCLC. A number of concerns, especially treatment-related toxicity associated with the use of immunotherapy agents, have also come to light. Nevertheless, efforts are underway to meet these challenges and optimize the application of immunotherapy in lung cancer.
Intensive research at several levels is steadily paving the path towards conceiving an ideal immunotherapy regimen for lung cancer. Presently, immune-checkpoint inhibitors are being assessed in combination with chemotherapy and in dual-immune checkpoint inhibitor regimens, in an attempt to achieve better clinical efficacy and toxicity profile. Several new immunotherapy agents are also under investigation, including tremelimumab and durvalumab. Concurrently, new immune-checkpoint pathways are being identified to act as targets for the next generation of immunotherapy agents.
Though the upcoming treatment strategies involving immune-checkpoint inhibitors are promising in terms of achieving superior clinical efficacy than present standard of care therapy, it is important to strike the right balance with treatment-related toxicity. Efforts made towards optimizing the case-selection process will provide a major footing in the direction of accomplishing this goal. Clearly defined criteria for positivity of available biomarkers would be vital in avoiding therapies that are unlikely to be of any benefit in a given scenario. Additionally, the development of new and more reliable biomarkers would also go a long way in enhancing clinical outcomes with various immunotherapy agents.
The above efforts directed at advancing immunotherapy for application in NSCLC have crafted the most dynamic landscape thus far in cancer therapeutics. However, considering the current scenario, it may be argued that immunotherapy does appear to have the potential to outperform and replace chemotherapy as the primary treatment modality for lung cancer in not so distant future.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. Treatment of non-small cell lung cancer (NSCLC). J Thorac Dis 2013;5 Suppl 4:S389-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DB, Rioth MJ, Horn L. Immune checkpoint inhibitors in NSCLC. Curr Treat Options Oncol 2014;15:658-69. 10.1007/s11864-014-0305-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorsteinsson H, Alexandersson A, Oskarsdottir GN, et al. Resection rate and outcome of pulmonary resections for non-small-cell lung cancer: a nationwide study from Iceland. J Thorac Oncol 2012;7:1164-9. 10.1097/JTO.0b013e318252d022 [DOI] [PubMed] [Google Scholar]
- 5.Su S, Hu Y, Ouyang W, et al. The survival outcomes and prognosis of stage IV non-small-cell lung cancer treated with thoracic three-dimensional radiotherapy combined with chemotherapy. Radiat Oncol 2014;9:290. 10.1186/s13014-014-0290-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicenas S, Zaliene A, Atkocius V. Treatment outcome of locally advanced stage IIIA/B lung cancer. Medicina (Kaunas) 2009;45:452-9. [PubMed] [Google Scholar]
- 7.Walker P, Rosenman J, Arastu H, et al. Oligometastatic Non-Small Cell Lung Cancer Treated With Curative Intent. Int J Radiat Oncol Biol Phys 2014;90:S48 10.1016/j.ijrobp.2014.08.239 [DOI] [Google Scholar]
- 8.Hung JY, Durrani HP, Aljumaily R, et al. Oligometastatic non-small cell lung cancer treated with curative intent. Alexandria, VA: ASCO, 2014. [Google Scholar]
- 9.Wanders R, Steevens J, Botterweck A, et al. Treatment with curative intent of stage III non-small cell lung cancer patients of 75years: a prospective population-based study. Eur J Cancer 2011;47:2691-7. 10.1016/j.ejca.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 11.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 12.Barlesi F, Pujol JL, Daures JP. Should chemotherapy (Cx) for advanced non-small cell lung cancer (NSCLC) be platinum-based? A literature-based meta-analysis of randomized trials. J Clin Oncol 2005;24:673s. [DOI] [PubMed] [Google Scholar]
- 13.Seder CW, Allen MS, Cassivi SD, et al. Stage IIIA non-small cell lung cancer: morbidity and mortality of three distinct multimodality regimens. Ann Thorac Surg 2013;95:1708-16. 10.1016/j.athoracsur.2013.02.041 [DOI] [PubMed] [Google Scholar]
- 14.Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. 10.1016/S0025-6196(11)60735-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disis ML. Mechanism of action of immunotherapy. Semin Oncol 2014;41 Suppl 5:S3-13. 10.1053/j.seminoncol.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974-82. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 1996;183:2533-40. 10.1084/jem.183.6.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011;332:600-3. 10.1126/science.1202947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley JL, Mao M, Kobayashi S, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A 2002;99:11790-5. 10.1073/pnas.162359999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science 2006;313:1972-5. 10.1126/science.1131078 [DOI] [PubMed] [Google Scholar]
- 21.Linsley PS, Brady W, Urnes M, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991;174:561-9. 10.1084/jem.174.3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrizosa DR, Gold KA. New strategies in immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2015;4:553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. Alexandria, VA: ASCO, 2014. [Google Scholar]
- 25.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev 2003;14:265-73. 10.1016/S1359-6101(03)00025-X [DOI] [PubMed] [Google Scholar]
- 26.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther 2012;11:1062-70. 10.1158/1535-7163.MCT-11-0677 [DOI] [PubMed] [Google Scholar]
- 27.Linch SN, McNamara MJ, Redmond WL. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front Oncol 2015;5:34. 10.3389/fonc.2015.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrissey KM, Yuraszeck T, Li CC, et al. Immunotherapy and novel combinations in oncology: current landscape, challenges, and opportunities. Clin Transl Sci 2016;9:89-104. 10.1111/cts.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dictionary ND. National Cancer Institute at the National Institutes of Health. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug
- 31.Raedler LA. Keytruda (Pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits 2015;8:96. [PMC free article] [PubMed] [Google Scholar]
- 32.Raedler LA. Opdivo (Nivolumab): second PD-1 inhibitor receives FDA approval for unresectable or metastatic melanoma. Am Health Drug Benefits 2015;8:180. [PMC free article] [PubMed] [Google Scholar]
- 33.Argiris A, Gillison M, Ferris R, et al. A randomized, open-label, phase 3 study of nivolumab in combination with ipilimumab vs extreme regimen (cetuximab+ cisplatin/carboplatin+ fluorouracil) as first-line therapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck-CheckMate 651. Ann Oncol 2016;27:1016TiP.
- 34.Spigel DR, Reckamp KL, Rizvi NA, et al. A phase III study (CheckMate 017) of nivolumab (NIVO; anti-programmed death-1 [PD-1]) vs docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:8009. [Google Scholar]
- 35.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 37.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 38.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 2011;17:6958-62. 10.1158/1078-0432.CCR-11-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sul J, Blumenthal GM, Jiang X, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist 2016;21:643-50. 10.1634/theoncologist.2015-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312-22. 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 43.Ferris R, Blumenschein G, Fayette J, et al. Further evaluations of nivolumab (nivo) versus investigator’s choice (IC) chemotherapy for recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): CheckMate 141. J Clin Oncol 2016;34:abstr 6009.
- 44.Seiwert TY, Haddad RI, Gupta S, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): preliminary results from KEYNOTE-012 expansion cohort. J Clin Oncol 2015;33:abstr lba6008.
- 45.Moskowitz CH, Ribrag V, Michot JM, et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013). Blood 2014;124:290. [Google Scholar]
- 46.Chen R, Zinzani P, Fanale M, et al. Pembrolizumab for relapsed/refractory classical Hodgkin lymphoma (R/R cHL): phase 2 KEYNOTE-087 study. J Clin Oncol 2016;34:abstr 7555.
- 47.Ansell S, Armand P, Timmerman JM, et al. Nivolumab in patients (pts) with relapsed or refractory classical Hodgkin lymphoma (R/R cHL): clinical outcomes from extended follow-up of a phase 1 study (CA209-039). Blood 2015;126:583. [Google Scholar]
- 48.Younes A, Santoro A, Zinzani P, et al. Checkmate 205: Nivolumab (nivo) in classical Hodgkin lymphoma (cHL) after autologous stem cell transplant (ASCT) and brentuximab vedotin (BV)–A phase 2 study. J Clin Oncol 2016;34:7535. [Google Scholar]
- 49.Kaufman H, Russell J, Hamid O. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic Merkel cell carcinoma previously treated with chemotherapy: Results of the phase 2 JAVELIN Merkel 200 trial. J Clin Oncol 2016;34:abstr 9508.
- 50.Massard C, Gordon M, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), a PD-L1 antibody, in urothelial bladder cancer. J Clin Oncol 2016;34:abstr 4502. [DOI] [PMC free article] [PubMed]
- 51.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 52.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 54.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 55.Socinski M, Creelan B, Horn L, et al. NSCLC, metastaticCheckMate 026: A phase 3 trial of nivolumab vs investigator's choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1)− positive NSCLC. Ann Oncol 2016;27:LBA7._PR. 10.1093/annonc/mdw435.39 [DOI] [Google Scholar]
- 56.Mok T, Wu YL, Watson PA, et al. Phase 3 KEYNOTE-042 trial of pembrolizumab (MK-3475) versus platinum doublet chemotherapy in treatment-naive patients (pts) with PD-L1–positive advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr TPS8105.
- 57.Hall R, Gadgeel S, Garon E. Phase 3 study of platinumbased chemotherapy with or without pembrolizumab for first-line metastatic, nonsquamous non-small cell lung carcinoma (NSCLC): KEYNOTE-189. J Clin Oncol 2016;34:abstr TPS9104.
- 58.Mok TSK, Cappuzzo F, Jotte RM, et al. 356TiPPhase III clinical trials of atezolizumab in combination with chemotherapy in chemotherapy-naive patients with advanced NSCLC. Ann Oncol 2015;26:ix106 10.1093/annonc/mdv528.13 [DOI] [Google Scholar]
- 59.Mok T, Schmid P, Arén O, et al. 192TiP: NEPTUNE: A global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab combination therapy versus standard of care (SoC) platinum-based chemotherapy in the first-line treatment of patients (pts) with advanced or metastatic NSCLC. J Thorac Oncol 2016;11:S140-1. 10.1016/S1556-0864(16)30301-X27198329 [DOI] [Google Scholar]
- 60.Planchard D, Shtivelband M, Shi K, et al. A phase III study of MEDI4736 (M), an anti-PD-L1 antibody, in monotherapy or in combination with Tremelimumab (T), versus standard of care (SOC) in patients (pts) with advanced non-small cell lung cancer (NSCLC) who have received at least two prior systemic treatment regimens (ARCTIC). J Clin Oncol 2015;33:abstr tps8104.
- 61.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N Engl J Med 2015;372:2006-17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hellmann M, Gettinger S, Goldman J, et al. CheckMate 012: safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. J Clin Oncol 2016;34:abstr 3001.
- 63.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 1997;3:682-5. 10.1038/nm0697-682 [DOI] [PubMed] [Google Scholar]
- 64.Bartkowiak T, Curran MA. 4-1BB agonists: multi-potent potentiators of tumor immunity. Front Oncol 2015;5:117. 10.3389/fonc.2015.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sznol M, Hodi F, Margolin K, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J Clin Oncol 2008;26:abstr 3007.
- 66.Ansell SM, Northfelt DW, Flinn I, et al. Phase I evaluation of an agonist anti-CD27 human antibody (CDX-1127) in patients with advanced hematologic malignancies. J Clin Oncol 2014;32:3024. [Google Scholar]


