Summary
Beckwith-Wiedemann syndrome (BWS) is a rare paediatric overgrowth disorder. Associated macroglossia is a feature of many children with BWS and is felt to be a risk factor for obstructive sleep apnoea (OSA). Sleep-disordered breathing is highly variable in this population. The relationship between degree of macroglossia or other genotypic or phenotypic factors and OSA severity has not been established. The natural history of OSA in this population is unknown; a variety of conservative and surgical therapies have been used to treat OSA in children with BWS but none have been studied systematically. Tongue reduction is the mainstay of surgical therapy for macroglossia associated with BWS, but limited data are available regarding its efficacy in treating OSA or its effect on speech and swallowing. More research is needed to better identify which children with BWS are at risk for OSA and the most effective treatment for these patients.
Keywords: Sleep apnea, obstructive, child, infant, macroglossia, syndrome
Introduction
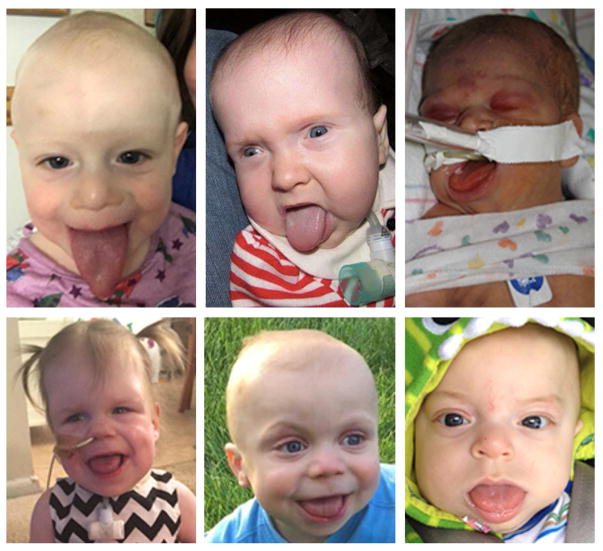
Beckwith-Wiedemann syndrome (BWS) is a paediatric overgrowth and cancer predisposition disorder with an incidence of at least 10,500 live births1. Clinical features can be variable based on the mosaic nature of the genetic and epigenetic causes of BWS on chromosome 11 as described below, leading to the characterization of the range of features under the broader domain of the ‘11p Overgrowth Spectrum.’ Classic BWS features include macroglossia, omphalocele, macrosomia, hemihypertrophy, distinct facies (ear creases, midface hyperplasia, facial nevus flammeus), hypoglycemia, and embryonal tumours.1 Even focusing on one feature such as macroglossia, there is a clinical range of presentations (Figure 1). It has also become increasing apparent that clinical features correlate with the genetic cause of BWS.
Figure 1. Spectrum of macroglossia in Beckwith-Wiedemann Syndrome.
Variation in degree of macroglossia in patients requiring tongue reduction.
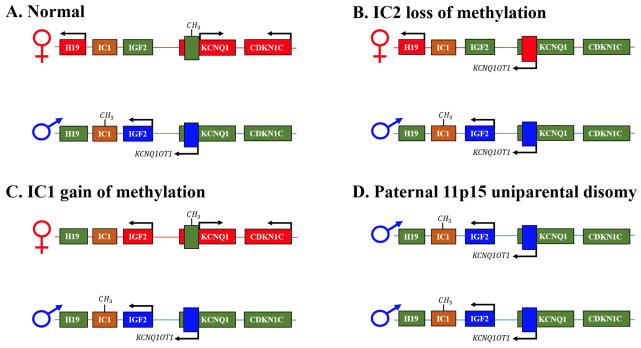
BWS is caused by genetic or epigenetic changes on chromosome 11p15 (Figure 2). There are several imprinted genes on chromosome 11p15 that are normally expressed in a parent of origin specific manner and regulated by two imprinting control regions. These genes all control growth and include (H19, insulin-like growth factor 2 (IGF2), and CDKN1C. The most common cause of BWS is loss of methylation at imprinting control region 2, KCNQ1OT1:TSS-DMR 2 (IC2) and these patients are more likely to have macroglossia2. The second most common cause of BWS is paternal uniparental isodisomy for part or all of chromosome 11 (pUPD11) and these patients demonstrate the largest spectrum of clinical features can have macroglossia. Gain of methylation at imprinting control region 1, H19/IGF2:IG-DMR (IC1) and maternally inherited mutations in CDKN1C also cause BWS, but less commonly present with macroglossia.2
Figure 2. Genetic and epigenetic causes of Beckwith-Wiedemann Syndrome.
A. Normal imprinting at chromosome 11p15.5. Methylation is indicated by CH3 on the maternal or paternal chromosome and arrows above genes indicate that a gene is on. Normally, the first region is methylated on the paternal chromosome and IGF2 is paternally expressed and H19 is maternally expressed. For the second region, the maternal chromosome is methylated and CDKN1C is maternally expressed. B. Loss of methylation at IC2 leads to loss of CDKN1C expression and expression of KCNQ1OT1. C. Gain of methylation at IC1 leads to loss of H19 expression and expression of IGF2 from both chromosomes. C. Paternal Uniparental Isodisomy leads to loss of H19 expression, expression of IGF2 from both chromosomes and loss of CDKN1C expression. Mutations in CDKN1C also occur and lead to absence of CDKN1C expression.
Evaluation and management of children with BWS
Any child with a feature suggestive of BWS, should be evaluated by a clinical geneticist. Genetic testing for methylation at IC2 and IC2 and microarray analysis for detection of pUPD11 is recommended. Testing affected tissue is most informative and the genetic or epigenetic changes may not be present in every tissue type, however testing is most often performed in blood or saliva as these are easily accessible samples. Early management of children with BWS focuses on evaluation for hypoglycemia and macroglossia. Children with prolonged hypoglycemia should be referred to endocrinology for evaluation and follow-up. Children with macroglossia should be referred to pulmonology, plastic surgery, and feeding specialists for evaluation. Due to the increased risk for embryonal tumours in BWS, tumour screening with alpha-feto protein blood measurements and abdominal ultrasounds should be initiated at the time of diagnosis. It is recommended that tumour screening be monitored by oncologists or geneticists specializing in cancer predisposition. For children with hemihyperplasia, orthopedics evaluation is recommended when the children are beginning to walk.
The tongue as a risk factor for OSA
Macroglossia has been identified as a risk factor for OSA.3 An absolute or relative increase in tongue size can narrow the retroglossal airway and lead to upper airway obstruction. This relationship has been demonstrated in a variety of high-risk adult and paediatric populations. For example, in children with Pierre Robin sequence, mandibular hypoplasia leads to glossoptosis, and results in upper airway obstruction at the tongue base. Surgical procedures such as mandibular distraction osteogenesis can increase the size of the retroglossal airway relative to the tongue, effectively treating OSA.4 Similarly, in patients with Down syndrome, tongue size is one risk factor for OSA. In a study using upper airway magnetic resonance imaging (MRI), Guimaraes and colleagues showed that patients with Down syndrome had relative macroglossia compared to healthy controls.5 In another MRI study, macroglossia was seen in 74% of children with Down syndrome who had persistent OSA following adenotonsillectomy.6 Tongue size has been shown to be significantly greater in obese adults with OSA compared with BMI-matched adults who do not have OSA.7 One case-control study of over 120 participants showed regional increases in the size of the tongue base due to fat deposits.8 Interestingly, this relationship did not hold for obese adolescents.9
While macroglossia is a feature of BWS, there have been no studies correlating tongue size with OSA severity or risk in children with BWS. Various techniques have been used to assess the size of the tongue and its relationship to the airway in children with BWS. Imaging studies may be indicated to determine the extent of macroglossia. In one series of two children, MRI and CT were used to confirm macroglossia and document the location of upper airway obstruction.10 Bronchoscopy may be used to visualvisualise the location of the tongue in the airway.
Risk of OSA in children with BWS
Polysomnography is the gold standard for evaluation of OSA in all children as it uses a standardised multichannel recording. However, it is not clear which children with BWS should be evaluated with polysomnography or at what age. There are no prospective studies assessing OSA in infants or children with BWS, but there are multiple small case series that include polysomnography in paediatric patients with BWS. One study reported on two children aged five and seven years old with BWS who were evaluated with polysomnography and were found to have moderate-severe OSA.11 In another study, two children with BWS were assessed with polysomnography and found to have apnoea indexes of 12.5/hour and 28.1/hour, one with significant desaturation.10 Another report of two children with BWS included a 16-month-old child who required tracheostomy for upper airway obstruction and a 14-year-old with an apnoea hypopnea index of 10/hour.12 One report described upper airway resistance syndrome in three of five patients with BWS who were studied with polysomnography with none having significant OSA.13 A retrospective review reported the evaluation of three young children with macroglossia and BWS.14 This report included a 13-month-old patient who had severe desaturation on an incomplete polysomnogram that was unable to assess for OSA, and two 20-month-olds with AHI of 3/hour and 7.2/hour, neither with significant desaturation.
The largest series of children with BWS, by Follmar and colleagues, is a single-center 30-year retrospective study that included 118 patients with BWS. Medical record review estimated a 48% prevalence of sleep-disordered breathing in this cohort, including 15 diagnosed with OSA by polysomnography, 16 who had airway obstruction during infancy, and 26 where snoring was reported.15 Of note, this study found no significant difference in the presence of sleep-disordered breathing among children with BWS who had macroglossia or underwent tongue reduction. Although available data are limited to mostly very small retrospective series and one large single-center retrospective study, it appears that while there is a high prevalence of OSA in children with BWS, there is significant variability in sleep-disordered breathing in this population. Based on the available data, the genotypic and phenotypic risk factors for OSA in children with BWS are not clear.
Treatment of OSA in children with BWS
Conservative management
As in other patients with OSA, treatment may include conservative or medical therapies as well as surgery. Data regarding successful treatment are again limited to case series and retrospective studies. In patients with macroglossia, conservative treatment might include positioning other than supine to avoid tongue-based airway obstruction, but there is little evidence of success with this intervention. Of the 15 children with BWS found to have OSA in the series published by Follmar et al, none were successfully treated with positioning, although it is not clear in how many treatment with positioning was actually attempted.15 One case report described OSA symptoms being avoided in the prone position in a 3-month-old with BWS, but no polysomnogram was performed.16 While side-lying or prone positioning may be useful in a subset of children with BWS who have obstruction from macroglossia, more research is needed to determine for which patients this treatment is appropriate. The natural history of OSA in infants and children with BWS is unknown, and the safety of watchful waiting has not been studied.
Continuous positive airway pressure (CPAP) is a highly effective treatment in children with OSA, including those with conditions such as Down syndrome17 and micrognathia18 where obstruction at the tongue base is felt to play a role in OSA. CPAP is recommended in children with Hunter syndrome, which also often includes macroglossia. However, there is little published about the success of CPAP in children with BWS. One series reported successful treatment of OSA using CPAP in three children with BWS as well as one patient who failed CPAP and went on to have surgery.15 While CPAP is likely effective in some children with BWS, its use has not been studied in children with BWS or macroglossia to identify which patients are good candidates for this treatment.
Surgical management
Tongue reduction
The mainstay of macroglossia treatment in children with BWS is tongue reduction surgery, both to relieve moderate to severe upper airway obstruction and to potentially improve speech and feeding.19 Additional indications for surgery include relief of dental malocclusion from tongue’s impact on teeth and for cosmetic reasons. Most studies consider normal resting tongue position as the primary outcome and report that the goal of the procedure is to reduce the tongue to as close to normal size as possible so that functionally it sits behind the lower dental arch at rest, yet can wet the lips on protrusion. Tongue reduction surgeries have existed for many years for various indications, and for an uncommon procedure, there are myriad techniques reported. Surgical techniques include anterior, peripheral, or central reduction,11,13 and the incisions may be V-shaped, W-shaped, stellate, or keyhole, among others.20,21 In addition to traditional scalpel-based techniques, surgeons have used radiofrequency coblation,12 ultrasonic dissection, and other modalities.19 No studies have compared success rates of the various surgical techniques in terms of complications, recovery time, or functional outcomes such as OSA, speech, or swallowing. One technique may be used over another because of the type of macroglossia present (hemihypertrophy versus global hypertrophy), or surgeon preference due to functional or aesthetic outcome or safety profile. Additional study of the various variables related to surgical reduction is warranted
Just as there is no standard technique for tongue reduction, timing is also highly variable, with surgery taking place any time from infancy to adulthood. Regrowth of tongue tissue has been reported, sometimes requiring a second debulking procedure.16,22 It is not clear whether this is dependent on the surgical technique. For this reason, some have advocated waiting until children are at least six months old to proceed with tongue reduction surgery.
There are no prospective studies of OSA outcomes following tongue reduction surgery. However, there are a number of small retrospective case series that evaluated OSA postoperatively (Table 1). The largest series, by Follmar et al, reported that of the 15 patients who were diagnosed with OSA, four underwent tongue reduction.15 Of these, three had resolution of OSA, and one had persistent OSA that resolved following adenotonsillectomy. No polysomnographic results are reported in that study. In a series of three children who underwent tongue reduction for OSA, polysomnogram following surgery showed improved OSA in all three, including resolved OSA in one patient.11 Kamata and colleagues report two cases of children with BWS and macroglossia were found to have obstructive apnoea following repair of omphalocele.10 Following central tongue resection and division of the frenulum linguae, polysomnography showed that apnoea had resolved in both (apnoea index of 0.3/hour in one and ‘apnoea events disappeared’ in the other). Maturo et al report success in treating three children with BWS and macroglossia who had obstructive apnoea using a submucosal minimally invasive lingual incision.12 In that series, a 16-month-old patient was able to be decannulated following the procedure and the AHI of two older children were reduced from 10/hour to less than one per hour following surgery.
Table 1.
Summary of evidence for tongue reduction as OSA treatment in BWS
| Study | n | Technique | Pre-operative respiratory findings | Post-operative respiratory findings | Notes |
|---|---|---|---|---|---|
| Mixter11 1993 | 3 | Central ‘W’ | 1 with moderate-severe OSA, 2 with severe OSA | One with mild-moderate OSA, 1 with mild OSA, 1 with no OSA | Minimal improvement in articulation disorders; 2 required tracheostomy |
| Kamata10 (2005) | 2 | 1 anterior resection, 1 central resection; both: division of frenulum | Severe OSA | No OSA | Both patients were preterm infants with omphalocele |
| Maturo12 (2006) | 3 | Submucosal minimally-invasive lingual excision | 1 with tracheostomy 2 with AHI=10/hr | 1 decannulated AHI for other two = 0.2/hour and 0.9/hr | |
| Follmar15 (2014) | 4 (118 in series) | Not reported | ‘OSA’ | ‘successfully treated’ | Unclear if PSGs performed in these patients |
Tongue reduction has also been used to treat OSA other populations with macroglossia. One small study suggested that tongue reduction with lingual tonsillectomy may be effective treatment of OSA in non-obese children with Down syndrome.23 A study of 15 adults with OSA showed that tongue reduction improved OSA in some patients but not others.24
Other indications for tongue reduction include feeding and swallowing difficulty and the tongue is of critical importance in speech, and these functional outcomes have been reported in some of the small surgical series. Some studies have shown that reducing the size of the tongue does not impair speech, while others have reported that speech generally does not improve following surgery.11,25 In a series of 23 children with BWS who underwent anterior wedge resection, one was reported to have speech problems following surgery.13 Interestingly, in this study, although 39% had feeding problems and 13% had swallowing difficulties pre-operatively, post-operative feeding outcomes are not reported. A study of 10 consecutive children with BWS who had tongue reduction assessed drooling and feeding problems pre- and post-operatively.26 All had feeding problems before surgery and had normal eating and drinking behaviors at a 3-month post-operative visit and a longer-term follow-up. There was also reported improvement with drooling as tongue position improved postoperatively.
While complication rate likely varies depending on the technique, the variety of techniques and small sample sizes of most studies makes determining the rate of complication difficult. In general, surgical complications of tongue reduction include bleeding, wound dehiscence, tongue edema which can cause airway obstruction, and wound infection.27
Other surgery
A variety of other surgeries have been used to treat upper airway obstruction in children with BWS, depending on the severity of symptoms. For infants with very severe obstruction, tracheostomy remains the mainstay of therapy, and by bypassing the obstruction completely is highly successful at treating OSA.11,12,15 Other surgical interventions may be used either in conjunction with tongue reduction or separately, and may include tonsillectomy, adenoidectomy, uvulectomy, tongue-lip adhesion, and/or lingual frenectomy. While there have not been any studies that specifically aimed to determine the efficacy of these airway surgeries on OSA in children with BWS, there are reports of their success.15,28
Conclusions
BWS is a rare, complex genetic condition that causes embryonal tumours, hemihyperplasia and macroglossia, among other findings, but the phenotype is highly variable. Available evidence suggests that children with BWS are at increased risk for OSA. Macroglossia seems to be the primary risk factor for OSA in this population, but a correlation between tongue size and OSA severity has not been established. The constellation of sleep-disordered breathing and presentation of children with BWS is highly variable, from infants who present with neonatal respiratory distress to children who present with milder findings later in childhood. There are a variety of treatment options for OSA in children with BWS, including conservative and surgical therapies. Success has been reported in treating OSA with tongue reduction, but larger studies are needed to better understand which patients benefit most from this procedure, if there are different outcomes from different surgical techniques, and the impact on speech and swallowing.
Research Directions.
The lack of available evidence regarding the risk factors for and optimal treatment of OSA in children with BWS highlights the need for systematic studies of OSA in this population. Because of the retrospective nature of the available literature, findings are subject to referral bias. The small sample size of the available studies and lack of standardised approach make pooling data between studies difficult. Specifically, research in the following areas is needed:
Prospective studies that include polysomnograms on all children with a diagnosis of BWS to assess the prevalence of OSA and genotype/phenotype risk factors, and other factors, such as age.
Prospective studies that include pre- and post-operative polysomnograms on children with BWS undergoing tongue reduction or other surgical intervention.
Studies that include pre- and post-treatment polysomnograms for conservative therapies such as continuous positive airway pressure and positioning.
Larger published series of tongue reduction techniques to allow for comparison between procedures
Imaging studies that identify the contribution of macroglossia to OSA in children with BWS.
Larger studies that assess functional outcomes such as speech and swallowing in children with BWS, and outcomes from tongue reduction.
Educational aims.
The reader will be able
To discuss the prevalence of obstructive sleep apnea (OSA) in children with Beckwith-Wiedemann syndrome (BWS)
To discuss the relationship between macroglossia and OSA
To review treatment options for OSA in children with BWS
To emphasize the need for prospective studies of OSA in children with BWS
Practice points.
Beckwith-Wiedemann syndrome is a rare genetic syndrome that requires a multidisciplinary team that may include genetics, oncology, endocrinology, orthopedics, plastic surgery, and sleep medicine.
Infants and children with BWS are at increased risk for OSA, likely due to macroglossia, and should be screened for sign of sleep-disordered breathing.
Tongue reduction is the most commonly reported surgical treatment for OSA in children with BWS; there are a variety of techniques depending on the type of macroglossia.
The current evidence base for the evaluation and treatment of OSA in infants and children with BWS is mostly based on retrospective series.
Acknowledgments
This work was made possible by the generous funding support by the National Institutes of Health (KL2 TR001879 01 C.M.C.; K08 CA193915 J.M.K.), a Parker B. Francis fellowship award (C.M.C.), the Alex’s Lemonade Stand Foundation (J.M.K.), and St. Baldrick’s Foundation (J.M.K.)
Footnotes
Conflict of Interest Statement
No conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Mussa A, Di Candia S, Russo S, Catania S, De Pellegrin M, Di Luzio L, et al. Recommendations of the Scientific Committee of the Italian Beckwith-Wiedemann Syndrome Association on the diagnosis, management and follow-up of the syndrome. Eur J Med Genet. 2016;59(1):52–64. doi: 10.1016/j.ejmg.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Brioude F, Lacoste A, Netchine I, Vazquez MP, Auber F, Audry G, et al. Beckwith-Wiedemann syndrome: growth pattern and tumor risk according to molecular mechanism, and guidelines for tumor surveillance. Horm Res Paediatr. 2013;80(6):457–465. doi: 10.1159/000355544. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Brooks LJ, Draper KA, Gozal D. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JA, Chung C, Paliga JT, Cielo C, Marcus CL, Lioy J, et al. Mandibular distraction osteogenesis for the treatment of neonatal tongue-based airway obstruction. J Craniofac Surg. 2015;26(3):634–641. doi: 10.1097/SCS.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 5.Guimaraes CV, Donnelly LF, Shott SR, Amin RS, Kalra M. Relative rather than absolute macroglossia in patients with Down syndrome: implications for treatment of obstructive sleep apnea. Pediatr Radiol. 2008;38(10):1062–1067. doi: 10.1007/s00247-008-0941-7. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly LF, Shott SR, LaRose CR, Chini BA, Amin RS. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. AJR Am J Roentgenol. 2004;183(1):175–181. doi: 10.2214/ajr.183.1.1830175. [DOI] [PubMed] [Google Scholar]
- 7.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 8.Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37(10):1639–1648. doi: 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab RJ, Kim C, Bagchi S, Keenan BT, Comyn FL, Wang S, et al. Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med. 2015;191(11):1295–1309. doi: 10.1164/rccm.201501-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamata S, Kamiyama M, Sawai T, Nose K, Usui N, Kawahara H, et al. Assessment of obstructive apnea by using polysomnography and surgical treatment in patients with Beckwith-Wiedemann syndrome. J Pediatr Surg. 2005;40(3):E17–19. doi: 10.1016/j.jpedsurg.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Mixter RC, Ewanowski SJ, Carson LV. Central tongue reduction for macroglossia. Plast Reconstr Surg. 1993;91(6):1159–1162. doi: 10.1097/00006534-199305000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Maturo SC, Mair EA. Submucosal minimally invasive lingual excision: an effective, novel surgery for pediatric tongue base reduction. Ann Otol Rhinol Laryngol. 2006;115(8):624–630. doi: 10.1177/000348940611500809. [DOI] [PubMed] [Google Scholar]
- 13.Kadouch DJ, Maas SM, Dubois L, van der Horst CM. Surgical treatment of macroglossia in patients with Beckwith-Wiedemann syndrome: a 20-year experience and review of the literature. Int J Oral Maxillofac Surg. 2012;41(3):300–308. doi: 10.1016/j.ijom.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Kansagra S, D’Cruz O, Noah TL, Vaughn BV. Sleep-disordered breathing in Beckwith-Wiedemann syndrome: three patients. Am J Med Genet A. 2012;158A(11):2956–2958. doi: 10.1002/ajmg.a.35628. [DOI] [PubMed] [Google Scholar]
- 15.Follmar A, Dentino K, Abramowicz S, Padwa BL. Prevalence of sleep-disordered breathing in patients with Beckwith-Wiedemann syndrome. J Craniofac Surg. 2014;25(5):1814–1817. doi: 10.1097/SCS.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 16.Davalbhakta A, Lamberty BG. Technique for uniform reduction of macroglossia. Br J Plast Surg. 2000;53(4):294–297. doi: 10.1054/bjps.1999.3311. [DOI] [PubMed] [Google Scholar]
- 17.Konstantinopoulou S, Tapia IE, Kim JY, Xanthopoulos MS, Radcliffe J, Cohen MS, et al. Relationship between obstructive sleep apnea cardiac complications and sleepiness in children with Down syndrome. Sleep Med. 2016;17:18–24. doi: 10.1016/j.sleep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Miller SD, Glynn SF, Kiely JL, McNicholas WT. The role of nasal CPAP in obstructive sleep apnoea syndrome due to mandibular hypoplasia. Respirology. 2010;15(2):377–379. doi: 10.1111/j.1440-1843.2009.01681.x. [DOI] [PubMed] [Google Scholar]
- 19.Kittur MA, Padgett J, Drake D. Management of macroglossia in Beckwith-Wiedemann syndrome. Br J Oral Maxillofac Surg. 2013;51(1):e6–8. doi: 10.1016/j.bjoms.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Kacker A, Honrado C, Martin D, Ward R. Tongue reduction in Beckwith-Weidemann syndrome. Int J Pediatr Otorhinolaryngol. 2000;53(1):1–7. doi: 10.1016/s0165-5876(00)00280-9. [DOI] [PubMed] [Google Scholar]
- 21.Van Lierde K, Galiwango G, Hodges A, Bettens K, Luyten A, Vermeersch H. Impact of tongue reduction on overall speech intelligibility, articulation and oromyofunctional behavior in 4 children with Beckwith-Wiedemann syndrome. Folia Phoniatr Logop. 2012;64(2):55–63. doi: 10.1159/000329569. [DOI] [PubMed] [Google Scholar]
- 22.Kopriva D, Classen DA. Regrowth of tongue following reduction glossoplasty in the neonatal period for Beckwith-Wiedemann macroglossia. J Otolaryngol. 1998;27(4):232–235. [PubMed] [Google Scholar]
- 23.Propst EJ, Amin R, Talwar N, Zaman M, Zweerink A, Blaser S, et al. Midline posterior glossectomy and lingual tonsillectomy in obese and nonobese children with down syndrome: Biomarkers for success. Laryngoscope. 2016 doi: 10.1002/lary.26104. [DOI] [PubMed] [Google Scholar]
- 24.Robinson S, Lewis R, Norton A, McPeake S. Ultrasound-guided radiofrequency submucosal tongue-base excision for sleep apnoea: a preliminary report. Clin Otolaryngol Allied Sci. 2003;28(4):341–345. doi: 10.1046/j.1365-2273.2003.00719.x. [DOI] [PubMed] [Google Scholar]
- 25.Morgan WE, Friedman EM, Duncan NO, Sulek M. Surgical management of macroglossia in children. Arch Otolaryngol Head Neck Surg. 1996;122(3):326–329. doi: 10.1001/archotol.1996.01890150096017. [DOI] [PubMed] [Google Scholar]
- 26.Shipster C, Morgan A, Dunaway D. Psychosocial, feeding, and drooling outcomes in children with Beckwith Wiedemann syndrome following tongue reduction surgery. Cleft Palate Craniofac J. 2012;49(4):e25–34. doi: 10.1597/10-232. [DOI] [PubMed] [Google Scholar]
- 27.Heggie AA, Vujcich NJ, Portnof JE, Morgan AT. Tongue reduction for macroglossia in Beckwith Wiedemann syndrome: review and application of new technique. Int J Oral Maxillofac Surg. 2013;42(2):185–191. doi: 10.1016/j.ijom.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Rimell FL, Shapiro AM, Shoemaker DL, Kenna MA. Head and neck manifestations of Beckwith-Wiedemann syndrome. Otolaryngol Head Neck Surg. 1995;113(3):262–265. doi: 10.1016/S0194-5998(95)70115-X. [DOI] [PubMed] [Google Scholar]