Abstract
Aim:
The aim of this study is to investigate the physical properties of conventional and resin-modified glass ionomer cements (GICs) compared to GICs supplemented with zinc oxide (ZnO) nanofiller particles at 5% (w/w).
Methods:
In this in vitro study, ZnO nanoparticles of different morphologies (nanospherical, nanorod, and nanoflower) were incorporated to glass ionomer powder. The samples were subjected to the flexural strength (n = 20) and surface hardness test (n = 12) using a universal testing machine and a Vickers hardness machine, respectively. Surface analysis and crystal structure of samples were performed with scanning electron microscope and X-radiation diffraction, respectively. The data were analyzed using one-way ANOVA, Shapiro–Wilk, and Tukey's tests (P < 0.05).
Results:
Flexural strength of glass ionomer containing nanoparticles was not significantly different from the control group (P > 0.05). The surface hardness of the glass ionomer containing nanospherical or nanoflower ZnO was significantly lower than the control group (P < 0.05). However, the surface hardness of glass ionomer containing nanorod ZnO was not significantly different from the control group (P = 0.868).
Conclusions:
Incorporation of nanospherical and nanoflower ZnO to glass ionomer decreased their surface hardness, without any changes on their flexural strength. Incorporation of nanorod ZnO particles caused no effect on the mechanical properties.
Keywords: Glass ionomer cements, mechanical properties, nanoparticles, zinc oxide
INTRODUCTION
Glass ionomer cements (GICs) are polymer-based composites invented in the early 1970s.[1] These cements have been become popular due to their fluoride releasing potential, adhesion to tooth structure anticariogenic properties, thermal compatibility, translucency, biocompatibility, and low toxicity which have to lead as luting, base, liners, and restorative materials[1,2,3,4,5,6] However, they have low fracture toughness.[7] Since the introduction of GICs, several alterations including the adjustments of powder/liquid ratio, modifications in the powder's formulation, and incorporation of filler-like particles and fibers[1] have been performed to increase their mechanical properties.[2,3]
The incorporation of the nanoparticle fillers to GICs has used to increase their mechanical and antibacterial properties. Zinc oxide (ZnO) as an important multifunctional semiconductor filler has many promising properties, namely, chemical stability, biocompatibility, and intrinsic (photo) catalytic bactericidal activity.[8,9,10,11] In addition, it has been reported that it showed nontoxic activity to human in low concentrations and is cost-effective.[10,11] Moreover, ZnO is extensively used in the formulations of oral hygiene products, especially toothpastes and mouth rinses, due to its antimicrobial and antibacterial properties against Streptococcus mutans and periodontal pathogens.[12]
These properties become more prominent following the application of nanosized ZnO; the higher the surface-to-volume ratio leads to the greater interaction of particles and organic molecules.[12,13] In this regard, incorporation of the ZnO nanoparticles to GICs can improve its physicomechanical properties as well as antibacterial activity.[14]
It has been reported that morphology of nanoparticle fillers is an important criterion which affects the mechanical properties[4] of GICs. The objective of this research was to investigate the surface hardness and flexural strength of restorative GIC modified with ZnO nanoparticle fillers in the form of nanospherical, nanorod, and nanoflower particles.
METHODS
In this experimental in vitro study, ZnO nanoparticles with three different morphologies (nanospherical, nanorod, and nanoflower) were synthesized and incorporated to a proprietary GIC (Fuji II conventional GIC). The description of groups was as follows:
Group 1 – GIC without nanoparticles (control group)
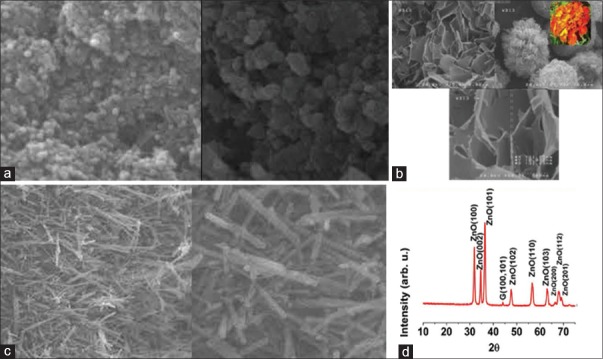
Group 2 – 5 wt% nanospherical ZnO particles were incorporated into GIC. The particles were measured < 100 nm in length, width, and height (mean size of 20 nm). They tended to stick together and form agglomerates [Figure 1a]
Group 3 – 5 wt% nanoflower ZnO particles were incorporated into GIC; these particles had a flower-shaped structure providing high surface area with low risk of agglomeration. The fabricated nanoflower particles had 2 nm petal thickness and 2 μ petal diameter [Figure 1b]
Group 4 – 5 wt% nanorod ZnO particles were incorporated into GIC. In these particles, one dimension was larger than the others. The rods had a round cross-section with <100 nm diameter. In this study, nanorods had a uniform thickness of <20 nm and length of over 1 μ [Figure 1c].
Figure 1.
(a) Scanning electron microscope micrograph of nanospherical ZnO particles; (b) scanning electron microscope micrograph of nanoflower zinc oxide particles; (c) scanning electron microscope micrograph of nanorod zinc oxide particles; (d) X-ray diffraction of zinc oxide nanoflowers
Preparation of zinc oxide nanostructures
Nanospherical and nanorods were synthesized by adding zinc acetate solution (0.1M) (Merck, Darmstadt, Germany) and sodium hydroxide solution (0.5M) (Merck, Darmstadt, Germany) through a hydrothermal method. A pH meter was placed in the zinc acetate solution, and sodium hydroxide solution was gradually added to prepare alkaline solutions (pH values = 9 and 11, respectively). The prepared solutions were autoclaved in screw-top vials at 150°C for 24 h. The synthesized powder was washed with deionized water for several times and dried at 100°C. Next, the samples were evaluated under a scanning electron microscope (SEM) to confirm the formation of ZnO nanospherical and ZnO nanorod particles.
For synthesis of nanoflower structure, urea was used as the surface agents to allow the growth of nanostructure in at least two dimensions; zinc nitrate (0.1M) (Zn [NO3]2.6H2O, 99.0%, Merck, Darmstadt, Germany) was dissolved in 25 mL of deionized distilled water and added to 20 mL of urea (0.1M) (CO [NH2]2, 99.99%, Merck, Darmstadt, Germany). Nanoflower ZnO particles were synthesized through the hydrothermal sedimentation process by autoclaving the mixture at 120°C for 6 h. The synthesized powder was washed with distilled water and dried at 100°C. The samples were then evaluated under SEM to ensure the formation of nanoflower particles.
A commercially available conventional cure GI restorative powder (Kavitan Plus; Lot number: 2015388, SpofaDental, Czech Republic) (code: GI) was blended in various proportions with TiO2 nanoparticles (Batch number: MKBC-4174, Sigma–Aldrich) with a particle size of 21 nm.
Preparation of samples
Experimental material powder was fabricated by mixing 5% (w/w) ZnO nanoparticles with the GIC powder. Nanoparticles powder and GIC powder were weighed on a digital scale (AL-104, Acculab, New York, USA) accurate to 0.0001 g. The restorative powder (totally 10% of the powder) and ZnO nanoparticles were mixed using a plastic spatula on a mixing paper. Next, the GIC powder was added again by twice the sum of the mixture (20% of the total amount of powder) to reach the weight of 5%. The recommended powder/liquid (P/L) ratio of 2.7/1 for Kavitan Plus was used in all of the prepared specimens.
Flexural strength
Twenty specimens (25 mm length × 2 mm thickness × 2 mm width) were prepared in rectangular-shaped stainless steel split mold (n = 5). The GIC powders of each group were mixed with its corresponding liquid according to the manufacturer's instructions. Subsequently, the mold was placed between two glass slides and was filled by the mixture. Two Mylar strips were used to prevent GIC from sticking to glass slides. A slight pressure was applied to extrude excess materials. After 15 min storage in 37°C and 80% relative humidity, the specimens were removed from the mold and stored in distilled water at 37°C for 24 h (Peco PL-455, Pooya Electronic Co., Iran).
Prior testing, the dimensions of the samples were measured by a digital caliper with 0.01 mm accuracy. The flexural strength test was performed based on the International Organization for Standardization standard 4049 using a universal testing machine (STM-20, Zwick Roell, Ulm, Germany) at a crosshead speed of 0.5 mm/min. Flexural strength was calculated using the following formula:
Σ =3FL/2bh2
Where F is the maximum load at fracture in N, L is the distance between the two supports in millimeters with 0.01 mm accuracy, b is the width of sample in millimeters, and h is the height of sample in millimeters.[15]
Sample characterization
The morphology of the samples was depicted using a SEM device (VEGA TESCAN II). The crystal structure of the ZnO samples was determined using X-ray diffraction (XRD) (Inel equinox 3000, equipped by a Cu-Kα radiation source (λ = 1.54056 Å) in the range of 20°–75° with step size of 0.1°).
Hardness test
Twelve specimens (6 mm in diameter and 2 mm in height) were prepared in rectangular-shaped stainless steel split mold (n = 3). The GIC powders of each group were mixed with its corresponding liquid according to the manufacturer's instructions. After that, the mold was filled by the mixture and covered with two Mylar strips at top and bottom (50 ± 3 μm thickness) and glass slides to remove excess materials. After setting, the specimens were removed from the mold and stored in distilled water at 37°C for 24 h before testing (Peco PL-455, Pooya Electronic Co., Iran). Afterward, the dimensions of the samples were measured twice by a digital caliper with 0.01 mm accuracy. Hardness was measured using a Vickers hardness device (an accuracy of 0.025 μ, HVS-1000, Laizhou Huayin Testing Inc., Taiwan). Ten hardness measurements were made for each specimen and averaged. The surface of each sample was first inspected at × 125 magnification, and a smooth area was selected. An indent was created in the chosen area using an indenter with 500 g load for 15 s. The size of the engraved indent was then measured at × 125 magnification, and the Vickers hardness number of the surface was calculated using the following formula: HV = 1.8544F/d2, where F is the load applied by the indenter and d is the mean diameter of the engraved diamond-shaped indent. Hardness test was performed 10 times for each sample at 10 different sites that placed 1.5 mm far from each other.
Statistical analysis
Data were analyzed using the Shapiro–Wilk test to assess the normality of the data, one-way ANOVA to compare the four groups in terms of flexural strength and surface hardness, and Tukey's test for pairwise comparisons (P < 0.05) (SPSS v12.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Figure 1a-c shows the SEM images of hydrothermally synthesized ZnO nanoparticles. Figure 1a shows the formation of low aspect ratio nanospherical-shaped particles with average size of about 25 nm. As can be seen, these nanoparticles are aggregated severely to each other due to the Van der Waals forces. By changing the acidity of hydrothermal bath to pH of 11, the nanoparticles tend to have preferential growth leading to high aspect ratio nanorods [Figure 1b]. Figure 1c shows a flower-like morphology with scaled flakes with the thickness of ~31 nm. The XRD patterns of ZnO nanoflower sample in Figure 1d show that nanoparticles are well crystallized in wurtzite hexagonal crystal structure without the existence of any impurities.
Flexural strength
The mean flexural strength in the four groups is presented in Table 1. Shapiro–Wilk test showed a normal distribution of the control (P = 0.99), nanospherical (P = 0.05), nanoflower (P = 0.29), and nanorod (P = 0.45) groups. There was a nonsignificant difference among experimental and control groups. Although the mean flexural strength in nanorod ZnO group was approximately three units higher than that in other groups, this difference was not statistically significant (P = 0.17) [Table 2].
Table 1.
Mean of the flexural strength and surface hardness of specimens in all groups
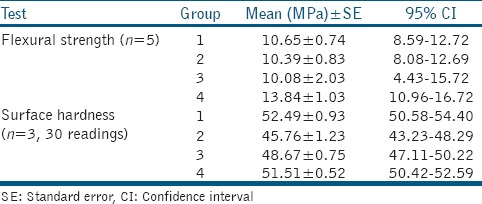
Table 2.
Pairwise comparison of surface hardness in all groups

Surface hardness results
The mean surface hardness in the four groups is presented in Table 2. Shapiro–Wilk test was first used to assess the normality of the surface hardness data in the control (P = 0.30), nanospherical (P = 0.97), nanoflower (P = 0.31), and nanorod (P = 0.27) groups. Significant differences were found in all experimental groups (P < 0.0001), except the difference between the experimental and control group (P > 0.05).
Tukey's test was applied for pairwise comparisons [Table 2]. The mean hardness of the control group was significantly higher than that of nanospherical (P < 0.001) and nanoflower (P = 0.017) ZnO groups, while no significant difference was noted in the mean hardness of control and nanorod ZnO groups (P > 0.05). Moreover, nanospherical ZnO group showed a significantly lower mean surface hardness than the nanorod ZnO group (P < 0.001). Other comparisons did not yield a significant difference.
DISCUSSION
GICs are a group of dental cements suitable for use in patients with high rates of caries formation due to the release of fluoride and appropriate chemical and micromechanical bonds to tooth structure.[16] Since their introduction, GICs have undergone several modifications; however, their low fracture toughness and strength remain as their major shortcomings.[2] This study sought to assess the flexural strength and surface hardness of Fuji II self-cure GICs containing 5 wt% ZnO nanoparticles in three different shapes.
Flexural test was used to investigate the strength of GIC-incorporated test groups. Crack propagation is the main reason for fracture of these materials, which is induced by application of tensile load.[17] For assessment of the mechanical properties of brittle dental materials such as dental cements, flexural strength test has gained some superiority over the compressive strength test. It was stated that the flexural strength test was the best method for assessment of mechanical properties of GICs. Compressive strength cannot be a suitable representative of the mechanical properties of these materials[18] since fracture in the matrix of GICs occurs as the result of shear and tensile loads in atomic scale.
Our results showed that the difference of flexural strength among tested materials was not statistically significantly; although 5 wt% nanorod ZnO group showed slightly higher values, it did not reach the significance level. We found that incorporation of ZnO nanoparticles did not enhance flexural strength; however, the effect of incorporation of nanorod ZnO particles on other properties requires further investigations. Garcia-Contreras et al., in 2015, showed that addition of TiO2 nanoparticles in 3 wt% and 5 wt% concentrations to conventional GICs decreased their flexural strength; they explained that this finding may be due to nonuniform and nonhomogeneous distribution of nanoparticles in GI powder and inefficient bonds between nanoparticles and polyacrylic ionomer.[19]
An interesting finding in our study was that the mean flexural strength of GIC containing 5 wt% nanospherical ZnO particles was slightly lower than that of the control group, which was similar to the results of Garcia-Contreras et al.[19] This finding may be attributed to the spherical shape of nanoparticles and their agglomeration. The spherical-shaped nanoparticles used in our study measured smaller than 100 nm in length, width, and height (mean size of 20 nm). Due to the presence of Van der Waals forces, they tended to stick together and form agglomerates [Figure 1a]; therefore, a high volume of particles cannot be bonded to the cement matrix. Owing to this limitation, the cement is weak at the site of presence of agglomerates and cracks initiate at these sites.[20]
Another finding of our study was the lower mean flexural strength of GIC containing 5 wt% nanoflower ZnO than that of other groups. In our study, these particles had a flower-shaped structure providing high surface area with low risk of agglomeration. The fabricated nanoflower particles had 2 nm petal thickness and 2 μ petal diameter [Figure 1b]. This particular morphology completely prevents agglomeration.[20] However, lower flexural strength in this group may be due to the microstructural defects in the petals and weak bonds between the molecules at these sites, resulting in inefficient bonds between the petals and the cement matrix. Large diameter of petals may be another reason for low flexural strength since it causes porosities.[20] The mean flexural strength of nanorod ZnO particles was slightly (but not significantly) higher than that of other groups. In these particles, one dimension was larger than the others. The rods had a round cross-section with < 100 nm diameter. In this study, nanorods had a uniform thickness of < 20 nm and length of over 1 μ [Figure 1c]. Nanorod ZnO particles have very high tensile strength due to the presence of covalence and ionic bonds in their structure and have considerable electrical conductivity. Nanorod particles have a lower risk of agglomeration than spherical nanoparticles and are better distributed in the matrix. They form transverse Van der Waals bonds, which may prevent crack propagation and increase flexural strength. They also increase the strength by filling the porosities in the material's structure.[20]
The most commonly used methods for assessment of surface hardness include Knoop, Vickers, and Brinell's hardness tests. In this study, the Vickers hardness test was used. According to Wang et al.,[21] Vickers hardness test is more commonly used test for the measurement of microhardness in brittle and highly fragile materials such as ceramics. Brinell's method is often used for measurement of hardness of metals and Knoop's method is used for measurement of enamel hardness.[22]
The current results showed that the mean surface hardness of GICs containing nanospherical and nanoflower ZnO particles was significantly lower than that of the control group while the difference between the nanorod and control groups was not significant. Similarly, Garcia-Contreras et al. showed that addition of TiO2 nanoparticles decreased the surface hardness of GICs. This reduction was attributed to the absence of glass particles in the surface. In other words, nanoparticles were not distributed homogeneously in the cement and formed agglomerates on the surface in such a way that nanoparticle agglomerates had mainly occupied the surface.[19] In contrast to our study, Elsaka et al. demonstrated that the surface hardness of GIC containing 3 wt% TiO2 was increased nonsignificantly compared to the control group; this can be due to the interactions within the matrix, which led to higher reactions between the liquid (acid) and nanoparticles. On the other hand, the increase of the weight percentage of nanoparticles (5 and 7 wt%) in their study leads to the reduction of the surface hardness of the GICs; this can increase the risk of their agglomeration and consequent reduction in mechanical properties such as surface hardness.[23] In addition, ZnO nanoparticles are softer than glass particles,[24] and their agglomeration on the surface might increase the chances of their indentations. In this case, the hardness value displayed by the tester drops which results in reduction in the final hardness value. Furthermore, accumulation of agglomerates on the surface, inefficient reaction between nanoparticles and cement liquid, and formation of porosities (particularly in use of nanoflower particles) can all decrease the mechanical properties of the cement. Further, microscopic studies at the indentation sites can provide useful information in this respect.
As seen in Table 1, the effect of incorporation of ZnO nanoparticles on the mean flexural strength of GIC was lower than its effect on the mean surface hardness. In other words, addition of 5 wt% nanospherical and nanoflower ZnO to GIC caused approximately 10% reduction in surface hardness compared to the control group while it caused no change in flexural strength. However, the same result was not obtained for nanorod particles. Thus, it can be concluded that incorporation of ZnO nanoparticles does not cause a significant change in flexural strength but decreases the surface hardness. In other words, incorporation of ZnO nanoparticles decreased one mechanical property while it had no effect on the other.
As a final point, GICs containing ZnO may be used in areas of high-risk caries, i.e., under orthodontic brackets and for Class II restorations as a base/liner to benefit from their antibacterial activities in gingival margins of the restorations.
Considering the above-mentioned points, future studies are recommended to assess the efficacy of incorporation of salinizing agents such as poly dimethyl silane for silanization and decreasing the agglomeration of nanospherical particles as well as their optimal weight percentage.
CONCLUSIONS
Within the limitations of this in vitro study, we found that incorporation of nanospherical and nanoflower ZnO particles to GIC did not change its flexural strength. However, incorporation of nanorod ZnO particles to GIC slightly (but not significantly) increased its flexural strength. Addition of nanospherical and nanoflower ZnO particles to GIC significantly decreased its surface hardness. Addition of nanorod ZnO particles slightly (but not significantly) decreased the surface hardness.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wilson AD, McLean JW. Glass-ionomer Cement. 2nd ed. Chicago: Quintessence Books; 1988. [Google Scholar]
- 2.Khoroushi M, Keshani F. A review of glass-ionomers: From conventional glass-ionomer to bioactive glass-ionomer. Dent Res J (Isfahan) 2013;10:411–20. [PMC free article] [PubMed] [Google Scholar]
- 3.Culbertson BM. New polymeric materials for use in glass-ionomer cements. J Dent. 2006;34:556–65. doi: 10.1016/j.jdent.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Zoergiebel J, Ilie N. Evaluation of a conventional glass ionomer cement with new zinc formulation: Effect of coating, aging and storage agents. Clin Oral Investig. 2013;17:619–26. doi: 10.1007/s00784-012-0733-1. [DOI] [PubMed] [Google Scholar]
- 5.Iazzetti G, Burgess JO, Gardiner D. Selected mechanical properties of fluoride-releasing restorative materials. Oper Dent. 2001;26:21–6. [PubMed] [Google Scholar]
- 6.Raggio DP, Tedesco TK, Calvo AF, Braga MM. Do glass ionomer cements prevent caries lesions in margins of restorations in primary teeth.: A systematic review and meta-analysis? J Am Dent Assoc. 2016;147:177–85. doi: 10.1016/j.adaj.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Ilie N, Hickel R, Valceanu AS, Huth KC. Fracture toughness of dental restorative materials. Clin Oral Investig. 2012;16:489–98. doi: 10.1007/s00784-011-0525-z. [DOI] [PubMed] [Google Scholar]
- 8.Roulet JF. Benefits and disadvantages of tooth-coloured alternatives to amalgam. J Dent. 1997;25:459–73. doi: 10.1016/s0300-5712(96)00066-8. [DOI] [PubMed] [Google Scholar]
- 9.Ozak ST, Ozkan P. Nanotechnology and dentistry. Eur J Dent. 2013;7:145–51. [PMC free article] [PubMed] [Google Scholar]
- 10.Lu X, Zhang B, Wang Y, Zhou X, Weng J, Qu S, et al. Nano-ag-loaded hydroxyapatite coatings on titanium surfaces by electrochemical deposition. J R Soc Interface. 2011;8:529–39. doi: 10.1098/rsif.2010.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan TN, Buckner T, Sheng J, Baldeck JD, Marquis RE. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol. 2004;19:31–8. doi: 10.1046/j.0902-0055.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 12.Yu JX, Li TH. Distinct biological effects of different nanoparticles commonly used in cosmetics and medicine coatings. Cell Biosci. 2011;1:19. doi: 10.1186/2045-3701-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–84. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed Hamouda I. Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res. 2012;26:143–51. doi: 10.7555/JBR.26.20120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy KP. International Organization for Standardization (ISO) standards as viewed from a US perspective. J Acoust Soc Am. 2005;118:1841–2. [Google Scholar]
- 16.Berg JH, Croll TP. Glass ionomer restorative cement systems: An update. Pediatr Dent. 2015;37:116–24. [PubMed] [Google Scholar]
- 17.Lohbauer U. Dental glass ionomer cements as permanent filling materials? – Properties, limitations and future trends. Materials. 2009;3:76–96. [Google Scholar]
- 18.Prosser HJ, Powis DR, Wilson AD. Glass-ionomer cements of improved flexural strength. J Dent Res. 1986;65:146–8. doi: 10.1177/00220345860650021101. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Sakagami H, Morales-Luckie RA, Nakajima H, et al. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl Oral Sci. 2015;23:321–8. doi: 10.1590/1678-775720140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kołodziejczak-Radzimska A, Jesionowski T. Zinc oxide-from synthesis to application: A Review. Materials (Basel) 2014;7:2833–81. doi: 10.3390/ma7042833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, D'Alpino PH, Lopes LG, Pereira JC. Mechanical properties of dental restorative materials: Relative contribution of laboratory tests. J Appl Oral Sci. 2003;11:162–7. doi: 10.1590/s1678-77572003000300002. [DOI] [PubMed] [Google Scholar]
- 22.Chuenarrom C, Benjakul P, Daosodsai P. Effect of indentation load and time on knoop and vickers microhardness tests for enamel and dentin. Mater Res. 2009;12:473–6. [Google Scholar]
- 23.Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J Dent. 2011;39:589–98. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Battez AH, González R, Viesca J, Fernández J, Fernández JD, Machado A, et al. CuO, ZrO 2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear. 2008;265:422–8. [Google Scholar]