Abstract
Background
Appendiceal cancer is a rare disease, which has proven difficult to study in prospective trials. Cytoreductive surgery with HIPEC is an established therapy for peritoneal dissemination from appendiceal cancer. The optimal chemotherapeutic agent to use in the HIPEC is not clear. Mitomycin has long been the utilized, however our previous phase I experience, and European retrospective studies suggest oxaliplatin as an alternative. Therefore, we initiated a multicenter randomized trial to compare mitomycin to oxaliplatin HIPEC for appendiceal cancer.
Study Design
Patients with mucinous appendiceal neoplasms with evidence of peritoneal dissemination underwent cytoreductive surgery and HIPEC using a closed technique for 120 minutes. Patients were randomized intraoperatively to HIPEC using mitomycin(40mg) or oxaliplatin(200mg/M2). Follow up included daily blood counts and toxicity assessments.
Results
121 analytic patients were accrued to the trial over 6 years at 3 sites. The cases were 57% female, with an average age of 55.3 years(range 22-82). The disease was low grade in 77% and high grade in 23%. There were no significant differences in hemoglobin or platelet counts. The WBC was significantly lower in the mitomycin group between postoperative days 5-10. Overall and disease free survival at 3 years were similar at 83.7% and 66.8% for mitomycin and 86.9% and 64.8% for oxaliplatin.
Conclusions
This represents the first completed prospective randomized trial for cancer of the appendix, and shows that multicenter trials for this disease are feasible. Both mitomycin and oxaliplatin are associated with minor hematologic toxicity. However, mitomycin has slightly higher hematologic toxicity and lower QOL than oxaliplatin in HIPEC. Consequently, oxaliplatin may be preferred in patients with leukopenia and mitomycin preferred in patients with thrombocytopenia due to prior chemotherapy.
Keywords: appendiceal cancer, pseudomyxoma, chemotherapy, oxaliplatin, mitomycin, HIPEC
Introduction
Appendiceal cancer represents a rare neoplasm which has been increasing in incidence1. There are no established screening systems for appendiceal cancer, so presents with peritoneal dissemination it all too frequently 1,2. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become an accepted and promising therapy for cases of peritoneal dissemination from appendiceal primary lesions. There is great variability in the observed outcomes based on histologic type, tumor grade and disease volume, with the best survival benefit observed in peritoneal surface disease (PSD) from low grade appendiceal (LGA) primary lesions. However, even within the LGA group, there is significant variability, thus all appendiceal cancer is not equal.3-6
Currently, both mitomycin and oxaliplatin are used as the active chemotherapy agents for HIPEC from appendiceal and colorectal cancer. Mitomycin has long been the standard agent for HIPEC at most large centers. These agents both have large molecular weight; which results in higher intraperitoneal concentrations with lower systemic levels, making them particularly useful for intraperitoneal therapy7-9. We have previously performed a phase 1 trial of oxaliplatin for HIPEC and found the maximally tolerated dose in a 120 minute perfusion to be 200mg/M2 10. Retrospective analyses have reached differing conclusions on which agent is superior 7,11-13. To date, there have been no prospective randomized trials for cancer of the appendix at any stage.
Herein we report the results of a multi-center, open-label, randomized phase II trial comparing the toxicity profiles of oxaliplatin and mitomycin c, delivered via HIPEC, in patients with PSD from primary appendiceal tumors.
Methods
This multi-center, open-label, randomized trial compared the toxicity profiles of oxaliplatin and mitomycin c delivered via HIPEC in patients with PSD from primary appendiceal tumors. The secondary objectives of this trial were to compare complications, quality of life, survival and time to progression in patients treated with oxaliplatin vs. mitomycin in this setting.
Institutional Review Board approval was obtained. The trial was registered with the NCT (#1580410) and investigational drug approval was obtained for oxaliplatin (NCI-2009-00947). Data analysis included demographics, age, race, gender, Eastern Cooperative Oncology Group (ECOG) performance status, R status of resection, type of malignancy, histologic grade, nodal status, co-morbidities, preoperative or postoperative chemotherapy, volume of peritoneal disease, morbidity, mortality, and survival. Appendiceal primaries were grouped in cohorts based on histologic grade (low or high)14 and were further sub-classified based on lymph nodal status. Appendiceal cancers with neuroendocrine features were excluded. Eligibility criteria for the study were: were histologic or cytologic diagnosis of peritoneal carcinomatosis, complete recovery from prior systemic chemotherapy treatment, resectable or resected primary lesion, debulkable peritoneal disease, no extra-abdominal disease, ECOG performance status ≤2, no prior major cytoreductive surgery and adequate renal, liver and marrow function. The presence of peripheral liver metastases, if readily resectable, was not considered a contraindication. Exclusion criteria included; peripheral neuropathy, prior radiation or investigational therapy, HIV hepatitits or tuberculosis infection, and metastatic disease outside the abdomen. All patients had a complete history and physical, tumor markers, and CT of the chest, abdomen and pelvis before CRS/HIPEC procedures. See CONSORT diagram, see Figure 1.
Figure 1.

CONSORT flow diagram.
The CRS/HIPEC procedure was conducted as previously described by our group using the closed abdominal technique15, 16. Briefly, the HIPEC was preceded by a complete cytoreduction with a goal of resection of all gross tumor. A generous midline incision was utilized for all explorations. Resection of the peritoneum (where needed) was by stripping it off the abdominal wall, combined with multivisceral resections (such as splenectomy, large and small bowel resection, hysterectomy, etc.), were utilized for maximal tumor debulking as determined by intraoperative findings.
Patients were cooled to a core temperature of about 34 to 35° C by passive measures (i.e., not warming airway gases or intravenous solutions) during cytoreduction. After the completion of cytoreductive surgery, peritoneal perfusion catheters were placed as per institutional standard practice. Probes were utilized to monitor perfusate and core temperatures. The abdominal skin incision was temporarily closed with a running suture to prevent leakage of peritoneal perfusate. A perfusion circuit was established (typically with 3 L of crystalloid solution). The goal flow rate was at least one liter per minute. The outflow catheters were drained into a reservoir containing a coarse filter for debris and to reduce foaming. The heated chemotherapy solution was added and circulated through the institution's standard perfusion equipment. The abdomen was gently massaged throughout the perfusion to improve drug distribution to all peritoneal surfaces.
In subjects randomized to the oxaliplatin arm, the agent was added to the perfusate once outflow temperatures exceed 39°C at a dose of 200 mg/M2. Subjects randomized to the mitomycin C arm received 30 mg of mitomycin C once outflow temperatures exceed 39°C. An additional 10 mg of mitomycin C was added to the perfusate 60 minutes into the HIPEC. The maximum inflow temperature of 42.5°C was tolerated during the perfusion, with the target outflow temperature being 40°C. The total perfusion time for both agents was 120 minutes. Following the perfusion, the peritoneum was washed out with 3 liters of perfusate and the peritoneum passively drained. The skin was then reopened, and the cannulas removed under direct vision. The abdomen was then definitively closed after completions of required anastomoses or stomas.
The general version of the Functional Assessment of Cancer Therapy (FACT-G) is a 27-item self-report questionnaire that measures QOL in cancer patients. The FACT-C is the FACT-G plus the 9-item colon subscale. We chose the colon subscale because it includes items that best address symptoms associated with peritoneal carcinomatosis (PC), and there is currently no FACT subscale specifically designed for PC patients. The FACT consists of four subscales measuring physical (PWB), functional (FWB), social/family (SFWB), and emotional well-being (EWB). A trial outcome index (TOI) representing PWB + FWB + the C subscale was used as a measure of treatment impact on physical symptoms and functioning.
After surgery, patients were followed with daily complete blood counts during their admission and at outpatient follow-up on postoperative day 30. Patients with WBC <4.0 were given G-CSF at 5μg/kg daily until the WBC exceeded 10. Transfusions were delivered per surgeon's discression. Surgical morbidity and mortality were recorded according to the CTCAE 3.0 classification17 and Clavien-Dindo18 systems. R0 and R1 resections were grouped together as complete cytoreductions. Cytoreductions with residual macroscopic disease were characterized as R2 and subdivided based on the size of residual disease (R2a ≤ 5mm, R2b ≤ 2 cm, R2c > 2cm)19.
Participants were randomized to receive either 200 mg/M2 of oxaliplatin or 40 mg of mitomycin C via HIPEC, see figure 1. Samples of normal and malignant peritoneum were collected pre- and post-treatment for analysis. Participants will be followed for toxicity starting 24 hours after the end of surgery to 30 days after surgery, and for progression and survival until 3 years after surgery. Patients were followed at least every 6 months with CT or MRI imaging, blood counts, functions and a CEA level.
Descriptive statistics including medians and ranges for continuous data and frequencies and percentages for categorical data were calculated. Fisher's exact tests were used assess for statistically significant differences in categorical variables and Wilcoxon rank sum tests were used to analyze group differences in continuous variables. Overall survival (OS) was calculated from the date of CRS/HIPEC (or first CRS/HIPEC in cases where a patient underwent more than one procedure) to the last known date of follow-up or the date of death. Survival was estimated using the Kaplan-Meier (product-limit) method. Group comparisons of OS were performed using the approximate chi-square statistic for the log-rank test. Cox's proportional hazards models were fit to assess univariate and multivariate relationships through regression analysis. Statistical significance was defined as a p value < 0.05. All analyses were performed using SAS 9.3, Cary, North Carolina.
Results
A total of 121 eligible patients were recruited to the trial between July 6, 2009 and October 15, 2015. An additional 9 patients were consented, were not evaluable, due to disease that could not be adequately debulked thereby precluding HIPEC. Demographics are shown in Table 1. Of the evaluable patients, 57% were female and 43% male. The average age at diagnosis was 54.1±13.0 years with a range of 21.9-81.7. The average age at the time of surgery was 55.3 ±13.3 years with a range of 22.0-81.8. The median follow up after surgery was 3.5 years. The patients were 92% Caucasian, 7% African American and 1% Asian. Patient recruitment was from Wake Forest in 80%, M.D. Anderson 13%, and University of Pittsburgh 7% of the cases respectively. The average peritoneal carcinomatosis index scores were similar with 18.0 in the mitomycin group vs. 17.9 in the oxaliplatin group. The appendiceal tumor was low grade in 71% and high grade in 29%. Resection (R) scores were similar between groups with 54% and 46% of achieving R0/1 and R2 resections in the mitomycin group vs. 51% and 49% respectively for the oxaliplatin group. We plan on reporting the quality of life data from this trial under separate cover.
Table 1. Cohort Characteristics.
| Characteristic | Mitomycin group (n=61) | Oxaliplatin group (n=60) | p Value |
|---|---|---|---|
| Follow-up, y | 3.2 | 3.9 | 0.14 |
| Sex, female, n (%) | 36 (59) | 33 (55) | 0.72 |
| Race, n (%) | 55 (90) | 56 (93) | 0.74 |
| Age, y, n (%) | 54.6 (14.0) | 55.2 (12.5) | 0.83 |
| PCI, n (%) | 18.0 (10.0) | 17.9 (9.4) | 0.94 |
| Resection status, n (%) | |||
| R0 | 13 (21) | 14 (23) | 0.35 |
| R1 | 20 (33) | 23 (38) | |
| R2a | 24 (39) | 15 (25) | |
| R2b | 4 (7) | 6 (10) | |
| R2c | 0 | 2 (3) | |
| Site, n (%) | 0.58 | ||
| MD Anderson | 5 (8) | 8 (13) | |
| University of Pittsburgh | 6 (10) | 4 (7) | |
| Wake Forest University | 50 (82) | 48 (80) | |
| Pathology, n (%) | 0.54 | ||
| Low grade | 40 (68) | 42 (74) | |
| High grade | 19 (32) | 15 (26) |
PCI, percutaneous coronary intervention.
There was no significant difference in rate of preoperative chemotherapy administration between groups 10% for mitomycin and 20% for oxaliplatin groups, p=.26. Preoperative chemotherapy was administered more frequently in patients with high grade disease patients, 38% vs. 6% of low grade disease participants, p<.05.
The white blood cell (WBC) K/mm3, platelet K/mm3 counts and hemoglobin g/dL levels are shown in figures 2-4. The WBC counts are similar at baseline and show the expected increase on the first 2 postoperative days. On day 5 the WBC is significantly lower in the mitomycin group and remained so through postoperative day 10, but returned to a level similar to the oxaliplatin group on outpatient follow up on postoperative day 30. G-CSF was needed in 13% of the oxaliplatin group and 21% of the mitomycin group, p=.072. The overall CTCAE toxicity for any level of leukocytopenia is significantly different between the 2 arms, p=0.0359, with the mitomycin having more (predominantly grade 1). However, when considering only grade 3-4 toxicity, the difference between groups is not significant (p=0.67).
Figure 2.
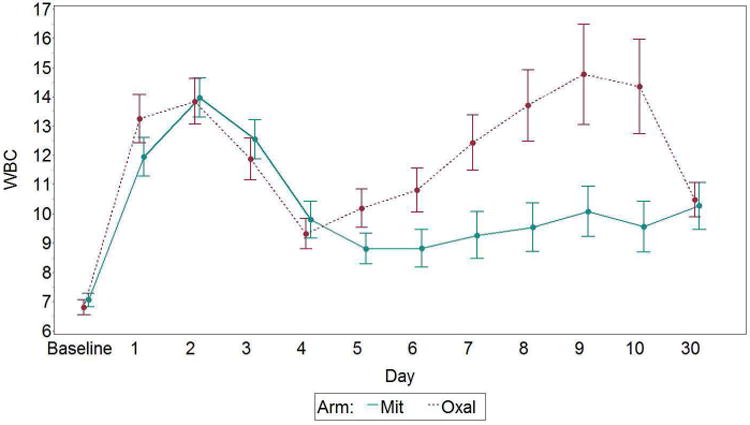
White blood cell count by day and arm, with standard error bars. Mit, mitomycin; Oxal, oxaliplatin. p<.01 on postoperative days 5, 6, 7, 8, 9, and 10.
Figure 4.
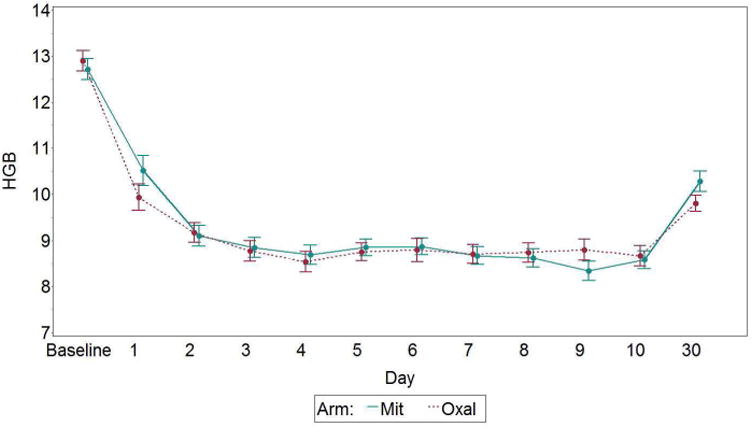
Hemoglobin (HGB) by day and arm, with standard error bars. Mit, mitomycin; Oxal, oxaliplatin.
The platelet counts are shown in figure 3 and are similar at baseline and postoperative days 1 and 3. However, by days 3-8 the counts are lower in the oxaliplatin group by differences ranging between 26 and 68,000K/mm3. The difference between groups is significant on postoperative days 5-6, p<.05. The hemoglobin levels are similar at baseline (12.7g/dL) and have the anticipated fall after surgery, which was similar between groups (-3.0 vs -2.5 g/dL) for both the oxaliplatin and mitomycin arms, as shown in figure 4. There were no differences in toxicity related to thrombocytopenia by CTCAE criteria, and only 3 grade 3-4 toxicities with 1 in the oxaliplatin group and 2 in the mitomycin group. Red cell transfusions were administered to 32% in the oxaliplatin group and 49% in the mitomycin group, p=.064.
Figure 3.
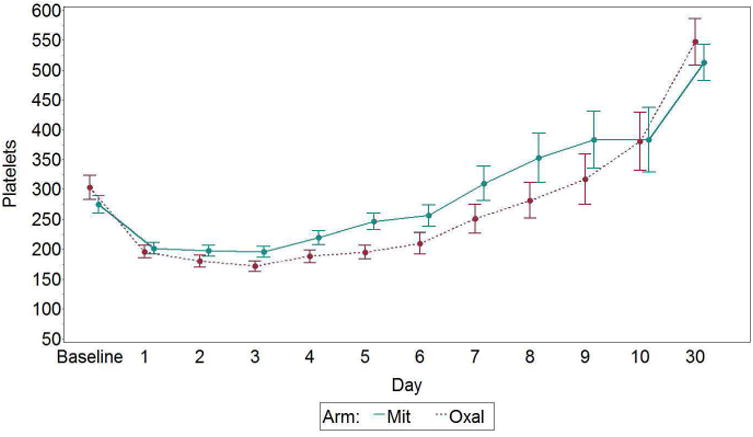
Platelets by day and arm, with standard error bars. Mit, mitomycin; Oxal, oxaliplatin. p<.05 on postoperative days 5 and 6.
The surgical complications using the Clavien Dindo18 classification stratified by group is shown in table 2. The 30 day mortality overall was 4.96%, with 3 deaths in each group. The complications were evenly distributed between groups. Thirty day Clavien Dindo (III/IV/V) major morbidity for the entire cohort was 33.3% for the oxaliplatin group and 32.7% for the mitomycin group. There was no difference in the rate of ICU admission, 38% vs 32%, or length of ICU stay, the median number of days spent in the intensive care unit was 3 vs.3 for mitomycin and oxaliplatin groups. The median length of hospital stay was similar between groups, 10. vs 8.0 days for mitomycin and oxaliplatin groups respectively, p=.57. There were no differences in 30 day readmission rates between groups, mitomycin group 14 (23%), oxaliplatin group 8 (13%) p=0.24. The 30 and 90 day mortality rates were similar between groups with 1.6 and 5% at 30 days, and 3.3 and 5% at 90 days for mitomycin and oxaliplatin respectively, p=.36 and .68.
Table 2. Complications after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy.
| Clavien Dindo arm of study | |||
|---|---|---|---|
| Frequency | Mitomycin 40 mg via IPHC, n (%), n=61 | Oxaliplatin 200 mg/m2 via IPHC, n (%), n=60 | Total |
| 0 | 7 (11.5) | 1 (1.7) | 8 |
| I | 5 (8.20) | 14 (23.3) | 19 |
| II | 29 (47.5) | 25 (41.7) | 54 |
| III | 0 (0.00) | 1 (1.7) | 1 |
| IIIa | 9 (14.8) | 9 (15.0) | 18 |
| IIIb | 3 (4.9) | 4 (6.7) | 7 |
| IV | 1 (1.6) | 1 (1.7) | 2 |
| IVa | 4 (6.6) | 1 (1.7) | 5 |
| IVb | 1 (1.6) | 1 (1.7) | 2 |
| V | 1 (1.6) | 3 (5.0) | 6 |
IPHC, intraperitoneal hyperthermic chemotherapy.
Short term outcomes of quality of life scores were similar between groups overall. However scores were better in the oxaliplatin group for physical well being (24.2 vs 22.4, p=.015) and emotional well being (19.4 vs 18.0,p=.048) through 1 year after surgery. Full quality of life data will be reported in another manuscript.
Overall survival and progression free survival was similar between groups throughout the study period, see figure 5. However, stratification of survival by histologic grade14 and group, as shown in figures 5 and 6, confirms the expected significant difference between low and high grade disease (p<.0001)18. To better clarify the impact of the HIPEC on outcome, figure 7 shows the similar overall survival only in those patients having an R0 and R1 resection stratified by grade and group. The 5 year survival is in excess of 95% with low grade tumors and complete (R0/1) resections, and the difference between low and high grade lesions is highly significant, p<.0001.
Figure 5.

(A) Overall survival and (B) progression-free survival. MMC, Mitomycin c; Oxal, oxaliplatin.
Figure 6.

Overall survival in (A) low grade and (B) high grade patients. Progression-free survival in (C) low grade and (D) high grade patients. MMC, Mitomycin c; Oxal, oxaliplatin.
Figure 7.

Overall survival. MMC Mitomycin c; Oxal, oxaliplatin.
Discussion
The rarity and variety of appendiceal primary lesions makes study of this disease difficult. Although the incidence has been increasing for over a decade,1 several issues have made completing clinical trials difficult20. While prospective randomized trials have been attempted in the past (NCT00052962), none, at any stage, had completed planned accrual. To the best of our knowledge, this is the first completed prospective randomized trial for cancer of the appendix at any stage. It is also the first completed HIPEC trial in the Western Hemisphere. Even with 3 large and experienced centers accruing, this trial took 6 years to complete accrual, which highlights the challenges in this setting. This also proves that such trials can be completed, and opens the door for additional trials for appendiceal cancer patients.
The optimal agent to use for intraperitoneal chemotherapy has yet to be identified. Mitomycin c has been the standard agent for decades6. However, oxaliplatin has substantially greater efficacy when used systemically for a variety of gastrointestinal neoplasms. The use of oxaliplatin has been of great interest in European centers, and is a standard agent there. The most commonly cited dose of oxaliplatin has been 460 mg/M2 with a 30 minute perfusion, frequently with intravenous 5-fluorouracil and leukovorin21. The 30 minute perfusion has been challenged as being too short for full efficacy. There has been a phase 1 trial of oxaliplatin with a 120 minute perfusion, which found a maximally tolerated dose of 200mg/M2 10. Retrospective analyses have reached differing conclusions on which agent is superior 7, 11-13. Our previous retrospective trial, as well as a report from Holland,7 found no difference between mitomycin and oxaliplatin11, an Australian report suggested oxaliplatin to have a better survival for colorectal primary,13 and a group report from the American Society for Peritoneal Surface Malignancies suggested mitomycin might be a better agent12. Therefore, we chose to compare a standard dose of mitomycin to a dose of oxaliplatin compatible with a longer (2 hour) perfusion time.
The data show that mitomycin results in statistically more leukopenia than oxaliplatin in a 2 hours perfusion. There was a trend toward greater use of white cell count support with G-CSF in the mitomycin group. Oxaliplatin resulted in somewhat lower platelet counts during the first week after the HIPEC. Although statistically significant, the impact of these differences on clinical outcome is minimal. In terms of toxicity, complications and survival, it suggests that unless patients have preoperative leukopenia (favoring oxaliplatin) or thrombocytopenia (favoring mitomycin), the choice of agent may be decided by institutional experience/preference with the agents or cost.
Complications following cytoreductive surgery and HIPEC have always been a concern for patients and surgeons embarking on treatment of peritoneal surface disease. Appendiceal cancer has been the leading indication for cytoreductive surgery and HIPEC since its original description22. Peritoneal dissemination from appendiceal mucinous cancer has been treated with CRS/HIPEC for many years, with acceptable and decreasing morbidity and mortality 6,15,16,20. As previously reported, surgical complications were associated with increased volume of disease, pre-existing medical comorbidities, poor functional status, and suboptimal nutrition.10,11 However, in this randomized trial surgical high grade complications were not significantly impacted by choice of mitomycin or oxaliplatin in the perfusate.
Complete cytoreduction has long been known to be a key prognostic factor for both low grade and high grade appendiceal cancer in predicting improved OS and PFS. This is confirmed in this randomized trial. This is in agreement with prior reports that have demonstrated that long term survival is possible, even for high grade disease, if a complete cytoreduction is obtained.3-6-15,16,20
The data also confirms that for low grade completely resected (R0/1/R2a) disease, systemic chemotherapy is unlikely to improve long term survival. Although this study did not control for receipt of preoperative or postoperative systemic chemotherapy, considering the low rates of response and the excellent outcomes with cytoreductive surgery and HIPEC, we see a very limited role for systemic treatment in this setting. High grade disease, on the other hand, had much poorer outcomes, and such cases should be considered for systemic preoperative and/or postoperative chemotherapy23,24,25.
The equivalent overall and progression free survival with either the oxaliplatin or mitomycin in the perfusion during HIPEC implies similar anti-neoplastic efficacy. However, the contrary hypothesis must be entertained; specifically, that neither agent has efficacy in this setting. Although it has previously been attempted for appendiceal cancer, no trial has compared aggressive cytoreduction with and without HIPEC. The Protige 7 trial which compares cytoreductive surgery with or without HIPEC for peritoneal metastases from colon cancer, has completed accrual in France and addresses this important question as it relates to colonic primary lesions21,26. However, the findings in that trial, may not be applicable in this setting since the appendiceal lesions are remarkably different on genomic analysis27,28 despite the appendix being anatomically part of the colon. Further, retrospective studies with and without intraperitoneal adjuvants have shown advantages for intraperitoneal adjuvants29.
The current study represents a sizeable cohort of a very rare disease, and is the first randomized trial of its kind, it has several limitations which must be considered. The selection of sites for this study was limited to institutions which have been through the lengthy learning curve, and results at less experienced centers may not be as good8. This study was not powered sufficiently to be able to define small differences in survival, and a much larger trial might be able to define a survival difference, if such as difference if exists.
In conclusion, oxaliplatin and mitomycin have slightly different hematologic toxicity, but similar complications rates in a 2 hour HIPEC. Patients with baseline thrombocytopenia may better be treated with oxaliplatin, while those with preoperative leukopenia may better be treated with mitomycin. This study also clearly confirms that randomized clinical trials of CRS/HIPEC are feasible for patients with PSD in general30,31 and for those with appendiceal primary lesions specifically. It is through such prospective randomized trials that the numerous questions related to this therapy can be answered.
Acknowledgments
The authors gratefully acknowledge the contributions of Joan L. Feder, Donna Angel and Kathleen C. Perry to this trial and manuscript.
Support: Wake Forest University Comprehensive Cancer Center Biostatistics shared resource funded via the NCI grant award P30CA012197 and the Orin Smith Family foundation.
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 129th Annual Meeting, Hot Springs, VA, December 2017.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marmor S, Portschy PR, Tuttle TM, Virnig BA. The rise in Appendiceal Cancer Incidence 2000-2009. J Gastrointestinal Surgery. 2015;19:743–750. doi: 10.1007/s11605-014-2726-7. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi AN, Mishra G, Levine EA. Adenocarcinoma of the appendix is rarely detected by colonoscopy. Journal of Gastrointestinal Surgery. 2009;13:668–675. doi: 10.1007/s11605-008-0774-6. [DOI] [PubMed] [Google Scholar]
- 3.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Journal of Clinical Oncology y. 2012;30:2449–56. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 4.Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 Patients treated with cytoreductive surgery and intraperitoneal chemotherapy. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2010;36:456–62. doi: 10.1016/j.ejso.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Annals of surgical oncology. 2008;15:526–34. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 6.Stewart JH, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624–34. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 7.Hompes D, D'Hoore A, Wolthuis A, et al. The use of oxaliplatin or mitomycin c in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparitive study. 2014;109:527–532. doi: 10.1002/jso.23546. [DOI] [PubMed] [Google Scholar]
- 8.van Ruth S, Mathot R, Sparidans R, et al. Population pharmacokinetics and pharmacodynamics of mitomycin during intraoperative hyperthermic intraperitoneal chemotherapy. Clinical Pharmacokinetics. 2004;43:131–143. doi: 10.2165/00003088-200443020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kusamara s, Elias D, Baratti D, et al. Durgs carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surgical Oncology. 2008;98:247–252. doi: 10.1002/jso.21051. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JH, Shen P, Russell GB, et al. A Phase I Trial of Oxaliplatin for Intraperitoneal Hyperthermic Chemoperfusion for the Treatment of Peritoneal Surface Dissemination From Colorectal and Appendiceal Cancer. Annals of Surgical Oncology. 2008;15:2137–2145. doi: 10.1245/s10434-008-9967-1. NIHMSid is 482983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Votanopoulos KI, Ihemelandu C, Shen P, et al. A Comparison of Hematologic Toxicity Profiles after Heated Intraperitoneal Chemotherapy with Oxaliplatin and Mitomycin-C. Journal of Surgical Research. 2013;179:E133–139. doi: 10.1016/j.jss.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prada-Villaverde A, Esquivel J, Lowy AM, et al. The American Society of Peritoneal Surface malignanies evaluation of HIPEC with mitomycin c versus oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. Journal of Surgical Oncology. 2014;110:770–785. doi: 10.1002/jso.23728. [DOI] [PubMed] [Google Scholar]
- 13.Leung v, Hua YR, Liauw, Morris DL. Oxaliplatin versus mitomycin for HIPEC in colorectal cancer with peritoneal carcinomatosis. European Journal of Surgical Oncology. 2017;43:144–149. doi: 10.1016/j.ejso.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Bradley RF, Stewart JH, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. The American journal of surgical pathology. 2006;30:551–9. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 15.Levine EA, Stewart JHt, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 204:943–53. doi: 10.1016/j.jamcollsurg.2006.12.048. discussion 953-5, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Levine EA, Stewart JH, Shen P, et al. Intraperitoneal Chemotherapy for Peritoneal Surface Malignancy: Experience with 1,000 Patients. Journal of the American College of Surgeons. 2014;218:573–587. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute: Cancer Therapy Evaluation Program. Common terminology criteria for adverse events v3.0 (CTCAE) Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery. 2004;240:205–13. 205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Joint Commission on Cancer Cancer Staging Manual. 7th. Springer; New York: 2010. pp. 13–14. [Google Scholar]
- 20.Votanopoulos KI, Russell G, Randle RW, et al. Peritoneal Surface Disease (PSD) from Appendiceal Cancer Treated with Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Overview of 481 Cases. Annals of Surgical Oncology. 2015;22:1274–1279. doi: 10.1245/s10434-014-4147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias D, Lefevre JH, Chevalier J, et al. Complete Cytoreductive Surgery Plus Intraperitoneal Chemohyperthermia With Oxaliplatin for Peritoneal Carcinomatosis of Colorectal Origin. Journal of Clinical oncology. 2009;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 22.Spratt JS, Adcock RA, Sherrill W, Travathen S. Hyperthermic peritoneal perfusion system in canines. Cancer Res. 1980;40:253–255. [PubMed] [Google Scholar]
- 23.Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Annals of oncology official journal of the European Society for Medical Oncology / ESMO. 23:652–8. doi: 10.1093/annonc/mdr279. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugarbaker PH, Bijelic L, Chang D, et al. Neoadjuvant FOLFOX chemotherapy in 34 consecutive patients with mucinous peritoneal carcinomatosis of appendiceal origin. J Surg Oncol. 2010;102:576–81. doi: 10.1002/jso.21679. [DOI] [PubMed] [Google Scholar]
- 25.Blackham AU, Swett K, Eng C, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyuperthermic intraperitoneal chemotherapy. Journal of Surgical Oncology. 2014;109:740–745. doi: 10.1002/jso.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias D, Quenet F, Goere D. Current status and future directions I the treatment of peritoneal dissemination from colorectal carcinoma. Surgical Oncology Clinics of North America. 2012;21:611–623. doi: 10.1016/j.soc.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Levine EA, Blazer DG, Kim MK, et al. Gene Expression Profiling of Peritoneal Metastases from Appendiceal and Colon Cancer Demonstrates Unique Biologic Signatures and Predicts Patient Outcomes. Journal of the American College of Surgeons. 2012;214:599–607. doi: 10.1016/j.jamcollsurg.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine EA, Votanopoulos KI, Qasem SA, et al. Prognostic Molecular Subtypes of Low-Grade Cancer of the Appendix. Journal of the American College of Surgeons. 2016;222:493–504. doi: 10.1016/j.jamcollsurg.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schomas DA, Miller RC, Donohue JH, et al. Intraperitoneal treatment for peritoneal mucinous carcinomatosis of appendiceal origin after operative management, long term follow up of the Mayo Clinic experience. Annals of Surgery. 2009;249:588–595. doi: 10.1097/SLA.0b013e31819ec7e3. [DOI] [PubMed] [Google Scholar]
- 30.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 31.Cashin PH, Mahteme H, Spang N, et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomized trial. European Journal of Cancer. 2016;53:155–162. doi: 10.1016/j.ejca.2015.09.017. [DOI] [PubMed] [Google Scholar]


