Abstract
Background
African American adolescent females are at elevated risk for unintended pregnancy and sexually transmitted infections (STIs). Dual protection (DP) is defined as concurrent prevention of pregnancy and STIs. This can be achieved by abstinence, consistent condom use, or the dual methods of condoms plus an effective non-barrier contraceptive. Previous clinic-based interventions showed short-term effects on increasing dual method use, but evidence of sustained effects on dual method use and decreased incident pregnancies and STIs are lacking.
Methods/Design
This manuscript describes the 2GETHER Project. 2GETHER is a randomized controlled trial of a multi-component intervention to increase dual protection use among sexually active African American females aged 14–19 years not desiring pregnancy at a Title X clinic in Atlanta, GA. The intervention is clinic-based and includes a culturally tailored interactive multimedia component and counseling sessions, both to assist in selection of a DP method and to reinforce use of the DP method. The participants are randomized to the study intervention or the standard of care, and followed for 12 months to evaluate how the intervention influences DP method selection and adherence, pregnancy and STI incidence, and participants’ DP knowledge, intentions, and self-efficacy.
Discussion
The 2GETHER Project is a novel trial to reduce unintended pregnancies and STIs among African American adolescents. The intervention is unique in the comprehensive and complementary nature of its components and its individual tailoring of provider-patient interaction. If the trial interventions are shown to be effective, then it will be reasonable to assess their scalability and applicability in other populations.
Keywords: contraception, reproductive health, unintended pregnancy, sexually transmitted infections, adolescents, dual protection
Introduction
Unintended pregnancies and sexually transmitted infections (STI) represent serious, yet preventable, public health problems. In the United States (US), more than 75% of all pregnancies among adolescents aged 15–19 years are unintended [1], leading to abortion [1] and negative outcomes for mothers and children [2–7]. US adolescents aged 14–19 years old also have a high prevalence of STIs, with 8.2% having one or more of chlamydia, gonorrhea, trichomonas or herpes simplex virus type 2 [8]. These infections can lead to complications with lasting sequelae [9–16]. African American adolescents, particularly those in the South, have higher birth rates and STI rates than adolescents of other racial and ethnic groups [8, 17–20].
Unintended pregnancy overwhelmingly results from not using contraception, or from not using it correctly and consistently, as opposed to contraceptive failure [21]. The most commonly used birth control methods by US adolescents are condoms, followed by oral contraceptive pills (OCP) [22], both of which are challenging to use correctly and consistently. Barrier methods, namely condoms, are the only contraceptives effective at preventing STI transmission, but are associated with a higher rate of pregnancy than non-barrier methods with typical use [23, 24]. OCPs, which must be taken daily, also are less effective under typical use than long acting reversible contraceptives (LARCs), specifically hormonal and non-hormonal intrauterine devices (IUDs) and hormonal implants [23]. LARCs are also known as user-independent methods because they require no effort to use after placement, and are the most effective reversible methods available [25]. However, among adolescents using contraceptives in the United States, only 4.3% use LARCs [26].
Dual protection (DP) is the concurrent prevention of unintended pregnancy and STIs. It can be achieved through abstinence, consistent condom use, or dual method use of condoms plus an effective non-barrier contraceptive [27]. Condoms plus LARCs are recommended by many public health and medical organizations as the most effective DP strategy to prevent both unintended pregnancy and STIs [28–30]. While LARCs and other hormonal contraceptive methods have excellent acceptance and tolerability profiles [31–33], it is vital that providers place the needs and preferences of the woman over their desire to promote specific methods, especially in populations that have historically been subject to racially-based reproductive injustice [34, 35]. Furthermore, some studies among adolescents [36, 37], and African American adolescents specifically, have shown that use of moderately or highly effective non-barrier contraceptive methods may be inversely related to condom use [38–40].
The only randomized controlled trial focused on dual method use for adolescents that we identified was a trial among African American and Latino adolescents; it combined a video promoting safer sex behaviors and individual counseling to work with participants to create a plan to reduce STI risk among those who were using or planning to use hormonal contraception. At 3 month follow up, average instances of unprotected sex were reduced, but there was no effect at 12 months [41]. Another trial of an intervention that included adolescents similarly showed a temporary effect in initiating dual method use for dual protection [42]. However, neither these nor other studies of counseling to promote dual method use have shown decreases in STIs and unintended pregnancy [41–44]. This may be due, in part, to failure to adhere to the chosen methods [43, 45]. Formative research we conducted with African American adolescents aged 14–19 years in Atlanta indicated that planning for sex and use of dual methods or condoms alone were associated with many factors, including family and social support, concerns about side effects, male partners’ attitudes, relationship status, changes to the relationship and previous experiences of STIs or pregnancy [46, 47]. Adolescents’ decisions about DP require balancing social, personal and relationship factors that may compete with the desire for and the ability to use DP [46]. Many of these factors will continue to change after the selection of a DP strategy, presenting new challenges to adherence to the chosen strategy. To sustain the effects of interventions promoting DP, it may be necessary to help individuals build skills for overcoming challenges to using their DP strategy and providing support as their circumstances and goals change.
This paper describes the design of “2GETHER: The Dual Protection Project,” an innovative, multi-component clinic intervention addressing the high rates of STDs and unintended pregnancy among African American adolescent females served by a teen health clinic in Atlanta, Georgia by increasing dual protection selection and adherence. This patient-centered project focuses on selection of a dual protection strategy (dual method use, correct and consistent condom use or abstinence) to prevent pregnancy and STIs that fits the individual needs and goals of the participant and interactive skill-building to apply that strategy in her unique context.
Methods
Study overview
This study is called “2GETHER: The Dual Protection Project.” It is an unblinded, two-arm randomized controlled trial to determine the effect of a multi-component, clinic-based behavioral intervention on the primary outcomes of increased adoption of and adherence to an effective DP strategy, and decreased incidence of pregnancy, chlamydia, gonorrhea and trichomonas infection in the intervention as compared with the control group. Secondary study outcomes include levels of DP knowledge; DP strategy intentions; self-efficacy related to contraceptive and condom use; STI testing; and self-efficacy and frequency of partner communication about reproductive health.
African American female adolescent (14–19 years old) participants are followed for 12 months with study outcomes assessed at enrollment and follow up visits (6 and 12 months after enrollment), data collection calls (3 and 9 months after enrollment), and via medical chart abstraction for any clinic visits initiated by the participants.
An effective DP strategy is one that can concurrently prevent pregnancy and STIs. This may be achieved by abstinence from sexual intercourse; dual method use of condoms plus an effective non-barrier contraceptive; or correct and consistent use of condoms. While some previous studies have focused on encouraging dual method use only, this study includes additional approaches to achieving DP including correct and consistent condom use and abstinence to balance the importance of selecting the most efficacious strategy with need to support participants in being successful with the strategy that works for them.
Study population, location and personnel
Young women are being recruited from the Grady Health System Teen Services Clinic for reproductive health care located in Atlanta, GA. The Teen Services Clinic receives Title X Family Planning funding and employs medical providers, nurses, and adult and adolescent health educators to provide teen-centered health care and counseling for adolescent and young adult male and female patients. Services include birth control; gynecological care; pregnancy, STI and HIV testing; STI treatment; HPV vaccines; and sports physicals. Participants in this study must be female; be 14–19 years old at enrollment; self-identify as African American or black or of mixed race with African American or black; have had vaginal sex with a male partner at least once in last 6 months; not be currently pregnant (verified by urine pregnancy test); desire to avoid pregnancy for at least 12 months; be HIV negative by self-report; plan to be in the study area for the next 12 months; be willing to participate in all scheduled study visits and tests; be competent to participate in consenting or assenting process per recruiter evaluation; and be willing to provide contact information.
Ethics approval has been obtained from the CDC and Emory University Institutional Review Boards, and has been approved by the Grady Health Systems Research Oversight Committee.
Enrollment and randomization
After consenting, participants give a urine sample for pregnancy and STI (chlamydia, gonorrhea and trichomonas) testing; HIV testing is not provided as part of the study, but may occur during routine clinical care. Participants then complete an Audio Computer Assisted Self-Interviewing (ACASI)-administered questionnaire–Self-administration has been shown to reduce response bias on surveys [48–50]. The baseline ACASI questionnaire collects information about demographic characteristics, current and past contraceptive use, current and past condom use, history of STIs, sexual behaviors, and core mediators and moderators of DP selection and use (e.g. pregnancy intentions, perceptions and attitudes about pregnancy and STDs, relationship characteristics, substance abuse, physical and emotional abuse history and more).
If a participant has a negative pregnancy test, she is randomized to either the intervention or control arm using permuted block randomization. A 1:1 assignment ratio between the study arms is used to maximize efficiency in detecting a difference between the study arms. Randomization and concealment of allocation is accomplished by the use of sequentially-numbered, sealed, opaque envelopes containing the group assignment. Staff and healthcare providers are not blinded to assignment.
The pregnancy and STI tests, as well as a condensed version of the enrollment ACASI, are completed at the six and 12 months study visits. Participants in both groups are called 3 and 9 months after enrollment to collect information about contraceptive use, condom use, STIs, and sexual behaviors.
Intervention
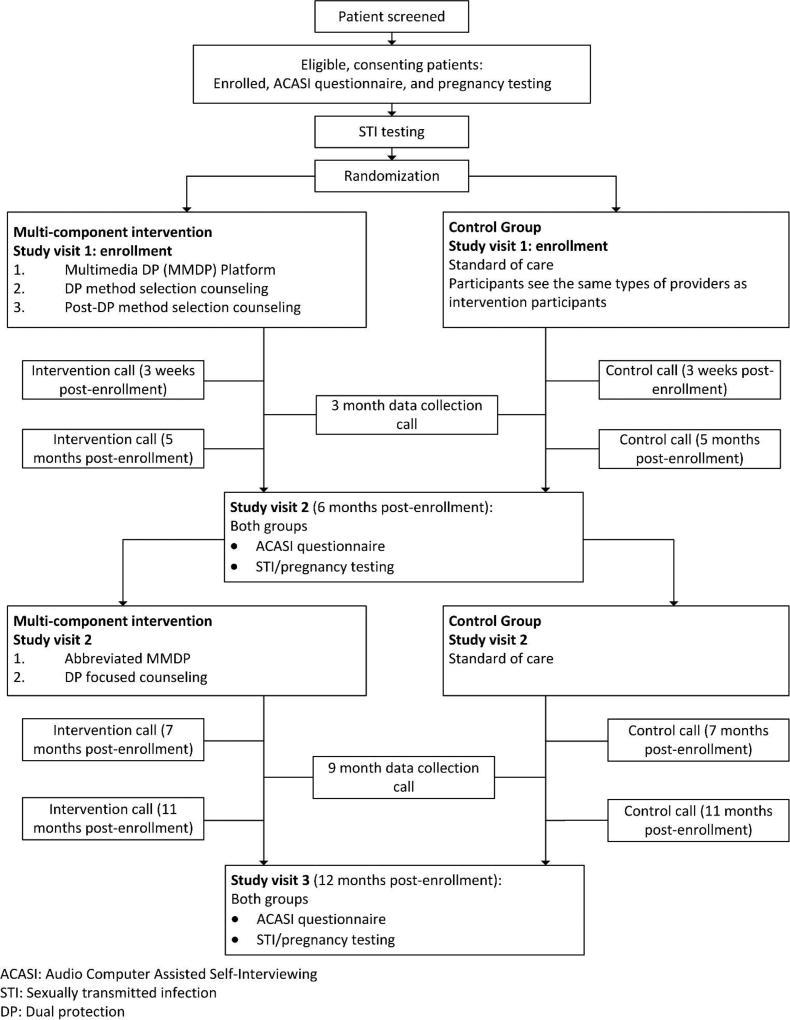
The intervention studied in the 2GETHER Project is designed to address key barriers and facilitators of effective DP strategy selection and adherence, specifically those identified in formative research we conducted with members of this population [46, 47]. This formative research included a cross-sectional questionnaire and focus group discussions with adolescents and providers, and semi-structured interviews with adolescents which informed this intervention’s focus on attitudes towards STIs and pregnancy as well as addressing barriers to contraception and condom use. The study schema is depicted in Figure 1.
Figure 1.
2GETHER study overview. Flow diagram displays recruitment through study completion.
ACASI: Audio Computer Assisted Self-Interviewing
STI: Sexually transmitted infection
DP: Dual protection
On the day of enrollment, participants randomized to the intervention group engage in a three-component clinic visit and intervention: a) multi-media dual protection (MMDP) platform, b) one-on-one DP method selection counseling session, and c) one-on-one post-method selection counseling session to reinforce DP method use. The control group follows the clinic’s standard of care for a reproductive health visit. To equalize exposure to medical providers between groups, participants in the control group see the same types of providers as those in the intervention group, but they do not receive the MMDP and are not counseled using the provider guides. Both the intervention and control groups have access to the same clinical services, which includes the full range of contraceptive options, STI counseling, testing and treatment, and other related services. The standard of care provided at the clinic is aligned with recommendations from the Medical Eligibility Criteria, Selected Practice Recommendations and Providing Quality Family Planning Services from the Centers for Disease Control and Prevention and the Office of Population Affairs [51] and adheres to the Program Requirements for Title X Funded Family Planning Projects [52].
Findings from our formative studies guided the creation of the MMDP platform. These identified that participants placed a high value on experiential learning, believed multiple myths and misperceptions about sexual health and contraception, were concerned about side effects associated with non-barrier contraception and, generally, had not heard of “dual protection” but could deduce what it meant [46]. The MMDP begins with a series of vignettes delivered by young African American female actors regarding relationships, STI prevention, pregnancy prevention, and DP use. The vignettes are “role model stories” that show how adolescents can identify and overcome barriers to DP use. The content of the MMDP consists of information relevant to making decisions about DP and addresses specific concerns identified during the formative study with this population. The MMDP explores these issues, providing examples of young African American females dealing with diverse situations – including some directly taken from focus group responses – and successfully choosing and implementing the DP strategy that works best for them individually.
In addition to the vignettes, the MMDP contains a true/not true game to address common myths and misconceptions about pregnancy, contraception and STIs. It also contains informational slides on contraceptive methods that provide opportunities for detailed exploration about methods regarding efficacy, ease of use, privacy, pros and cons, common side effects and tips for managing these. The MMDP contains a visual aid for comparing DP strategies according to how well they could work to concurrently prevent pregnancy and STI prevention. The concept of condom use being better when used together with a LARC or other hormonal contraceptive is introduced. Lastly, the MMDP includes reflection questions about what a participant may want from a contraceptive method, her future plans, and her current condom use and level of communication with her partners. The answers to these reflection questions are used to tailor the DP method selection and post-DP method selection counseling sessions that follow the MMDP.
After completing the MMDP, the participant engages in a one-on-one DP method selection counseling session with a medical provider. Responses to questions on the MMDP are incorporated into a guide received by providers upon participant completion of the MMDP. This guide is used as a starting point for further discussion and is designed to encourage the participant to explore her goals and feelings regarding pregnancy, STIs, her relationship, and her desires regarding DP. The provider works interactively with the participant to select a DP strategy that she feels will work best for her using information introduced during the MMDP (e.g., how well the strategy could work, condoms as important for STI prevention and even better when used with another non-barrier method, side effect management) as well as the participant’s own assessment of her pregnancy and STI risk and importance for DP. After she has selected the DP strategy that works best for her, the patient receives any necessary medical care (e.g., STI testing, prescription for or placement of a method).
After selecting a DP strategy, participants engage in a one-on-one post-DP method selection counseling session with a nurse educator, addressing skills and strategies to promote consistent use of the DP approach the participant selected. This session is highly interactive, involves a variety of activities (e.g., role plays, condom use demonstrations), and is framed around the participant’s life goals and how being successful with DP could support her in reaching these goals. It focuses on DP adherence and application to the participant’s unique context including communicating with her partner(s) about DP. It encourages the participant to not only predict challenges she may have in using her DP strategy but also to identify potential solutions to those challenges for herself. Finally, participants are encouraged to return to the clinic for ongoing DP support or if she’d like to change her DP strategy.
The counseling sessions with both the medical provider and nurse educator use motivational interviewing techniques to facilitate the discussions around DP. The counseling in this intervention does not strictly follow the motivational interviewing protocol as described by Miller [53], but is based on similar principles. These include empathetic listening, fostering intrinsic motivation for change, and developing discrepancies between patient’s behavior and their goals. To put these principles in practice, the counseling sessions utilize techniques including reflective listening, open-ended questions and affirming statements, while avoiding assigning blame or giving directions without the patient’s request. All providers and educators conducting counseling are trained with a focus on these principles before beginning work on the intervention. During training, counseling sessions are taped and feedback provided to ensure application of the principles and techniques as stated. Ongoing training and supervision meetings are conducted with nurse educators and providers.
All participants return to the clinic approximately 6 months after the Enrollment visit for Study Visit 2. After data collection, intervention participants receive an abbreviated booster version of the MMDP focused on DP strategies and strategy adherence followed by a one-on-one health education and counseling session with the health educator to reinforce skills for correct and consistent use of the DP strategy. Control participants follow the clinic’s standard of care.
Intervention group participants also receive booster counseling sessions from the nurse educator via phone four times over the 12 month follow-up period. In these sessions, nurse educators work interactively with participants to support ongoing correct and consistent DP use by discussing successes, challenges and plans for the future. Control group participants receive calls from study staff on the same schedule to remind them of their appointments and to update their contact information.
Participants from both groups have access to the clinic outside of the study visits as needed, and are specifically encouraged to return to the clinic for concerns about their DP strategy, and if they are thinking about switching their DP strategy or have STI symptoms.
Non-planned visits to the clinic follow the clinic’s standard of care. In case of an STI diagnosis, this includes providing information about the diagnosis, treatment, partner notification, prevention of future STIs and condom use, and future STI testing. For visits related to either an STI diagnosis or to switch contraceptive method, intervention group participants receive counseling reinforcing key messages about DP in addition to counseling provided according the standard of care. For contraceptive changes specifically, participants receive specialized counseling that attempts to assess and address her reasons for dissatisfaction with her current method, and if indicated, assist the participant in choosing the alternate DP strategy that is most effective while meeting her individual needs and desires. When possible, the participants see the same provider they saw during their enrollment visit.
Participant communication
Loss to follow up is a major issue affecting the power and validity of RCTs [54, 55]. This is especially true when the intervention plans to follow an adolescent population, as borne out by previous efforts to test interventions that promote dual method use [41]. As such, this study uses a broad spectrum of communication options, according to the participant’s preference, to establish and maintain contact with participants and reduce loss to follow up. These include texting, email, phone calls and social media (e.g. Facebook, Kik, etc.). Many of these methods are mobile phone based, taking advantage of teens’ high levels of mobile phone ownership and engagement [56].
Statistical methods
Power analysis and sample size
We plan to enroll 710 participants, randomized in a 1:1 ratio to the intervention arm and the control arm. This study is powered based on the number of participants needed to compare the incidence of unintended pregnancy, chlamydia, gonorrhea, and trichomonas infection in the two study arms given the baseline incidence of these outcomes. The calculations use the method described by Lachin and Foulkes [57] for a two-sided log-rank test (α=0.05). Using an estimated loss to follow-up rate of 35% and an expected incidence of 35% (249 events) in the control arm, a total sample size of 710 would allow us to detect a hazard ratio of 0.65 with 80% power and two-sided. This would correspond to an incidence of 24.4% (173 events) in the intervention arm.
Analysis plan
Primary outcomes
An intent-to-treat analysis will be used to assess the effect of the intervention on each primary outcome, including selection of an effective DP strategy, self-reported consistent use of an effective DP strategy during the previous 3 months and first occurrence of pregnancy or an STD (chlamydia infection, gonorrhea infection or trichomonas infection). To evaluate the efficacy of the behavioral intervention in increasing selection of an effective DP strategy, compared to standard clinical practice, the endpoint measured will be selection of an effective DP strategy, defined as either abstinence, an effective non-barrier contraceptive method (hormonal contraceptive or copper IUD) used with condoms, or correct and consistent condom use only. The difference in the proportion of participants in each study arm selecting an effective DP strategy, as measured at each scheduled data collection call or visit (intervention, 3, 6, 9 and 12 months), will be tested using Pearson’s chi-square tests and modeled as a binary outcome using generalized linear mixed models to account for repeated measures and to evaluate confounding by demographic, behavioral, and clinical characteristics. It will also be modeled as a multinomial outcome to evaluate differences in the proportions of participants choosing each type of DP strategy.
To assess the intervention’s effect on adherence to an effective DP strategy, the proportions of participants self-reporting consistent use of an effective DP strategy during the last 3 month time period and those self-reporting use of DP strategy at last sex will be compared using Pearson’s chi-square tests at each scheduled follow-up data collection call or visit (3, 6, 9 and 12 months). The mean estimated proportion of time adherent will be calculated at each scheduled data collection time point and compared by study arm using Wilcoxon rank-sum tests. These endpoints will also be modeled using generalized linear mixed models to account for repeated measures and to evaluate for confounding.
To evaluate the efficacy of the behavioral intervention in decreasing incidence of pregnancy and STIs compared to standard clinical practice, we will use the time from study enrollment to the first occurrence of pregnancy, chlamydia infection, gonorrhea infection or trichomonas infection, as a single time-to-event outcome by date of lab test. These tests will occur at each scheduled follow-up visit (6 and 12 months), but may also occur at unscheduled interim visits. If participants have received STI and/or pregnancy tests elsewhere during the study period, study staff will request they fill out a medical records request to include in the analysis. A 2-sided log-rank test will be used to compare the incidence in the two arms. The Kaplan-Meier method will be used to estimate the cumulative incidence of a biologic event by 12 months in each study arm. Cox proportional hazards regression will be used to estimate a hazard ratio and confidence interval comparing the study arms. Numerous demographic, behavioral, and clinical variables will be collected through the ACASI questionnaire, and evaluated as potential confounders which may need to be included in a multivariable model to calculate an adjusted hazard ratio for the intervention.
Secondary outcomes
Secondary outcomes will include levels of knowledge related to contraception and STIs, intentions to use dual protection to prevent pregnancy and STIs, frequency of communication with partner on reproductive health topics, and self-efficacy related to contraceptive use; condom use; STI testing; and communication with partners. These outcomes will be measured at follow-up data collection calls and visits (at 3, 6, 9 and 12 months of follow up). Secondary outcomes will be assessed by study arm using appropriate summary measures (e.g., proportions, means, and percentiles), compared using Pearson’s chi-square tests for categorical outcomes or Wilcoxon rank-sum tests for continuous outcomes, and modeled using generalized linear mixed models to account for repeated measures.
Discussion
Previous studies of behavioral interventions designed to increase the use of DP (via dual method use exclusively or including consistent condom use) that have included US adolescents have consisted of an intervention at enrollment and again during follow up within the next two months. These interventions have shown promising effects on selection and short-term use of dual methods [41, 42, 44]. Despite this, those reporting long-term (12 months or longer) results have not consistently shown sustained effects [41, 42], and none have demonstrated an effect on biological outcomes (STIs and/or unintended pregnancy) [41–44], although they may have lacked a large enough sample to do so. While some of these trials focused on dual methods for DP, our study tries to achieve DP through a wider variety of methods. Ultimately, the goal of these interventions and of our own is to prevent unintended pregnancy and STI through dual protection.
The 2GETHER Project is designed to respond to the needs of the individuals in the community served, with the clinic becoming more comprehensive and engaged in addressing an individual participant’s needs and goals. The population our intervention targets is young, and their lives – along with the factors that influence their decisions regarding DP – are frequently changing. Important themes identified in formative research include the importance of relationship factors and experiential learning when making decisions about DP. These themes led to the creation of the MMDP and the hands-on and interactive nature of the intervention counseling.
This trial uses multiple components to provide relevant information and to promote the selection of an effective DP strategy. Instead of a discrete intervention inserted into a routine visit, the components of this intervention inform each interaction between various clinic providers and the patient, and they are personalized in response to information provided by participants. This patient-centered approach transforms the clinic experience by supporting the DP choice made by the participant, by being proactive in engaging with participants after the initial encounter, by using several approaches simultaneously, by maintaining consistent messaging throughout and by acknowledging that personal contexts that foster DP use can change over time, as can one’s DP strategy. It is designed to maintain the short-term improvements in DP strategy selection and adherence seen in prior studies and set the stage for long term prevention of pregnancy and STIs. This trial also benefits the community by expanding the clinic’s capacity to provide clinical care, irrespective of the efficacy of the studied intervention.
The multi-component nature of this intervention means that we do not have the ability to test whether any individual component is the driver for the measured effect of the overall intervention. However, the multi-component nature of the study is also its strength, as we expect that the components will work synergistically to improve reproductive health outcomes in the population studied. This intervention should be adaptable to other locations and populations. Individual components, especially the MMDP, but also the provider guides, are readily applicable to other clinics serving similar populations and can be adapted to other populations, although additional research would be necessary to demonstrate their efficacy. Other strengths of this study include the randomized controlled trial design and the enrollment of a well-defined, high-risk population. If recruitment goals are met, our study will have the largest cohort of high-risk young adults engaged in an intervention designed to promote dual protection. The comprehensive nature of the intervention and the sustained engagement with participants has the potential to effect long term improvements in the reproductive health of its target population as well as the sample size to demonstrate its significance.
There is a clear and urgent need to identify and test novel interventions designed to enhance DP uptake and its consistent use among African American adolescent females in the Southern United States, a population with particularly high rates of STIs and unintended pregnancies. If successful, this intervention can be applicable to other settings that serve African-American and other groups of adolescents at high risk, to enhance DP rates, and ultimately reduce unintended pregnancy and STI rates among this vulnerable population.
Trial status
This trial is currently open and in active enrollment.
Abbreviations
- ACASI
Audio Computer Assisted Self-Interviewing
- DMPA
Depot Medroxyprogesterone Acetate
- DP
Dual Protection
- HIV
Human Immunodeficiency Virus
- IUD
Intrauterine Device
- LARC
Long Acting Reversible Contraceptive
- MMDP
Multi-media dual protection interactive program
- OCP
Oral Contraceptive Pill
- STI
Sexually Transmitted Infection
Footnotes
CDC Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843–52. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Angelo DV, Gilbert BC, Rochat RW, Santelli JS, Herold JM. Differences between mistimed and unwanted pregnancies among women who have live births. Perspectives on sexual and reproductive health. 2004;36:192–7. doi: 10.1363/psrh.36.192.04. [DOI] [PubMed] [Google Scholar]
- 3.Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the well-being of children and families. National Academies Press; 1995. [PubMed] [Google Scholar]
- 4.Baydar N. Consequences for children of their birth planning status. Fam Plann Perspect. 1995:228–45. [PubMed] [Google Scholar]
- 5.Joyce TJ, Kaestner R, Korenman S. The effect of pregnancy intention on child development. Demography. 2000;37:83–94. [PubMed] [Google Scholar]
- 6.Moore KA, Myers DE, Morrison DR, Nord CW, Brown B, Edmonston B. Age at first childbirth and later poverty. J Res Adolesc. 1993;3:393–422. doi: 10.1207/s15327795jra0304_5. [DOI] [PubMed] [Google Scholar]
- 7.Leadbeater BJ. School outcomes for minority-group adolescent mothers at 28 to 36 months postpartum: a longitudinal follow-up. J Res Adolesc. 1996 [PubMed] [Google Scholar]
- 8.Forhan SE, Gottlieb SL, Sternberg MR, Xu F, Datta SD, McQuillan GM, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics. 2009;124:1505–12. doi: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]
- 9.Wiesenfeld HC, Sweet RL, Ness RB, Krohn MA, Amortegui AJ, Hillier SL. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32:400–5. doi: 10.1097/01.olq.0000154508.26532.6a. [DOI] [PubMed] [Google Scholar]
- 10.Tjiam KH, Zeilmaker GH, Alberda TA, van Heijst BY, de Roo JC, Polak-Vogelzang AA, et al. Prevalence of antibodies to Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma hominis in infertile women. Genitourin Med. 1985;61:175–8. doi: 10.1136/sti.61.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunham RC, Maclean IW, Binns B, Peeling RW. Chlamydia trachomatis: Its role in tubal infertility. J Infect Dis. 1985;152:1275–82. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- 12.Bouyer J, Coste J, Shojaei T, Pouly J-L, Fernandez H, Gerbaud L, et al. Risk Factors for Ectopic Pregnancy: A Comprehensive Analysis Based on a Large Case-Control, Population-based Study in France. Am J Epidemiol. 2003;157:185–94. doi: 10.1093/aje/kwf190. [DOI] [PubMed] [Google Scholar]
- 13.Weström L. Influence of sexually transmitted diseases on sterility and ectopic pregnancy. Obstet Gynecol Surv. 1986;41:56–7. [PubMed] [Google Scholar]
- 14.Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of Pathology. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: Results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Topics in HIV medicine : a publication of the International AIDS Society, USA. 2004;12:104–7. [PubMed] [Google Scholar]
- 17.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2013. Natl Vital Stat Rep. 2015:1–68. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. U.S. Department of Health and Human Services; Atlanta, GA: 2011. [Google Scholar]
- 19.Torrone E, Papp J, Weinstock H, Control CfD, Prevention Prevalence of chlamydia trachomatis genital infection among persons aged 14–39 years–United States, 2007–2012. MMWR Morb Mortal Wkly Rep. 2014;63:834–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Kost K, Henshaw S, Carlin L. US teenage pregnancies, births and abortions: National and state trends and trends by race and ethnicity. NY: Guttmacher Institute; 2010. [Google Scholar]
- 21.Frost JJ, Darroch JE, Remez L. Improving contraceptive use in the United States. Issues in brief (Alan Guttmacher Institute) 2007:1–8. [PubMed] [Google Scholar]
- 22.Martinez G, Copen CE, Abma JC. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2006–2010 national survey of family growth. Vital Health Stat. 2011;23:1–35. [PubMed] [Google Scholar]
- 23.Fu H, Darroch JE, Haas T, Ranjit N. Contraceptive failure rates: new estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999:56–63. [PubMed] [Google Scholar]
- 24.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004;82:454–61. [PMC free article] [PubMed] [Google Scholar]
- 25.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavanaugh ML, Jerman J, Finer LB. Changes in Use of Long-Acting Reversible Contraceptive Methods Among U.S. Women, 2009–2012. Obstet Gynecol. 2015;126:917–27. doi: 10.1097/AOG.0000000000001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. US Department of Health and Human Services; 2006. [PubMed] [Google Scholar]
- 28.Gavin L, Moskosky S, Carter M, Curtis K, Glass E, Godfrey E. Providing quality family planning services. MMWR. 2014;63:1–54. [PubMed] [Google Scholar]
- 29.Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2012;120:983–8. doi: 10.1097/AOG.0b013e3182723b7d. [DOI] [PubMed] [Google Scholar]
- 30.Ott MA, Sucato GS, Braverman PK, Adelman WP, Alderman EM, Breuner CC, et al. Contraception for adolescents. Pediatrics. 2014;134:e1257–e81. doi: 10.1542/peds.2014-2300. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: A prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577–82. doi: 10.1016/s0002-9378(98)70047-x. [DOI] [PubMed] [Google Scholar]
- 32.Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007;76:267–72. doi: 10.1016/j.contraception.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Mestad R, Secura G, Allsworth JE, Madden T, Zhao Q, Peipert JF. Acceptance of long-acting reversible contraceptive methods by adolescent participants in the Contraceptive CHOICE Project. Contraception. 2011;84:493–8. doi: 10.1016/j.contraception.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JA. Celebration Meets Caution: Long Acting Reversible Contraception (LARC)’s Boons, Potential Busts, and the Benefits of a Reproductive Justice Approach. Contraception. 2014;89:237–41. doi: 10.1016/j.contraception.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez AM, Fuentes L, Allina A. Women or LARC First? Reproductive Autonomy and the Promotion of Long-Acting Reversible Contraceptive Methods. Perspectives on sexual and reproductive health. 2014;46:171–5. doi: 10.1363/46e1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santelli JS, Kouzis AC, Hoover DR, Polacsek M, Burwell LG, Celentano DD. Stage of behavior change for condom use: the influence of partner type, relationship and pregnancy factors. Fam Plann Perspect. 1996:101–7. [PubMed] [Google Scholar]
- 37.Kaplan D, Feinstein R, Fisher M, Klein J, Olmedo L, Rome E, et al. Condom use by adolescents. Pediatrics. 2001;107:1463–9. doi: 10.1542/peds.107.6.1463. [DOI] [PubMed] [Google Scholar]
- 38.Darney PD, Callegari LS, Swift A, Atkinson ES, Robert AM. Condom practices of urban teens using Norplant contraceptive implants, oral contraceptives, and condoms for contraception. Am J Obstet Gynecol. 1999;180:929–37. doi: 10.1016/s0002-9378(99)70664-2. [DOI] [PubMed] [Google Scholar]
- 39.Santelli JS, Davis M, Celentano DD, Crump AD, Burwell LG. Combined use of condoms with other contraceptive methods among inner-city Baltimore women. Fam Plann Perspect. 1995;27:74–8. [PubMed] [Google Scholar]
- 40.Pazol K, Kramer MR, Hogue CJ. Condoms for dual protection: patterns of use with highly effective contraceptive methods. Public Health Rep. 2010;125:208–17. doi: 10.1177/003335491012500209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roye C, Perlmutter Silverman P, Krauss B. A brief, low-cost, theory-based intervention to promote dual method use by black and Latina female adolescents: a randomized clinical trial. Health Educ Behav. 2007;34:608–21. doi: 10.1177/1090198105284840. [DOI] [PubMed] [Google Scholar]
- 42.Peipert JF, Redding CA, Blume JD, Allsworth JE, Matteson KA, Lozowski F, et al. Tailored intervention to increase dual-contraceptive method use: a randomized trial to reduce unintended pregnancies and sexually transmitted infections. Am J Obstet Gynecol. 2008;198:630.e1–e8. doi: 10.1016/j.ajog.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen R, Albright J, Garrett JM, Curtis KM. Pregnancy and STD prevention counseling using an adaptation of motivational interviewing: a randomized controlled trial. Perspect Sex Reprod Health. 2007;39:21–8. doi: 10.1363/3902107. [DOI] [PubMed] [Google Scholar]
- 44.Exner TM, Mantell JE, Hoffman S, Adams-Skinner J, Stein ZA, Leu C-S. Project REACH: a provider-delivered dual protection intervention for women using family planning services in New York City. AIDS Care. 2011;23:467–75. doi: 10.1080/09540121.2010.516335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peipert JF, Zhao Q, Meints L, Peipert BJ, Redding CA, Allsworth JE. Adherence to dual-method contraceptive use. Contraception. 2011;84:252–8. doi: 10.1016/j.contraception.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray CC, Hatfield-Timajchy K, Kraft JM, Bergdall AR, Habel MA, Kottke M, et al. In their own words: Romantic relationships and the sexual health of young African American women. Public Health Rep. 2013;128:33–42. doi: 10.1177/00333549131282S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottke M, Whiteman MK, Kraft JM, Goedken P, Wiener J, Kourtis AP, et al. Use of Dual Methods for Protection from Unintended Pregnancy and Sexually Transmitted Diseases in Adolescent African American Women. J Pediatr Adolesc Gynecol. 2015 doi: 10.1016/j.jpag.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gribble JN, Miller HG, Rogers SM, Turner CF. Interview mode and measurement of sexual behaviors: Methodological issues. J Sex Res. 1999;36:16–24. doi: 10.1080/00224499909551963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metzger DS, Koblin B, Turner C, Navaline H, Valenti F, Holte S, et al. Randomized controlled trial of audio computer-assisted self-interviewing: Utility and acceptability in longitudinal studies. Am J Epidemiol. 2000;152:99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 50.Tourangeau R, Smith TW. Asking sensitive questions: The impact of data collection mode, question format, and question context. Public Opin Q. 1996;60:275–304. [Google Scholar]
- 51.Gavin L, Moskosky S, Carter M, Curtis K, Glass E, Godfrey E, et al. Providing quality family planning services. MMWR Morbidity & Mortality Weekly Report. 2014;63:1–54. [PubMed] [Google Scholar]
- 52.Program Requirements for Title X Funded Family Planning Projects. Department of Health and Human Services. Office of Population Affairs; 2014. [Google Scholar]
- 53.Miller WR. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. DIANE Publishing; 1995. [Google Scholar]
- 54.Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ. 2012;344:e2809. doi: 10.1136/bmj.e2809. [DOI] [PubMed] [Google Scholar]
- 55.Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child. 2008;93:458–61. doi: 10.1136/adc.2007.127316. [DOI] [PubMed] [Google Scholar]
- 56.Lenhart A. Pew Research Center: Teen, Social Media and Technology Overview 2015. 2015 [Google Scholar]
- 57.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42:507–19. [PubMed] [Google Scholar]