Abstract
Social structure influences animal societies on various levels (e.g., relatedness, behaviour). In ants, both the number of matings per queen and the number of queens per colony can vary strongly. While workers from both monogynous and polygynous colonies often fight fiercely, in supercolonies (an extreme form of polygyny comprising thousands of queens in spatially separated but interconnected nests), non-nestmates interact peacefully. Studies on social and behavioural polymorphism within ant species can help elucidate their influence on genetic diversity and behaviour and the factors triggering variation in social structure and behaviour. Here, we reveal a behavioural and social polymorphism comprising monogyny with and without internest aggression in Tetramorium alpestre sampled in Tyrol, Austria. The social polymorphism is based on genetic and behavioural evidence and contrasts with the supercolonial organisation known from another location in Austria (Carinthia), 150 km away. Microsatellite genotyping using eight polymorphic loci revealed monogyny-monandry and high intranest pairwise relatedness. Interestingly, various experimental one-on-one worker encounters revealed only occasional aggressive behaviour between monogynous colonies, and thus a behavioural polymorphism. Mantel tests revealed a significant negative correlation between spatial distance and relatedness, while worker behaviour was not correlated with relatedness or spatial distance. These results indicate that behaviour might be influenced by other factors – for example, the experience of workers, ecological, chemical, and/or genetic factors not characterised in this study. However, workers distinguished nestmates from non-nestmates also when aggression was lacking. We hypothesise an adaptive value of reduced aggression. We speculate that the non-aggressive and partly aggressive encounters observed represent different options in the social structure of T. alpestre, the non-aggressiveness possibly also promoting supercolony development. The social and behavioural polymorphisms observed offer opportunities to identify the factors triggering these changes and thus further explore the behavioural and social polymorphism of this ant species.
KEY WORDS: aggression, behaviour analyses, Formicidae, microsatellites, social structure, Tetramorium alpestre
INTRODUCTION
Social structure varies greatly among animal societies. Within each society, the number of reproductive individuals shapes its genetic diversity (Hughes et al. 2008). Ants are among the most abundant and ubiquitous organisms in the world (Alonso 2009). In some ant species, social structure varies, which is termed social polymorphism (Gyllenstrand et al. 2002). Gynes either mate once (monandry) or multiply (polyandry), and males may mate with one or several gynes (Heinze 2008). Colonies are headed by one single queen (monogyny) or by several queens (polygyny; Schmid-Hempel & Crozier 1999), and monogynous and polygynous colonies can live in either one nest (monodomy) or two or more nests simultaneously (polydomy; Crozier & Pamilo 1996).
Supercolonies are an extreme form of polygyny and polydomy. They are extensive cooperative units with many queens and very many workers integrated harmoniously over several square metres to many square kilometres (Crozier & Pamilo 1996; Giraud et al. 2002; Steiner et al. 2009). Supercoloniality is often considered key to the success of invasive ant species (Holway et al. 2002), and supercolonies have been thoroughly studied over the last decades (Tsutsui et al. 2000; Giraud et al. 2002; Pedersen et al. 2006; Leniaud et al. 2011; Huszar et al. 2014; Kennedy et al. 2014). However, the factors triggering their emergence remain largely unknown (Suarez & Suhr 2012).
Beside social polymorphism, behavioural polymorphism can also be observed within ant species describing variation in behaviour. Workers belonging to the same supercolony behave peacefully towards each other, although they may derive from different nests often several hundreds to thousands of kilometres apart (Giraud et al. 2002), while workers of monogynous and polygynous colonies usually fight fiercely when non-nestmates are encountered. However, non-aggressive behaviour between workers from monogynous colonies can also be observed (Steiner et al. 2007 and references therein), and severe aggression occurs between supercolonies (Giraud et al. 2002), indicating that this behaviour is a variable trait.
In this pilot study, we investigate the Alpine ant Tetramorium alpestre (Steiner et al. 2010), which is known to be supercolonial in Carinthia (Steiner et al. 2003) and thought to form monogynous-monodomous colonies in Tyrol, based on field observations (Fig. 1; F.M. Steiner et al. unpublished data). Clear evidence of monogyny-monodomy in Tyrol and thus confirmation of a social polymorphism within the species would make T. alpestre a highly suitable study organism for exploring the ecological and genetic factors triggering transitions from monogyny to polygyny and eventually supercoloniality. We therefore investigated various nests in Tyrol using behavioural analysis and microsatellite genotyping, addressing four questions: (1) Do T. alpestre nests differ in their social structure? (2) Do nests differ in their behaviour in terms of aggression between nests? (3) Are worker behaviour, internest averages of pairwise relatedness, and geographic distance correlated? (4) Do workers that are non-aggressive towards workers from other nests discriminate nestmates from non-nestmates?
Fig. 1.
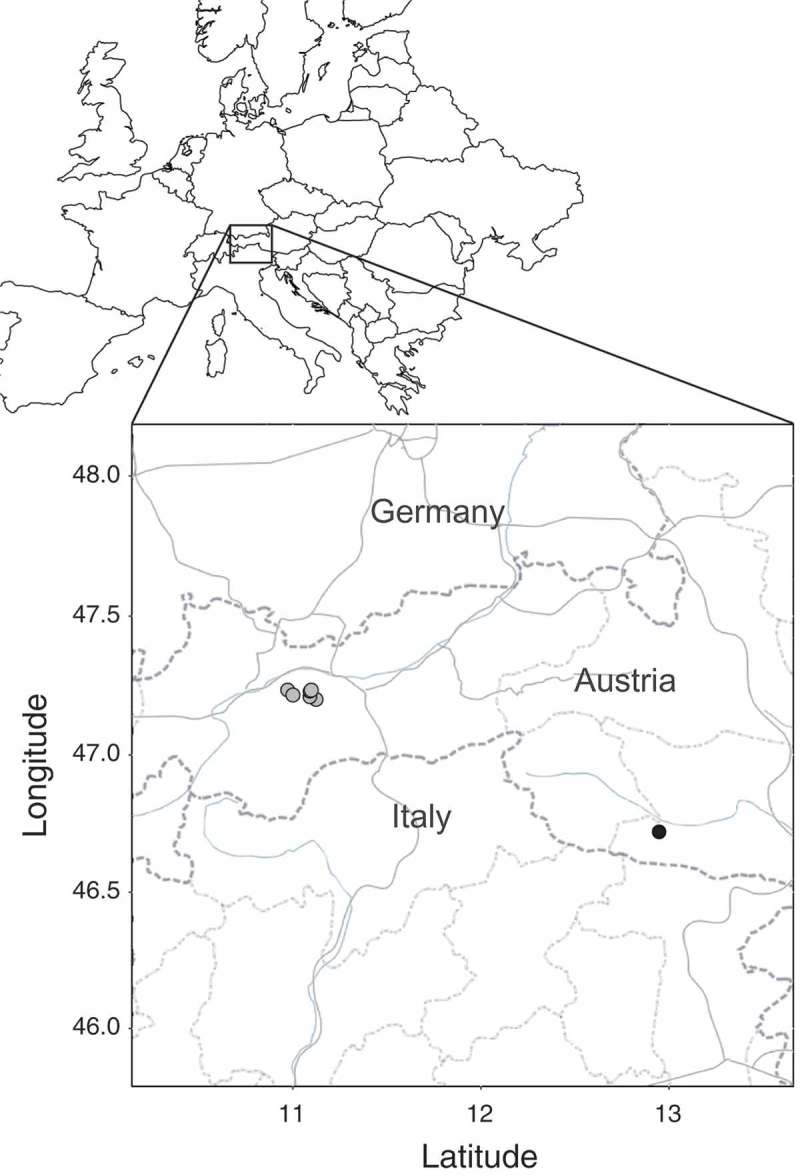
Map of the sampled nests in Tyrol (grey points) and of the location of the known supercolony in Carinthia (black point).
MATERIALS AND METHODS
Fieldwork and worker maintenance
In 2011, workers were collected from 11 nests in Tyrol, Austria (Supplementary Table 1), with distances among nests ranging from 9 m to 9 km (Supplementary Table 2). Workers were killed in 96% ethanol for genetic analyses or collected alive for aggression tests. See Supplementary online material for all details on fieldwork and worker maintenance.
Microsatellite genotyping and allele analyses
DNA was extracted from 12 workers per nest, that is, a total of 132 workers using the GenElute™ Mammalian Genomic DNA Extraction kit (Sigma-Aldrich, Saint Louis, USA) following the manufacturer’s protocol. The DNA extracts were amplified using primers for nine microsatellites (Steiner et al. 2008). For all details on microsatellite genotyping and allele analyses, see Supplementary online material.
MICROCHECKER v2.2.3 (Van Oosterhout et al. 2004) was used to test for null alleles. Arlequin v3.5 (Excoffier & Lischer 2010) was used to calculate an exact probability test for deviations from linkage equilibrium (LE) and Hardy-Weinberg equilibrium (HWE) using the Markov chain method with default parameters. To decrease the effect of relatedness of individuals, two subsets consisting of either four or one randomly chosen worker(s) per nest were used for these tests (Qian et al. 2012). Arlequin was further used to calculate observed (HO) and expected (HE) heterozygosities. The distribution of genetic differentiation was analysed among all nests. The fixation index (FST) and the inbreeding coefficient (FIS) were calculated as an average over all loci with 10,000 permutations. The FST matrix for pairwise nest-comparisons was analysed for significant genetic differentiation between nests. GenAlEx v6.502 (Peakall & Smouse 2012) was used to calculate pairwise geographic distances between nests. For all statistics involving multiple pairwise comparisons, a Bonferroni–Holm correction (Holm 1979; Rice 1989) was performed.
A non-detection error (NDE) for each nest was calculated following Boomsma and Ratnieks (1996). The NDE is the probability that two males share the same genotype at all loci by chance, which might cause underestimation of queen-mating frequencies. A non-sampling error (NSE) for each nest was calculated following Foster et al. (1999), using a proportion of offspring of P = 0.10. NSE estimates whether sampling size is sufficient to detect all males siring offspring.
COLONY v2.0.6.2 (Jones & Wang 2010) was used to infer sibship using workers’ individual multilocus genotypes. Based on these results, the effective number of matings per queen corrected for sample size (M e,p) was calculated following Nielsen et al. (2003). The intranest and internest averages of pairwise relatedness (r ww) were calculated in GenAlEx using the algorithms of Queller and Goodnight (1989). Based on these results, the effective number of queens (f) was calculated following Pamilo (1991).
One-on-one encounters and behaviour analyses
For the behaviour assays, one-on-one encounters were chosen because they represent encounters of single workers foraging distantly from the nest (Roulston et al. 2003), thus mimicking natural conditions. Encounters were performed following Giraud et al. (2002) with modifications (see Supplementary online material for details on one-on-one encounters). Four replicates of each pairwise intra- and internest combination were filmed for 3 min (Roulston et al. 2003). The films were examined in slow motion, and the observer had access to the information of the origin of workers. Such a situation is considered problematic by some researchers, as it might influence the observation and introduce bias (van Wilgenburg & Elgar 2013). However, the observer had no information on the relatedness between workers (Frizzi et al. 2015). The behaviours scored were ignoring (0), being next to each other without contact (1), antennation (2), food exchange or cleaning (3), avoiding (4), mandible threatening (5), biting (6), and fighting (7). Levels 4–7 were categorised as aggressive. The behaviours scored were adapted from those used in similar experimental designs (Giraud et al. 2002; Steiner et al. 2003; Boulay et al. 2007; Charbonneau et al. 2015).
For the aggression-index calculations, only aggressive behaviours were used, while all non-aggressive behaviours were excluded, similar to Boulay et al. (2007). Two indices were calculated: (1) aggression index (AI, modified from d’Ettorre & Heinze 2005); and (2) mean maximum aggression index (MMAI, Vogel et al. 2009). For AI, the frequency of each observed aggressive behaviour per worker was multiplied with its respective scoring level (4–7) and their sum divided by 180, as there were 180 records (one per second). The arithmetic mean of the four replicates was calculated. For MMAI, the arithmetic mean of the highest aggression values observed in each encounter over the four replicates was calculated.
In all statistical analyses, an alpha level of 0.05 was used. Before applying any t-test, an f-test was performed to check for differences in variances. If variances were significantly different, a modified t-test using a Satterthwaite approximation accounting for heteroscedasticity was used. To check for differences in behaviour between intranest and internest encounters, two-sided t-tests were calculated in R (R Development Core Team 2016) using the mean behaviour frequencies.
Mantel and partial mantel tests and recognition test
Mantel tests were calculated to check for correlations between the geographic distance and the behaviour indices (A) AI and, separately, (B) MMAI, internest average of pairwise relatedness and (C) AI and, separately, (D) MMAI, and (E) internest average of pairwise relatedness and geographic distance. Partial Mantel tests (‘Pearson method’) were calculated to check for correlations between geographic distance, internest average of pairwise relatedness, and the behaviour indices AI and, separately, MMAI while controlling for the effect of each of the variables. The Mantel and partial Mantel tests were performed with 9999 permutations using the ‘biotools’ and the ‘ecodist’-package in R, respectively. To determine the power of the Mantel tests for different effect sizes, sensitivity power analyses were performed using the ‘mantelPower’-function in the ‘biotools’-package in R. For these analyses, the effect sizes were set to range from 0.10 to 1.00 incrementing by 0.01, and the respective power was calculated for each effect size (Supplementary Table 3). To assist the interpretation of our results, effect sizes calculated from our data were then compared with the effect sizes that would have yielded significant results (P < 0.05) with power set to 0.8, a standard threshold for power analyses. A Spearman rank correlation was performed to check for an association between the intranest average of pairwise relatedness and the mean aggression level of each nest (AI, and, separately, MMAI). A two-sided t-test was calculated in R using the total antennation time from intranest and non-aggressive internest encounters to detect differences regarding worker discrimination.
RESULTS
Genetic analyses
For both subsets (four workers and one worker from each nest), MICROCHECKER revealed the possible presence of null alleles in locus 55a, which thus was excluded from further analyses. After correction for multiple comparisons, no locus deviated from HWE or departed significantly from LE for both subsets. The eight loci yielded a total of 68 alleles in the 132 workers genotyped. The mean HO value was significantly higher than HE (two-sided t-test, t 136.73 = 7.74; P < 0.001 for all nests). Among all nests, the global FST value for internest genetic differentiation was 0.37 (P < 0.001); the global FIS value for genetic inbreeding was not significant (– 0.58, P = 1.00). After Bonferroni–Holm correction, all pairwise-nest comparisons were significant regarding their FST values (internest genetic differentiation), which ranged from 0.20 to 0.52 (data not shown).
COLONY assigned 11 monogynous-monandrous colonies to the 11 nests sampled and calculated a number of 11 queens (Q est) and 11 males (M est). A M e,p value of 1.00 for all nests confirmed that queens mated only once. The NDE ranged from 2.02 × 10–8 to 9.97 × 10–37 for all nests. The NSE was 1.62 × 10–3 for all nests. The averages of pairwise intranest and internest relatedness values ranged from 0.62 to 0.82 and from – 0.39 to 0.31, respectively (Fig. 2E; Supplementary Table 4). Negative relatedness values can be observed if, for example, compared individuals differ in their allele frequencies. Based on the calculation of the effective number of queens (f, Supplementary Table 1) and the average pairwise intranest relatedness values, each nest was inferred to have one single queen.
Fig. 2.
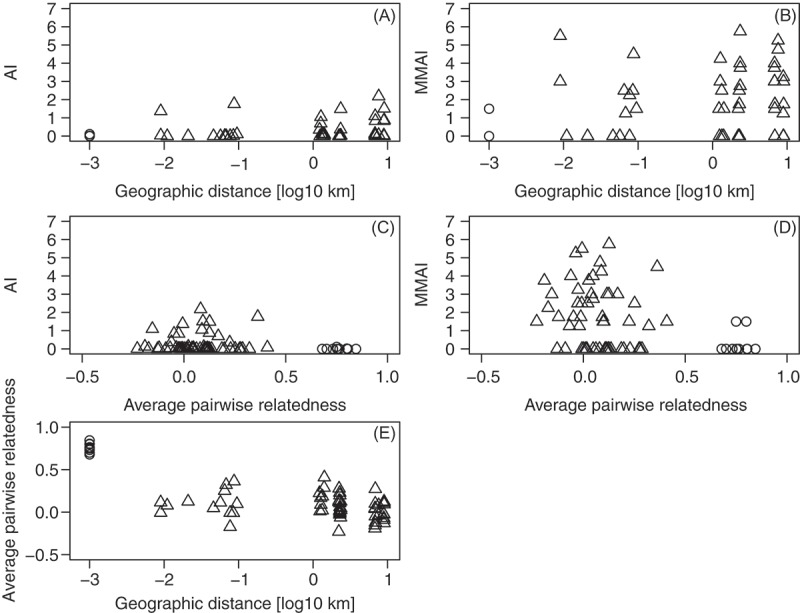
(A) Aggression index values (AI, modified from d’Ettorre & Heinze 2005) and (B) mean maximum aggression index values (MMAI) plotted against log10 transformed geographic distance, (C) AI and (D) MMAI plotted against the intranest and internest averages of pairwise relatedness values, and (E) intranest and internest averages of pairwise relatedness plotted against the log10 transformed geographic distance. Dots and triangles represent intranest and internest values, respectively. In plots showing the geographic distance, the intranest comparisons are set at – 3 on the x-axis, while internest comparisons are shown at their respective distance. Relatedness values were calculated using the algorithm of Queller and Goodnight (1989).
Aggression tests and behaviour statistics
In the 44 intranest encounters, AI and MMAI ranged from 0.00 to 0.11 and from 0.00 to 1.50, respectively. In the 220 internest encounters, AI and MMAI ranged from 0.00 to 2.18 and from 0.00 to 5.75, respectively (Fig. 2A-B; Supplementary Tables 5–6). Both the AI and MMAI values significantly increased from intranest to internest encounters (two-sided t-tests, AI, t 55.90 = – 3.88, P < 0.001; MMAI, t 46.38 = – 5.02, P < 0.001; Fig. 3). In internest encounters, both non-aggressive (level 0–3) and aggressive (level 4–7) behaviours were detected. In seven of 220 internest encounters, workers were fighting throughout the observation. In 62 encounters, workers were fighting briefly and stopped fighting after some time. In the remaining 151 of 220 encounters, no aggression was observed. However, workers of all colonies reacted at least briefly with aggressive behaviour in at least one pairing. The mean frequencies of behaviour levels 1, 2, 4, 5, 6, and 7 (being next to each other, antennation, avoiding, mandible threatening, biting, and fighting) were significantly higher in internest than in intranest encounters (two-sided t-test, level 1, t 110.10 = – 2.02, P = 0.046; level 2, t 271.02 = – 5.83, P < 0.001; level 4, t 507.24 = – 2.90, P = 0.004; level 5, t 493.45 = – 4.19, P < 0.001; level 6, t 459.88 = – 3.39, P < 0.001; and level 7, t 439.00 = – 3.51, P < 0.001), while the mean frequency of behaviour levels 0 and 3 (ignoring and food exchange) were significantly lower in internest than in intranest encounters (two-sided t-test, level 0, t 304.85 = 7.60, P < 0.001; level 3, t 89.34 = 3.61, P < 0.001, Fig. 4; all t-tests remained significant after Bonferroni–Holm correction).
Fig. 3.
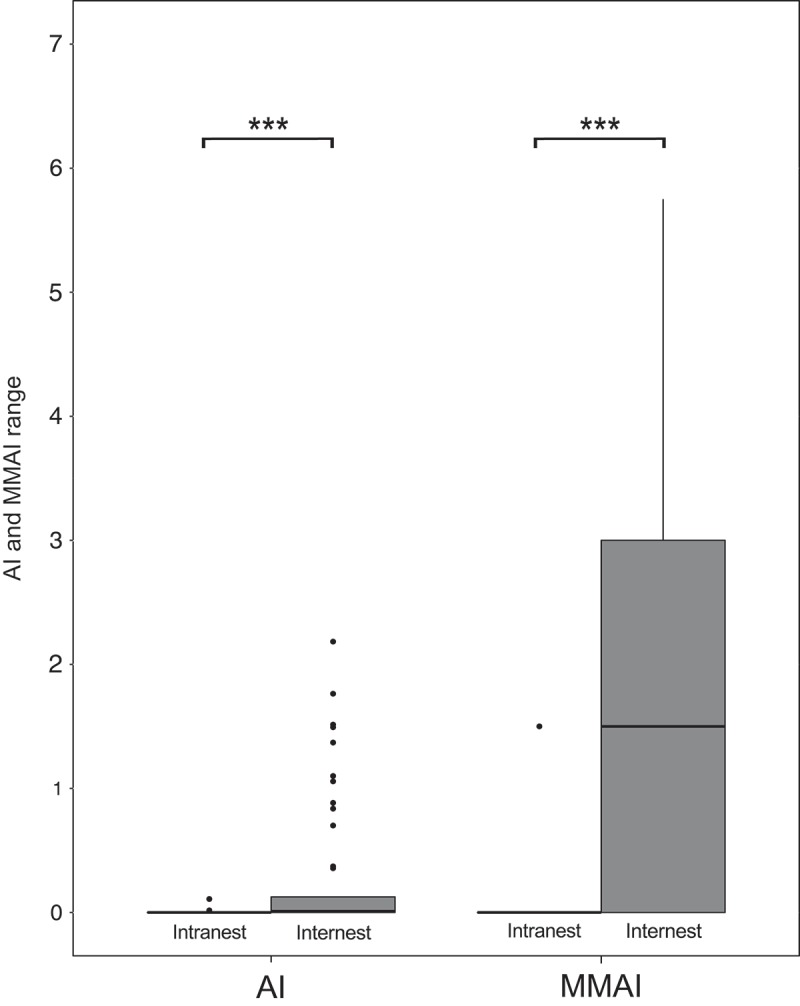
Aggression index (AI) values and mean maximum aggression index (MMAI) values from Tyrol on different organisational scales. Intranest and internest represent the aggression indices observed within and between nests, respectively. Whiskers represent the lowest and highest data still within 1.5 interquartile range of the lower and upper quartile, respectively, and dots represent outliers beyond the 1.5 interquartile range. Asterisks represent significant differences between intranest and internest behaviour levels (two-sided t-tests, *** = P < 0.001).
Fig. 4.
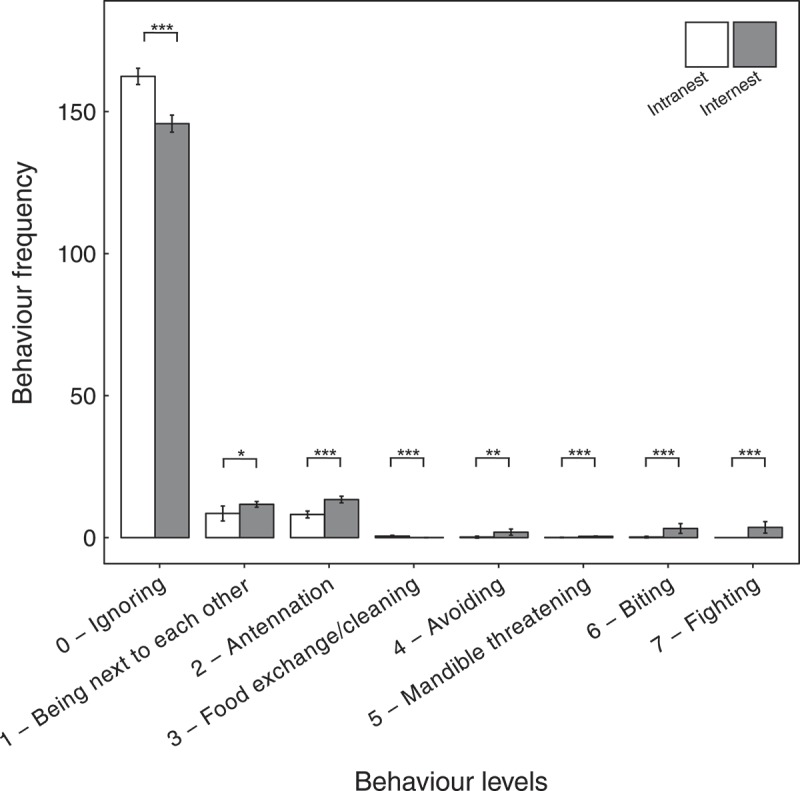
Mean frequency ± 1.96 × standard error of the mean (SE) of all seven behaviours observed. White and grey bars represent intranest and internest encounters, respectively. Asterisks represent significant differences between behaviour levels (two-sided t-tests, * = P < 0.05, ** = P < 0.01, *** = P < 0.001).
Mantel and partial mantel tests and recognition test
Geographic distance had no influence on AI (Mantel test A, r = 0.16, P = 0.35, Fig. 2A) and MMAI (Mantel test B, r = 0.11, P = 0.54, Fig. 2B). Internest averages of pairwise relatedness were not correlated with AI and MMAI (AI, Mantel test C, r = – 0.03, P = 0.87; MMAI, Mantel test D, r = – 0.20, P = 0.14, Fig. 2C–D). Geographic distance was significantly negatively correlated with the average of pairwise relatedness (Mantel test E, r = – 0.31, P < 0.05, Fig. 2E). The partial Mantel tests revealed no significant correlations between the internest averages of pairwise relatedness and the two behaviour indices AI and, separately MMAI, when controlling for geographic distance (partial Mantel test, AI, r = – 0.08, P = 0.58; MMAI, r = – 0.17, P = 0.20) or between the geographic distance and AI and, separately MMAI, when controlling for internest averages of pairwise relatedness (partial Mantel test, AI, r = 0.17, P = 0.33; MMAI, r = 0.05, P = 0.81). The effect sizes of the sensitivity power analyses ranged between ≥ 0.30 and ≥ 0.47 (Supplementary Table 3). There was no significant correlation between the intranest averages of pairwise relatedness and any of the two aggression indices, AI and MMAI (Spearman rank correlation, AI, ρ = 0.24, P = 0.49; MMAI, ρ = 0.30, P = 0.37). Workers distinguished nestmates from non-nestmates, in that total antennation times were significantly shorter in intranest than non-aggressive internest encounters (two-sided t-test, t 183.62 = – 5.69, P < 0.001).
DISCUSSION
The genetic and behavioural data analysed here allow inferences on the social structure, the relationship between behaviour, relatedness, and geographic distance, the aggression level of workers, and the ability of non-aggressive workers to discriminate nestmates from non-nestmates. In all nests except one, the maximum number of alleles per locus was three, and one of these alleles was seen in all diploid workers of the same nest, likely originating from one single male, as expected under monogyny-monandry in a haplodiploid species. In the one nest mentioned, one new allele was detected, likely being a recent mutation (Schlick-Steiner et al. 2015). In the nests, HO was significantly higher than HE, and we assume that unrelated gynes and males mated, leading to an increased heterozygosity and differing allele frequencies. The high global FST value (0.37) further indicates strong genetic differentiation among nests (Wright 1978). In combination with the high, significant pairwise FST values between nests (0.20–0.52; data not shown), limited or no gene flow can be inferred in our data, which in turn might increase genetic differentiation among nests. The FST value obtained here is comparable with those in various other studies applying a similar spatial sampling design ranging from several metres to kilometres, for example on Anoplolepis gracilipes (0.23, among colonies within a sampling region, Drescher et al. 2007), Cataglyphis emmae (0.27, 44 nests in a 1156 m2 plot, Jowers et al. 2013), and Formica exsecta (0.72, between pastures along a 6-km transect, Liautard & Keller 2001). It thus seems likely that mated T. alpestre gynes disperse and colonise distant new habitats as expected under monogyny (Heinze 2008), thus leading to increased genetic differentiation between nests.
The monogyny-monandry pattern was confirmed via the COLONY analysis, the high intranest average of pairwise relatedness (~ 0.75, Supplementary Table 4) in all nests, and the effective number of queens (f) of approximately one in all nests. The low internest averages of pairwise relatedness indicated that most workers were unrelated (Fig. 2E; Supplementary Table 4). The low M e,p corroborated that queens had mated only once. The low NDE values indicated that all genotypes of all siring males were likely to have been detected, and the low NSE values that the overall sample size had probably been sufficient to detect all siring males. Based on these results, we assume that one nest represents one colony. Regarding Question (1), the results revealed monogynous-monandrous colonies in Tyrol, thus differing from the supercolony detected in Carinthia (Steiner et al. 2003). Furthermore, the results affirm a social polymorphism in T. alpestre, which is, besides T. moravicum with macrogynous-monogynous and microgynous-polygynous colonies (Schlick-Steiner et al. 2005), the second socially polymorphic species known from Palearctic Tetramorium.
While the observer of the behaviour encounters principally had access to the information of the origin of workers, we consider it unlikely that an observer bias had been introduced (van Wilgenburg & Elgar 2013). Among the authors of recent behaviour studies, there were both observers blind (Jongepier et al. 2015; Purcell et al. 2016; Yagound et al. 2016) and observers not blind about the origin of workers (Kleeberg & Foitzik 2016; Parmentier et al. 2016; Ślipiński & Żmihorski 2016). The aggression indices AI and MMAI yielded different values (Figs 2A–B and 3). AI integrates behaviour over time and is more balanced, in that brief aggressive interactions caused, for example, by disturbance (Huszar et al. 2014) do not strongly affect AI. MMAI uses the mean of the most aggressive behaviour observed and helps detecting if any aggression occurs. Thus, for MMAI to be high it suffices that workers attack each other at least briefly. The two indices were here used to detect both long-lasting and brief aggressive behaviours. The behavioural variation observed within nest pairings was likely due to individual responses of T. alpestre workers.
In the one-on-one internest encounters, we observed both aggressive and non-aggressive behaviour (Fig. 4). Regarding Question (2), we reveal that the nests sampled differ behaviourally. Moreover, the behavioural polymorphism is affirmed, as in only seven of 220 internest encounters, workers fought throughout the observation, while aggression was present but ceased in 62 encounters and was completely absent in 151 encounters, indicating that behaviour towards non-nestmates is a rather variable trait in T. alpestre. Aggressive behaviour was more likely to occur in internest than intranest encounters (Fig. 4), as workers generally attack non-nestmates to protect the nest against intruders (d’Ettorre & Lenoir 2009). Brief aggression observed in the first seconds of an encounter, however, might be a result of disturbance (Huszar et al. 2014) indicating that workers eventually recognised each other and stopped aggressive behaviour. Also the average of pairwise intranest relatedness had no influence on the average aggressive behaviour (Spearman rank correlation) implying that aggression, if occurring at all, seems not to depend on intranest relatedness, at least for this pilot study. As far as we know, peaceful behaviour between non-nestmates of monogynous colonies has been rarely observed in 24 species, for example in Allomerus decemarticulatus, A. octoarticulatus (Grangier et al. 2008), Lasius austriacus (Steiner et al. 2007 and references therein), L. flavus (Steinmeyer et al. 2012), and Monomorium pharaonis (Schmidt et al. 2010). In monogynous T. alpestre nests, peaceful behaviour might be explained by two hypotheses: (1) The colonies could stay monogynous on the long run, but workers might avoid aggression as it is time and energy consuming and linked to injury and mortality (Davies & Houston 1984; Cole 1986; Crozier 1987). The energy saved could be used for colony growth and reproduction. For example, the underground-living ant species L. austriacus tends mealybugs for honeydew; thus, foraging seems to be less important, and aggression can be reduced (Steiner et al. 2007). We assume that T. alpestre is similar in its trophic ecology and that, in principle, the same theory might apply. (2) The colonies might represent an intermediate state where aggression is being reduced, but polygyny and, eventually, supercoloniality have not yet been established (cf. Steiner et al. 2007). Regarding the unresolved transition from multicoloniality to supercoloniality, T. alpestre seems to be an ideal study species. Further research is needed to evaluate the two hypotheses and to unveil factors triggering transitions in social structure, behaviour, and genetic make-up of colonies.
We detected that neither the internest averages of pairwise relatedness nor the geographic distance correlated with worker behaviour (Mantel test). Only geographic distance and the internest averages of pairwise relatedness were significantly negatively correlated. Also the partial Mantel tests, controlling for an effect of another variable, revealed no significant correlation. Addressing Question (3), the results reveal that, at least in this pilot study in Tyrol, no significant correlation involving the behaviour was detectable, while with increasing geographic distance the internest averages of pairwise relatedness decreased. This decrease of pairwise relatedness is not unexpected as the distance among the most distant colonies was approximately 9 km. The sensitivity power analyses revealed that, with our sample size, alpha level, and power, correlation coefficients ranging between 0.30 and 0.47 would have yielded significant results (Supplementary Table 3), suggesting that moderate to strong correlations should have been detected despite the moderate sample size of this pilot study. At least for this study, the variation in behaviour seems not to be substantially spatially or genetically determined (unless the neutral variation detected by microsatellites is not representative of the variation encoding aggressive behaviour). Rather, the behavioural variation observed might depend on (i) the context as suggested, for example, for L. austriacus (Steiner et al. 2007), or (ii) the experience of workers in previous encounters with non-nestmates (Van Wilgenburg et al. 2010), or (iii) environmentally derived cuticular hydrocarbon cues (Liang & Silverman 2000). Furthermore, recent studies regarding correlations between behaviour and, for example, spatial distance revealed contrasting results: in some cases, spatial distance and aggressive behaviour were correlated (Pirk et al. 2001; Benedek & Kobori 2014; Frizzi et al. 2015; Fournier et al. 2016), while in others, no correlation was observed (Langen et al. 2000; van Wilgenburg 2007; Martin et al. 2012), corroborating the general need for further studies. A correlation known from other studies is one between aggressive behaviour and recognition cues (e.g. cuticular hydrocarbons, CHCs) allowing workers to discriminate nestmates from non-nestmates (Guerrieri et al. 2009; Fürst et al. 2012; Martin et al. 2012; Tsutsui 2013; di Mauro et al. 2015; Larsen et al. 2016). Recognition cues are genetically and/or environmentally determined (d’Ettorre & Lenoir 2009), but nestmate recognition seems not to be influenced by the social origin (monogyny vs polygyny) of workers (Rosset et al. 2007; Helanterä et al. 2011; Chirino et al. 2012). As there are only quantitative differences in CHCs of conspecific species, workers have to detect those small differences to correctly discriminate between nestmates and non-nestmates (di Mauro et al. 2015). Here, we did not analyse CHCs of T. alpestre, but we speculate that CHC profiles might be similar in Tyrolean nests, possibly leading to reduced aggressive behaviour as observed, for example, in Formica exsecta (Martin et al. 2012). However, analyses of CHCs are needed to evaluate this hypothesis for T. alpestre. Moreover, further studies are needed focusing on context-dependent behaviour and worker experience using specific behaviour assays, on chemical assays regarding chemical (dis)similarity using gas chromatography-mass spectrometry, and on comparative genomics and transcriptomics to identify and characterise potential variation in coding regions.
We detected that in non-aggressive encounters between non-nestmates, the total antennation time was significantly increased compared with intranest encounters, implying that workers distinguished nestmates from non-nestmates. This allows us to address Question (4): As mentioned above, a reduction in aggression probably increases time spent on tasks beneficial for the colony, such as nest maintenance and foraging. Lacking aggression is often associated with reduced recognition ability, for example in invasive species (Giraud et al. 2002). Although aggression was partly absent in T. alpestre, workers distinguished nestmates from non-nestmates, which was also observed in Formica paralugubris (Holzer et al. 2006) and in L. austriacus (Steiner et al. 2007), indicating that lacking aggression does not necessarily imply a loss of recognition ability.
Biography
P. Krapf and L. Russo contributed equally to the manuscript as first authors. F.M. Steiner and B.C. Schlick-Steiner contributed equally to the manuscript as senior authors.
Funding Statement
This work was supported by the University of Innsbruck and the Austrian Science Fund under grant number P23409 awarded to B.C. Schlick-Steiner. P. Krapf was funded by the association for the support of South-Tyrolean students at the University of Innsbruck.
ACKNOWLEDGEMENTS
We thank Stefanie Fischnaller and Clemens Folterbauer for help during laboratory work, Thomas Dejaco, Rüdiger Kaufmann, Martin-Carl Kinzner, Anna Kluibenschedl, Herbert C. Wagner, and two anonymous reviewers for helpful input on the manuscript.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed at https://doi.org/10.1080/03949370.2017.1343868
References
- Alonso LE. 2009. Ant conservation: current status and a call to action In: Lach L, et al., editors. Ant ecology. Oxford (UK): Oxford University Press; p. 59–74. [Google Scholar]
- Benedek K, Kobori OT.. 2014. ‘Nasty neighbour’ effect in Formica pratensis Retz. (Hymenoptera: Formicidae). North West J Zool. 10:245–250. [Google Scholar]
- Boomsma JJ, Ratnieks FLW. 1996. Paternity in eusocial Hymenoptera. Philos Trans R Soc Lond B. 351:947–975. [Google Scholar]
- Boulay R, Cerdá X, Simon T, Roldan M, Hefetz A. 2007. Intraspecific competition in the ant Camponotus cruentatus: should we expect the ‘dear enemy’ effect? Anim Behav. 74:985–993. [Google Scholar]
- Charbonneau D, Hillis N, Dornhaus A. 2015. ‘Lazy’ in nature: ant colony time budgets show high ‘inactivity’ in the field as well as in the lab. Insectes Soc. 62:31–35. [Google Scholar]
- Chirino MG, Gilbert LE, Folgarait PJ. 2012. Behavioral discrimination between monogyne and polygyne red fire ants (Hymenoptera: Formicidae) in their native range. Ann Entomol Soc Am. 105:740–745. [Google Scholar]
- Cole BJ. 1986. The social behavior of Leptothorax allardycei (Hymenoptera, Formicidae): time budgets and the evolution of worker reproduction. Behav Ecol Sociobiol. 18:165–173. [Google Scholar]
- Crozier RH. 1987. Genetic aspects of kin recognition: concepts, models, and synthesis In: Fletcher DJC, Michener CD, editors. Kin recognition in animals. New York (NY): John Wiley; p. 55–73. [Google Scholar]
- Crozier RH, Pamilo P. 1996. Evolution of social insect colonies, sex allocation and kin-selection. Oxford (UK): Oxford University Press. [Google Scholar]
- d’Ettorre P, Heinze J. 2005. Individual recognition in ant queens. Curr Biol. 15:2170–2174. [DOI] [PubMed] [Google Scholar]
- d’Ettorre P, Lenoir A.. 2009. Nestmate recognition In: Lach L, et al., editors. Ant ecology. Oxford (UK): Oxford University Press; p. 194–209. [Google Scholar]
- Davies NB, Houston AI. 1984. Territory economics In: Krebs JR, Davies NB, editors. Behavioural ecology: an evolutionary approach. Sunderland: Sinauer Associates; p. 148–169. [Google Scholar]
- di Mauro G, Perez M, Lorenzi MC, Guerrieri FJ, Millar JG, d’Ettorre P. 2015. Ants discriminate between different hydrocarbon concentrations. Front Ecol Evol. 3:133. [Google Scholar]
- Drescher J, Blüthgen N, Feldhaar H. 2007. Population structure and intraspecific aggression in the invasive ant species Anoplolepis gracilipes in Malaysian Borneo. Mol Ecol. 16:1453–1465. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 10:564–567. [DOI] [PubMed] [Google Scholar]
- Foster KR, Seppa P, Ratnieks FLW, Thoren PA. 1999. Low paternity in the hornet Vespa crabro indicates that multiple mating by queens is derived in vespine wasps. Behav Ecol Sociobiol. 46:252–257. [Google Scholar]
- Fournier D, de Biseau JC, De Laet S, Lenoir A, Passera L, Aron S. 2016. Social structure and genetic distance mediate nestmate recognition and aggressiveness in the facultative polygynous ant Pheidole pallidula . PLoS ONE. 11:e0156440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzi F, Ciofi C, Dapporto L, Natali C, Chelazzi G, Turillazzi S, Santini G. 2015. The rules of aggression: how genetic, chemical and spatial factors affect intercolony fights in a dominant species, the Mediterranean acrobat ant Crematogaster scutellaris . PLoS ONE. 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst MA, Durey M, Nash DR. 2012. Testing the adjustable threshold model for intruder recognition on Myrmica ants in the context of a social parasite. Proc R Soc Lond B. 279:516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud T, Pedersen JS, Keller L. 2002. Evolution of supercolonies: the Argentine ants of southern Europe. Proc Natl Acad Sci USA. 99:6075–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangier J, Orivel J, Negrini M, Dejean A. 2008. Low intraspecific aggressiveness in two obligate plant-ant species. Insectes Soc. 55:238–240. [Google Scholar]
- Guerrieri FJ, Nehring V, Jorgensen CG, Nielsen J, Galizia CG, d’Ettorre P. 2009. Ants recognize foes and not friends. Proc R Soc Lond B. 276:2461–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllenstrand N, Gertsch PJ, Pamilo P. 2002. Polymorphic microsatellite DNA markers in the ant Formica exsecta . Mol Ecol Notes. 2:67–69. [Google Scholar]
- Heinze J. 2008. The demise of the standard ant (Hymenoptera: Formicidae). Myrmecol News. 11:9–20. [Google Scholar]
- Helanterä H, Lee YR, Drijfhout FP, Martin SJ. 2011. Genetic diversity, colony chemical phenotype, and nest mate recognition in the ant Formica fusca . Behav Ecol. 22:710–716. [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat. 6:65–70. [Google Scholar]
- Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ. 2002. The causes and consequences of ant invasions. Annu Rev Ecol Syst. 33:181–233. [Google Scholar]
- Holzer B, Chapuisat M, Kremer N, Finet C, Keller L. 2006. Unicoloniality, recognition and genetic differentiation in a native Formica ant. J Evol Biol. 19:2031–2039. [DOI] [PubMed] [Google Scholar]
- Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. 2008. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science. 320:1213–1216. [DOI] [PubMed] [Google Scholar]
- Huszar DB, Larsen RS, Carlsen S, Boomsma JJ, Pedersen JS. 2014. Convergent development of ecological, genetic, and morphological traits in native supercolonies of the red ant Myrmica rubra . Behav Ecol Sociobiol. 68:1859–1870. [Google Scholar]
- Jones OR, Wang JL. 2010. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour. 10:551–555. [DOI] [PubMed] [Google Scholar]
- Jongepier E, Kleeberg I, Foitzik S. 2015. The ecological success of a social parasite increases with manipulation of collective host behaviour. J Evol Biol. 28:2152–2162. [DOI] [PubMed] [Google Scholar]
- Jowers MJ, Leniaud L, Cerda X, Alasaad S, Caut S, Amor F, Aron S, Boulay RR. 2013. Social and population structure in the ant Cataglyphis emmae . PLoS ONE. 8:e72941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P, Uller T, Helanterä H. 2014. Are ant supercolonies crucibles of a new major transition in evolution? J Evol Biol. 27:1784–1796. [DOI] [PubMed] [Google Scholar]
- Kleeberg I, Foitzik S. 2016. The placid slavemaker: avoiding detection and conflict as an alternative, peaceful raiding strategy. Behav Ecol Sociobiol. 70:27–39. [Google Scholar]
- Langen TA, Tripet F, Nonacs P. 2000. The red and the black: habituation and the dear-enemy phenomenon in two desert Pheidole ants. Behav Ecol Sociobiol. 48:285–292. [Google Scholar]
- Larsen J, Nehring V, d’Ettorre P, Bos N. 2016. Task specialization influences nestmate recognition ability in ants. Behav Ecol Sociobiol. 70:1433–1440. [Google Scholar]
- Leniaud L, Hefetz A, Grumiau L, Aron S. 2011. Multiple mating and supercoloniality in Cataglyphis desert ants. Biol J Linn Soc. 104:866–876. [Google Scholar]
- Liang D, Silverman J. 2000. ‘You are what you eat’: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile . Naturwissenschaften. 87(9):412–416. [DOI] [PubMed] [Google Scholar]
- Liautard C, Keller L. 2001. Restricted effective queen dispersal at a microgeographic scale in polygynous populations of the ant Formica exsecta . Evolution. 55:2484–2492. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Vitikainen E, Drijfhout FP, Jackson D. 2012. Conspecific ant aggression is correlated with chemical distance, but not with genetic or spatial distance. Behav Genet. 42:323–331. [DOI] [PubMed] [Google Scholar]
- Nielsen R, Tarpy DR, Reeve HK. 2003. Estimating effective paternity number in social insects and the effective number of alleles in a population. Mol Ecol. 12:3157–3164. [DOI] [PubMed] [Google Scholar]
- Pamilo P. 1991. Evolution of colony characteristics in social insects. II. Number of reproductive individuals. Am Nat. 138:412–433. [Google Scholar]
- Parmentier T, Dekoninck W, Wenseleers T. 2016. Survival of persecuted myrmecophiles in laboratory nests of different ant species can explain patterns of host use in the field (Hymenoptera: Formicidae). Myrmecol News. 23:71–79. [Google Scholar]
- Peakall R, Smouse PE. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics. 28:2537–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen JS, Krieger MJB, Vogel V, Giraud T, Keller L. 2006. Native supercolonies of unrelated individuals in the invasive Argentine ant. Evolution. 60:782–791. [PubMed] [Google Scholar]
- Pirk P, Neumann P, Moritz RFA, Pamilo P. 2001. Intranest relatedness and nestmate recognition in the meadow ant Formica pratensis (R.). Behav Ecol Sociobiol. 49:366–374. [Google Scholar]
- Purcell J, Zahnd S, Athanasiades A, Türler R, Chapuisat M, Brelsford A. 2016. Ants exhibit asymmetric hybridization in a mosaic hybrid zone. Mol Ecol. 25:4866–4874. [DOI] [PubMed] [Google Scholar]
- Qian ZQ, Schlick-Steiner BC, Steiner FM, Robson SKA, Schluns H, Schluns EA, Crozier RH. 2012. Colony genetic structure in the Australian jumper ant Myrmecia pilosula . Insectes Soc. 59:109–117. [Google Scholar]
- Queller DC, Goodnight KF. 1989. Estimating relatedness using genetic-markers. Evolution. 43:258–275. [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2016. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; Available from: http://www.R-project.org/ [Google Scholar]
- Rice WR. 1989. Analyzing tables of statistical tests. Evolution. 43:223–225. [DOI] [PubMed] [Google Scholar]
- Rosset H, Schwander T, Chapuiset M. 2007. Nestmate recognition and levels of aggression are not altered by changes in genetic diversity in a socially polymorphic ant. Anim Behav. 74:951–956. [Google Scholar]
- Roulston TH, Buczkowski G, Silverman J. 2003. Nestmate discrimination in ants: effect of bioassay on aggressive behavior. Insectes Soc. 50:151–159. [Google Scholar]
- Schlick-Steiner BC, Arthofer W, Moder K, Steiner FM. 2015. Recent insertion/deletion (reINDEL) mutations: increasing awareness to boost molecular-based research in ecology and evolution. Ecol Evol. 5:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlick-Steiner BC, Steiner FM, Sanetra M, Heller G, Stauffer C, Christian E, Seifert B. 2005. Queen size dimorphism in the ant Tetramorium moravicum (Hymenoptera, Formicidae): morphometric, molecular genetic and experimental evidence. Insectes Soc. 52:186–193. [Google Scholar]
- Schmid-Hempel P, Crozier RH. 1999. Polyandry versus polygyny versus parasites. Philos Trans R Soc Lond B. 354:507–515. [Google Scholar]
- Schmidt AM, d’Ettorre P, Pedersen JS. 2010. Low levels of nestmate discrimination despite high genetic differentiation in the invasive pharaoh ant. Front Zool. 7:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ślipiński P, Żmihorski M. 2016. Changes in the speed of ants as a result of aggressive interactions. Insectes Sci. doi: 10.1111/1744-7917.12354 [DOI] [PubMed] [Google Scholar]
- Steiner FM, Arthofer W, Schlick-Steiner BC, Crozier RH, Stauffer C. 2008. Twenty four new microsatellite markers in two invasive pavement ants, Tetramorium sp.E and T. tsushimae (Hymenoptera: Formicidae). Conserv Genet. 9:757–759. [Google Scholar]
- Steiner FM, Crozier RH, Schlick-Steiner BC.. 2009. Colony structure In: Lach L, et al., editors. Ant ecology. Oxford (UK): Oxford University Press; p. 177–193. [Google Scholar]
- Steiner FM, Schlick-Steiner BC, Buschinger A. 2003. First record of unicolonial polygyny in Tetramorium cf. caespitum (Hymenoptera, Formicidae). Insectes Soc. 50:98–99. [Google Scholar]
- Steiner FM, Schlick-Steiner BC, Moder K, Stauffer C, Arthofer W, Buschinger A, Espadaler X, Christian E, Einfinger K, Lorbeer E, et al. 2007. Abandoning aggression but maintaining self-nonself discrimination as a first stage in ant supercolony formation. Curr Biol. 17:1903–1907. [DOI] [PubMed] [Google Scholar]
- Steiner FM, Seifert B, Moder K, Schlick-Steiner BC. 2010. A multisource solution for a complex problem in biodiversity research: description of the cryptic ant species. Tetramorium alpestre sp.n. (Hymenoptera: Formicidae). Zool Anz. 249:223–254. [Google Scholar]
- Steinmeyer C, Pennings PS, Foitzik S. 2012. Multicolonial population structure and nestmate recognition in an extremely dense population of the European ant Lasius flavus . Insectes Soc. 59:499–510. [Google Scholar]
- Suarez AV, Suhr EL. 2012. Ecological and evolutionary perspectives on ‘supercolonies’: a commentary on Moffett. Behav Ecol. 23:937–938. [Google Scholar]
- Tsutsui ND. 2013. Dissecting ant recognition systems in the age of genomics. Biol Letters. 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui ND, Suarez AV, Holway DA, Case TJ. 2000. Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci USA. 97:5948–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P. 2004. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes. 4:535–538. [Google Scholar]
- van Wilgenburg E. 2007. The influence of relatedness, neighbourhood and overall distance on colony mate recognition in a polydomous ant. Ethology. 113:1185–1191. [Google Scholar]
- Van Wilgenburg E, Clémencet J, Tsutsui ND. 2010. Experience influences aggressive behaviour in the Argentine ant. Biol Lett. 6:152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg E, Elgar MA. 2013. Confirmation bias in studies of nestmate recognition: A cautionary note for research into the behaviour of animals. PLoS ONE. 8:e53548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Pedersen JS, d’Ettorre P, Lehmann L, Keller L. 2009. Dynamics and genetic structure of Argentine ant supercolonies in their native range. Evolution. 63:1627–1639. [DOI] [PubMed] [Google Scholar]
- Wright S. 1978. Evolution and the genetics of populations. Vol. 4. Variability within and among natural populations. Chicago (IL): University of Chicago Press. [Google Scholar]
- Yagound B, Crowet M, Leroy C, Poteaux C, Âline NC. 2016. Interspecific variation in neighbour–stranger discrimination in ants of the Neoponera apicalis complex. Ecol Entomol. 42:125–136. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


