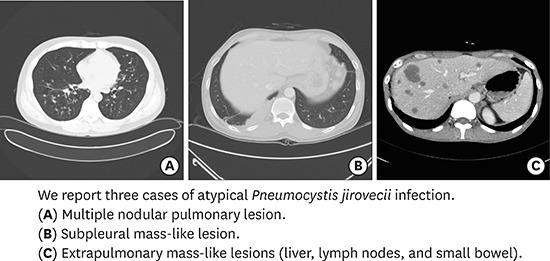
Abstract
Advances in the treatment and prevention of Pneumocystis jirovecii infection (PJI) in human immunodeficiency virus (HIV) patients decreased incidence and mortality dramatically, however, it may be associated with an increased frequency of unusual manifestation such as cystic formation, pneumothorax, focal infiltration, nodular formation, or extrapulmonary lesions. We report three cases of PJI with atypical manifestations. Each case demonstrates different clinical features: multiple nodular pulmonary lesion (32-year-old man with abnormal chest X-ray finding), subpleural mass-like lesion (43-year-old man with left visual loss and right pleuritic chest pain), and extrapulmonary mass-like lesions in the liver, lymph nodes, and small bowel (39-year-old man with cough, sputum, and dyspnea). P. jirovecii was confirmed in all 3 cases and they were treated well. It is necessary to understand that PJI shows variable clinical features.
Keywords: Pneumocystis jirovecii, Atypical, Nodular, Extrapulmonary, AIDS, HIV
Graphical Abstract
INTRODUCTION
Pneumocystis jirovecii infection (PJI) often occurs in human immunodeficiency virus (HIV) infected patients with less than 200 cells/mm3 of CD4+ T cell count as well as other immunocompromised patients such as those with cancer and transplant recipients.1 Since the primary prophylaxis strategy was widely accepted, the incidence of PJI has decreased dramatically.2 However, it still remains a leading cause of opportunistic infection in HIV patients. According to domestic research, 15.6% of all HIV infected patients have experienced more than one episode of PJI.3 Furthermore, advances in the treatment and prevention of PJI in HIV patients may be associated with an increased frequency of unusual manifestation.4 Typical computed tomography (CT) findings of PJI is diffuse ground glass opacity in both lungs, however atypical features such as cystic formation, pneumothorax, focal infiltration, nodular or mass-like formation or extrapulmonary mass-like lesions can also be observed.4 To our best knowledge, few cases of atypical PJI have been reported to date in Korea.5,6,7 We report three cases of PJI with atypical presentations; each case demonstrates different radiologic features.
CASE DESCRIPTION
Case 1
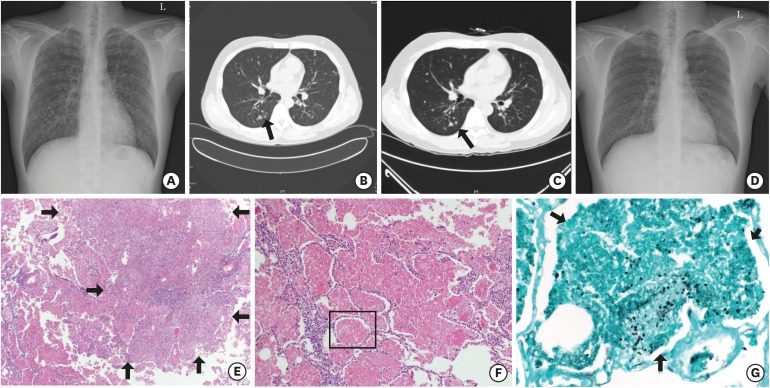
A 32-year-old man visited a hospital with abnormal chest X-ray findings from routine medical check-up in 2014. On initial chest X-ray and CT, disseminated multiple granulomatous nodules with cavitation were observed on whole lung field (Fig. 1A and B). He was revealed to be HIV positive with western blot assay; his initial CD4+ T lymphocyte count was 56/mm3 and HIV ribonucleic acid (RNA) was 410,000 copies/mL on polymerase chain reaction (PCR) test. Antigen test for Cryptococcus was negative but P. jirovecii was detected from bronchoalveolar lavage (BAL) specimen on PCR test. Accordingly, we started intravenous trimethoprim/sulfamethoxazole (TMP/SMX) and intravenous amphotericin B, suspecting PJI combined with fungal infection. However, there was no improvement in pulmonary nodules at follow-up chest X-rays. On the 13th day of hospitalization, he received open lung biopsy by video-assisted thoracoscopic surgery. Histopathologic examination observed the necrotizing granulomas in lung parenchymal tissue and alveolar spaces filled with pink, foamy exudate containing oval shaped fungal cysts on hematoxylin and eosin (H & E) and gomori methenamine-silver (GMS) stain (Fig. 1E-G). Bacteria, acid-fast bacilli or other fungal species were not observed. Subsequently, TMP/SMX treatment was maintained and amphotericin B was discontinued. After 2 weeks of TMP/SMX therapy, we changed the regimen to oral clindamycin plus primaquine because leukopenia and thrombocytopenia developed. A total of 21 days of antibiotics therapy against PJI was maintained. After completion of PJI therapy, the size of multiple cavitary nodules was decreased at follow-up chest CT (Fig. 1C), and he was finally discharged on hospital day 34. At outpatient clinic, we observed gradual disappearance of multiple nodules on follow-up X-rays (Fig. 1D).
Fig. 1.
Findings of the 32-year-old man with multiple pulmonary nodules (case 1). (A, B) Simple chest X-ray and chest CT on admission day show disseminated multiple nodules with some cavitation (arrow) on whole lung field. (C) Chest CT on hospital day 29 shows decreased size of multiple nodules with disappearance of cavitary lesions (arrow). (D) Simple chest X-ray after 16 months from discharge shows previous multiple nodules disappeared. (E-G) Histopathologic feature of lung specimen shows necrotizing granulomatous lesions (arrows) and pink, foamy exudate (E, F: H & E stain; × 100, × 200, respectively) containing oval shaped fungal cysts (arrows) within alveolar space (G: GMS stain; × 400).
CT = computed tomography, H & E = hematoxylin and eosin, GMS = gomori methenamine-silver.
Case 2
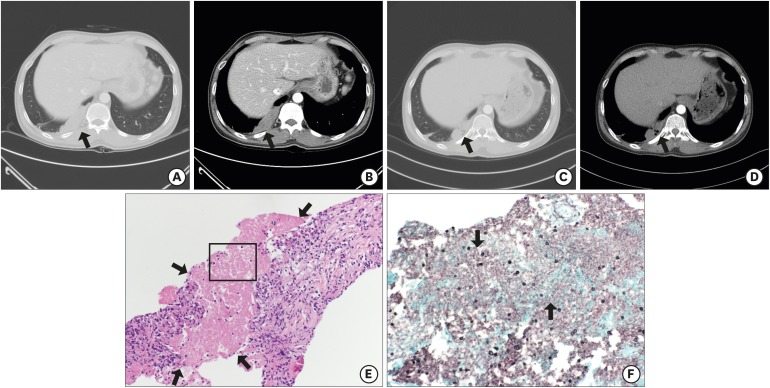
A 43-year-old man visited a hospital with loss of vision in the left eye and right pleuritic chest pain which began 15 days prior in 2015. He was a known HIV patient but had voluntarily stopped highly active antiretroviral therapy (HAART) since 3 years prior. His initial CD4+ T lymphocyte count was 20/mm3 and HIV RNA was 57,900 copies/mL. Retinal hemorrhage was compatible with cytomegalovirus (CMV) retinitis at fundoscopic examination, thus we started intravenous ganciclovir therapy subsequently. Additionally, a 13.35 cm2 of lobulated homogeneously enhancing mass-like lesion on right lower lobe with mild filling defect of right descending pulmonary artery was observed at initial chest CT (Fig. 2A and B). We started enoxaparin under the diagnosis of pulmonary infarction. On the 11th day of hospitalization, aggravation of retinal hemorrhage and retinal detachment were observed at fundoscopic examinations; enoxaparin was stopped on hospital day 11 and vitrectomy with silicon oil insertion was performed on hospital day 14. On hospital day 23, follow-up chest CT showed that the initial mass-like lesion was augmented (18.30 cm2), and he underwent fine needle aspiration biopsy (FNAB). Histopathologic examination revealed the fibrotic granulomatous lesions with amorphous bubbly necrotic area which contained oval shaped fungal cysts on H & E and GMS stain (Fig. 2E and F). We started oral TMP/SMX subsequently. After 2 weeks, we changed the regimen to oral clindamycin plus primaquine because the patient experienced visual hallucination as a side effect of TMP/SMX. A total of 28 days of antibiotics therapy against PJI was maintained and he was finally discharged on hospital day 63. At outpatient clinic, we observed decreased mass size (5.87 cm2) on follow-up chest CT (Fig. 2C and D).
Fig. 2.
Findings of the 43-year-old man with subpleural mass-like lesion (case 2). (A, B) Chest CT on admission day shows a 13.35 cm2 lobulated homogeneously enhancing mass-like lesion (arrows) on right lower lobe. (C, D) Chest CT shows the mass size decreased to 5.87 cm2 (arrows) after one month from discharge. (E, F) Histopathologic feature of lung specimen shows fibrotic granulomatous lesions with necrotic amorphous bubbly area (arrows) (E: H & E stain; × 200) which contained oval shaped fungal cysts (arrows) (F: GMS stain; × 400).
CT = computed tomography, H & E = hematoxylin and eosin, GMS = gomori methenamine-silver.
Case 3
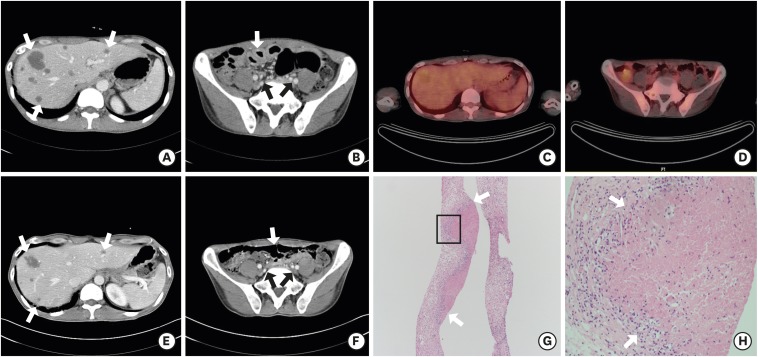
A 39-year-old man visited a university hospital with cough, sputum and dyspnea that began 14 days prior in 2014. On initial simple X-ray and chest CT, diffuse ground-glass opacity was observed on whole lung field. In addition, multiple hepatic nodules, enlarged intra-abdominal lymph nodes, and diffuse jejunal wall thickening were found incidentally (Fig. 3A and B). He was revealed to be HIV positive with western blot assay; his initial CD4+ T lymphocyte count was 20/mm3 and HIV RNA was 57,900 copies/mL. P. jirovecii was detected on sputum PCR and we started intravenous TMP/SMX subsequently. For differential diagnosis of hepatic nodules, intra-abdominal lymph node enlargement and jejunal wall thickening from other diseases such as lymphoma, he underwent FNAB of the liver and received a positron emission tomography (PET)-CT on hospital day 7 and 10, respectively. PET-CT showed no increased metabolic uptake (Fig. 3C and D). On histopathology of liver biopsy, necrotizing granuloma without evident organisms was found on H & E and GMS stain (Fig. 3G and H). Under the diagnosis of extrapulmonary manifestation of PJI, we determined to treat him with TMP/SMX only and observe the clinical progress. On follow-up CT performed on hospital day 17, a significant improvement was observed in the aforementioned lesions (Fig. 3E and F). He received TMP/SMX for a total of 3 weeks, and was finally discharged on hospital day 28. Abnormal findings disappeared on follow-up CT at outpatient clinic.
Fig. 3.
Findings of the 39-year-old man with extrapulmonary mass-like lesions (case 3). (A, B) Abdominal CT on admission day shows multiple hepatic nodules (arrows), multiple small lymph nodes in upper abdominal region (arrows), and diffuse jejunal wall thickening (arrows). (C, D) PET-CT on hospital day 10 shows no increased metabolic uptake on mentioned lesions, and diffuse jejunal wall thickening. (E, F) Abdominal CT on hospital day 17 shows significant improvement in mentioned areas (arrows). (G, H) Histopathologic feature of liver specimen shows granuloma with ill-formed necrosis (arrows) (H & E stain; × 200, × 400, respectively).
CT = computed tomography, PET-CT = positron emission tomography-computed tomography, H & E = hematoxylin and eosin.
DISCUSSION
PJI was reported to show variable atypical findings; 10%–34% of PJI patients show cystic pulmonary lesions, 3%–5% present nodular lesions, 18% accompany enlarged lymph nodes, and approximately 2%–3% have extrapulmonary manifestations.4,8 Our first two cases (case 1 and 2) represent nodular or mass-like formation in PJI. Most nodular or mass-like PJI have been found among HIV patients, but rarely, are also observed in patients with hematologic malignancy, solid cancer, or transplant recipients who undergo immunosuppressant therapy.5,9 As shown in our cases, lesions may vary in size from miliary (2–3 mm in diameter) to larger nodules (≥ 1 cm in diameter), and number from solitary to multiple nodules or masses. Previous data presented that the solitary pulmonary nodule was approximately 19% among nodular or mass-like PJI cases.4,9,10
Several hypotheses have been postulated to explain the mechanism of this rare manifestation: exposure time to Pneumocystis surface glycoproteins, absence of IgA Pneumocystis antibodies, and prior Pneumocystis exposure were hypothesized to be linked to nodular formation9,11 while microorganism factors (e.g., genotypes) are not likely to be associated with it.12 Above all, change in CD4+ T cell hypothesis has been most widely accepted.9,11 Nodular or mass-like PJI cases are more common in patients whose immunity has recently recovered such as after using antiretroviral agents, or stopping steroid support.9
As described in case 3, PJI can disseminate beyond the lungs by both lymphatic and hematogenous routes. It could affect almost every organ except a few sites such as prostate and joint spaces: lymph nodes represent the most common site of infection followed by spleen, liver, and bone marrow.8,13 Half of the patients of extrapulmonary PJI had a single site involvement and slightly less than half of them with HIV infection had revealed to have concomitant pulmonary involvement.13 The definitive mechanism of extrapulmonary PJI has yet to be identified. Previous studies indicate that PJI with atypical presentation can occur in patients who receive inhaled pentamidine, which was used commonly to prevent PJI in HIV patients; it probably relates to poor systemic distribution of the inhaled drug, which makes it ineffective in the prevention of extrapulmonary infection.14 Another possible explanation is the dysgammaglobulinemia which may permit migration of parasites from the lung to extrapulmonary sites.13
Histological examination is imperative for proper diagnosis of both nodular or mass-like and extrapulmonary PJI. Symptoms and images are nonspecific, varying with the site of PJI involvement.8,13 As for nodular or mass-like PJI, pathogens usually are not detected through sputum examination or BAL. It may be due to the presence of the organism inside the granuloma rather than free in the alveoli.9,15 Furthermore, it is almost impossible to distinguish pulmonary nodular or mass-like lesions in PJI from other nodular or mass forming diseases such as lymphoma, Aspergillus infection, Cryptococcus infection, or Nocardia infection.5
According to reports to date, both nodular or mass-like and extrapulmonary PJI respond well to TMP/SMX treatment, like conventional PJI.5,8,12,13 The treatment duration is yet to be established. We suggest three weeks of treatment with secondary prophylaxis thereafter as conventional PJI would be enough. Further investigation about treatment is required.
In conclusion, PJI can present with various clinical and radiological features. When an immunocompromised patient presents with unidentified manifestation such as nodular or mass-like lesions, PJI should be considered.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Pai H. Investigation: Kim B, Park SS. Writing - original draft: Kim B. Writing - review & editing: Pai H, Kim B, Kim J, Park SS.
References
- 1.The Korean Society for AIDS. Clinical guidelines for the treatment and prevention of opportunistic infections in HIV-infected Koreans. Infect Chemother. 2012;44(3):93–139. doi: 10.3947/ic.2016.48.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furrer H, Egger M, Opravil M, Bernasconi E, Hirschel B, Battegay M, et al. Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1 infected adults treated with combination antiretroviral therapy. Swiss HIV Cohort Study. N Engl J Med. 1999;340(17):1301–1306. doi: 10.1056/NEJM199904293401701. [DOI] [PubMed] [Google Scholar]
- 3.Kim JM, Cho GJ, Hong SK, Chang KH, Chung JS, Choi YH, et al. Epidemiology and clinical features of HIV infection/AIDS in Korea. Yonsei Med J. 2003;44(3):363–370. doi: 10.3349/ymj.2003.44.3.363. [DOI] [PubMed] [Google Scholar]
- 4.Boiselle PM, Crans CA, Jr, Kaplan MA. The changing face of Pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999;172(5):1301–1309. doi: 10.2214/ajr.172.5.10227507. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Shin KE, Lee JH. Single nodular opacity of granulomatous Pneumocystis jirovecii pneumonia in an asymptomatic lymphoma patient. Korean J Radiol. 2015;16(2):440–443. doi: 10.3348/kjr.2015.16.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim EJ, Yoo SJ, Kang GH, Hong MY, Hong JS, Ryu DS, et al. A case of multiple pulmonary nodular Pneumocystis jirovecii pneumonia in an acquired immune deficiency syndrome patient. Infect Chemother. 2012;44(1):40–43. [Google Scholar]
- 7.Kim HW, Heo JY, Lee YM, Kim SJ, Jeong HW. Unmasking granulomatous Pneumocystis jirovecii pneumonia with nodular opacity in an HIV-infected patient after initiation of antiretroviral therapy. Yonsei Med J. 2016;57(4):1042–1046. doi: 10.3349/ymj.2016.57.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Telzak EE, Cote RJ, Gold JW, Campbell SW, Armstrong D. Extrapulmonary Pneumocystis carinii infections. Rev Infect Dis. 1990;12(3):380–386. doi: 10.1093/clinids/12.3.380. [DOI] [PubMed] [Google Scholar]
- 9.Hartel PH, Shilo K, Klassen-Fischer M, Neafie RC, Ozbudak IH, Galvin JR, et al. Granulomatous reaction to Pneumocystis jirovecii: clinicopathologic review of 20 cases. Am J Surg Pathol. 2010;34(5):730–734. doi: 10.1097/PAS.0b013e3181d9f16a. [DOI] [PubMed] [Google Scholar]
- 10.Barrio JL, Suarez M, Rodriguez JL, Saldana MJ, Pitchenik AE. Pneumocystis carinii pneumonia presenting as cavitating and noncavitating solitary pulmonary nodules in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986;134(5):1094–1096. doi: 10.1164/arrd.1986.134.5.1094. [DOI] [PubMed] [Google Scholar]
- 11.Otahbachi M, Nugent K, Buscemi D. Granulomatous Pneumocystis jiroveci pneumonia in a patient with chronic lymphocytic leukemia: A literature review and hypothesis on pathogenesis. Am J Med Sci. 2007;333(2):131–135. doi: 10.1097/00000441-200702000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Totet A, Duwat H, Daste G, Berry A, Escamilla R, Nevez G. Pneumocystis jirovecii genotypes and granulomatous pneumocystosis. Med Mal Infect. 2006;36(4):229–231. doi: 10.1016/j.medmal.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Raviglione MC. Extrapulmonary pneumocystosis: the first 50 cases. Rev Infect Dis. 1990;12(6):1127–1138. doi: 10.1093/clinids/12.6.1127. [DOI] [PubMed] [Google Scholar]
- 14.Edelstein H, McCabe RE. Atypical presentations of Pneumocystis carinii pneumonia in patients receiving inhaled pentamidine prophylaxis. Chest. 1990;98(6):1366–1369. doi: 10.1378/chest.98.6.1366. [DOI] [PubMed] [Google Scholar]
- 15.Bondoc AY, White DA. Granulomatous Pneumocystis carinii pneumonia in patients with malignancy. Thorax. 2002;57(5):435–437. doi: 10.1136/thorax.57.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]