Abstract
Controlled assembly of two-dimensional (2D) supramolecular organic frameworks (SOFs) has been demonstrated through a binary strategy in which 1,4-bis-(4-(3,5-dicyano-2,6-dipyridyl)pyridyl)naphthalene (2), generated in situ by oxidative dehydrogenation of 1,4-bis-(4-(3,5-dicyano-2,6-dipyridyl)dihydropyridyl)naphthalene (1), is coupled in a 1:1 ratio with terphenyl-3,3′,4,4′-tetracarboxylic acid (3; to form SOF-8), 5,5′-(anthracene-9,10-diyl)diisophthalic acid (4; to form SOF-9), or 5,5′-bis-(azanediyl)-oxalyl-diisophthalic acid (5; to form SOF-10). Complementary O–H···N hydrogen bonds assemble 2D 63-hcb (honeycomb) subunits that pack as layers in SOF-8 to give a three-dimensional (3D) supramolecular network with parallel channels hosting guest DMF (DMF = N,N′-dimethylformamide) molecules. SOF-9 and SOF-10 feature supramolecular networks of 2D → 3D inclined polycatenation of similar hcb layers as those in SOF-8. Although SOF-8 suffers framework collapse upon guest removal, the polycatenated frameworks of SOF-9 and SOF-10 exhibit excellent chemical and thermal stability, solvent/moisture durability, and permanent porosity. Moreover, their corresponding desolvated (activated) samples SOF-9a and SOF-10a display enhanced adsorption and selectivity for CO2 over N2 and CH4. The structures of these activated compounds are well described by quantum chemistry calculations, which have allowed us to determine their mechanical properties, as well as identify their soft deformation modes and a large number of low-energy vibration modes. These results not only demonstrate an effective synthetic platform for porous organic molecular materials stabilized solely by primary hydrogen bonds but also suggest a viable means to build robust SOF materials with enhanced gas uptake capacity and selectivity.
Short abstract
A family of 2D hydrogen-bonded binary supramolecular organic frameworks show enhanced gas adsorption and selectivity that are regulated by structural polycatenation.
Introduction
Porous organic molecular solids have been proposed as a new platform of chemical materials. They tend to show low framework density resulting from being composed of light elements (typically limited to H, C, N, O, and B), and their synthesis can be achieved via controlled assembly using concepts from crystal engineering.1−5 Various applications of porous organic molecular solids, including in catalysis, medicine, molecular sensing, storage, and separation,6−10 depend on achieving designed architectures with predictable pore structures and the necessary functionality. However, the construction of porous organic molecular solids presents challenges in the delivery of required properties, including stable and permanent porosity, tailored pore structure, and functionality.
Supramolecular organic frameworks (SOFs),11−18 which are structurally mimetic of the well-known metal organic frameworks (MOFs),19−23 define porous crystalline molecular solids built from organic constituents assembled through supramolecular interactions. SOFs have shown promising applications in gas storage and separation.24,25 The prevailing strategy for the construction of SOFs involves crystallization of a single organic constituent from an appropriate solvent system by exploiting supramolecular interactions such as hydrogen bonds, halogen bonds, and/or π···π interactions.26−30 Given the “soft” and “flexible” nature of supramolecular interactions, as well as the numerous donating/accepting groups present, prediction of structures and controlled assembly of hydrogen-bonded SOFs in such single-component systems can be challenging.30,31 We have now developed a binary design strategy,25 which requires two types of organic constituents possessing separate complementary hydrogen bond donating and acceptor groups. The organic constituents should be chemically and geometrically adaptable to give intermolecular interactions that are optimized and balanced for assembly of binary SOF materials.32
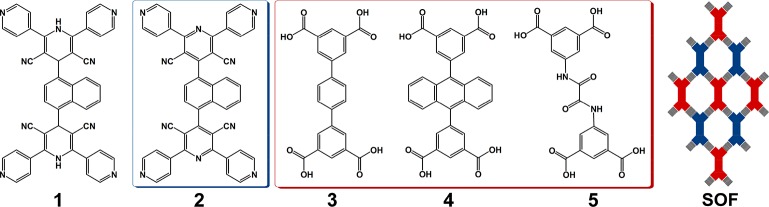
The importance of dimensionality and well-defined in-plane crystallinity of two-dimensional (2D) materials is now well established,28,29 and structural diversity of 2D molecular networks comes not only from the molecular assembly but also from the different packing arrangements possible for these nets.29,33 In contrast to 3D SOFs,12−18 2D SOFs assembled solely through hydrogen bonds rarely exhibit both structural robustness and significant adsorption capacity in the solid state. With this in mind, we sought to construct thermally and chemically stable 2D SOF materials for selective adsorption of carbon dioxide (CO2) using a new binary strategy. 1,4-Bis-(4-(3,5-dicyano-2,6-dipyridyl)dihydropyridyl)naphthalene (1) has been designed as a precursor for the generation of 1,4-bis-(4-(3,5-dicyano-2,6-dipyridyl) pyridyl)naphthalene (2) via in situ oxidative dehydrogenation.25,34 We report that the combination of 2 with the tetracarboxylic acids terphenyl-3,3′,4,4′-tetracarboxylic acid, 3, 5,5′-(anthracene-9,10-diyl)diisophthalic acid, 4, or 5,5′-bis-(azanediyl)-oxalyl-diisophthalic acid, 5, leads to the assembly of 2D binary SOF materials (Scheme 1). The exo-pyridyl (acceptors) and carboxyl (donors) groups on two separate organic constituents provide complementary and directional hydrogen bonding interactions for the assembly of porous structures with decorating phenyl, anthryl, or oxamide groups. Quantum chemistry calculations are used to determine the energetic and mechanical stability of the various frameworks synthesized here, as well as to analyze their elastic moduli and vibrational properties.
Scheme 1. Views of the Organic Components 1–5 and a Representative 63-hcb Net Observed in SOF8–10.
Experimental Section
Chemicals and General Methods
Commercially available reagents and organic solvents were used as received without further purification. 3-Amino-3-(4-pyridinyl)-propionitrile, naphthalene-1,4-dicarbaldehyde, terphenyl-3,3′,4,4′-tetracarboxylic acid, 5,5′-bis-(azanediyl)-oxalyl-diisophthalic acid, and 5,5′-(anthracene-9,10-diyl)diisophthalic acid were prepared according to the reaction procedures previously described in the literature; naphthalene-1,4-dicarbaldehyde was synthesized using an adapted method reported previously.35,36
Elemental analyses (C, H, and N) were performed on a CE-440 elemental analyzer. Infrared (IR) spectra were recorded with a PerkinElmer Spectrum One instrument as KBr pellets in the range 400–4000 cm–1, or on a Nicolet is5 FT-IR spectrophotometer in the range 550–4000 cm–1 using attenuated total reflectance (ATR) mode. 1H NMR spectra were recorded on a Bruker DPX-400 spectrometer. Thermal gravimetric analyses (TGA) were performed under a flow of N2 (20 mL min–1) with a heating rate of 10 °C min–1 using a TA SDT-600 thermogravimetric analyzer. Powder X-ray diffraction (PXRD) measurements were carried out at room temperature on a PANalytical X’Pert PRO diffractometer using Cu Kα radiation (λ = 1.5418 Å) and generator settings of 40 kV, 40 mA, at a scan speed of 0.02°/s and a step size of 0.005° in 2θ. CO2, N2, and CH4 isotherms were recorded using an IGA gravimetric adsorption apparatus (Hiden) at the University of Nottingham in a clean ultra-high-vacuum system with a diaphragm and turbo pumping system. Before measurement, about 60 mg of solvent-exchanged sample was loaded into the sample basket within the adsorption instrument and then degassed under dynamic vacuum at 100 °C for 24 h to obtain fully desolvated samples.
Synthesis of 1,4-Bis-(4-(3,5-dicyano-2,6-dipyridyl)dihydropyridyl)naphthalene (1)
3-Amino-3-(4-pyridinyl)-propionitrile (580 mg, 4.0 mmol) and naphthalene-1,4-dicarbaldehyde (184 mg, 1.0 mmol) were added to acetic acid (10 mL) under N2 and the reaction mixture refluxed at 120 °C for 48 h. The light yellow precipitate of 1 was collected by filtration and washed with hot acetic acid, EtOH, and distilled water and dried in air. Yields: ca. 51%. 1H NMR (DMSO-d6): 10.51 (s, 2H, dihydropyridyl-NH); 8.77 (d, J = 5.9 Hz, 8H, Py-H); 8.68 (dd, J = 9.9, 5.4 Hz, 2H, naphthyl-H); 7.99 (d, J = 5.8 Hz, 2H, naphthyl-H); 7.76 (dd, J = 6.5, 3.2 Hz, 2H, naphthyl-H); 7.71 (dd, J = 12.7, 9.8 Hz, 8H, Py-H); 5.93 (s, 2H, dihydropyridyl-CH) ppm. IR (KBr, υmax, cm–1): 2206 (s), 1643 (m), 1578 (w), 1550 (m), 1475 (s), 1406 (m), 1390 (m), 1339 (w), 1290 (s), 1221 (w), 1189 (w), 1153 (m), 1068 (w), 996 (m), 851 (s). Elemental analysis for C44H26N10 (found/calcd): C, 74.02/76.07; H, 3.69/3.77; N, 19.78/20.16%.
Synthesis of SOF8–10
1,4-Bis-(4-(3,5-dicyano-2,6-dipyridyl)dihydropyridyl)naphthalene (1) (35 mg, 0.05 mmol) and terphenyl-3,3′,4,4′-tetracarboxylic acid (3) (21 mg, 0.05 mmol) were added into 3 mL of DMF. The reaction mixture was transferred into a 15 mL pressure tube and heated in oil bath at 90 °C and autogenous pressure for 3 days. Orange crystals were collected by filtration and washed with cold DMF to give the pure phase of SOF-8 (yield: ca. 32%). SOF-9 and SOF-10 were prepared in similar reactions using 5,5′-bis-anthrathene-diisophthalic acid (4) (26 mg, 0.05 mmol) and 5,5′-bis-(azanediyl)-oxalyl-diisophthalic acid (5) (21 mg, 0.05 mmol) instead of 3 (in yields of ca. 29% and ca. 36%, respectively). The composition and amount of included solvent molecules in SOF8–10 were determined by elemental analyses, TGA results, and the electron densities calculated from the PLATON/SQUEEZE routine,37 which give optimized formulae of [(C22H14O8)·(C44H22N10)]·3DMF (SOF-8), [(C30H18O8)·(C44H22N10)]·7DMF (SOF-9), and [(C18H12N2O10)·(C44H22N10)]·6DMF (SOF-10). Elemental analysis for SOF-8 (C75H57N13O11; found/calcd): C, 68.77/68.44; H, 4.09/4.36; N, 13.04/13.83%; for SOF-9 (C95H89N17O15, found/calcd): C, 66.02/66.77; H, 5.20/5.25; N, 13.76/13.93%; for SOF-10 (C80H76N18O16, found/calcd): C, 61.42/62.17; H, 4.88/4.96; N, 15.99/16.31%. IR (KBr, υmax, cm–1) for SOF-8: 1943 (w), 1707 (s), 1662 (s), 1608 (m), 1529 (s), 1426 (w), 1390 (s), 1318 (m), 1248 (s), 1145 (w), 1101 (w), 1016 (m), 904 (w), 845 (m), 766 (m), 692 (w), 609 (w), 574 (w), 500 (w); SOF-9: 1944 (w) 1712 (s), 1601 (m), 1529 (s), 1447 (w), 1418 (w), 1393 (m), 1269 (s), 1114 (w), 1061 (w), 1022 (w), 840 (m), 792 (m), 762 (s), 690 (m), 656 (m), 608 (w), 574 (w), 507 (w); SOF-10: 1923 (w), 1709 (s), 1670 (s), 1597 (w), 1530 (s), 1440 (m), 1384 (s), 1322 (w), 1255 (s), 1097 (m), 1064 (m), 1019 (m), 939 (w), 839 (m), 778 (m), 688 (m), 592 (m), 502 (m).
Sample Activation
DMF molecules within the pores of SOF8–10 were exchanged with acetone, and the acetone-exchanged samples were degassed under dynamic vacuum at 100 °C for 24 h to afford the activated desolvated samples SOF8a–10a. Thermal stability of as-prepared SOF8–10 was evaluated by thermogravimetric analysis (TGA, Figure S1). The phase purity of as-prepared SOF8–10 and activated SOF8a–10a was confirmed by powder X-ray diffraction (PXRD, Figures S2–S4). SOF-9a and SOF-10a possess highly robust frameworks, while structural collapse occurs during activation of SOF-8 to afford amorphous SOF-8a.
Crystallography
Single crystal X-ray data were collected on an Rigaku Oxford Diffraction SuperNovaII Atlas X-ray diffractometer at the University of Nottingham. Details of the data collection are included in the CIF files. Structures of SOF8–10 were solved by direct methods and developed by difference Fourier techniques, using the SHELXL software package (Table S1).38 Hydrogen atoms of the ligands were placed geometrically and refined using a riding model. The unit cell volume of SOF8–10 included a large region of disordered solvent which could not be modeled as discrete atomic sites. We therefore employed PLATON/SQUEEZE37 to calculate the contribution of the solvent region to the diffraction and thereby produced a set of solvent-free diffraction intensities. The solvent molecules of SOF8–10 are included in the unit cell contents and in all parameters derived from these. Despite multiple attempts, the limited crystal size and stability of SOF-8 restricted the quality of the resulting data set, but this did not preclude the identification of the framework structure. Topological analysis and polycatenation identification of SOF-9 and SOF-10 were performed using ToposPro software.39
Results and Discussion
Structural Description
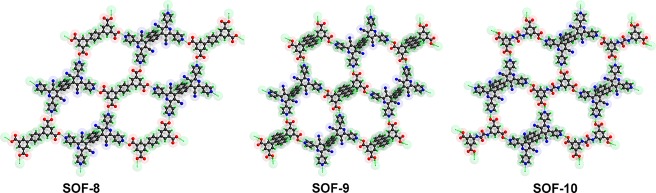
In a typical procedure, reaction of 1 and 3 in a 1:1 molar ratio in dimethylformamide (DMF) at 90 °C resulted in the formation of light-yellow prismatic crystals of SOF-8 after 72 h. Single crystal X-ray diffraction reveals that SOF-8 crystallizes in the monoclinic space group P21/m (Table S1), and features a 2D hydrogen-bonded network structure in which 1 has undergone a structural transformation into 2 through oxidative dehydrogenation (Scheme 1). The exo-pyridyl and carboxyl groups on 2 and 3 contribute to the complementary O–H···N hydrogen bonds that direct the self-assembly process. Each molecule of 2 interacts with four neighboring molecules of 3 through strong primary hydrogen bonds (OcarboxylH···Npyridyl, 2.562/2.568 Å; Table S2) to form a 2D honeycomb layer (63-hcb) supramolecular organic framework with windows of ca. 10.1 Å × 15.9 Å (Figure 1, left). The 2D 63-hcb layers stack in a parallel fashion and give rise to an overall 3D supramolecular structure with one-dimensional cyano-decorated channels (Figure S5, Supporting Information). It should be noted that the naphthalene moieties on 2 are disordered in symmetric positions, resulting in them pointing up and down into the windows of the 2D layers. In this way, neighboring 2D layers in SOF-8 interact with each other via π···π interactions.
Figure 1.
Views of the hcb layers in SOF8–10.
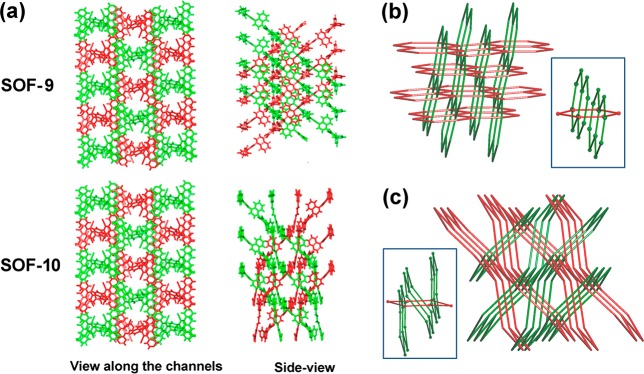
Single crystal X-ray analysis confirms that SOF-9 crystallizes as a 1:1 mixture of 2 and 4 in the orthorhombic space group Pba2 (Table S1). The 2D honeycomb layer subunit in SOF-9 is built by primary O–H···N hydrogen bonds between exo-pyridyl and carboxyl groups on 2 and 4 (Figure 1, middle), hydrogen bonds characterized by O···N distances in the range 2.548–2.633 Å, and approximately linear O–H···N angles (Table S2). In comparison with SOF-8, windows of the 2D layers in SOF-9 are more regular, with dimensions of ca. 12.0 Å × 12.3 Å. Packing of the 2D layers generates two sets of hcb layers,40−43 resulting in a 3D inclined polycatenated framework (Figure 2a,b). The dihedral angle between these two sets of layers is 79.6°. Each window of a layer is polycatenated with four other windows from two different layers via Hopf links.39,44 Alternatively, it can be described that each window encircles two edges passing through it (Figure 2b, inset).
Figure 2.
(a) Packing patterns of the polycatenated SOF-9 and SOF-10; parallel layers are presented in the same color. (b) Topological representation of the polycatenated hcb layers in SOF-9 and (c) SOF-10 (insets: views of the Hopf links).
X-ray crystallography confirms that SOF-10 crystallizes in the orthorhombic space group Pbc21 (Table S1) and comprises 2 and 5 in a 1:1 ratio (Scheme 1). A 2D honeycomb layer with windows of ca. 12.1 Å × 14.2 Å is constructed by complementary O–H···N hydrogen bonds between exo-pyridyl and carboxyl groups on 2 and 5, respectively (OcarboxylH···Npyridyl, 2.547–2.627 Å; Figure 1, right; Table S1, Supporting Information). Packing of the 2D layers results in a 3D inclined polycatenated framework similar to that of SOF-9, consisting of two sets of equivalent hcb layers (Figure 2a,c). The dihedral angle between these two sets of layers is 59.5°. Each window of a layer is polycatenated with six windows from three different layers via Hopf links. Alternatively, each window can be described as encircling the three edges passing through it (Figure 2c, inset). It should be noted that the O–H···N hydrogen bonds in SOF8–10 are considerably shorter than the ordinary H-bond (2.70–3.00 Å), and they are thus considered as double charge-assisted H-bonds, comprised of strong 1/2–D···H+···A1/2– interactions (D = H-bond donor; A = H-bond acceptor).45,46
Adsorption and Selectivity
The total solvent-accessible volume of SOF8–10 after removal of guest DMF molecules was estimated to be ca. 39.2%, 34.2%, and 29.9%, respectively, as calculated using the PLATON/VOID routine.37 It is not surprising that nonentangled SOF-8 has the largest potential molecular voids, and the decrease of molecular voids for SOF-9 and SOF-10 is attributable to framework interpenetration. However, structural collapse occurs upon removal of guest DMF molecules from SOF-8 (Figures S1 and S2, Supporting Information). In contrast, the polycatenated SOF-9 and SOF-10 display excellent thermal stability, as evaluated by thermogravimetric analysis (TGA) and powder X-ray diffractions (PXRD) (Figures S1, S3, and S4, Supporting Information), and they retain their structural integrity and crystallinity upon solvent exchange as well as upon the removal of guest molecules.
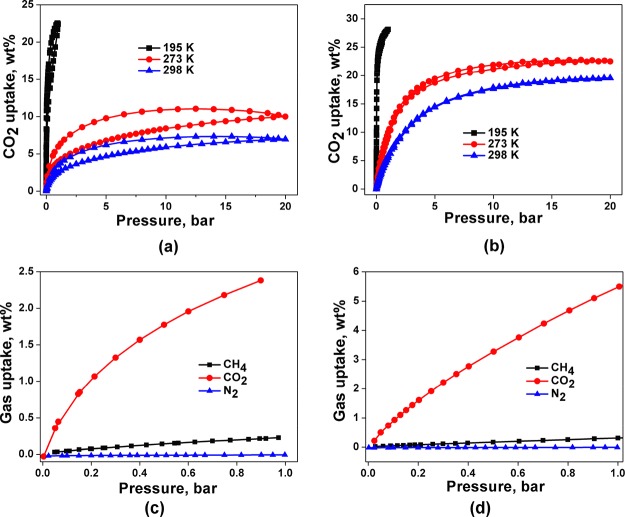
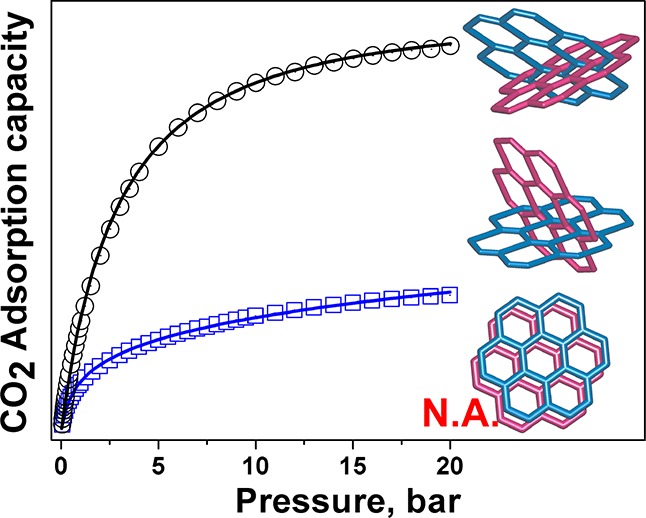
Encouraged by the above observations, we have confirmed the permanent porosity of the activated samples SOF-9a and SOF-10a by gas adsorption studies. The results clearly show that SOF-9a and SOF-10a exhibit selective adsorption for CO2 over N2 and CH4. The CO2 adsorption isotherms of SOF-9a and SOF-10a recorded at 195, 273, and 298 K (Figure 3a,b) reveal type-I sorption behavior and surface areas of 181.5 and 221.1 m2 g–1, respectively, comparable to the results for some hydrogen-bonded organic framework and organic molecular solids with intrinsic porosity (Table S3).6,11,26,47 At 1 bar, SOF-9a shows reversible CO2 adsorption capacities of 4.09 wt % (0.93 mmol g–1) and 2.43 wt % (0.55 mmol g–1) at 273 and 298 K, respectively (Figure 3a). High pressure (20 bar) CO2 adsorption studies of SOF-9a give the total capacities of 10.0 wt % (2.27 mmol g–1) and 6.96 wt % (1.58 mmol g–1) at 273 and 298 K, respectively (Figure 3a), comparable to the CO2 adsorption capacity of TBC[4]DHQ at a higher pressure (35 bar) despite SOF-9a having a considerably smaller surface area.6,48 At 1 bar, SOF-10a shows reversible CO2 adsorption capacities of 9.28 wt % (2.11 mmol g–1) and 5.67 wt % (1.29 mmol g–1) at 273 and 298 K, respectively (Figure 3b). High pressure (20 bar) CO2 adsorption studies of SOF-10a give the total capacities of 22.49 wt % (5.11 mmol g–1) and 19.55 wt % (4.44 mmol g–1) at 273 and 298 K (Figure 3b), respectively. The CO2 adsorption capacity of SOF-10a is superior to that of porous organic molecular solids with comparable surface area, as well as to that of the single-component SOF-1a that shows a much higher surface area (474 m2 g–1). It is notable that the CO2 adsorption capacity of SOF-10a is comparable to that of the only other binary SOF material documented so far, SOF-7a (Table S3), which has a surface area more than 4 times that of SOF-10a. The enhanced CO2 adsorption capacity of SOF-10a is attributable to the presence of oxamide groups, which function as favorable binding sites for guest CO2 molecules.25,49−52 The heat of adsorption for CO2 (Qst) was calculated via the Clausius–Clapeyron equation using CO2 isotherms at 273 and 298 K (Figures S6 and S7, Supporting Information) and was found to be 31.9 kJ mol–1 for SOF-9a and 27.1 kJ mol–1 for SOF-10a) (Figure S8, Supporting Information), which are slightly higher values than previously reported for single-component SOF materials but comparable to many MOF materials (25–35 kJ mol–1).13,15,24,26,27,53,54
Figure 3.
Adsorption/desorption isotherms for CO2 in SOF-9a (a) and SOF-10a (b) at 195 K (black), 273 K (red), and 298 K (blue) in the pressure range 0–20 bar; selectivity of CO2 adsorption over CH4 and N2 at 298 K for SOF-9a (c) and SOF-10a (d).
In order to evaluate the CO2 adsorption selectivity, uptake of CH4 by SOF-9a and SOF-10a was tested at pressures of up to 20 bar (Figure S9) at 273 and 298 K. SOF-9a shows CH4 uptake of 1.42 wt % (0.89 mmol g–1, 273 K) and 0.73 wt % (0.46 mmol g–1, 298 K) at 20 bar, whereas SOF-10a displays significantly higher CH4 uptake of 3.40 wt % (2.13 mmol g–1, 273 K) and 2.74 wt % (1.71 mmol g–1, 298 K) at the same pressure (Figure S7). Notably, the CH4 adsorption capacity of SOF-10a at 298 K is comparable to those of SOF-1a (1.43 mmol·g–1) and SOF-7a (1.71 mmol·g–1). In comparison with the selectivity of CO2 over CH4 calculated for SOF-1a and SOF-7a from the Henry’s Law, SOF-9a and SOF-10a exhibit higher CO2/CH4 selectivity than SOF-1a but lower selectivity than SOF-7a (Table S4). The order of CO2 adsorption is SOF-1a < SOF-9a < SOF-10a < SOF-7a, whereas the CO2 selectivity follows an order of SOF-1a < SOF-10a < SOF-9a < SOF-7a.
Quantum Chemical Modeling
In order to gain more insight into these new 2D SOF materials, and to understand why SOF-9 and SOF-10 are, like SOF-7,25 stable upon guest removal while SOF-8 is not, we analyzed these structures by means of quantum chemistry calculations, at the density functional theory (DFT) level. Starting from the guest-free crystallographic structures, geometry optimization was performed first to check that these materials, together with SOF-7, are well described at this level of theory, and then focused on their energetic and mechanical stabilities, as well as on vibrational properties. The relaxed structures are in excellent agreement with those obtained by single crystal X-ray crystallography, with deviations below 2% (even below 1% for SOF-8 and SOF-10) in either volume or individual cell parameters, as shown in Table S5. Intermolecular binding via OcarboxylH···Npyridyl bonds is well reproduced, with O···H···N distances between 2.52 and 2.57 Å, although slightly shorter than observed in the experimental structures for SOF-9 and SOF-10.
For each of the relaxed structures, the binding energy (or formation enthalpy) has been estimated by subtracting from the total energy (appropriately weighted) that of its constituting tectons. These binding energies have been found to be all fairly large, between −429 and −438 kJ mol–1 (per carboxylate-based constituent). It should be noted that these refer to the formation of SOF materials in the absence of solvents; we expect the formation enthalpies of these materials in DMF to be significantly smaller, since a rather large binding energy of DMF to carboxylate-based constituents (calculated binding energy as −65 to −80 kJ mol–1, depending on the constituent) is essentially lost when this constituent binds to an exo-pyridyl group.
The mechanical properties of these SOF materials have also been investigated by means of computing their second-order elastic stiffness tensors (also known as elastic constants). This has proven to be a reliable indicator of mechanical stability and potential for crystal-to-crystal phase transitions in metal–organic frameworks and other framework materials.55−57 The resulting bulk, Young’s, and shear moduli are listed in Table S6. All materials have reasonably large bulk moduli, between 9.0 and 9.5 GPa. Their response to isostatic pressure is however unusual, with (small) negative linear compressibility in specific directions. Whereas spatially averaged Young’s moduli E, ranging from 5.8 GPa in SOF-7 to 9.0 GPa in SOF-10, do not differ much from one material to another, their response to directional strains is instructive. Some materials are particularly soft with respect to specific deformation modes, especially the shear moduli which can be very low, Gmin = 0.21 GPa for SOF-7 and 0.41 GPa for SOF-8. Both materials are also sensitive to specific compression modes with Emin of the order of 1 GPa or below. For instance, the soft shearing mode of SOF-8 corresponds to sliding of a 2D honeycomb layer with respect to neighboring layers. Its counterpart in SOF-7 is similar, implicating relative motions of neighboring constituents, although the situation is slightly more complex since neighboring coordination layers are intertwined.25 The higher stability of SOF-8 over SOF-7 to shearing deformations, from these results, seems inconsistent with higher stability of SOF-7 to desolvation, which might induce some local deformations. An important difference, however, is that SOF-7 consists of intertwined networks (as do SOF-9 and SOF-10), which may result in a higher stability to large deformations (beyond the elastic regime), compared to the essentially layered SOF-8. A similar behavior has been observed, for example, in the family of MUF-8 and MUF-9 metal–organic frameworks with controlled partial interpenetration.58
Vibration modes of SOF-7 and SOF-8 have been further analyzed. For the latter, a large number of low-frequency modes are found: eight modes with frequencies ω < 30 cm–1 (not including three purely translational modes), and about 50 modes spread quite regularly over the range of 30–100 cm–1. An analysis of the former eight gives the following trends:
-
(a)
In two of these modes, constituents vibrate as (almost) rigid units, and their motion involves significant deformations in the O–H···N units (shortening/elongation of O–H or N···H bonds).
-
(b)
In the two other modes, constituents from the same coordination layer vibrate in phase, but such that neighboring layers slide with respect to each other. This induces local deformations, e.g., bending of C–C–C angles at tertiary carbons, and relative motions of neighboring aromatic cycles coupled by π···π interactions.
-
(c)
The remaining four modes have more complex structures, involving significant bending of C–C–C angles at tertiary carbons and/or of dihedrals centered on single C–C bonds between aromatic rings.
Similarly, SOF-7 has a large number of low-frequency vibration modes, but fewer (in comparison to SOF-8) in the ω < 20 cm–1 range. Moreover, modes of the type (c), connected to the polycatenated nature of the framework, are again absent for SOF-7. This qualitative difference, as well as the larger population of vibration modes in SOF-8 at ambient temperature, may explain its instability upon desolvation.
Finally, vibrational mode calculations at finite (positive and negative) pressures have been performed in order to determine the thermal expansion coefficients αV = V–1 (dV/dT). We obtained the values αV = 15.7 MK–1 for SOF-7, and a remarkably low αV = 2.2 MK–1 for SOF-8. The latter value is due to the presence, among low-energy vibration modes, of several modes that tend to soften (decrease in frequency) under compression; i.e., these modes alone would result in negative thermal expansion (NTE). This makes it likely that NTE will be encountered in further SOF materials, which opens further perspectives for applications of these materials.
Conclusion
In summary, we have demonstrated a rational design strategy to prepare 2D binary supramolecular organic framework (SOF) materials assembled via complementary hydrogen bonding interactions. The SOF assembly is tunable through rational design of organic constituents with hydrogen bond donating and accepting groups. The porous supramolecular organic frameworks SOF8–10 display similar 2D hydrogen-bonded 63-hcb layers with various packing modes: SOF-8 features parallel packing of 2D layers, whereas SOF-9 and SOF-10 possess 2D → 3D inclined polycatenation of 63-hcb layers. The higher chemical and thermal stability for SOF-9 and SOF-10, which are stable up to 300 °C, have been achieved through structural polycatenation. Further enhancement of adsorption capacity in activated samples of SOF-10a depends greatly on the introduction of favorable binding sites (the oxamide group in this case) for guest CO2 molecules. Therefore, appropriate functionalization of the organic constituents in SOF materials favors not only the stability of the materials but also their gas adsorption capacity and selectivity. Quantum chemistry calculations suggest a connection between this stability and the existence of soft deformation modes (especially shearing modes) and low-energy vibration modes. The latter may in particular account for the instability of SOF-8 upon guest removal, and also for unusual thermal expansion properties. These results further confirm that our binary design strategy is effective for the synthesis of highly stable and robust functional porous supramolecular organic framework materials with potentials for storage and separation. Moreover, enhanced CO2 adsorption and selectivity has been achieved by structural regulation via controlled assembly of 2D SOF materials into polycatenated structures.
Acknowledgments
We thank the EPSRC (EP/I011870), ERC (Advanced Grant AdG 226593), the 973 program (Grant 2014CB845605), the NSFC (Grants 91622114, 21520102001, and 21521061), the Strategic Priority Research Program (Grants XDB20000000 and XDA09030102) of the Chinese Academy of Sciences for funding. J.L. acknowledges financial support from the International Science and Technology Cooperation and Exchange Project of Fujian Agriculture and Forestry University (Grant No. KXGH17010). F.T. and F.-X.C. acknowledge access to high-performance computing platforms provided by a GENCI grant (A0030807069).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.cgd.8b00153.
Gas adsorption isotherms, gas uptake data and analysis, and quantum chemistry calculations, tables, and figures (PDF)
Accession Codes
CCDC 1818274–1818276 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Notes
Correspondence and requests for materials should be addressed to F-X.C., R.C., and M.S.
Supplementary Material
References
- Brändle M.; Sauer J. Acidity differences between inorganic solids induced by their framework structure. A combined quantum mechanics/molecular mechanics ab initio study on zeolites. J. Am. Chem. Soc. 1998, 120, 1556–1570. 10.1021/ja9729037. [DOI] [Google Scholar]
- Yang Z.; Xia Y.; Mokaya R. Enhanced hydrogen storage capacity of high surface area aeolite-like carbon materials. J. Am. Chem. Soc. 2007, 129, 1673–1679. 10.1021/ja067149g. [DOI] [PubMed] [Google Scholar]
- Hao G.-P.; Li W.-C.; Qian D.; Lu A.-H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. 10.1002/adma.200903765. [DOI] [PubMed] [Google Scholar]
- Hao G.-P.; Li W.-C.; Qian D.; Wang G.-H.; Zhang W.-P.; Zhang T.; Wang A.-Q.; Schüth F.; Bongard H.-J.; Lu A.-H. Structurally designed synthesis of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J. Am. Chem. Soc. 2011, 133, 11378–11388. 10.1021/ja203857g. [DOI] [PubMed] [Google Scholar]
- Hudson M. R.; Queen W. L.; Mason J. A.; Fickel D. W.; Lobo R. F.; Brown C. M. Unconventional, highly selective CO2 adsorption in zeolite SSZ-13. J. Am. Chem. Soc. 2012, 134, 1970–1973. 10.1021/ja210580b. [DOI] [PubMed] [Google Scholar]
- Msayib K. J.; Book D.; Budd P. M.; Harris K. D. M.; Helliwell M.; Tedds S.; Warren J. E.; Xu M. C.; McKeown N. B.; Chaukura N.; Walton A. Nitrogen and hydrogen adsorption by an organic microporous crystal. Angew. Chem., Int. Ed. 2009, 48, 3273–3277. 10.1002/anie.200900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewin A.; Cooper A. I. Porous organic polymers: distinction from disorder?. Angew. Chem., Int. Ed. 2010, 49, 1533–1535. 10.1002/anie.200906827. [DOI] [PubMed] [Google Scholar]
- Ben T.; Pei C.; Zhang D.; Xu J.; Deng F.; Jing X.; Qiu S. Gas storage in porous aromatic frameworks (PAFs). Energy Environ. Sci. 2011, 4, 3991–3999. 10.1039/c1ee01222c. [DOI] [Google Scholar]
- Bezzu C. G.; Carta M.; Tonkins A.; Jansen J. C.; Bernardo P.; Bazzarelli F.; McKeown N. B. A spirobifluorene-based polymer of intrinsic microporosity with improved performance for gas separation. Adv. Mater. 2012, 24, 5930–5933. 10.1002/adma.201202393. [DOI] [PubMed] [Google Scholar]
- Ding S.-Y.; Wang W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 2013, 42, 548–568. 10.1039/C2CS35072F. [DOI] [PubMed] [Google Scholar]
- Sozzani P.; Bracco S.; Comotti A.; Ferretti L.; Simonutti R. Methane and carbon dioxide storage in a porous van der Waals crystal. Angew. Chem., Int. Ed. 2005, 44, 1816–1820. 10.1002/anie.200461704. [DOI] [PubMed] [Google Scholar]
- He Y.; Xiang S.; Chen B. A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 2011, 133, 14570–14573. 10.1021/ja2066016. [DOI] [PubMed] [Google Scholar]
- Mastalerz M.; Oppel I. M. Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem., Int. Ed. 2012, 51, 5252–5255. 10.1002/anie.201201174. [DOI] [PubMed] [Google Scholar]
- Luo X.-Z.; Jia X.-J.; Deng J.-H.; Zhong D.-C.; Liu H.-J.; Wang K.-J.; Zhong D.-C. A microporous hydrogen-bonded organic framework: exceptional stability and highly selective adsorption of gas and liquid. J. Am. Chem. Soc. 2013, 135, 11684–11687. 10.1021/ja403002m. [DOI] [PubMed] [Google Scholar]
- Li P.; He Y.; Guang J.; Weng L.; Zhao J. C.-G.; Xiang S.; Chen B. A homochiral microporous hydrogen-bonded organic framework for highly enantioselective separation of secondary alcohols. J. Am. Chem. Soc. 2014, 136, 547–549. 10.1021/ja4129795. [DOI] [PubMed] [Google Scholar]
- Yamamoto A.; Hamada T.; Hisaki I.; Miyata M.; Tohnai N. Dynamically deformable cube-like hydrogen-bonding networks in water-responsive diamondoid oorous organic salts. Angew. Chem., Int. Ed. 2013, 52, 1709–1712. 10.1002/anie.201208153. [DOI] [PubMed] [Google Scholar]
- Li P.; He Y.; Zhao Y.; Weng L.; Wang H.; Krishna R.; Wu H.; Zhou W.; O’Keeffe M.; Han Y.; Chen B. A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem., Int. Ed. 2015, 54, 574–577. 10.1002/anie.201410077. [DOI] [PubMed] [Google Scholar]
- Wang H.; Li B.; Wu H.; Hu T.-L.; Yao Z.; Zhou W.; Xiang S.; Chen B. A flexible microporous hydrogen-bonded organic framework for gas sorption and separation. J. Am. Chem. Soc. 2015, 137, 9963–9970. 10.1021/jacs.5b05644. [DOI] [PubMed] [Google Scholar]
- Zhang J. P.; Chen X. M. Optimized acetylene/carbon dioxide sorption in a dynamic porous crystal. J. Am. Chem. Soc. 2009, 131, 5516–5521. 10.1021/ja8089872. [DOI] [PubMed] [Google Scholar]
- Sumida K.; Rogow D. L.; Mason J. A.; McDonald T. M.; Bloch E. D.; Herm Z. R.; Bae T.-H.; Long J. R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- Yang S.; Lin X.; Lewis W.; Suyetin M.; Bichoutskaia E.; Parker J.; Tang C. C.; Allan D. R.; Rizkallah P. J.; Hubberstey P.; Champness N. R.; Thomas K. M.; Blake A. J.; Schröder M. A partially interpenetrated metal-organic framework for selective hysteretic sorption of carbon dioxide. Nat. Mater. 2012, 11, 710–716. 10.1038/nmat3343. [DOI] [PubMed] [Google Scholar]
- Yang S.; Sun J.; Ramirez-Cuesta A. J.; Callear S. K.; David W. I. F.; Anderson D.; Newby R.; Blake A. J.; Parker J. E.; Tang C. C.; Schröder M. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat. Chem. 2012, 4, 887–849. 10.1038/nchem.1457. [DOI] [PubMed] [Google Scholar]
- Zhu X.-D.; Zhang K.; Wang Y.; Long W.-W.; Sa R.-J.; Liu T.-F.; Lü J. Fluorescent metal organic framework (MOF) as a highly sensitive and quickly responsive chemical sensor for the detection of antibiotics in simulated wastewater. Inorg. Chem. 2018, 57, 1060–1065. 10.1021/acs.inorgchem.7b02471. [DOI] [PubMed] [Google Scholar]
- Yang W.; Greenaway A.; Lin X.; Matsuda R.; Blake A. J.; Wilson C.; Lewis W.; Hubberstey P.; Kitagawa S.; Champness N. R.; Schröder M. Exceptional thermal stability in a supramolecular organic framework: porosity and gas storage. J. Am. Chem. Soc. 2010, 132, 14457. 10.1021/ja1042935. [DOI] [PubMed] [Google Scholar]
- Lü J.; Perez-Krap C.; Suyetin M.; Alsmail N. H.; Yan Y.; Yang S.; Lewis W.; Bichoutskaia E.; Tang C. C.; Blake A. J.; Cao R.; Schröder M. A robust binary supramolecular organic framework (SOF) with high CO2 adsorption and selectivity. J. Am. Chem. Soc. 2014, 136, 12828–12831. 10.1021/ja506577g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Kim Y.; Yoon M.; Lim S.; Park S. M.; Seo G.; Kim K. Highly selective carbon dioxide sorption in an organic molecular porous material. J. Am. Chem. Soc. 2010, 132, 12200–12202. 10.1021/ja105211w. [DOI] [PubMed] [Google Scholar]
- Tian J.; Ma S.; Thallapally P. K.; Fowler D.; McGrail B. P.; Atwood J. L. Cucurbit[7]uril: an amorphous molecular material for highly selective carbon dioxide uptake. Chem. Commun. 2011, 47, 7626–7628. 10.1039/c1cc12689j. [DOI] [PubMed] [Google Scholar]
- Zhang K.-D.; Tian J.; Hanifi D.; Zhang Y.; Sue A. C.-H.; Zhou T.-Y.; Zhang L.; Zhao X.; Liu U.; Li Z.-T. Toward a single-layer two-dimensional honeycomb supramolecular organic framework in water. J. Am. Chem. Soc. 2013, 135, 17913–17918. 10.1021/ja4086935. [DOI] [PubMed] [Google Scholar]
- Hisaki I.; Nakagawa S.; Ikenaka N.; Imamura Y.; Katouda M.; Tashiro M.; Tsuchida H.; Ogoshi T.; Sato H.; Tohnai N.; Miyata M. A series of layered assemblies of hydrogen-bonded, hexagonal networks of C3-symmetric π-conjugated molecules: A potential motif of porous organic materials. J. Am. Chem. Soc. 2016, 138, 6617–6628. 10.1021/jacs.6b02968. [DOI] [PubMed] [Google Scholar]
- Martí-Rujas J.; Colombo L.; Lü J.; Dey A.; Terraneo G.; Metrangolo P.; Pilati T.; Resnati G. Hydrogen and halogen bonding drive the orthogonal self-assembly of an organic framework possessing 2D channels. Chem. Commun. 2012, 48, 8207–8209. 10.1039/c2cc33682k. [DOI] [PubMed] [Google Scholar]
- Bhogala B. R.; Basavoju S.; Nangia A. Three-component carboxylic acid-bipyridine lattice inclusion host. Supramolecular synthesis of ternary cocrystals. Cryst. Growth Des. 2005, 5, 1683–1686. 10.1021/cg058012p. [DOI] [Google Scholar]
- Huang Y.-G.; Shiota Y.; Wu M.-Y.; Su S.-Q.; Yao Z.-S.; Kang S.; Kanegawa S.; Li G.-L.; Wu S.-Q.; Kamachi T.; Yoshizawa K.; Ariga K.; Hong M.-C.; Sato O. Superior thermoelasticity and shape-memory nanopores in a porous supramolecular organic framework. Nat. Commun. 2016, 7, 11564. 10.1038/ncomms11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. J.; Long D.-L.; Champness N. R.; Hubberstey P.; Schröder M. New approaches to the analysis of high connectivity materials: Design frameworks based upon 44- and 63-subnet tectons. Acc. Chem. Res. 2005, 38, 335–348. 10.1021/ar040174b. [DOI] [PubMed] [Google Scholar]
- Lü J.; Han L.-W.; Alsmail N. H.; Blake A. J.; Lewis W.; Cao R.; Schröder M. Control of assembly of dihydropyridyl and pyridyl molecules via directed hydrogen bonding. Cryst. Growth Des. 2015, 15, 4219–4224. 10.1021/acs.cgd.5b00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaate A.; Roy P.; Godt A.; Lippke J.; Waltz F.; Wiebcke M.; Behrens P. Modulated synthesis of Zr-based metal-organic frameworks: from nano to single crystals. Chem. - Eur. J. 2011, 17, 6643–6651. 10.1002/chem.201003211. [DOI] [PubMed] [Google Scholar]
- Hauptvogel I. M.; Biedermann R.; Klein N.; Senkovska I.; Cadiau A.; Wallacher D.; Feyerherm R.; Kaskel S. Flexible and hydrophobic Zn-based metal-organic framework. Inorg. Chem. 2011, 50, 8367–8374. 10.1021/ic200937u. [DOI] [PubMed] [Google Scholar]
- Spek A. L. Structure validation in chemical crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, 65, 148–155. 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Blatov V. A.; Shevchenko A. P.; Proserpio D. M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. 10.1021/cg500498k. [DOI] [Google Scholar]
- Carlucci L.; Ciani G.; Proserpio D. M. Polycatenation, polythreading and polyknotting in coordination network chemistry. Coord. Chem. Rev. 2003, 246, 247–289. 10.1016/S0010-8545(03)00126-7. [DOI] [Google Scholar]
- Proserpio D. M. Topological crystal chemistry: polycatenation weaves a 3D web. Nat. Chem. 2010, 2, 435–436. 10.1038/nchem.674. [DOI] [PubMed] [Google Scholar]
- Zentner C. A.; Lai H. W. H.; Greenfield J. T.; Wiscons R. A.; Zeller M.; Campana C. F.; Talu O.; FitzGerald S. A.; Rowsell J. L. C. High surface area and Z′ in a thermally stable 8-fold polycatenated hydrogen-bonded framework. Chem. Commun. 2015, 51, 11642–10645. 10.1039/C5CC04219D. [DOI] [PubMed] [Google Scholar]
- Lai H. W. H.; Wiscons R. A.; Zentner C. A.; Zeller M.; Rowsell J. L. C. Supramolecular assembly of tris(4-carboxyphenyl)arenes: Relationship between molecular structure and solid-state catenation motifs. Cryst. Growth Des. 2016, 16, 821–833. 10.1021/acs.cgd.5b01416. [DOI] [Google Scholar]
- Hisaki I.; Ikenaka N.; Gomez E.; Cohen B.; Tohnai N.; Douhal A. Hexaazatriphenylene-based hydrogen-bonded organic framework with permanent porosity and single-crystallinity. Chem. - Eur. J. 2017, 23, 11611–11619. 10.1002/chem.201701893. [DOI] [PubMed] [Google Scholar]
- Gilli P.; Pretto L.; Bertolasi V.; Gilli G. Predicting hydrogen-bond strengths from acid-base molecular properties. The pKa slide rule: toward the solution of a long-lasting problem. Acc. Chem. Res. 2009, 42, 33–44. 10.1021/ar800001k. [DOI] [PubMed] [Google Scholar]
- Lü J.; Han L.-W.; Lin J.-X.; Liu T.-F.; Cao R. Rare Case of a Triple-stranded molecular braid in an organic cocrystal. Cryst. Growth Des. 2010, 10, 4217–4220. 10.1021/cg100577t. [DOI] [Google Scholar]
- Lü J.; Cao R. Porous organic molecular frameworks with extrinsic porosity: A platform for carbon storage and separation. Angew. Chem., Int. Ed. 2016, 55, 9474–9480. 10.1002/anie.201602116. [DOI] [PubMed] [Google Scholar]
- Thallapally P. K.; McGrail B. P.; Atwood J. L.; Gaeta C.; Tedesco C.; Neri P. Carbon dioxide capture in a self-assembled organic nanochannels. Chem. Mater. 2007, 19, 3355–3357. 10.1021/cm0709121. [DOI] [Google Scholar]
- Zheng B.; Bai J.; Duan J.; Wojtas L.; Zaworotko M. J. Enhanced CO2 binding affinity of a high-uptake rht-type metal-organic framework decorated with acylamide groups. J. Am. Chem. Soc. 2011, 133, 748–751. 10.1021/ja110042b. [DOI] [PubMed] [Google Scholar]
- Yuan D. Q.; Zhao D.; Sun D. F.; Zhou H.-C. An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem., Int. Ed. 2010, 49, 5357–5361. 10.1002/anie.201001009. [DOI] [PubMed] [Google Scholar]
- Park J.; Li J.-R.; Chen Y.-P.; Yu J.; Yakovenko A. A.; Wang Z. U.; Sun L.-B.; Balbuena P. B.; Zhou H.-C. A versatile metal-organic framework for carbon dioxide capture and cooperative catalysis. Chem. Commun. 2012, 48, 9995–9997. 10.1039/c2cc34622b. [DOI] [PubMed] [Google Scholar]
- Alsmail N. H.; Suyetin M.; Yan Y.; Cabot R.; Krap C. P.; Lü J.; Easun T. L.; Bichoutskaia E.; Lewis W.; Blake A. J.; Schröder M. Analysis of high and selective uptake of CO2 in an oxamide containing {Cu2(OOCR)4}-based metal-organic framework. Chem. - Eur. J. 2014, 20, 7317–7324. 10.1002/chem.201304005. [DOI] [PubMed] [Google Scholar]
- Bourrelly S.; Llewellyn P. L.; Serre C.; Millange F.; Loiseau T.; Férey G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. 10.1021/ja054668v. [DOI] [PubMed] [Google Scholar]
- Loiseau T.; Lecroq L.; Volkringer C.; Marrot J.; Férey G.; Haouas M.; Taulelle F.; Bourrelly S.; Llewellyn P. L.; Latroche M. MIL-96, a porous aluminum trimesate 3D structure constructed from a hexagonal network of 18-membered rings and μ3-oxo-centered trinuclear units. J. Am. Chem. Soc. 2006, 128, 10223–10230. 10.1021/ja0621086. [DOI] [PubMed] [Google Scholar]
- Bouëssel du Bourg L.; Ortiz A. U.; Boutin A.; Coudert F.-X. Thermal and mechanical stability of zeolitic imidazolate frameworks polymorphs. APL Mater. 2014, 2, 124110–124119. 10.1063/1.4904818. [DOI] [Google Scholar]
- Ortiz A. U.; Boutin A.; Coudert F.-X. Prediction of flexibility of metal-organic frameworks CAU-13 and NOTT-300 by first principles molecular simulations. Chem. Commun. 2014, 50, 5867–5870. 10.1039/c4cc00734d. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Jiang X.; Kim S. T.; Alahakoon S. B.; Hou X.; Zhang Z.; Thompson C. M.; Smaldone R. A.; Ke C. An elastic hydrogen-bonded cross-linked organic framework for effective iodine capture in water. J. Am. Chem. Soc. 2017, 139, 7172–7175. 10.1021/jacs.7b03204. [DOI] [PubMed] [Google Scholar]
- Ferguson A.; Liu L.; Tapperwijn S. J.; Perl D.; Coudert F.-X.; Van Cleuvenbergen S.; Verbiest T.; van der Veen M. A.; Telfer S. G. Controlled partial interpenetration in metal-organic frameworks. Nat. Chem. 2016, 8, 250–257. 10.1038/nchem.2430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.