Abstract
Glutathione reductase (GR; EC 1.6.4.2) activity was assayed in bundle sheath and mesophyll cells of maize (Zea mays L. var H99) from plants grown at 20°C, 18°C, and 15°C. The purity of each fraction was determined by measuring the associated activity of the compartment-specific marker enzymes, Rubisco and phosphoenolpyruvate carboxylase, respectively. GR activity and the abundance of GR protein and mRNA increased in plants grown at 15°C and 18°C compared with those grown at 20°C. In all cases GR activity was found only in mesophyll fractions of the leaves, with no GR activity being detectable in bundle sheath extracts. Immunogold labeling with GR-specific antibodies showed that the GR protein was exclusively localized in the mesophyll cells of leaves at all growth temperatures, whereas GR transcripts (as determined by in situ hybridization techniques) were observed in both cell types. These results indicate that post-transcriptional regulation prevents GR accumulation in the bundle sheath cells of maize leaves. The resulting limitation on the capacity for regeneration of reduced glutathione in this compartment may contribute to the extreme chilling sensitivity of maize leaves.
Glutathione (γ-glutamyl cysteinyl Gly) is a versatile regulator of cell metabolism and function (Rennenberg, 1982). Essential for plant growth and development, this antioxidant is a key cellular redox component that functions in the regulation of gene expression and the cell cycle (for review, see Noctor et al., 1998). The reduced glutathione (GSH)/oxidized glutathione (GSSG, or glutathione disulfide) redox couple is involved in the expression of defense genes (Dron et al., 1988; Wingate et al., 1988), in sulfur metabolism by regulation of sulfur uptake in the roots (Herschbach and Rennenberg, 1994; Lappartient and Touraine, 1996), in the detoxification of xenobiotics through GSH S-transferases (Lamoreaux and Rusness, 1993; Marrs, 1996), and in the redox control of cell division (Russo et al., 1995; Sanchez-Fernandez et al., 1997).
The enzyme glutathione reductase (GR; EC 1.6.4.2) is pivotal to the function of the glutathione system in eukaryotic cells (Noctor et al., 1998). This flavoprotein oxidoreductase catalyzes the reduction of GSSG to GSH in a NADPH-dependent reaction. GR has a central role in maintaining GSH within the cellular environment, particularly during stress. Most, if not all, stresses include an oxidative stress component (Wise and Naylor, 1987; Hodgson and Raison, 1991; McKersie, 1991; Prasad et al., 1994; Wise, 1995) that leads to tissue damage if antioxidative defenses are insufficient. Chilling sensitivity in maize (Zea mays) leaves has been linked to the antioxidant status of the cells (Doulis et al., 1997), while interspecific variations in cold tolerance have been correlated with antioxidant capacity (Jahnke et al., 1991; Kocsy et al., 1996; Prasad, 1996, 1997). In addition, chilling has been shown to cause H2O2 accumulation in the leaves of cereals including maize (Okuda et al., 1991; Kingston-Smith et al., 1998). GR is considered to be an important factor limiting the degree of photodamage experienced by maize leaves upon exposure to chilling temperatures (Jahnke et al., 1991; Massacci et al., 1995; Hodges et al., 1997; Leipner et al., 1997; Fryer et al., 1998).
Maize is one of the most important crops worldwide. Since it originated in tropical regions, it is not surprising that it is particularly sensitive to low-temperature stress. The optimal growth temperature for maize is between 20°C and 30°C. In northern Europe and other areas, suboptimal temperatures that cause chilling-induced damage are frequently encountered early in the mornings. The combination of high light intensities and low temperatures, such as those experienced on cold but sunny mornings in the spring, can cause dramatic damage to young maize seedlings (Fryer et al., 1998). Stress tolerance has therefore become a major selection criterion in maize breeding programs. The damage caused to mature and developing leaves by low-temperature stress occurs primarily in the chloroplasts, leading to inhibition of photosynthesis and premature senescence (Nie and Baker, 1991; Nie et al., 1992, 1995). Studies on the relationships between CO2 assimilation, photosynthetic electron transport, and antioxidant enzyme activities in field-grown maize suggest that the donation of electrons to oxygen by the photosynthetic electron transport chain is increased by growth at low temperatures (Fryer et al., 1998).
The differential partitioning of antioxidants between photosynthetic cell types may be central to the inherent low-temperature sensitivity of maize (Doulis et al., 1997; Burgener et al., 1998) and to the sensitivity of proteins in the bundle sheath cells to oxidative damage (Kingston-Smith and Foyer, 2000). Maize has a specialized leaf anatomy that encompasses the C4 photosynthetic cycle in addition to the C3 pathway (Furbank and Foyer, 1988). The initial steps of CO2 assimilation in the mesophyll cells are spatially separated from the enzymes of the Benson-Calvin cycle in the bundle sheath cells (Hatch and Osmond, 1976; Furbank and Foyer, 1988; Furbank and Taylor, 1995). Similarly, components of the antioxidant system are differentially distributed between the bundle sheath and the mesophyll cells in maize leaves (Doulis et al., 1997). GR activity has been found to be almost exclusively localized in the mesophyll cells, whereas ascorbate peroxidase and superoxide dismutase are largely absent from the mesophyll fraction. The enzymes of assimilatory sulfate reduction and GSH synthesis are also differentially compartmented between the bundle sheath and mesophyll cells (Burgener et al., 1998). ATP sulfurylase and adenosine 5′-phosphosulfate sulfotransferase are localized in the bundle sheath cells, whereas GSH synthetase, Cys, γ-glutamyl-Cys, and GSH are found mainly in the mesophyll cells (Burgener et al., 1998).
The differential partitioning of antioxidants between bundle sheath and mesophyll cells has been explained in terms of the availability of reducing power and NADPH, because the bundle sheath cells are depleted in NADPH-producing capacity (Furbank and Foyer, 1988; Doulis et al., 1997). GR activity requires NADPH, so it is not surprising that it is localized in the mesophyll cells, where the availability of NADPH is sufficient for catalysis. The molecular mechanisms that determine this cell-specific partitioning of GR activity in maize leaves are largely unexplored, and the localization of the GR protein and GR mRNA in maize leaves is unknown. The aim of this work was to elucidate factors determining the intercellular distribution of GR between bundle sheath and mesophyll cells in maize leaves and to determine whether these phenomena are related to the low-temperature sensitivity of many maize genotypes.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Maize (Zea mays L. var H99) plants were grown for 4 to 5 weeks in Fitotron growth chambers (SGC 660/C/PPL, Sanyo, Loughborough, UK) at optimal (20°C) and suboptimal (18°C and 15°C) temperatures, with a 16-h photoperiod and a PPFD of 300 μmol m−2 s−1.
Whole Leaf, Bundle Sheath, and Mesophyll Extraction
Whole leaf, bundle sheath, and mesophyll extracts were prepared from leaves from maize plants grown at 20°C, 18°C, and 15°C. For whole leaf extracts, the midrib was removed and the remaining leaf segments were frozen in liquid N2. The homogenized, frozen material was ground in ice-cold extraction buffer consisting of 100 mm Bicine buffer (pH 7.8), 1 mm EDTA, 5 mm MgCl2, 0.1% (v/v) Triton X-100, 1 mm bensamidine, 1 mm phenylmethylsulfonyl fluoride (PMSF), 10 mm leupeptin, and 5 mm dithiothreitol (DTT).
The mesophyll sap-extrusion technique of Leegood (1985) was used for rapid extraction of the mesophyll fraction. Ice-cold extraction buffer was used to obtain the mesophyll sap from the maize leaf segment. Segments were rolled once, as described by Doulis et al. (1997). The mesophyll sap, collected under vacuum, was centrifuged at full speed for 5 min in a microfuge (Microfuge E, Beckman Instruments, Fullerton, CA) and the supernatant was used immediately for enzyme assays.
Bundle sheath extracts were prepared by rolling the maize leaf segments several times, followed by washing in distilled water. The tissue remaining following rolling to extrude the mesophyll sap was frozen in liquid N2. Extraction was performed under the same conditions as for whole leaves. In all cases, the purity of the fractions was determined by measuring the bundle sheath and mesophyll marker enzymes Rubisco and phosphoenolpyruvate carboxylase (PEPC), respectively. Maximal Rubisco activity was determined according to the method of Parry et al. (1988). PEPC activity was measured according to the method of Wong and Davies (1973). Chlorophylls and proteins were determined in whole leaf, bundle sheath, and mesophyll extracts according to the methods of Arnon (1949) and Bradford (1976), respectively.
GR Activity and Protein Assays
GR activity was determined spectrophotometrically at 340 nm in a reaction mixture containing 50 mm Tris-HCl buffer (pH 7.6), 1 mm GSSG, and 10 mm NADPH, as described by Foyer and Halliwell (1976), and on 11% non-denaturing polyacrylamide gels according to the method of Halliwell and Foyer (1978). Following activity staining, proteins were transferred to nitrocellulose and probed with an antibody against a chloroplastic pea GR (Edwards et al., 1990).
RNA Extraction and Northern-Blot Analysis
Total RNA was extracted from whole leaves at a ratio of 1:4 (grams of tissue:milliliters of phenol) according to the method of Ougham and Davies (1990). Total RNA (50 μg) was separated electrophoretically in 1% (w/v) agarose gels containing 0.6 m formaldehyde and transferred to a nitrocellulose membrane with 1.5 m sodium chloride and 1.5 m sodium citrate (10× SSC, pH 7.0) as transfer buffer. The membrane was baked at 80°C for 2 h and incubated at 65°C for 2 h in a prehybridization buffer containing 0.25 m sodium phosphate (pH 7.4), 0.75 m sodium chloride, 25 mm EDTA, 1% (w/v) SDS, 5% Denhardt's reagent, and 50% (v/v) formamide. Chloroplastic maize GR and maize β-tubulin probes were prepared with [α-32P]dCTP by the primer extension method of Feinberg and Vogelstein (1983). Hybridization was carried out at 65°C for 18 to 24 h in the same buffer solution as for prehybridization. After hybridization, membranes were washed three times for 15 min with 2× SSC (for GR) or 0.1× SSC (for β-tubulin) at 65°C, and then exposed to x-ray film (X-OMAT, Kodak, Rochester, NY) with an intensifying screen for 3 d. GR and β-tubulin transcripts were quantified by densitometry. The assay was always within the linear range of detection as determined with a range of different concentrations of total RNA.
Immunogold Labeling
Maize leaves excised from 5-week-old plants grown at 20°C were fixed and embedded in LR White resin as described by Vandenbosch et al. (1989). Sections (90 nm thick) were collected on gold mesh grids and incubated for 1 h in a blocking solution containing 1% bovine serum albumin (BSAc, Aurion, Wageningen, The Netherlands) in PBS (pH 7.4) and 0.1% (v/v) Tween 20. Sections were immunolabeled with a chloroplastic pea GR polyclonal antibody in the same blocking solution for 1 h using preimmune serum as a control. Grids were washed in PBS three times for 10 min and then incubated with goat anti-rabbit IgG conjugated to 15 nm gold particles for 1 h, followed by washing in PBS and distilled water. After counterstaining of sections for 5 min in uranyl acetate and for 30 s in alkaline Pb citrate, grids were examined in a transmission electron microscope (JEM-1200 EA, JEOL, Herts, UK) at 80 kV.
In Situ Hybridization
In situ hybridization was carried out according to the method of Coen et al. (1990). The probe for in situ hybridization was labeled with digoxigenin-11-rUTP using the Boehringer nucleic acid labeling kit (Roche Diagnostics, East Sussex, UK). pBluescript (Stratagene, La Jolla, CA) vector containing a chloroplastic maize GR fragment of 1 kb was linearized with a restriction enzyme that cuts the flanking polylinker region further from the T7 promoter (BamHI), and about 1 μg was used as a template to synthesize digoxigenin-labeled RNA using T7 polymerase (no unlabeled rUTP was used in the reaction). Maize leaves were fixed in 4% (v/v) formaldehyde, embedded in wax, and sections were prepared for in situ hybridization according to the method of Jackson (1991).
The RNA probe was subjected to alkali hydrolysis by heating at 60°C for 1 h in 100 mm carbonate buffer (pH 10.2), and about 4% of each labeling reaction in 40 μL of hybridization buffer (Ingham et al., 1985) was used as a probe for each slide and incubated at 50°C overnight. Slides were washed in several changes of 2× SSC, 50% (v/v) formamide at 50°C, followed by two rinses with 0.5 m NaCl, 10 mm Tris-HCl (pH 7.5), and 1 mm EDTA, and treated with 20 μg/mL RNAase A in this buffer at 37°C for 30 min. The slides were then washed again in 2× SSC, 50% (v/v) formamide for 1 h, and finally washed several times in 130 mm NaCl and 10 mm sodium phosphate (pH 7.0) and stored for 1 to 3 d in this buffer at 4°C.
Immunological detection of the hybridized probe was carried out as described in the digoxigenin-nucleic acid detection kit with some modifications. Slides were incubated with gentle agitation for 45 min in 0.5% (w/v) blocking agent (Roche Diagnostics) in 100 mm Tris-HCl and 150 mm NaCl (pH 7.5), followed by 45 min in 1% (w/v) bovine serum albumin, 0.3% (v/v) Triton X-100, 100 mm Tris-HCl, and 150 mm NaCl (pH 7.5) (buffer A). This was followed by a 1-h incubation in dilute antibody-conjugate (1:2,500) in buffer A and four washes of 20 min each in buffer A without antibody. Slides were briefly washed in 100 mm Tris-HCl, 100 mm NaCl, and 50 mm MgCl2 (pH 9.5), and incubated for 1 to 3 d in 0.34 mg/mL nitroblue tetrazolium salt and 0.175 mg/mL 5-bromo-4-chloro-3-indoyl phosphate toluidinium salt in 100 mm Tris-HCl, 100 mm NaCl, and 50 mm MgCl2 (pH 9.5). The color reaction was stopped with 10 mm Tris-HCl and 1 mm EDTA (pH 8.0), and sections were passed through an ethanol series and Histoclear before mounting in Entellan (Merck, Dorset, UK).
RESULTS
GR was measured in whole leaf, bundle sheath, and mesophyll extracts from maize plants grown at 20°C (Table I). As previously reported by Doulis et al. (1997), GR activity was mainly found in the mesophyll fraction of maize leaves. The degree of contamination in each of the different cell extracts was evaluated by measuring the marker enzymes Rubisco and PEPC for bundle sheath and mesophyll cells, respectively. There was a low level of contamination of the bundle sheath extracts by mesophyll proteins, as indicated by PEPC activity (Table I). GR activity in the isolated bundle sheath fraction was lower than PEPC activity. If the degree of contamination by PEPC is used as a correction factor, then no GR activity is detectable in the bundle sheath fractions.
Table I.
GR, Rubisco, and PEPC activities in whole leaves (WL), bundle sheath (BS), and mesophyll (M) cell extracts from maize plants grown at 20°C
| Enzyme | WL | BS | M | BS/WL | M/WL |
|---|---|---|---|---|---|
| GRa | 2.1 ± 0.2 | 0.56 ± 0.15 | 11.0 ± 3.6 | 0.26 | 5.16 |
| Rubiscoa | 167.7 ± 11.1 | 220.2 ± 13.5 | 27.8 ± 5.0 | 1.31 | 0.17 |
| PEPCa | 104.6 ± 8.3 | 27.8 ± 6.2 | 1745 ± 123 | 0.26 | 16.7 |
Values are means ± sd (n = 6) and are expressed as micromoles per minute per milligram of protein (GR) and nanomoles per minute per milligram of protein (Rubisco and PEPC).
The data shown in Table I suggest that GR activity is exclusively localized in the mesophyll cells of maize leaves from plants grown at 20°C. As shown later in this manuscript, this view is supported by immunolocalization studies. To determine whether the distribution of GR between these cell types changes in plants grown at suboptimal temperatures, GR activity was measured in whole leaf, bundle sheath, and mesophyll extracts of plants grown at 18°C and 15°C (Table II). Foliar GR activity increased as growth temperature decreased, being 2-fold higher in plants grown at 15°C than 20°C. In all cases, GR activity was only detected in leaf mesophyll fractions but not in the bundle sheath extracts (data not shown). Enzyme activity increased strongly in mesophyll extracts of plants grown at 18°C and 15°C. Compared with 20°C, GR activity was increased by 9- and 5-fold (on both a chlorophyll and a protein basis) at 18°C and 15°C, respectively.
Table II.
GR activity in whole-leaf (WL) and mesophyll (M) cell extracts from maize plants grown at different temperatures
| Temperature | WL | M | WL | M |
|---|---|---|---|---|
| μmol min−1 mg−1 chlorophylla | μmol min−1 mg−1 proteina | |||
| 20°C | 21.5 ± 1.9 | 84.1 ± 0.8 | 2.4 ± 0.3 | 12.5 ± 1.0 |
| 18°C | 27.1 ± 0.7 | 123.3 ± 1.6 | 3.9 ± 0.6 | 29.3 ± 1.7 |
| 15°C | 41.9 ± 4.1 | 756.2 ± 18.9 | 6.1 ± 0.4 | 67.1 ± 3.9 |
Values are means ± sd (n = 6).
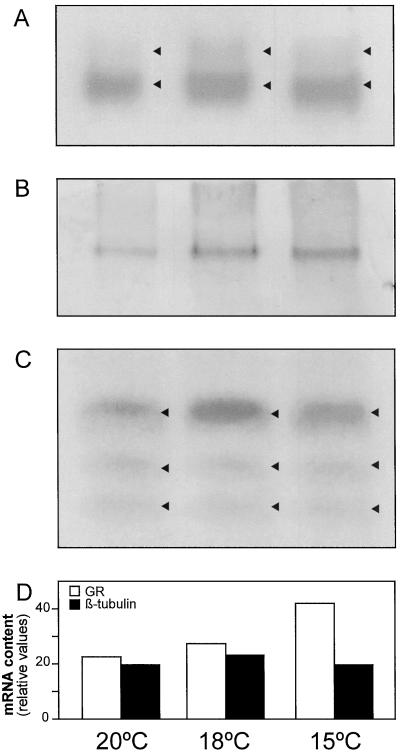
GR isoenzyme patterns were analyzed in maize leaves from plants grown at different temperatures (Fig. 1A). Two bands of GR activity were detected on non-denaturing polyacrylamide gels. The major band corresponds to the chloroplastic isoform, while the minor band is probably the cytosolic GR isoform. The activity of both GR isoforms increased equally at low temperatures by values similar to those observed when GR activity was measured spectrophotometrically (Table II). An antibody for the chloroplastic GR isoform from pea, which was known to have 10 times less affinity for the cytosolic isoform (Edwards et al., 1990), was used to identify the maize chloroplastic GR. Only one band of chloroplastic GR protein was found in foliar extracts at all growth temperatures (Fig. 1B). Chloroplastic GR increased at 18°C and 15°C, compared with 20°C. This increase was observed in GR activity at each temperature (Table II).
Figure 1.
Effect of suboptimal temperatures on GR activity (A), protein content (B), and mRNA levels (C and D). GR activity staining, western blot, and northern blots were carried out in leaf extracts (50 μg of protein) and leaf total RNA (50-μg) fractions from maize plants grown at 20°C, 18°C, and 15°C. Arrowheads indicate the position of GR isoforms and mRNAs. All three bands of GR were quantified by densitometry and compared with β-tubulin, which was used as a control for RNA loading.
GR isoforms (Fig. 1A) and GR protein (Fig. 1B) were quantified with a densitometer. The assays were always within the linear range of detection, as determined using a range of different concentrations of leaf extracts with equal protein amounts. Both in native gels (Fig. 1A) and in western blots (Fig. 1B), wells were loaded with equal amounts (on a protein basis) of whole leaf extracts from plants grown at the three temperatures. Based on this, it appears that both GR isoforms and GR protein increase at low temperatures in maize leaves. We have no evidence to assume that the extra band found on GR activity gels is a cytosolic isoform. However, we performed the detection of GR activity on gels using negative controls (incubation of the gel in the same reaction mixture without GSSG, the substrate of GR) to determine the specificity of the signal, and neither band showed activity in the absence of GSSG. GR activity is associated with chloroplasts, mitochondria, peroxisomes, and cytosol, and has a distribution of approximately 77%, 2%, 1%, and 20%, respectively (Edwards et al., 1990; Jimenez et al., 1997). Based on this knowledge and on the specificity of the activity, we suggest that the extra band is a cytosolic GR.
Total foliar RNA was analyzed using a chloroplastic GR cDNA from maize as a probe (Fig. 1C). A major band of GR mRNA and two extra smaller bands were detected. The major band corresponded to the chloroplastic GR, while the smaller bands were identified as cytosolic GR mRNAs by hybridization of the filter with a pea cytosolic GR cDNA (data not shown). Both chloroplastic and cytosolic GR transcripts increased about 2-fold in plants grown at 15°C compared with 20°C and 18°C. The densitometric values for β-tubulin and GR transcripts (Fig. 1D) show the control of RNA loading and the increase of GR mRNA at low temperatures. These results suggest that a coordinated stimulation of GR activity, GR protein synthesis, and GR transcripts may occur in maize leaves grown at low temperatures.
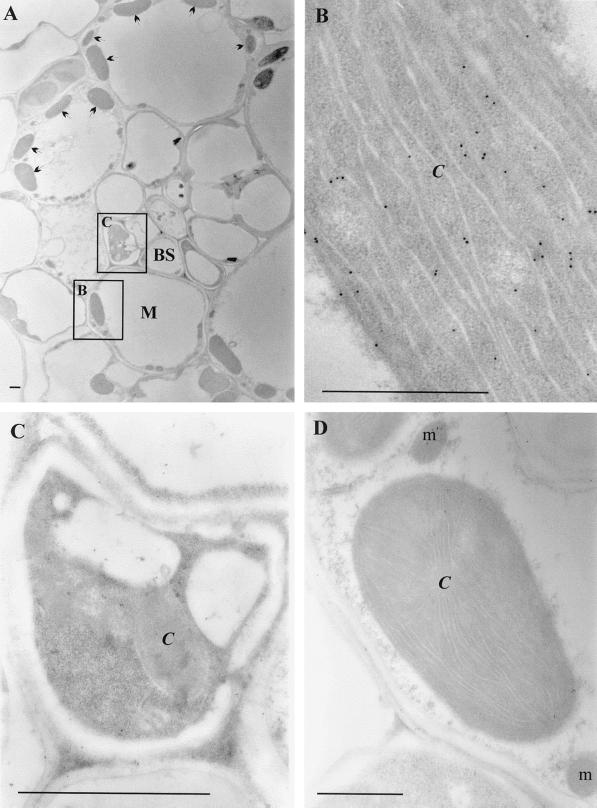
To explore the molecular basis for the absence of GR from the bundle sheath cells, the distribution of GR protein and mRNA between the bundle sheath and mesophyll cells was examined. Immunogold labeling of GR was performed using the pea chloroplastic GR antibody (Fig. 2). Electron micrographs of transverse maize leaf sections show the structure of the maize leaf (Fig. 2A). Bundle sheath cells are organized around the vascular tissue and surrounded by larger mesophyll cells with abundant chloroplasts. GR protein was exclusively localized in the mesophyll cells and was concentrated in the chloroplasts (Fig. 2B). In contrast, GR protein could not be detected in the bundle sheath cells (Fig. 2C). No gold particles were found in sections incubated with the preimmune serum control (Fig. 2D).
Figure 2.
Immunogold localization of GR in maize leaves. A to C, Sections incubated with GR antibody (1:100 dilution). D, Sections incubated with preimmune serum control. C, Chloroplast; BS, bundle sheath cells; M, mesophyll cells; and m, mitochondrion. Arrows indicate the position of chloroplasts in the mesophyll cells. Squares in A indicate the types of tissues examined in B and C. Bars = 1 μm.
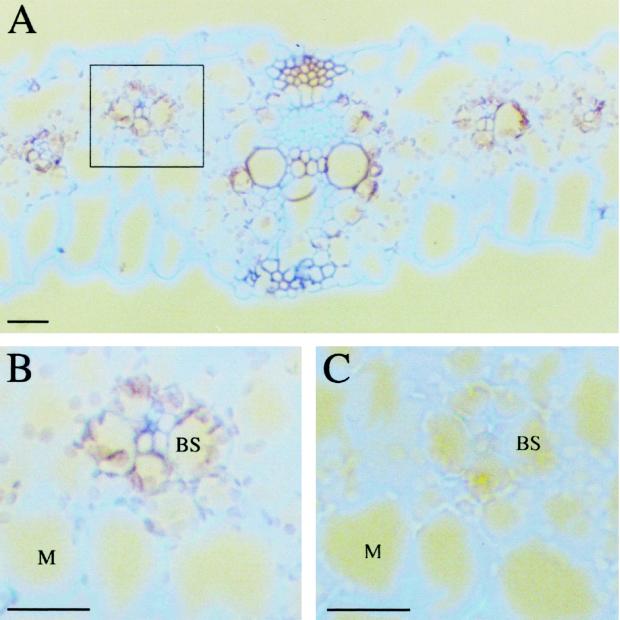
To analyze the distribution of GR mRNA between maize leaf compartments, complementary RNA labeled with digoxigenin, synthesized from a subclone of a chloroplastic GR cDNA from maize using T7 polymerase, was used to probe transverse maize leaf sections (Fig. 3). GR mRNA was present in both bundle sheath and mesophyll cells of maize leaves (Fig. 3, A and B). No GR mRNA was detected in maize leaf sections probed with the sense strand of GR probe, indicating the specificity of the detected signal (Fig. 3C). The signal was detected in both cell types with similar intensity and was localized around the chloroplasts. This could be simply due to the fact that at this stage of maize leaf development, cells consist of a large vacuole that compresses the cytosol and the rest of organelles against the plasma membrane. This diminishes signals from smaller cellular compartments. In contrast, the chloroplasts, which are among the largest organelles of the plant cell, are clearly visible.
Figure 3.
In situ hybridization of transverse maize leaf sections with GR. Maize leaf sections were probed with digoxigenin-labeled antisense (A and B) and sense (C) RNA and viewed under dark-field illumination. Signal is observed as a pale brown color in both bundle sheath (BS) and mesophyll (M) cells of maize sections. The square in A indicates the type of tissue examined in B and C. Bars = 10 μm.
DISCUSSION
Post-transcriptional regulation of gene expression may involve pre-mRNA processing, nucleocytoplasmic transport, translation efficiency, transcript stability, or protein modification and turnover. Such regulation can occur at specific stages during plant development, such as embryogenesis and germination. In many cases there is little correlation between observed changes in protein synthesis and mRNA abundance (Cohen and Mayfield, 1997; for review, see Gallie, 1993). Environmental stress can also affect protein synthesis. The expression of stress-specific messages is induced following stresses such as heat shock, dessication, nutrient starvation, hypoxia, dark-induced senescence, or pathogen attack, while the translation of many other transcripts is repressed (Dhindsa and Cleland, 1975; Skadsen and Scandalios, 1987; Scott and Oliver, 1994; Fennoy and Bailey-Serres, 1995; Gallie et al., 1995; Mittler et al., 1998).
GR protein was exclusively found in the mesophyll cells, while GR transcripts were detected in both bundle sheath and mesophyll cells. GR gene expression is therefore post-transcriptionally regulated in the bundle sheath cells. The presence of GR transcripts in both cell types suggests the occurrence of GR pre-mRNA processing and transport to the cytoplasm. Regulation at the level of transcript stability is therefore improbable, although it cannot be completely ruled out. A differential mechanism of protein modification and turnover may be present in the bundle sheath and mesophyll cells. Low-temperature- and methyl-viologen-mediated oxidative damage and protein oxidation were found to be restricted to the bundle sheath cells of maize leaves (Kingston-Smith and Foyer, 2000). The rate of GR breakdown and degradation by proteases could be faster than the rate of protein synthesis in the bundle sheath than the mesophyll cells. However, if this was the case, it still should have been possible to detect GR protein in bundle sheath cells, if at a much lower frequency.
Regulation of GR by protein translation is indicated by this study. In dark-grown maize leaves, the catalase isoenzyme CAT-2 is absent even though CAT-2 mRNA is present in polysomes, suggesting that the mRNA itself has undergone a modification that renders it incompetent for translation (Skadsen and Scandalios, 1987). Similarly, an increase in the steady-state level of transcripts encoding cytosolic ascorbate peroxidase (APX) was induced during programmed cell death in tobacco leaves, while the level of the cytosolic APX protein declined, probably by a mechanism of suppression of translation elongation (Mittler et al., 1998).
Translational activity changes substantially during plant development. High rates of translation are found in seeds at the early to mid-developmental stages, which then become nearly quiescent in the mature seed and active again during the initiation of the germination program (for review, see Gallie, 1993). In maize, GR protein translation appears to be repressed in a specific cell type, the bundle sheath. This control of protein abundance may be related to the metabolic activity of the cells and their reductive capacity (Doulis et al., 1997; Pastori et al., 2000). The bundle sheath chloroplasts are restricted in their capacity for noncyclic electron flow and NADPH formation, which provokes a net NADPH deficit in the bundle sheath cells (Furbank and Foyer, 1988; Doulis et al., 1997). NADPH availability or the NADPH-NADP+ ratio could be key factors regulating GR translation in maize leaves. High NADPH-NADP+ ratios may favor GR translation in the mesophyll, whereas low ratios in the bundle sheath cells limit or repress GR translation.
The differential regulation of GR expression in bundle sheath and mesophyll cells of maize may contribute to the sensitivity of maize to low temperatures. Both GR activity and protein content increased in maize leaves of plants grown at suboptimal temperatures. At 15°C, GR activity was significantly enhanced but only in the mesophyll extracts. No GR activity was found in the bundle sheath fractions of leaves from plants grown at 20°C, 18°C, and 15°C.
The increase of both chloroplastic and cytosolic GR isoforms at low temperatures suggests that each isoform is important in protection against the oxidative stress provoked by growth at suboptimal temperatures in maize. These results suggest that coordinated stimulation of GR activity, GR protein synthesis, and GR mRNA occurs in the mesophyll cells of maize leaves at all growth temperatures used in this study.
We have also observed that high GSH-GSSG ratios are maintained in maize leaves at low temperatures (data not shown). High GSH-GSSG ratios confer high antioxidant capacities that enable the mesophyll cells to cope with low temperatures and other stresses. GR could determine the antioxidant capacity of the maize leaves by controlling the GSH-GSSG ratios in the mesophyll cells (Doulis et al., 1997; Burgener et al., 1998). Furthermore, the GSH-GSSG ratio may function as an ubiquitous regulatory signal (Noctor and Foyer, 1998). In the absence of GR, GSSG formed in the bundle sheath cells must travel to the mesophyll to be re-reduced, as suggested by Doulis et al. (1997). The bundle sheath cells depend entirely on the import of GSH from the mesophyll cells to keep a high antioxidant capacity. The presence of APX and superoxide dismutase activities in the bundle sheath cells gives them the ability to detoxify H2O2 and superoxide, but their capacity to regenerate ascorbate and GSH is restricted. Ascorbate regeneration in these cells must occur by MDHAR activity or by non-enzymic reduction, since GR and DHAR (Doulis et al., 1997; Pastori et al., 2000) are present only in the mesophyll cells.
Both GSH and GSSG may function as signal molecules in the hypersensitive response (Dron et al., 1988; Wingate et al., 1988). Similarly, GSH-responsive elements on the promoters of glutathione S-transferase genes and in genes involved in the synthesis of phytoalexins have been identified (for review, see Noctor and Foyer, 1998). However, the cellular GSH-GSSG ratio is probably more important in the regulation of defense-related gene expression than the absolute amounts of either form (Mehdy, 1994), since it was a regulatory significance in many cellular processes (Noctor and Foyer, 1998). GR may be a central determinant of overall cellular redox state involving redox signaling for the expression of specific genes in optimal and stress conditions. The limitations on the regulation of such signaling pathways in bundle sheath cells in situ caused by the absence of GR may render this tissue and, therefore, maize leaves sensitive to low temperatures.
ACKNOWLEDGMENTS
The authors wish to thank Drs. Gary Creissen and Frank Van Breusegem for the maize GR cDNA, Dr. Brian Wells for helpful technical advice on immunocytochemistry, and Dr. Desmond Bradley for generous help and advice on in situ hybridization.
Footnotes
This work was funded by the European Commission (AIR1–CT92–0205, Engineering Stress Tolerance in Maize) and by an European Economic Community Research Training Fellowship (FAIR CT–965055 to G.P.).
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgener M, Suter M, Jones S, Brunold C. Cyst(e)ine is the transport metabolite of assimilated sulfur from bundle-sheath to mesophyll cells in maize leaves. Plant Physiol. 1998;116:1315–1322. doi: 10.1104/pp.116.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliot R, Murphy G, Carpenter R. floricaula: a homeotic gene required for flower development in Antirrinum majus. Cell. 1990;63:1311–1322. doi: 10.1016/0092-8674(90)90426-f. [DOI] [PubMed] [Google Scholar]
- Cohen A, Mayfield S. Translational regulation of gene expression in plants. Curr Opin Biotechnol. 1997;8:189–194. doi: 10.1016/s0958-1669(97)80101-2. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Cleland RE. Water stress and protein synthesis. I. Differential inhibition of protein synthesis. Plant Physiol. 1975;55:778–781. doi: 10.1104/pp.55.4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulis A, Debian N, Kingston-Smith A, Foyer C. Characterization of chilling sensitivity in maize: differential localization of antioxidants in maize leaves. Plant Physiol. 1997;114:1031–1037. doi: 10.1104/pp.114.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M, Clouse SD, Dixon RA, Lawton MA, Lamb CJ. Glutathione and fungal elicitor regulation of a plant defense promoter in electroporated protoplasts. Proc Natl Acad Sci USA. 1988;85:6738–6742. doi: 10.1073/pnas.85.18.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Rawsthorne S, Mullineaux P. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativumL.) Planta. 1990;180:278–284. doi: 10.1007/BF00194008. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabelling DNA-restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fennoy SL, Bailey-Serres J. Post-transcriptional regulation of gene expression in oxygen-deprived roots of maize. Plant J. 1995;7:287–295. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Foyer C, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR. Relationships between CO2 assimilation, photosynthetic electron transport and active O2metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 1998;116:571–580. doi: 10.1104/pp.116.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Foyer CH. C4 plants as valuable model experimental systems for the study of photosynthesis. New Phytol. 1988;109:265–277. [Google Scholar]
- Furbank RT, Taylor WC. Regulation of photosynthesis in C3 and C4 plants: a molecular approach. Plant Cell. 1995;7:797–807. doi: 10.1105/tpc.7.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR. Posttranscriptional regulation of gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:77–105. [Google Scholar]
- Gallie DR, Cadwell C, Pitto L. Heat shock disrupts cap and poly(A) tail function during translation and increase mRNA stability of introduced reporter mRNA. Plant Physiol. 1995;108:1703–1713. doi: 10.1104/pp.108.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Foyer C. Properties and physiological function of glutathione reductase purified from spinach leaves by affinity chromatography. Planta. 1978;139:9–17. doi: 10.1007/BF00390803. [DOI] [PubMed] [Google Scholar]
- Hatch MD, Osmond CB. Compartmentalization and transport in C4 photosynthesis. In: Stocking CR, Heber U, editors. Encyclopedia of Plant Physiology, New Series. Vol. 3. Berlin: Springer-Verlag; 1976. pp. 144–184. [Google Scholar]
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on the net uptake of sulfate and sulfate transport in tobacco plants. J Exp Bot. 1994;45:1069–1076. [Google Scholar]
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J Exp Bot. 1997;48:1105–1113. [Google Scholar]
- Hodgson RAJ, Raison JK. Superoxide production by thylakoids during chilling and its implication in the susceptibility of plants to chilling-induced photoinhibition. Planta. 1991;183:222–228. doi: 10.1007/BF00197792. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Howard KR, Ish-Horowicz D. Transcription pattern of the Drosophila segmentation gene hairy. Nature. 1985;318:439–445. [Google Scholar]
- Jackson DP. In situ hybridization in plants. In: Bowles DJ, Gurr SJ, McPhereson M, editors. Molecular Plant Pathology: A Practical Approach. Oxford: Oxford University Press; 1991. pp. 163–174. [Google Scholar]
- Jahnke LS, Hull MR, Long SP. Chilling stress and oxygen metabolising enzymes in Zea mays and Zea diploperennis. Plant Cell Environ. 1991;14:97–104. [Google Scholar]
- Jimenez A, Hernandez J, del Rio L, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–284. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston-Smith AH, Foyer CH (2000) Bundle sheath proteins are more sensitive to oxidative damage than those of the mesophyll in maize leaves exposed to paraquat and low temperatures. J Exp Bot 51 (in press) [PubMed]
- Kingston-Smith AH, Harbinson J, Foyer CH. Acclimation of photosynthesis and antioxidant metabolism in maize (Zea mays) grown at sub-optimal temperatures. Plant Cell Environ. 1998;22:1071–1083. [Google Scholar]
- Kocsy G, Brunner M, Rüegsegger A, Stamp P, Brunold C. Glutathione synthesis in maize genotypes with different sensitivities to chilling. Planta. 1996;198:365–370. [Google Scholar]
- Lamoreaux GL, Rusness DG. Glutathione in the metabolism and detoxification of xenobiotics in plants. In: de Kok LJ, Stulen Y, Rennenberg H, Brunold C, Rauser WE, editors. Sulfur Nutrition and Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 221–237. [Google Scholar]
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulfurylase activity and sulfate uptake in intact canola. Plant Physiol. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood R. The intercellular compartmentalization of metabolites in leaves of Zea maysL. Planta. 1985;164:163–171. doi: 10.1007/BF00396078. [DOI] [PubMed] [Google Scholar]
- Leipner J, Fracheboud Y, Stamp P. Acclimation by sub-optimal growth temperatures diminishes photooxidative damage in maize leaves. Plant Cell Environ. 1997;20:366–372. [Google Scholar]
- Marrs K. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- Massacci A, Iannelli MA, Pietrini F, Loreto F. The effect of growth at low temperature on photosynthetic characteristics and mechanisms of photoprotection of maize leaves. J Exp Bot. 1995;46:119–127. [Google Scholar]
- McKersie BD. The role of oxygen free radicals in mediating freezing and dessication stress in plants. In: Pell EJ, Steffen KL, editors. Active Oxygen/Oxidative Stress and Plant Metabolism. Rockville, MD: American Society of Plant Physiologists; 1991. pp. 107–118. [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:442–467. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Feng X, Cohen M. Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during pathogen-induced programmed cell death in tobacco. Plant Cell. 1998;10:461–473. doi: 10.1105/tpc.10.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Baker NR. Modifications to thylakoid composition during development of maize leaves at low growth temperatures. Plant Physiol. 1991;95:184–191. doi: 10.1104/pp.95.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Long SP, Baker NR. The effects of development at sub-optimal growth temperatures on photosynthetic capacity and susceptibility to chilling dependent photoinhibition in Zea mays. Physiol Plant. 1992;85:554–560. [Google Scholar]
- Nie GY, Robertson EJ, Fryer MJ, Leech RM, Baker NR. Response of the photosynthetic apparatus in maize leaves grown at low temperature on transfer back to normal growth temperature. Plant Cell Environ. 1995;18:1–12. [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Kunert K-J, Rennenberg H, Foyer C. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Noctor G, Foyer C. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Okuda T, Matsuda Y, Yamanaka A, Sagisaka S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991;97:1265–1267. doi: 10.1104/pp.97.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougham H, Davies T. Leaf development in Lolium temulentum: gradients of RNA complement and plastid and non-plastid transcripts. Physiol Plant. 1990;79:331–338. [Google Scholar]
- Parry MAJ, Keys AJ, Foyer C, Furbank RT, Walker DA. Regulation of ribulose-1,5-bisphosphate carboxylase activity by activase system in lysed spinach chloroplasts. Plant Physiol. 1988;87:558–561. doi: 10.1104/pp.87.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori G, Foyer C, Mullineaux P (2000) Low temperature-induced changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. J Exp Bot 51 (in press) [PubMed]
- Prasad T. Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J. 1996;10:1017–1026. [Google Scholar]
- Prasad T, Anderson M, Martin BA, Steward CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK. Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiol. 1997;114:1369–1376. doi: 10.1104/pp.114.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg H. Glutathione metabolism and possible biological roles in higher plants. Phytochemistry. 1982;21:2771–2781. [Google Scholar]
- Russo T, Zambrano N, Eposito F, Ammendola R, Cimino F, Fiscella M, Jackman J, O'Connor PM, Anderson CW, Appella E. A p53-independent pathway for activation of WAF1/CIP1 expression following oxidative stress. J Biol Chem. 1995;270:29386–29391. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ. Cell proliferation and hair tip growth in the Arabidopsisroot are under mechanistically different forms of redox control. Proc Natl Acad Sci USA. 1997;94:2745–2750. doi: 10.1073/pnas.94.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HB, Oliver MJ. Accumulation and polysomal recruitment of transcript in response to desiccation and rehydration of the moss Tortula ruralis. J Exp Bot. 1994;45:577–583. [Google Scholar]
- Skadsen RW, Scandalios JG. Translational control of photo-induced expression of the Cat2catalase gene during leaf development in maize. Proc Natl Acad Sci USA. 1987;84:2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch K, Bradley D, Knox J, Perotto S, Butcher G, Brewin N. Common components of the infection thread matrix and the intracellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 1989;8:335–342. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate VPM, Lawton MA, Lamb CJ. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 1988;31:205–211. doi: 10.1104/pp.87.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RR. Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res. 1995;45:79–97. doi: 10.1007/BF00032579. [DOI] [PubMed] [Google Scholar]
- Wise RR, Naylor AW. Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987;83:278–282. doi: 10.1104/pp.83.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KF, Davies DD. Regulation of phosphoenolpyruvate carboxylase of Zea maysby metabolites. Biochem J. 1973;131:451–458. doi: 10.1042/bj1310451. [DOI] [PMC free article] [PubMed] [Google Scholar]