Abstract
Purpose
The Prostate Cancer Prevention Trial prostate cancer risk calculator was developed in a clinical trial cohort that does not represent men routinely referred for prostate biopsy. We assessed the generalizability of the Prostate Cancer Prevention Trial calculator in a cohort more representative of patients referred for consideration of prostate biopsy in American urology practice.
Materials and Methods
Patients undergoing prostate biopsy by 12 urologists at 5 sites were enrolled in an Early Detection Research Network cohort. The Prostate Cancer Prevention Trial risk calculator was validated by examining area underneath the receiver operating characteristic curve, sensitivity, specificity and calibration comparing observed vs predicted risk of prostate cancer detection.
Results
Cancer incidence was greater (43% vs 22%, p = 0.001) in the Early Detection Research Network validation cohort (645) compared to the Prostate Cancer Prevention Trial group (5,519). Early Detection Research Network participants were younger and more racially diverse, and had more abnormal digital rectal examinations and higher prostate specific antigen than Prostate Cancer Prevention Trial participants (all p <0.001). Cancer severity was worse in the Early Detection Research Network cohort than in the Prostate Cancer Prevention Trial (Gleason 7 or higher 60% vs 21%, p <0.001). Nevertheless, the Prostate Cancer Prevention Trial risk calculator was superior to prostate specific antigen alone for predicting cancer in the Early Detection Research Network (AUC 0.691 vs 0.655, p = 0.009) and calibration confirmed that the Prostate Cancer Prevention Trial risk score accurately predicted individual risks in the Early Detection Research Network cohort.
Conclusions
Differences between the Early Detection Research Network validation cohort and the Prostate Cancer Prevention Trial cohort underscore the importance of validating calculator performance in the multicenter urology practice setting. Our findings extend the applicability of the Prostate Cancer Prevention Trial calculator for measuring the risk of prostate cancer detection on biopsy to the routine American urology practice setting.
Keywords: biological markers, early detection of cancer, mass screening, prostate-specific antigen, clinical trials as topic
While digital rectal examination and PSA are commonly used in determining who may be at risk for prostate cancer and should undergo prostate biopsy, use of these tests alone can expose patients to unnecessary biopsy.1,2 Traditionally PSA has been treated as a dichotomous value with a specific cutoff point (PSA greater than 4.0 ng/ml or greater than 2.5 ng/ml), leading to a recommendation of prostate biopsy.3–5 Previously data from the PCPT were used to develop a prostate cancer risk calculator wherein PSA was considered with other factors including DRE results, age, race and family history of prostate cancer in a multivariable model predicting the probability of prostate cancer in an individual patient.6
A limitation of the PCPT risk calculator is that it was developed from a cohort of men with a PSA of 3.0 ng/ml or less, normal DRE and age 55 years or older at study enrollment.7 As a result the validity of the PCPT risk calculator to assess prostate cancer risk in men commonly referred for urological evaluation of abnormal prostate screening (performed in the primary care setting) is uncertain. Most of these patients have an increased PSA and/or an abnormal DRE, and many are younger than 55 years. These men were not represented in the cohort from which the PCPT prostate cancer risk calculator was developed.6
Therefore, we determined if the PCPT risk calculator is applicable in patients referred from primary care to urology practices for the assessment of abnormal prostate cancer screening results. Thus, we measured performance and validity of the PCPT risk calculator in a prospective, multiple urology practice cohort of men referred for consideration of initial prostate biopsy.
MATERIALS AND METHODS
Subjects
Patients referred for prostate biopsy by 12 urologists at 5 urology clinical practice sites in Eastern Massachusetts and Southeastern Michigan were prospectively enrolled before prostate biopsy in an institutional review board approved, National Cancer Institute Early Detection Research Network cohort study designed to evaluate biomarkers and risk factors for prostate cancer detection. Eligibility for this analysis was limited to patients undergoing their first prostate biopsy (645). Patients were enrolled after providing informed consent and before undergoing prostate biopsy. Findings on transrectal ultrasound guided biopsy and subsequent prostate biopsy histopathology results were collected.
Analyses
Evaluation of PCPT risk calculator performance was assessed by area under the receiver operating characteristic curve, sensitivity, specificity, and by calibration comparing PCPT calculator predicted vs observed risk of detecting prostate cancer on biopsy. Comparisons of baseline variables between the PCPT and EDRN cohorts were performed by the Wilcoxon test for continuous variables and by Fisher’s exact test or the chi-square test for categorical variables. Comparisons between PSA and the PCPT risks were tabulated in terms of operating characteristics of diagnostic tests including sensitivity, specificity, ROC and AUC. Sensitivity was defined as the proportion of cancer cases with diagnostic tests exceeding a cutoff, specificity as the proportion of noncancer cases with diagnostic tests less than or equal to the cutoff, the ROC curve as a plot of the false-positive rate (1-specificity) on the x-axis vs sensitivity on the y-axis for all cutoffs. AUCs were calculated as the Wilcoxon statistic, and statistical tests of differences between AUCs for the different diagnostic tests were performed via the nonparametric U-statistic method.8 Comparisons of sensitivities and specificities between tests were performed via asymptotic normal Wald statistics for comparison of 2 binomial proportions on the same sample.
Calibration was performed by computing PCPT risks of prostate cancer for each individual and comparing them to observed rates of prostate cancer in several ways. The average PCPT risk in the sample was compared to the proportion of prostate cancer cases, with calibration assessed as good if these 2 agreed. In addition, individuals were grouped according to deciles of PCPT risks observed in the validation set, and the proportions of patients with cancer (observed risks) in each group were compared to median PCPT risks in each group. The binomial formula was used to calculate 95% CI for all proportions and the sampling distribution of the mean to calculate a 95% CI for overall PCPT risk. The Pearson chi-square statistic was used to assess agreement between expected and observed prostate cancer rates according to PCPT risk deciles. Additional comparison between average PCPT risks and observed rates of prostate cancer were performed for subgroups. Cox’s measures of calibration and refinement were used by fitting univariate logistic regression of the cancer status of each individual to his PCPT risk.9,10 Calibration was considered good if the intercept equaled 0 and the slope for the PCPT risk equaled 1. Statistical tests were performed at the α = 0.05 (2-sided) level of statistical significance using the R statistical package (version 2.6.0, R Foundation for Statistical Computing).
RESULTS
Clinical Cohort
Characteristics of the EDRN clinical cohort are contrasted to those of the PCPT in table 1. A larger percentage of men in the EDRN cohort than in the PCPT cohort had abnormal DRE, increased PSA greater than 4.0 ng/ml or a family history of prostate cancer. The PCPT cohort was older, with nearly half of the men older than 70 years compared to less than 20% of the EDRN cohort, and less racially mixed than the EDRN cohort. The EDRN cohort contained more patients with cancer (43.4% vs 21.9% in the PCPT), proportionately more of whom had higher grade disease (60% high grade in the EDRN cohort vs 21.2% in the PCPT).
Table 1.
Participant characteristics
| EDRN | PCPT* | |||
|---|---|---|---|---|
| No. pts | 645 | 5,519 | ||
| No. pt age (%): | ||||
| 60 or Younger | 301 | (46.7) | 38 | (0.7) |
| 60–64 | 110 | (17.1) | 1,143 | (20.7) |
| 65–69 | 118 | (18.3) | 1,741 | (31.5) |
| 70 or Older | 116 | (18.0) | 2,597 | (47.1) |
| No. race (%): | ||||
| White | 559 | (86.7) | 5,276 | (95.6) |
| African-American | 47 | (7.3) | 175 | (3.2) |
| Other | 39 | (6.0) | 68 | (1.2) |
| No. family history (%):† | ||||
| No | 483 | (74.9) | 4,599 | (83.3) |
| Yes | 162 | (25.1) | 920 | (16.7) |
| No. ng/ml PSA (%): | ||||
| 2 or Less | 66 | (10.2) | 3,603 | (65.3) |
| 2–4 | 159 | (24.7) | 1,285 | (23.3) |
| Greater than 4 | 420 | (65.1) | 631 | (11.4) |
| No. DRE (%): | ||||
| Normal | 510 | (79.1) | 4,968 | (89.9) |
| Abnormal/suspicious | 135 | (20.9) | 551 | (10.1) |
| Median biopsy cores (% with indicated No. cores) | 12 | (81.1) | 6 | (84.5) |
| No. Ca (%) | 280 | (43.4) | 1,211 | (21.9) |
| No. Gleason 7–10 Ca (%) | 168 (26.0 overall, 60.0 Ca) | 257 (4.7 overall, 21.2 Ca) | ||
Differences between cohorts significant for each listed variable, p <0.0001 for each.
Number of biopsy cores in PCPT was proscribed by trial protocol.6
Whether a father, brother or son had prostate cancer.
Operating Characteristics
The AUC of the PCPT risk calculator for prostate cancer of 0.691 was a significant improvement in operating characteristics compared to PSA alone (AUC 0.655, p = 0.009) (see figure). Sensitivities and specificities at respective spectrums ranging from 80% to 98% are reported in tables 2 and 3. Improvement in predicting prostate cancer by the PCPT calculator compared to PSA alone is most notable at 80% specificity, where the sensitivity of the calculator is 47% compared to 35% for PSA alone.
Figure 1.
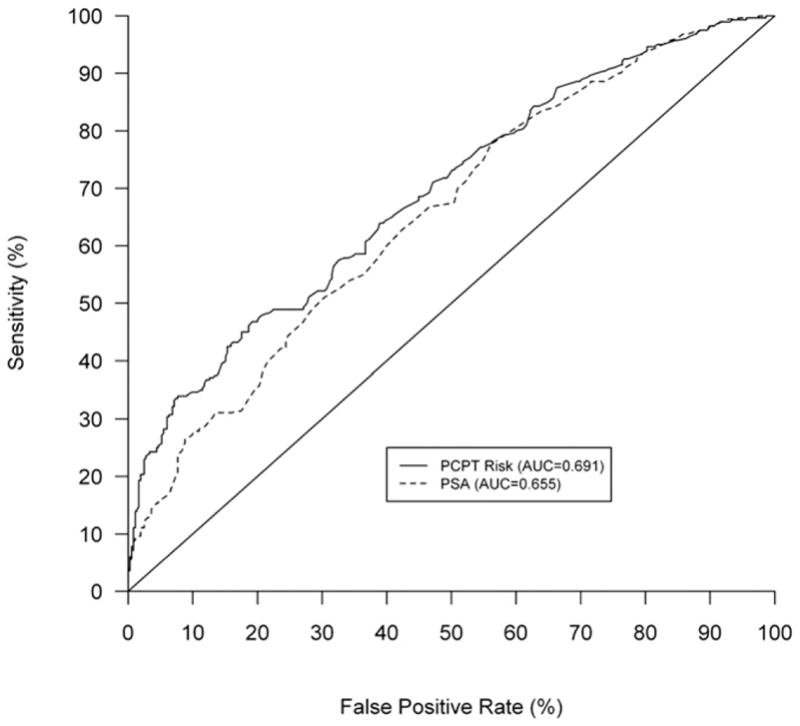
Performance of PCPT risk calculator compared to that of PSA alone for predicting prostate cancer in EDRN validation cohort depicted by ROC curve representation of sensitivity and specificity (AUC for PCPT calculator 0.691 vs AUC for PSA 0.655, p = 0.009).
Table 2.
Sensitivity for specificity
| 98% | 95% | 90% | 80% | |
|---|---|---|---|---|
| PCPT risk: | ||||
| Cutoff (%) | 66.3 | 61.7 | 54.8 | 48.4 |
| Sensitivity (%) | 20.4 | 25.4 | 34.6 | 47.1 |
| PSA: | ||||
| Cutoff (ng/ml) | 13.5 | 10.2 | 7.9 | 6.9 |
| Sensitivity (%) | 11.1 | 15.7 | 27.1 | 35.4 |
Above cutoff indicates a positive test.
Table 3.
Specificity for sensitivity
| 80% | 90% | 95% | 98% | |
|---|---|---|---|---|
| PCPT risk: | ||||
| Cutoff (%) | 38.0 | 33.9 | 30.8 | 26.3 |
| Specificity (%) | 40.3 | 28.5 | 18.6 | 10.1 |
| PSA: | ||||
| Cutoff (ng/ml) | 4.0 | 3.0 | 2.4 | 1.3 |
| Specificity (%) | 44.1 | 26.3 | 18.6 | 10.4 |
Above cutoff indicates a positive test.
Calibration
The average PCPT risk value for the 645 men in the EDRN cohort was 45.1% and closely agreed with the proportion of patients with cancer in the cohort (43.4%). Table 4 shows that the PCPT risk calculator was accurate for predicting risk across all patient subgroups. Risk was slightly underestimated in African-American men and men with a family history of prostate cancer. Underprediction vs overprediction of risk by the calculator occurred randomly across levels of risk (p = 0.10) (table 5). The logistic regression of prostate cancer on the log odds of the PCPT risk score revealed a nonstatistically significant intercept (α = −0.014, standard error 0.091, p = 0.87), confirming that the PCPT risks were appropriately calibrated to actual risks. The gradient for the log odds of PCPT risk was not statistically significantly different from unity (α = 1.291, standard error 0.159, p <0.0001), indicating that PCPT risks showed the correct trend of increasing with increasing actual risk.
Table 4.
Comparison of PCPT risks to actual risks in subgroups of the EDRN cohort
| No. | Prostate Ca (%) | Av PCPT Risk (%) | AUC (95% CI) | |
|---|---|---|---|---|
| All | 645 | 43.4 | 45.1 | 0.691 (0.650–0.732) |
| PSA 4 ng/ml or less | 224 | 28.1 | 33.6 | 0.638 (0.558–0.718) |
| PSA greater than 4 ng/ml | 421 | 51.5 | 51.1 | 0.656 (0.604–0.708) |
| Normal DRE | 510 | 39.8 | 41.8 | 0.622 (0.573–0.672) |
| Abnormal DRE | 135 | 57.0 | 57.4 | 0.839 (0.771–0.908) |
| White | 559 | 44.0 | 45.1 | 0.694 (0.650–0.737) |
| African-American | 47 | 51.1 | 45.4 | 0.697 (0.541–0.852) |
| Age 65 yrs or older | 234 | 51.3 | 49.9 | 0.649 (0.579–0.720) |
| Age younger than 65 yrs | 411 | 38.9 | 42.3 | 0.705 (0.654–0.755) |
| Family history | 162 | 50.0 | 46.8 | 0.650 (0.566–0.734) |
| No family history | 483 | 41.2 | 44.5 | 0.699 (0.651–0.746) |
Table 5.
Comparison of risks predicted by PCPT calculator to observed risk of prostate cancer in EDRN cohort
| % PCPT Predicted Risk Range* | No. EDRN Cohort With PCPT Risk in Range | % EDRN Cohort (actual observed) With Prostate Ca |
|---|---|---|
| 4.1–29.1 | 67 | 17.9 |
| 29.1–34.0 | 66 | 25.8 |
| 34.0–37.4 | 64 | 40.6 |
| 37.4–40.6 | 67 | 35.8 |
| 40.6–42.8 | 60 | 36.7 |
| 42.8–46.1 | 64 | 51.6 |
| 46.1–49.2 | 63 | 31.7 |
| 49.2–54.9 | 66 | 47.0 |
| 54.9–66.1 | 63 | 60.3 |
| 66.1–98.2 | 65 | 87.7 |
Greater than lower end of interval, less than or equal to upper end.
DISCUSSION
The PCPT prostate cancer risk calculator was developed based on 5,519 men from the placebo group of the PCPT, all of whom had prostate biopsy. The original cohort used to develop the PCPT calculator was limited to men in the control arm of the PCPT trial, who (based on trial eligibility) had no history of prostate cancer, were 55 years old or older, and had a normal DRE and a PSA of 3.0 ng/ml or less at study enrollment.6 The calculator (http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp) includes race/ethnicity, age, PSA, family history of prostate cancer, DRE and results of prior prostate biopsy as risk factors shown to have independent predictive value for predicting prostate cancer diagnosis.6,11
We assessed the PCPT calculator validity for clinical decisions in urology practices where men are considered for possible prostate biopsy due to abnormal screening results or other findings that precede referral to urology. The EDRN cohort better represented this clinical setting than did the PCPT cohort in 4 distinct and clinically relevant features that are related to the strict exclusion criteria of PCPT. 1) The PCPT excluded from study men younger than 55 years, and only 0.7% was younger than 60 years at biopsy, whereas 47% of patients in the EDRN cohort were younger than 60 years. 2) The PCPT excluded men with a PSA greater than 4.0 ng/ml or abnormal DRE at study initiation. Thus, only 11% of patients in the PCPT vs 65% in the EDRN cohort had a PSA before biopsy greater than 4.0 ng/ml. 3) Due to these PCPT exclusions the rate of cancer detection in the PCPT was 22%, whereas 43% of the EDRN cohort had cancer. In addition, a larger percentage of those patients diagnosed with cancer in the EDRN cohort (60%) than in the PCPT (21%) had Gleason score 7 or greater disease. 4) Patients in the PCPT were evaluated by sextant biopsy, whereas the EDRN cohort was evaluated by extended biopsy (usually 12 cores), which is more representative of contemporary urology practice. Despite these notable differences in patient characteristics between the PCPT cohort (based on which the risk calculator was developed) and men routinely referred for consideration of prostate biopsy as represented in the EDRN cohort, the PCPT calculator was accurate in predicting the risk of prostate cancer, attesting to its generalizability.
The relevance of tools such as the PCPT risk calculator for refining decision making has been increased by recent clinical trials whose findings suggest that routine use of PSA cutoffs has a limited survival benefit.1,2 Comparison of our validation EDRN cohort to the screening arms of these trials reveals greater similarities of our cohort with the screening arm of the ERSPC (where survival benefit was evident) than with the PLCO. In the screening arm of PLCO only 8% of subjects had a PSA less than 4 ng/ml, 61% with abnormal DRE and PSA underwent biopsy and there was no survival benefit with screening (although screening contamination in the control arm may have confounded the results).12 In contrast, the ERSPC showed a cumulative incidence of prostate cancer of 8.2% in the screening group and 4.8% in the control group, and an absolute risk difference of 0.71 deaths per 1,000 men.2 In the ERSPC although PSA based screening reduced the rate of death from prostate cancer by 20%, use of an absolute PSA cutoff was associated with a high risk of over diagnosis (requiring 1,410 men to be screened and an additional 48 with prostate cancer to be treated to prevent 1 death from prostate cancer). Although different from the PSA distribution in the PCPT, of interest the PSA distribution in the EDRN validation cohort was similar to that in the ERSPC, with 25% of ERSPC and 35% of EDRN participants having PSA less than 4 ng/ml (table 1).13 The ERSPC and PLCO studies in combination demonstrate the need to use additional factors beyond PSA and DRE alone in deciding whom to biopsy, and the PCPT risk calculator represents one such approach of combining PSA and DRE with other associated risk factors.
Despite these indications from the ERSPC and PLCO that absolute PSA thresholds may be suboptimal for population based screening decisions, few multicenter studies have developed and validated multivariable models for predicting the presence or absence of prostate cancer on biopsy.14–17 Karakiewicz et al developed and externally validated a nomogram to predict biopsy outcome in a cohort of men who underwent sextant biopsy in Hamburg, Germany and Montreal, Canada.15 However, subsequent studies questioned the generalizability of models developed in sextant biopsy settings to contemporary, more extended biopsy.14,16 Models predicting the probability of cancer on sextant biopsies in the Tyrol screening study did not consistently ascertain the significance of PSA in the model when validated in 2 American sites where a more extended (10-core) biopsy was used.17 Our validation study, in contrast, validates the PCPT calculator (that was developed in a cohort evaluated using sextant biopsy) in a cohort evaluated using extended, 12-core biopsy.18 A model for predicting prostate cancer risk based on patients biopsied in the ERSPC included only PSA, DRE and transrectal ultrasound findings to predict cancer risk, and neither family history retained an effect in the ERSPC model.13 To our knowledge the PCPT calculator is unique among predictive models validated in multi-center studies in having evaluated and confirmed a significant contribution of race and family history, in addition to PSA and findings on DRE.
The performance of the PCPT risk calculator in this EDRN validation cohort, as measured by AUC of the ROC (0.691), compares favorably to that found by Parekh et al, who reported an AUC of 0.655 in the relatively young, Hispanic dominated San Antonio Center for Biomarkers of Risk cohort.19 We observed a statistically significant improvement in operating characteristics of the PCPT risk calculator compared to PSA. The PCPT calculator appears advantageous compared to PSA alone by allowing improved sensitivity at higher levels of specificity, with an increase in sensitivity of 8% to 12% at cutoffs having 80% to 98% specificity (tables 2 and 3). However, there remains a need to develop predictive tools that would improve specificity at the upper range of sensitivity, when the PCPT calculator does not outperform PSA (table 3).
Our validation of the PCPT prostate cancer risk calculator in this multicenter EDRN cohort has limitations. Although the EDRN cohort was less dominated by white men than the PCPT (87% in EDRN vs 96% in PCPT), the representation of African-American men in the EDRN cohort was relatively low. Nevertheless, the EDRN cohort had a sufficient number of African-American men to verify that the PCPT risk calculator is calibrated for this risk group. In addition, the EDRN cohort was composed of patients referred to urologists to undergo biopsy based on abnormal prostate cancer screening results or other concerns and, thus, does not explore the validity of the calculator to assess prostate cancer risk in patients without abnormal DRE and with normal PSA as would be encountered in the primary care setting. However, the PCPT cohort represents just such a primary care setting. Validation of the calculator in the urology care setting complements the normal prostate screening setting of PCPT. Finally, the PCPT calculator and this validation do not take into account the possible usefulness of % free PSA, which may help further substratify prostate cancer risk,20 nor the usefulness of PSA velocity, which is the subject of considerable debate as an additional marker of risk.21–23
CONCLUSIONS
If expected longevity is adequate at patient evaluation, a biopsy has been generally recommended when a patient has a PSA greater than 2.5 ng/ml or 4.0 ng/ml, or a DRE suspicious for cancer. The PCPT risk calculator further specifies an individual patient specific overall risk of having prostate cancer by including other risk factors in addition to PSA and DRE. By more specifically substratifying prostate cancer risk the PCPT calculator may facilitate individualized decision making about whether to perform a biopsy. Our validation of the PCPT calculator in a multicenter cohort of men referred to urology practices for evaluation of abnormal prostate cancer screening indicates that the calculator can be reliably used in general urology practice to determine prostate cancer risk and thereby facilitate decisions regarding prostate biopsy.
Acknowledgments
Supported by the Early Detection Research Network, National Cancer Institute, National Institutes of Health Grant U01-CA113913 and U01-CA86402, and the San Antonio Cancer Institute (P30-CA54174).
Drs. William Dewolf, Andrew Wagner, Gary Kearney, Robert Eyre, Paul Church, Abraham Morgentaler, Steven Lazarou, Stephanie Meyers and Doug Scherr assisted in the enrollment of study subjects.
Abbreviations and Acronyms
- DRE
digital rectal examination
- EDRN
Early Detection Research Network
- ERSPC
European Randomized Screening for Prostate Cancer trial
- PCPT
Prostate Cancer Prevention Trial
- PLCO
Prostate, Lung, Colon, and Ovarian Screening Trial
- PSA
prostate specific antigen
References
- 1.Andriole GL, Crawford D, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 5.Punglia RS, D’Amico AV, Catalona WJ, et al. Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med. 2003;349:335. doi: 10.1056/NEJMoa021659. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 8.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837. [PubMed] [Google Scholar]
- 9.Cox DR. Two further applications of a model for binary regression. Biometrika. 1988;45:562. [Google Scholar]
- 10.Miller ME, Hui SL, Tierney WM. Validation techniques for logistic regression models. Stat Med. 1991;10:1213. doi: 10.1002/sim.4780100805. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Pauler Ankerst D, Chi C, et al. Prediction of prostate cancer for patients receiving finasteride: results from the Prostate Cancer Prevention Trial. J Clin Oncol. 2007;25:3076. doi: 10.1200/JCO.2006.07.6836. [DOI] [PubMed] [Google Scholar]
- 12.Andriole GL, Levin DL, Crawford ED, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst. 2005;97:433. doi: 10.1093/jnci/dji065. [DOI] [PubMed] [Google Scholar]
- 13.van den Bergh RC, Roobol MJ, Wolters T, et al. The Prostate Cancer Prevention Trial and European Randomized Study of Screening for Prostate Cancer risk calculators indicating a positive prostate biopsy: a comparison. BJU Int. 2008;102:1068. doi: 10.1111/j.1464-410X.2008.07940.x. [DOI] [PubMed] [Google Scholar]
- 14.Shariat SF, Karakiewicz PI, Margulis V, et al. Inventory of prostate cancer predictive tools. Curr Opin Urol. 2008;18:279. doi: 10.1097/MOU.0b013e3282f9b3e5. [DOI] [PubMed] [Google Scholar]
- 15.Karakiewicz PI, Benayoun S, Kattan MW, et al. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J Urol. 2005;173:1930. doi: 10.1097/01.ju.0000158039.94467.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun FK, Briganti A, Graefen M, et al. Development and external validation of an extended 10-core biopsy nomogram. Eur Urol. 2007;52:436. doi: 10.1016/j.eururo.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Porter CR, Gamito EJ, Crawford ED, et al. Model to predict prostate biopsy outcome in large screening population with independent validation in referral setting. Urology. 2005;65:937. doi: 10.1016/j.urology.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4. 0 ng per milliliter. N Engl J Med. 2004;350:2239. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 19.Parekh DJ, Ankerst DP, Higgins BA, et al. External validation of the Prostate Cancer Prevention Trial risk calculator. Urology. 2006;68:1152. doi: 10.1016/j.urology.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 21.Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215. [PMC free article] [PubMed] [Google Scholar]
- 22.D’Amico AV, Chen MH, Roehl KA, et al. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 23.Etzioni RD, Ankerst DP, Weiss NS, et al. Is prostate-specific antigen velocity useful in early detection of prostate cancer? A critical appraisal of the evidence. J Natl Cancer Inst. 2007;99:1510. doi: 10.1093/jnci/djm171. [DOI] [PubMed] [Google Scholar]


