Abstract
Background
The aim of this multicenter study was to evaluate the safety and efficacy of tolvaptan (TLV) in Korean patients with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH).
Methods
Of 51 enrolled patients with SIADH, 39 patients (16 female patients, aged 70.8 ± 11.3 years) were included in an intention to treat analysis. All patients received 15 mg/day as the initial dose, and the dose was then increased up to 60 mg/day (as needed) until day 4.
Results
Serum sodium increased significantly from baseline during the first 24 hours (126.8 ± 4.3 vs. 133.7 ± 3.8 mmol/L, P < 0.001), rose gradually between days 1 and 4 (133.7 ± 3.8 vs. 135.6 ± 3.6 mmol/L, P < 0.05), and then plateaued until day 11 (136.7 ± 4.5 mmol/L). The correlation between the change in serum sodium for the first 24 hours and initial serum sodium concentration was significant (r = −0.602, P < 0.001). In severe hyponatremia (< 125 mmol/L), the change was significantly higher (11.1 ± 4.8 mmol/L) than in moderate (6.4 ± 2.5 mmol/L, P < 0.05) or mild hyponatremia (4.3 ± 3.3 mmol/L, P < 0.01). In addition, logistic regression analysis showed that body weight (odds ratio [OR], 0.858; 95% confidence interval [CI], 0.775–0.976; P = 0.020) and body mass index (BMI) (OR, 0.692; 95% CI, 0.500–0.956; P = 0.026) were associated with rapid correction. No serious adverse events were reported, but in 13% of patients hyponatremia was overcorrected.
Conclusion
TLV is effective in correcting hyponatremia and well-tolerated in Korean patients with SIADH. However, those with low body weight, low BMI or severe hyponatremia, could be vulnerable to overcorrection with the initial dose of 15 mg TLV.
Keywords: Hyponatremia, Inappropriate ADH Syndrome, Tolvaptan
Graphical Abstract
INTRODUCTION
Tolvaptan (TLV), an orally active, non-peptide, selective vasopressin-2 receptor antagonist,1 is an effective treatment for euvolemic and hypervolemic hyponatremia, as proven by large randomized control trials (such as Study of Ascending Levels of Tolvaptan in Hyponatremia [SALT] and Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan [EVEREST]),2,3 and by other smaller studies worldwide.4,5,6,7 Nevertheless, the use of TLV for patients with hyponatremia due to liver cirrhosis was excluded by the US Food and Drug Administration in 2013 because the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) trial for autosomal dominant polycystic kidney disease found severe toxic liver injury in the TLV group,8 although this adverse event (AE) was not reported in the Safety and sodium Assessment of Long-term Tolvaptan With hyponatremia: A year-long, open-label Trial to gain Experience under Real-world conditions (SALTWATER) trial.9,10
In Korea and the US, TLV is indicated for patients with hyponatremia due to congestive heart failure or syndrome of inappropriate secretion of antidiuretic hormone (SIADH) when they have a serum sodium concentration of < 125 mmol/L that is symptomatic and resistant to fluid restriction. However, recent guidelines from the US and Europe for the treatment of patients with hyponatremia have differing opinions on the usage of vaptans, including TLV.11,12 The European guidelines consider the rapid correction of serum sodium and hepatotoxicity of vaptans as “concerning,” and many experts do not recommend the use of vaptans for patients with hyponatremia of any cause,12 although they are currently indicated for use in the US.11
These conflicting trial results, expert opinions and guidelines are potentially confusing for physicians considering the use of TLV, and, in addition, there are insufficient data on the use of vaptans in patients with euvolemic or hypervolemic hyponatremia in Korea. Therefore, the aim of this study was to evaluate the short-term efficacy and safety of TLV in Korean patients with hyponatremia caused by SIADH, and to identify any factors influencing the change in serum sodium concentration during the first 24 hours after administration of 15 mg TLV; we also wanted to determine whether Korean patients respond to TLV differently from patients of other nationalities.
METHODS
This study was a prospective, multicenter, phase IV, open-label, safety and efficacy trial of TLV. Patients were recruited from eight general hospitals in Korea between June 1, 2013 and December 31, 2014.
Patients
Eligible patients with SIADH were 20 years of age or older and had chronic, euvolemic hyponatremia (defined as serum sodium concentration < 135 mmol/L) without acute or chronic neurological symptoms, such as seizure, stupor or coma, and urine osmolality was inappropriately high (> 100 mOsm/L). Mild hyponatremia was defined as a serum sodium concentration between 130 and 134 mmol/L, moderate hyponatremia was between 129 and 125 mmol/L, and severe hyponatremia was less than 125 mmol/L. Ineligible diseases and conditions included: 1) hypovolemic hyponatremia, psychogenic polydipsia, head trauma, postoperative conditions, uncontrolled hypothyroidism or adrenal insufficiency; 2) cardiac surgery, myocardial infarction, sustained ventricular tachycardia or fibrillation, severe angina, cerebrovascular accident, or stroke during the previous three months; 3) systolic blood pressure (BP) < 90 mmHg, estimated glomerular filtration rate (eGFR) calculated using the modification of diet and renal disease equation (MDRD) of < 60 mL/min/1.73 m2; 4) urinary tract obstruction; 5) uncontrolled diabetes mellitus with a fasting glucose greater than 200 mg/dL; 6) progressive or episodic neurologic disease; 7) severe liver disease with transaminases more than three times the upper limit of normal (ULN); 8) malignancy with life expectancy less than 6 months; 9) patients who received any drugs interacting with CYP3A inhibitors (such as clarithromycin, ketoconazole, itraconazole, nefazodone and telithromycin); 10) pregnant or lactating women; and 11) drug-induced hyponatremia.
Study protocol
The enrolled patients received oral TLV once daily for up to 11 days. TLV was administered in an inpatient setting during 4 days without fluid restriction. All patients received the starting dose of 15 mg; then the dose was increased to 30 mg and then to 60 mg, as necessary, according to a regimen designed to slowly increase serum sodium concentration to 135 mmol/L or higher. If the serum sodium remained below 135 mmol/L and had increased by less than 5 mmol/L in the previous 24 hours, the dose was increased. If it rose above 145 mmol/L or increased by more than 12 mmol/L in 24 hours (or by more than 8 mmol/L during the first 8 hours of therapy), the next dose was either withheld or decreased, or the patient's fluid intake was increased. Patients were hospitalized for the first day of the study and discharged by day 4. No further change in dosage was permitted after day 4, and the dosage was maintained until day 11, at which point TLV administration was stopped until day 18. Overcorrection of serum sodium was defined as an increase of serum sodium exceeding 12 mmol/L over 24 hours or 8 mmol/L over 8 hours.
Study assessments
Patients were evaluated at baseline (0), then at 4, 8, and 24 hours after the first dose of TLV, and on days 4, 11, and 18. The primary outcomes were the rate of correction of serum sodium concentration over the first 24 hours of administration of TLV, and the average serum sodium concentrations on days 0, 4, 11 and 18. The secondary outcomes were 1) a comparison of the responses in patients with mild, moderate, and severe hyponatremia and 2) identification of any risk factors for rapid correction of serum sodium.
AEs were defined as overcorrection, or any new medical problems, or deterioration of the underlying medical condition. All AEs, including serious AEs (SAEs), and those potentially related to the administration of TLV, were evaluated to determine the proportion and number of patients reporting AEs.
Statistical analyses
Changes in serum sodium levels from baseline to 4, 8, 24 hours, and days 4, 11, and 18 were analyzed with Wilcoxon signed-rank test using the program IBM SPSS Statistics version 22.0 (IBM SPSS Statistics Inc., Chicago, IL, USA). We used the intention to treat (ITT) method, and therefore included data from patients who dropped out after the initial dosing in the analysis of changes in serum sodium concentration during the first 24 hours of administration of 15 mg TLV because our main focus was to determine the rate of correction of serum sodium concentration within the first 24 hours. Categorical variables were compared using Pearson's χ2 test. Correlation analysis was performed using Spearman's rank correlation coefficient. The Mann-Whitney U test, the Wilcoxon signed-rank test and the Kruskal-Wallis test were used to analyze subgroups. Logistic regression analysis was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Data are shown as means ± standard deviations (SDs), unless indicated otherwise. P values of less than 0.05 were considered significant.
Ethics statement
The current study was undertaken in accordance with the principles outlined in the Declaration of Helsinki. The Institutional Review Board of Hanyang University Guri Hospital (approval No. 2012-01-096) assessed the study protocol and ensured that a written informed consent was obtained from all patients, and the patients permitted that the data could be used in analysis regardless of withdrawal. Because this was an open-label study, patients were informed of potential side effects and AEs that might be caused by TLV, including dry mouth, thirst, polydipsia, pollakiuria, hypernatremia, hepatotoxicity, osmotic demyelination syndrome and etc.
RESULTS
Baseline characteristics
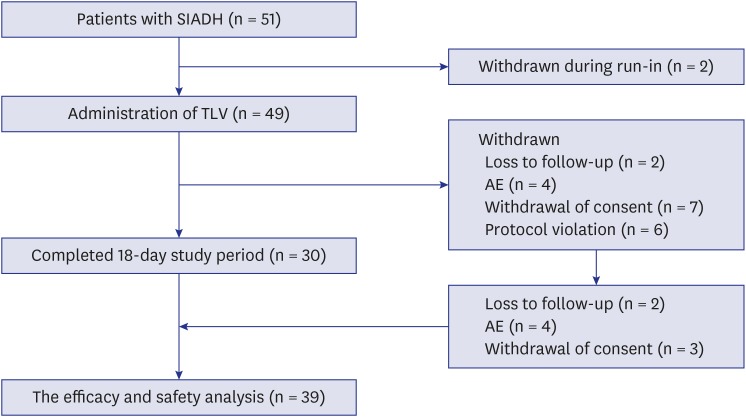
Of the 51 enrolled patients with chronic, euvolemic hyponatremia caused by SIADH, 30 completed both the 11-day treatment and the 7-day follow-up (Fig. 1). Two patients were withdrawn during run-in period, and 19 patients dropped out due to protocol violation (n = 6), loss to follow-up (n = 2), withdrawal of consent (n = 7), and AEs (n = 4) (Fig. 1). The data from 9 patients who dropped out were included in the ITT analysis of the demographic data and changes in serum sodium concentration during the first 24 hours after administration of 15 mg TLV (Table 1 and Fig. 1), because we collected data in these patients for more than 24 hours. Thus, data from 39 patients were analyzed. The mean age of the 39 patients included in the analysis was 70.8 ± 11.3 years, and 23 (59%) were male. Mean aspartate aminotransferase (AST; 22.9 ± 8.1 IU/L) and alanine aminotransferase (ALT; 19.3 ± 13.8) were within three-times ULN, and the mean creatinine level was 0.66 ± 0.22 mg/dL (Table 1). Other demographic data and baseline clinical features are shown in Table 1.
Fig. 1.
Flowchart of the enrollment and follow-up of patients.
SIADH = syndrome of inappropriate secretion of antidiuretic hormone, TLV = tolvaptan, AE = adverse event.
Table 1. Baseline characteristics of Korean patients with hyponatremia caused by SIADH, and changes in serum sodium concentration during the study period.
| Variables | Values (n = 39) | |
|---|---|---|
| Age, yr | 70.8 ± 11.3 (22–87) | |
| Height, cm | 160.9 ± 8.4 (146–176) | |
| Weight, kg | 57.1 ± 10.0 (40–95) | |
| BMI, kg/m2 | 22.1 ± 3.1 (14.7–32.5) | |
| Female (%) | 16 (41%) | |
| Cr, mg/dL | 0.66 ± 0.22 (0.3–1.1) | |
| AST, IU/L | ||
| AST (initial) | 22.9 ± 8.1 (21–55) | |
| AST (11 days) | 23.1 ± 5.7 (14–37) | |
| ALT, IU/L | ||
| ALT (initial) | 19.3 ± 13.8 (6–76) | |
| ALT (11 days) | 19.3 ± 10.8 (7–51) | |
| Na, mmol/L | ||
| Na (initial) | 126.8 ± 4.3 (116–134) | |
| Na (4 hours) | 129.6 ± 4.4a (121–137) | |
| Na (8 hours) | 132.7 ± 4.6b (124–141) | |
| Na (24 hours) | 133.7 ± 3.8c (127–143) | |
| Na (4 days) | 135.6 ± 3.6d (126–141) | |
| Na (11 days) | 136.7 ± 4.5 (120–142) | |
| Na (18 days) | 128.7 ± 8.8e (106–139) | |
| ΔNa, mmol/L | ||
| ΔNa (4 hours) | 2.8 ± 3.3 (−5–10) | |
| ΔNa (8 hours) | 5.9 ± 4.1 (−1–15) | |
| ΔNa (24 hours) | 6.8 ± 4.2 (−1–17) | |
| The changes of average AUC (4 days) | 8.1 ± 3.9 (2–16) | |
Data are presented as means ± SDs (range) or number (%).
SIADH = syndrome of inappropriate secretion of antidiuretic hormone, BMI = body mass index, Cr = creatinine, AST = aspartate aminotransferase, ALT = alanine aminotransferase, Na = serum sodium level, ΔNa = the change in serum sodium level, AUC = area under curve, SD = standard deviation.
aP < 0.001, 0 hours vs. 4 hours; bP < 0.001, 4 hours vs. 8 hours; cP < 0.05, 8 hours vs. 24 hours; dP < 0.05, 24 hours vs. 4 days; eP < 0.001, 11 days vs. 18 days.
From day 4 onwards, twenty-seven patients (69%) received 15 mg of TLV per day, nine patients (23%) received 30 mg of TLV per day, and three patients (8%) received 60 mg of TLV per day.
Serum sodium concentrations
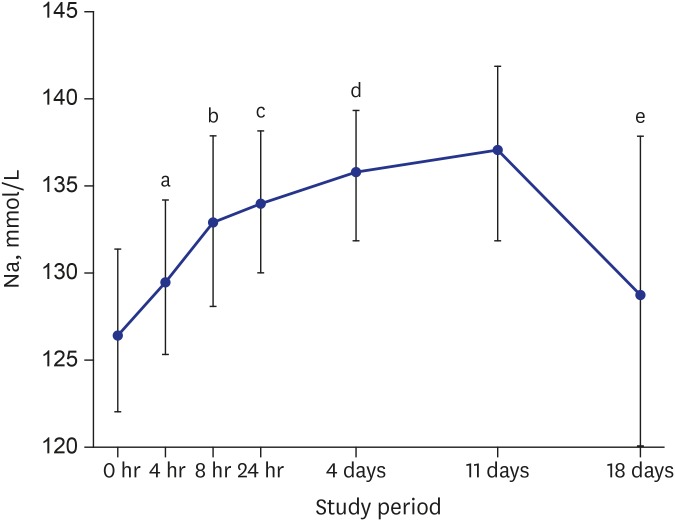
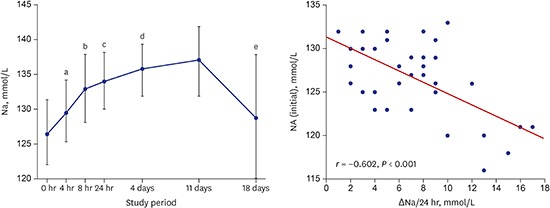
The serum sodium levels of all the patients increased significantly between baseline (126.8 ± 4.3 mmol/L) and 4 hours (129.6 ± 4.4 mmol/L, n = 39, P < 0.001), between 4 and 8 hours (132.7 ± 4.6 mmol/L, n = 39, P < 0.001), and between 8 and 24 hours (133.7 ± 3.8 mmol/L, n = 39, P < 0.05). Between 24 hours and day 4 the increase was more gradual (135.6 ± 3.6 mmol/L, n = 35, P < 0.05), and the level reached was maintained up to day 11 (136.7 ± 4.5 mmol/L, n = 32). At the follow-up visit on day 18, seven days after discontinuation of TLV, serum sodium levels had decreased to near-baseline levels (128.7 ± 8.8 mmol/L, n = 30, P = 0.342) (Table 1 and Fig. 2). The average increase in serum sodium level during the first 24 hours of administration of TLV was 6.8 ± 4.2 mmol/L, which agrees with the increase recommended by an expert panel and clinical practice guidelines.11,12
Fig. 2.
Mean (± SD) changes in Na during the study period.
SD = standard deviation, Na = serum sodium level.
a0 hours vs. 4 hours, P < 0.001; b4 hours vs. 8 hours, P < 0.001; c8 hours vs. 24 hours, P < 0.05; d24 hours vs. 4 days, P < 0.05; e11 days vs. 18 days, P < 0.001.
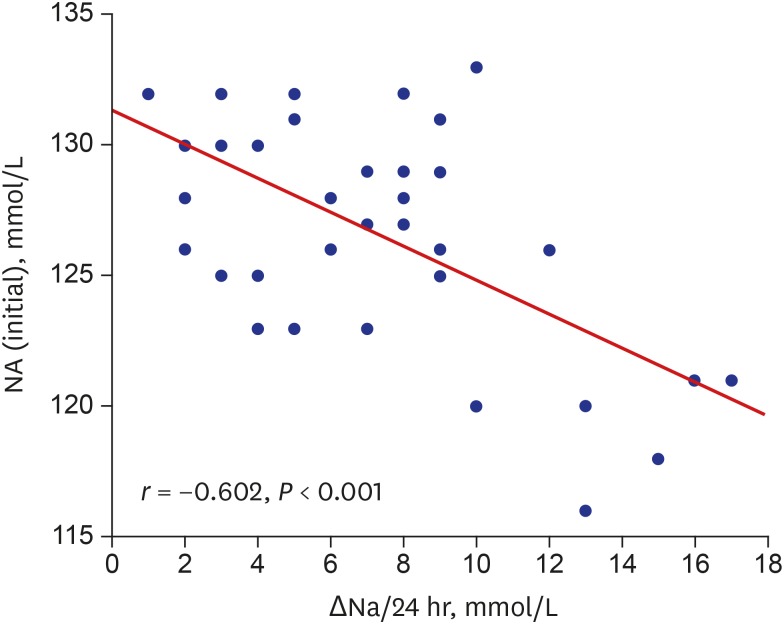
There was an inverse correlation between baseline serum sodium levels and changes in serum sodium levels over the first 24 hours (r = −0.602, P < 0.001, Fig. 3); the lower the initial serum sodium, the greater the increase in serum sodium with treatment with talvaptan.
Fig. 3.
Correlation of initial Na with ΔNa during the first 24 hours of administration of TLV (15 mg).
Na = serum sodium level, ΔNa = the change in serum sodium level, TLV = tolvaptan.
The change in serum sodium concentration during the first 24 hours in patients with severe hyponatremia at baseline (11.1 ± 4.8 mmol/L) was greater than in patients with mild (4.3 ± 3.3 mmol/L, P < 0.01) or moderate (6.4 ± 2.5 mmol/L, P < 0.05) hyponatremia (Table 2). In five (55%) patients out of nine with severe hyponatremia (serum Na < 125 mEq/L), the rate of change of serum sodium was more than 1 mmol per hour at 8 hours and 0.5 mmol per hour at 24 hours.
Table 2. Effect of TLV on serum sodium in patients with severe, moderate or mild hyponatremia.
| Variables | < 125 mmol/L (n = 9, 23%) | 125–129 mmol/L (n = 18, 46%) | 130–134 mmol/L (n = 12, 31%) | P value | |
|---|---|---|---|---|---|
| Age, yr | 67.1 ± 9.0 (59–85) | 70.1 ± 13.5 (22–87) | 74.7 ± 8.3 (58–84) | 0.115 | |
| Height, cm | 161.1 ± 9.5 (147–73) | 162.0 ± 7.4 (151–175) | 158.7 ± 9.4 (146–176) | 0.607 | |
| Weight, kg | 56.4 ± 7.7 (48–73) | 60.7 ± 11.4 (47–95) | 51.9 ± 6.9 (40–62) | 0.063 | |
| BMI, kg/m2 | 21.8 ± 2.8 (16.2–25.5) | 23.0 ± 3.1 (19.6–32.5) | 20.7 ± 3.1 (14.7–23.9) | 0.379 | |
| Female, % | 2 (22%) | 5 (28%) | 9 (75%) | 0.015 | |
| Na, mmol/L | |||||
| Na (initial) | 120.6 ± 2.4 (116–123) | 126.9 ± 1.5 (125–129) | 131.4 ± 1.3 (130–134) | < 0.001 | |
| Na (4 hours) | 125.3 ± 3.2 (121–131) | 129.6 ± 3.2 (124–135) | 132.9 ± 4.0 (125–137) | 0.001 | |
| Na (8 hours) | 128.6 ± 3.4 (124–135) | 132.9 ± 4.2 (128–140) | 135.6 ± 4.0 (129–141) | 0.002 | |
| Na (24 hours) | 131.7 ± 3.8 (127–138) | 133.4 ± 3.4 (128–138) | 135.7 ± 3.6 (132–143) | 0.124 | |
| Na (4 days) | 133.3 ± 3.9 (126–139) | 135.3 ± 3.4 (127–140) | 137.9 ± 2.4 (133–141) | 0.011 | |
| Na (11 days) | 132.7 ± 7.3 (120–142) | 137.2 ± 3.0 (131–142) | 138.3 ± 2.7 (134–142) | 0.143 | |
| Na (18 days) | 119.8 ± 10.6 (106–133) | 129.1 ± 8.3 (113–138) | 132.4 ± 5.9 (124–139) | 0.084 | |
| ΔNa, mmol/L | |||||
| ΔNa (4 hours) | 4.8 ± 3.6 (−1–10) | 2.7 ± 2.7 (−2–8) | 1.5 ± 3.4 (−5–5) | 0.154 | |
| ΔNa (8 hours) | 8.0 ± 4.8 (1–15) | 6.1 ± 3.8 (0–13) | 4.2 ± 3.4 (−1–9) | 0.181 | |
| ΔNa (24 hours) | 11.1 ± 4.8a,b (4–17) | 6.4 ± 2.5 (2–9) | 4.3 ± 3.3 (−1–10) | 0.005 | |
Data are presented as mean ± SD (range) or number (%).
TLV = tolvaptan, BMI = body mass index, Na = serum sodium level, ΔNa = the change in serum sodium level, SD = standard deviation.
aP < 0.05, < 125 mmol/L vs. 126–129 mmol/L; bP < 0.01, < 125 mmol/L vs. 130–134 mmol/L.
In logistic regression analysis, we compared the patients whose serum sodium levels increased by ≥8 mmol/L in the first 24 hours after administration of TLV (rapid group, n = 18) with those whose serum sodium levels increased by < 8 mmol/L (regular group, n = 21). Body weight (54.0 ± 8.9 kg vs. 60.0 ± 10.3 kg, P = 0.038), body mass index (BMI; 20.9 ± 2.6 vs. 23.1 ± 3.3, P = 0.022) and serum creatinine levels (0.56 ± 0.18 mg/dL vs. 0.73 ± 0.22 mg/dL, P = 0.020) were significantly lower, and eGFR (154.9 ± 61.2 vs. 107.6 ± 29.3, P = 0.007) was significantly greater in the former, but other parameters including age, height, sex, AST, ALT and initial serum sodium levels in baseline characteristics, were not significantly different. Logistic regression analysis to test the association between the rapid group and baseline characteristics, adjusted for age, sex and initial sodium level, revealed an association with BMI (OR, 0.692; 95% CI, 0.500–0.956; P = 0.026), body weight (OR, 0.858; 95% CI, 0.775–0.976; P = 0.02) and eGFR (OR, 1.030; CI, 1.006–1.054; P = 0.014) (Table 3).
Table 3. Logistic regression analysis for the association of the rapid group with baseline characteristics.
| Variables | Univariate | Multivariatea | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, yr | 0.927 (0.853–1.007) | 0.073 | ||
| Sex (Male) | 0.848 (0.235–3.060) | 0.802 | ||
| Na (initial), mmol/L | 0.854 (0.723–1.009) | 0.064 | ||
| eGFR, mL/min/1.73 m2 | 1.026 (1.005–1.047) | 0.014 | 1.030 (1.006–1.054) | 0.014 |
| Height, cm | 0.991 (0.918–1.070) | 0.820 | ||
| Body weight, kg | 0.901 (0.815–0.997) | 0.044 | 0.858 (0.775–0.976) | 0.020 |
| BMI, kg/m2 | 0.744 (0.557–0.995) | 0.046 | 0.692 (0.500–0.956) | 0.026 |
OR = odds ratio, CI = confidence interval, Na = serum sodium level, eGFR = estimated glomerular filtration rate, BMI = body mass index.
aAdjusted for age, sex, and initial Na.
AEs
The most common AEs were dry mouth (45%), thirst (26%), pollakiuria (18%) and overcorrection (13%). A total of four patients dropped out due to AEs: three withdrew because of nausea, and one developed severe overcorrection — an increase of 13 mmol/L within 8 hours of the first dose of TLV. However, between baseline and day 11 there were no significant changes in any patients with regard to level of AST (22.9 ± 8.1 IU/L vs. 23.1 ± 5.7 IU/L, respectively; P = 0.118) or ALT (19.3 ± 13.8 IU/L vs. 19.3 ± 10.8 IU/L, respectively; P = 0.070) (Table 1). Neither osmotic demyelination syndrome nor hypernatremia (serum sodium concentration > 145 mmol/L) was observed.
DISCUSSION
Recent systematic reviews and meta-analyses of randomized controlled trials (RCTs) of TLV for hyponatremia have concluded that TLV is effective, and without SAEs, in patients with euvolemic or hypervolemic hyponatremia.13,14,15 The findings of our study agree with those in other studies to some extent. The results of the present study also correspond with those from an randomized control trial of TLV in Chinese patients with hyponatremia caused by SIADH; changes in average area under the curve (AUC) of serum sodium levels from baseline to day 4 were very similar: 8.1 ± 3.6 mmol/L in the RCT from the Chinese study and 8.1 ± 3.9 mmol/L in the current study (Table 1), and SAEs, including hypernatremia (> 145 mmol/L serum sodium), ODS and abnormal liver function, did not occur in both studies (Table 1),6 except for the occurrence of overcorrection of serum sodium over the first 24 hours in 5 patients (13%) in the present study.
The results of the current study are not surprising, as many previous studies have already provided sufficient evidence for the efficacy of TLV; however, this is the first prospectively designed study on Korean patients with hyponatremia caused by SIADH. Despite the previous studies, expert opinions and guidelines for using vaptans to treat hyponatremia still vary11,12,16; some have warned against rapid correction of serum sodium by vaptans in patients with euvolemic hyponatremia.17,18,19,20 The current finding of overcorrection of serum sodium in 13% of patients seems to indicate appropriateness of such warnings.
Previous studies of TLV have focused on the changes in average AUCs of serum sodium levels on days 1 and 4 rather than on the rate of correction of serum sodium over the first 24 hours. Thus, our aim was to test the short-term efficacy and safety of TLV and to evaluate the rate of correction of serum sodium levels over the first 24 hours of administration of TLV. We also identified the risk factors for overcorrection of serum sodium levels.
The maximal effect of TLV at a dose of 15 mg occurred at 8 hours, as was the case in previous studies,21,22 and the average rate of increase in serum sodium seemed to be acceptable: 5.9 ± 4.1 mmol/L at 8 hours and 6.8 ± 4.2 mmol/L at 24 hours. However, a subgroup analysis comparing the rate of increase in serum sodium levels in patients with the degree of hyponatremia at baseline revealed that, in patients with severe hyponatremia, the increase in serum sodium levels at 24 hours was 11.1 ± 4.8 mmol/L (Table 2), and also the correlation analysis showed lower baseline serum sodium level significantly associated with greater changes of serum sodium level on TLV within the first 24 hours (Fig. 3). In all 3 groups of patients with hyponatremia, the serum sodium concentrations achieved at hour 24 were similar, greater than 130 mmol/L, but the rate of rise in serum sodium was much greater in those with the low baseline serum sodium than in the other two groups (Table 2).
Two small studies have focused on the overcorrection of serum sodium within the first 24 hours of treatment with 15 mg TLV in patients with hypervolemic or euvolemic hyponatremia.23,24 Castello et al.23 investigated the efficacy and safety of two different doses of TLV (7.5 and 15 mg) in patients with hypervolemic and euvolemic hyponatremia and reported that the group receiving TLV 15 mg showed a median increase in serum sodium of 12 mmol/L (range, 6–25 mmol/L) and this correction rate exceeded the values recommended by recent guidelines in 75% of patients, although no ODS was reported. In contrast, the group receiving TLV 7.5 mg dose had a median increase in serum sodium of 6 mmol/L (range, 1–11 mmol/L). Castello et al.23 also found that in patients with lower starting levels of serum sodium there was a tendency to see larger increases in serum sodium levels. The authors concluded that a daily dose of 7.5 mg TLV was both effective and safe, whereas 15 mg TLV had a high risk of overcorrection. Harbeck et al.24 retrospectively analyzed 37 patients with euvolemic hyponatremia, who received either 7.5 mg or 15 mg TLV. Two patients who received 15 mg TLV had an increase in serum sodium levels of 17 and 18 mmol/L within the first 24 hours. The authors therefore also recommended that the starting dose of TLV should be 7.5 mg in patients with SIADH.
The greater absolute increase in serum sodium in those with more severe hyponatremia may be explained by the greater effective volume expansion in these patients that would have resulted in greater suppression of proximal salt reabsorption and hence delivery of greater urine flow to the distal nephron where free water is generated. The muted response to vaptans is well known in hypervolemic hyponatremia such as congestive heart failure, in which low effective vascular volume stimulates proximal salt reabsorption with markedly reduced distal urine flow.25,26,27
Since the total number of circulating ADH that can be competitively inhibited by TLV must be proportionate to the body mass, the smaller body mass of Korean patients would dictate smaller drug doses. The high incidence of overcorrection with the dose of 15 mg TLV in our study further indicates that the currently recommended starting dosage for TLV is too large to Korean patients with SIADH.
Interestingly, in the SALT-1 study in the western population,2 using the same starting dose of TLV at 15 mg per day, the authors reported smaller changes in average AUC at day 4 (3.6 ± 2.7 mmol/L) and a larger starting body weight (78 ± 23 kg) than in studies of Asian populations. For example, AUCs at day 4 were 8.1 ± 3.6 mmol/L and 8.1 ± 3.9 mmol/L in the RCT from China and in the current study respectively, and body weight was 57.1 ± 10.0 kg in the current study but body weight data in the RCT from China were not available).6
Our logistic regression analysis, adjusted by age, sex and initial sodium level, showed that body weight and BMI were inversely associated with the rate of serum sodium correction (Table 3). However, creatinine and eGFR were significantly different between the regular and rapid group, and even associated with the correction rate in univariate logistic regression analysis. Nevertheless, those were excluded in multivariate logistic regression analysis, because the body frame, such as muscle mass, body weight and BMI, positively associated with creatinine level in patient without chronic kidney disease, and also creatinine levels inversely associated with eGFR in calculation of the MDRD equation. Thus, those were not considered as confounders.
The Ministry of Food and Drug Safety (MFDS, formerly known as the Korea Food & Drug Administration [KFDA]) currently permits the use of a regular dose of 15 mg TLV in patients with severe hyponatremia (serum sodium < 125 mmol/L) caused by SIADH. However, based on the current results, rapid correction or overcorrection of serum sodium levels may develop at this dosage. Therefore, we suggest that a starting dose of TLV 7.5 mg per day be used in the MFDS for Korean patients or patients with other ethnicity that has small body mass in treating hyponatremia caused by SIADH (tablets of 7.5 mg TLV are not currently available in Korea).
The present study has major weaknesses for a small sample size and a short duration, in proving statistical differences, and several other limitations. First, there was a gender imbalance in each subgroup due to the small sample size. Second, we were not able to assess the fluid status and urine electrolytes of patients, because standardization of processes for measuring urine output and electrolytes in each center was unsatisfactory. As a result, the mechanism of overcorrection of serum sodium cannot be determined in our study. Finally, we were not able to compare a dose of 7.5 mg TLV with 15 mg TLV. Further large-scale studies comparing a daily dose of 7.5 mg TLV with a daily dose of 15 mg TLV are required to establish the most effective and safest starting dose of TLV in Korean patients with hyponatremia caused by SIADH.
The advantage of the current study is that the efficacy and safety of TLV have now been tested in Korean patients with hyponatremia caused by idiopathic SIADH in a prospective, multicenter, single-arm, open-label study. Moreover, in difference from previous studies for TLV, the major strengths of this study are that the correction rate of serum sodium over the first 24 hours following the initial dose of TLV was focused and the risk factors for overcorrection was identified.
In conclusion, TLV is generally effective and well-tolerated, but overcorrection is quite common when the recommended starting dose is used for Korean patients with hyponatremia caused by SIADH. A starting dose of 15 mg TLV daily often results in overcorrection of serum sodium within the first 24 hours in patients with severe hyponatremia (< 125 mmol/L) and particularly in those with small body mass. Therefore, we suggest that a starting dose of a half tablet of 15 mg of TLV be used for Korean patients with SIADH.
Footnotes
Funding: This study was funded by Korea Otsuka Pharmaceutical Co., Ltd. in 2012, and the funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The funder was provided the opportunity to review a preliminary version of this manuscript for factual accuracy but the authors are solely responsible for final content and interpretation. The authors received no financial support or other form of compensation related to the development of the manuscript.
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Yi JH, Kim HJ, Kim GH, Kim SW. Data curation: Han SW, Kang KP, Kim HY, Choi HY, Ha SK, Kim GH, Kim YW, Jeong KH, Shin SK. Formal analysis: Yi JH, Han SW, Kim HJ. Writing - original draft: Han SW, Kim HJ. Visualization: Yi JH. Writing - review & editing: Yi JH, Han SW, Kim HJ.
References
- 1.Doggrell SA. Tolvaptan (Otsuka) Curr Opin Investig Drugs. 2004;5(9):977–983. [PubMed] [Google Scholar]
- 2.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Gottlieb SS, Udelson JE, Konstam MA, Czerwiec F, Ouyang J, et al. Vasopressin v(2) receptor blockade with Tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol. 2006;97(7):1064–1067. doi: 10.1016/j.amjcard.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 5.Verbalis JG, Adler S, Schrier RW, Berl T, Zhao Q, Czerwiec FS, et al. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol. 2011;164(5):725–732. doi: 10.1530/EJE-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Zhao JJ, Tong NW, Guo XH, Qiu MC, Yang GY, et al. Randomized, double blinded, placebo-controlled trial to evaluate the efficacy and safety of tolvaptan in Chinese patients with hyponatremia caused by SIADH. J Clin Pharmacol. 2014;54(12):1362–1367. doi: 10.1002/jcph.342. [DOI] [PubMed] [Google Scholar]
- 7.Verbalis JG, Ellison H, Hobart M, Krasa H, Ouyang J, Czerwiec FS, et al. Tolvaptan and neurocognitive function in mild to moderate chronic hyponatremia: a randomized trial (INSIGHT) Am J Kidney Dis. 2016;67(6):893–901. doi: 10.1053/j.ajkd.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 8.FDA Drug Safety Communication.: FDA limits duration and usage of Samsca (tolvaptan) due to possible liver injury leading to organ transplant or death. [Updated 2013]. [Accessed October 10, 2017]. http://www.fda.gov/drugs/drugsafety/ucm350062.htm.
- 9.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705–712. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10) Suppl 1:S1–S42. doi: 10.1016/j.amjmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, et al. Clinical practice guideline on diagnosis and treatment of hyponatremia. Nephrol Dial Transplant. 2014;29(Suppl 2):i1–i39. doi: 10.1093/ndt/gfu040. [DOI] [PubMed] [Google Scholar]
- 13.Bhandari S, Peri A, Cranston I, McCool R, Shaw A, Glanville J, et al. A systematic review of known interventions for the treatment of chronic nonhypovolaemic hypotonic hyponatraemia and a meta-analysis of the vaptans. Clin Endocrinol (Oxf) 2017;86(6):761–771. doi: 10.1111/cen.13315. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Fang D, Qian C, Feng H, Wang Y. The efficacy and safety of tolvaptan in patients with hyponatremia: a meta-analysis of randomized controlled trials. Clin Drug Investig. 2017;37(4):327–342. doi: 10.1007/s40261-016-0470-3. [DOI] [PubMed] [Google Scholar]
- 15.Nemerovski C, Hutchinson DJ. Treatment of hypervolemic or euvolemic hyponatremia associated with heart failure, cirrhosis, or the syndrome of inappropriate antidiuretic hormone with tolvaptan: a clinical review. Clin Ther. 2010;32(6):1015–1032. doi: 10.1016/j.clinthera.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Hoorn EJ, Zietse R. Diagnosis and treatment of hyponatremia: compilation of the guidelines. J Am Soc Nephrol. 2017;28(5):1340–1349. doi: 10.1681/ASN.2016101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Benedetto G, See M. Direct Healthcare Professional Communication on the risk of increases in serum sodium with tolvaptan (Samsca) which are too rapid. [Updated 2012]. [Accessed October 10, 2017]. http://www.mhra.gov.uk/home/groups/comms-ic/documents/websiteresources/con146921.pdf.
- 18.Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447–457. doi: 10.1159/000106456. [DOI] [PubMed] [Google Scholar]
- 19.Soupart A, Gross P, Legros JJ, Alföldi S, Annane D, Heshmati HM, et al. Successful long-term treatment of hyponatremia in syndrome of inappropriate antidiuretic hormone secretion with satavaptan (SR121463B), an orally active nonpeptide vasopressin V2-receptor antagonist. Clin J Am Soc Nephrol. 2006;1(6):1154–1160. doi: 10.2215/CJN.00160106. [DOI] [PubMed] [Google Scholar]
- 20.Decaux G. V2-antagonists for the treatment of hyponatraemia. Nephrol Dial Transplant. 2007;22(7):1853–1855. doi: 10.1093/ndt/gfm136. [DOI] [PubMed] [Google Scholar]
- 21.Kim SR, Hasunuma T, Sato O, Okada T, Kondo M, Azuma J. Pharmacokinetics, pharmacodynamics and safety of tolvaptan, a novel, oral, selective nonpeptide AVP V2-receptor antagonist: results of single- and multiple-dose studies in healthy Japanese male volunteers. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S5–S17. doi: 10.1007/s10557-011-6299-3. [DOI] [PubMed] [Google Scholar]
- 22.Yi S, Jeon H, Yoon SH, Cho JY, Shin SG, Jang IJ, et al. Pharmacokinetics and pharmacodynamics of oral tolvaptan administered in 15- to 60-mg single doses to healthy Korean men. J Cardiovasc Pharmacol. 2012;59(4):315–322. doi: 10.1097/FJC.0b013e318241e89c. [DOI] [PubMed] [Google Scholar]
- 23.Castello LM, Baldrighi M, Panizza A, Bartoli E, Avanzi GC. Efficacy and safety of two different tolvaptan doses in the treatment of hyponatremia in the Emergency Department. Intern Emerg Med. 2017;12(7):993–1001. doi: 10.1007/s11739-016-1508-5. [DOI] [PubMed] [Google Scholar]
- 24.Harbeck B, Lindner U, Haas CS. Low-dose tolvaptan for the treatment of hyponatremia in the syndrome of inappropriate ADH secretion (SIADH) Endocrine. 2016;53(3):872–873. doi: 10.1007/s12020-016-0912-y. [DOI] [PubMed] [Google Scholar]
- 25.Dunn FL, Brennan TJ, Nelson AE, Robertson GL. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973;52(12):3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uretsky BF, Verbalis JG, Generalovich T, Valdes A, Reddy PS. Plasma vasopressin response to osmotic and hemodynamic stimuli in heart failure. Am J Physiol. 1985;248(3 Pt 2):H396–H402. doi: 10.1152/ajpheart.1985.248.3.H396. [DOI] [PubMed] [Google Scholar]
- 27.Park GH, Lee CM, Song JW, Jung MC, Kim JK, Song YR, et al. Comparison of tolvaptan treatment between patients with the SIADH and congestive heart failure: a single-center experience. Korean J Intern Med. 2017 doi: 10.3904/kjim.2016.155. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]