Abstract
Low fat free mass index (FFMI) is an independent risk factor for mortality in Chronic Obstructive Pulmonary Disease (COPD) not typically measured during routine care. In the current study, we aimed to derive FFMPMA from the pectoralis muscle area (PMA) and assess whether low FFMIPMA is associated with all-cause mortality in COPD cases. To achieve our objectives, we used data from two independent COPD cohorts, ECLIPSE and COPDGene.
Two equal sized groups of COPD cases (N=759) from the ECLIPSE Study were used to derive and validate an equation to calculate the FFMPMA measured using bioelectrical impedance from PMA. Then in COPDGene, we applied the equation in COPD cases (n=3121) and assessed survival. Low FFMIPMA was defined using the Schols classification (FFMI<16 in men, FFMI<15 in women) and the 5th percentile normative values of FFMI from the UK Biobank.
The final regression model included PMA, weight, sex and height and had an adjusted R2 of 0.92 with FFM as the outcome. In the test group, the correlation between FFMPMA with FFM remained high (Pearson correlation=0.97). In COPDGene, COPD cases with low FFMIPMA had increased risk of death (HR:1.6, P<0.001).
We demonstrated COPD cases with low FFMIPMA have increased risk of death.
Introduction
Low fat-free mass index (FFMI) is an independent risk factor for mortality in Chronic Obstructive Pulmonary Disease (COPD) irrespective of lung function[1–3]. The FFMI, obtained by dividing fat-free mass (FFM) by height square (units: kg/m2), is an indirect marker of muscle that includes skeletal and non-skeletal muscle mass, organs, connective tissue and bone[4]. It is not, however, measured during routine care or typically in large epidemiological cohorts investigating COPD. FFM can be measured using indirect techniques such as air displacement plethysmography (ADP), bioelectrical impedance (BIA), skin-fold anthropometry (SFA), Dual X-ray Absorptiometry (DXA) [4, 5]. ADP is a reproducible measure of FFM but assumes that the density of lean tissue is identical in all patients[6, 7]. BIA and SFA are convenient but criticized for underestimating or overestimating FFM, respectively, in comparison with the current research standard DXA[5, 8]. DXA is a more expensive method using specialized equipment involving radiation exposure and necessitating highly trained technicians typically outside of COPD clinics[9].
Low FFMI has been defined using different cut-points depending on the population. In COPD cases, a FFMI less than 16 kg/m2 in male or 15 kg/m2 in female COPD cases is often used to define low FFMI[1, 10]. The European Working Group on Sarcopenia in Older People currently recommends using measures from age and sex stratified populations to define cut-points for low FFMI[11]. As FFMI decline accelerates with age and current cut-points may under-diagnose low FFMI in overweight and obese individuals[12, 13], it is important to take age and BMI into account in addition to gender when classifying low FFMI. Examples of this include reported reference values stratified by age, sex and BMI categories based on data from 186,975 healthy individuals aged 45 to 69 years of age recruited as part of the UK Biobank [13].
Chest computed tomography (CT) scans are increasingly employed during routine care and can be used to monitor muscle mass. In COPD, CT imaging of muscle groups, including intercostal muscles and mid-thigh area, has been used to assess low muscle mass and to predict adverse clinical outcomes[14, 15]. In patients with cancer, axial CT from the third lumbar vertebra (L3) region of the spine has been used to calculate FFMI[16–18]. We previously demonstrated pectoralis muscle area (PMA) is a reproducible measure of muscle mass that can be easily obtained from chest CT scans[19, 20]. Further, low PMA has been associated with increased risk of death[21] and muscle loss among cancer cases[22]. As low-dose CT for lung cancer screening has become part of the standard of care for high risk current and former smokers[23, 24], PMA can be obtained without increasing the cost or radiation exposure. However, the relationship between PMA and FFM has not been explored in detail among COPD cases. To do so would require a large cohort of COPD cases with both FFM measured and chest CT. Our goal was to derive FFM from PMA (FFMPMA) and assess whether low FFMIPMA is associated with all-cause mortality in COPD cases. To achieve our objectives, we compared PMA to FFM measured from BIA and used the relationship to derive a formula to calculate FFM from PMA (FFMPMA) measured in a large cohort of COPD cases from the ECLIPSE study. We then applied the formula in an independent cohort of COPD cases from COPDGene to examine the relationship between low FFMIPMA and all-cause mortality. We used FFMIPMA (as opposed with FFMPMA) to assess survival due to the established cut-points for defining low FFMI [1, 10].
Materials and Methods
Access to data was obtained and analyses performed after obtaining approval from the Institutional Review Boards at all participating centers in ECLIPSE and COPDGene, including Brigham and Women’s Hospital and University of Alabama at Birmingham. The ECLIPSE and COPDGene studies have been described in detail elsewhere [25, 26]. Briefly, ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) study (SCO104960, NCT00292552, www.eclipse-copd.com) recruited COPD patients and controls with a smoking history of ≥ 10 pack-years, aged 45-75 years from 46 centers across 12 countries(11). The COPDGene Study (NCT00608764, www.copdgene.org) enrolled ever smokers with and without COPD (Non-Hispanic Whites and African Americans), aged 45-80 with at least 10 pack-years of lifetime smoking history from 21 U.S. centers(14).
COPD was defined by the presence of Global Initiative for Chronic Obstructive Lung Disease (GOLD) lung function spirometry grades 2-4 with a post-bronchodilator FEV1/FVC less than 0.7 and FEV1 less than 80% predicted [27]. In ECLIPSE, the FFM was derived from BIA measured using the Bodystat 1500; Bodystat Ltd, Isle of Man, UK. The measured resistance was converted to FFM using the formula developed using data from 1087 COPD cases by Rutten et al 2010[28]:
Quantitative assessments of the PMA were obtained from a single axial slice above the aortic arch by a trained analyst using in-house software as described previously [19, 29]. Once the borders of the muscles were closed by manual tracing a second filtering step was applied to remove all intra-muscular voxels such as those representing adipose tissue that were outside of an tissue attenuation range of -50 to 90 Hounsfield Units. The aggregate PMA (in cm2) was calculated as the sum of the cross sectional area of right and left pectoralis major and minor muscles in that single slice. Quality control of the data involved 2 steps. All segmentations were visually inspected by pulmonologists with experience in lung imaging (AD, GW). Segmentation failures were identified (e.g. malposition of the arm, anatomic distortion due to prior chest wall surgery) and excluded. In a second step, the distributions of PMA values for each muscle group (right, left, major and minor muscles) were examined and the CT scans for individuals with muscle group values greater than 6SD from the median values were re-inspected and exclusion made if supported by scan re-inspection. One COPD case in ECLIPSE and one in COPDGene had duplicate chest CTs and in both cases the PMA from the first chest CT was arbitrarily selected for each duplicate and the second measurement excluded. Analyses in ECLIPSE was further restricted to COPD cases who had a chest CT within 60 days of FFM measured using BIA. A total of 98.0% of COPD cases in ECLIPSE with PMA measured on chest CT and phenotype data passed quality control and were included in the analyses. A total of 98.3% of COPD cases in COPDGene with chest CT and phenotype data including mortality information passed quality control and were included in the analyses.
All statistical analyses were performed using the program R. The ECLIPSE sample was randomly split into training and test groups each comprised of 759 COPD cases. The training group was used to develop a formula to predict FFMPMA and the test group was used to objectively test the performance of this formula. T-tests and chi-square tests were used to test for differences in general characteristics between the training and test groups prior to model building. In the training group, manual stepwise multiple regression analysis was performed with FFM as the dependent variable considering weight, sex and height as independent variables (P<0.05). The adjusted R2 correlation was used as a prediction model quality parameter. Variable selection was further assessed using automatic backwards and forwards stepwise multiple regression based on Akaike Information Criterion (AIC). In the test group, the Pearson correlation coefficient was used to assess the agreement between FFM from BIA with FFM derived from PMA. A Bland-Altman plot was used to check for systematic bias across the range of FFM values in the test group. FFMI was generated by dividing FFM by height squared in meters. FFMIPMA is used to differentiate FFMI derived from PMA on chest CT from FFMI measured using BIA throughout the manuscript.
Survival modeling in COPDGene, using the September 2016 mortality dataset, was performed using Kaplan-Meier curves and Cox proportional hazards regression. Cox proportional hazards models included adjustment for categorical variables sex, race, GOLD grade, number of comorbidities as well as the continuous measures of age and smoking duration (ATS pack-years). All covariates were collected at baseline. Race was based on self-report and GOLD grade by spirometry. Comorbidities were assessed by self-reported physician diagnosed cancer, congestive heart failure, coronary artery disease, diabetes, hypertension, heart attack, stroke and gastroesophageal reflux. Low FFMIPMA was defined using two criteria: the Schols classification and UK Biobank normative values. The Schols classification characterizes male COPD cases with FFMIPMA <16 and female COPD cases with FFMIPMA <15 as having low FFMIPMA[1]. UK Biobank normative values were used to classify low FFMIPMA when FFMIPMA was less than 5th percentile normative values for the appropriate age, sex and BMI categories in healthy individuals from the UK Biobank[13].
Results
To derive total body FFM from PMA, COPD cases from ECLIPSE were randomized into two equal sized test and training groups. The test and training groups did not differ significantly based on age, sex, weight, height, current smoking or lung function measurements (Table 1). The unadjusted R2 correlation between PMA and FFM was 0.38 in the training group of COPD cases (Table 2). The fully adjusted final model is depicted in equation 1:
| [1] |
Table 1.
Comparison of baseline characteristics between training and test groups of COPD cases in ECLIPSE. Unless otherwise denoted values are represented as median (interquartile range). Training and test groups did not differ significantly (P<0.05) for any characteristic listed in the table.
| Training | Test | |
|---|---|---|
| N | 759 | 759 |
| Sex (% Male) | 65 | 64 |
| Age | 64 (10) | 64 (10) |
| Weight (kg) | 75.3 (23.1) | 74.2 (23) |
| Height (m) | 1.69 (0.12) | 1.69 (0.13) |
| BMI | 26.3 (6.4) | 25.8 (6.9) |
| Current Smoking (%) | 35.8 | 35.4 |
| FEV1 % | 47.6 (26.1) | 47.1 (22.9) |
| FEV1/FVC | 0.57 (0.23) | 0.57 (0.22) |
Abbreviations: BMI – body mass index, FEV1pp – Forced Expiratory Volume in 1 second percent predicted, FEV1/FVC – Forced Expiratory Volume in 1 second / Forced Vital Capacity.
Table 2.
Manual stepwise model building in the training group of ECLIPSE COPD cases to generate formula to convert PMA to FFM.
| Independent Variable | Adjusted R2 | Beta | P-value |
|---|---|---|---|
| Model 1 | 0.38 | ||
| PMA (cm2) | 0.79 | <0.0001 | |
| Model 2 | 0.79 | ||
| PMA (cm2) | 0.4 | <0.0001 | |
| Weight (kg) | 0.41 | <0.0001 | |
| Model 3 | 0.90 | ||
| PMA (cm2) | 0.08 | <0.0001 | |
| Weight (kg) | 0.38 | <0.0001 | |
| Sex (ref: male) | 9.5 | <0.0001 | |
| Model 4 | 0.92 | ||
| PMA (cm2) | 0.09 | <0.0001 | |
| Weight (kg) | 0.35 | <0.0001 | |
| Sex (ref: male) | 7.5 | <0.0001 | |
| Height (cm) | 0.20 | <0.0001 |
Abbreviation: PMA – pectoralis muscle area, FFM – fat-free mass.
This equation included PMA, weight, sex and height and had an adjusted R2 correlation of 0.92 with total body FFM in the test group.
We then generated FFMPMA in the test group using Equation 1. In this subgroup, the FFM and FFMPMA were strongly related (R=0.973, 95% Confidence Interval (CI): 0.969-0.976, P<2.2×10−16) (Figure 1a). The correlation between FFM and FFMPMA was 0.935 (95% CI: 0.922-0.945, P<2.2×10−16) and 0.967 (95% CI: 0.958-0.974, P<2.2 × 10−16) between male and female COPD cases in the ECLIPSE test group, respectively (Supplementary Figure 2). The Bland-Altman plots (Figure 1b and Supplementary Figure 2) further supported excellent agreement between total body FFM and FFMPMA across the range of values in the test group. The mean difference between FFMPMA and total body FFM in the test group was -0.28 kg corresponding to −0.5% of FFM. The limits of agreement related to the mean difference between FFMPMA and FFM were ± 4.7 kg.
Figure 1.
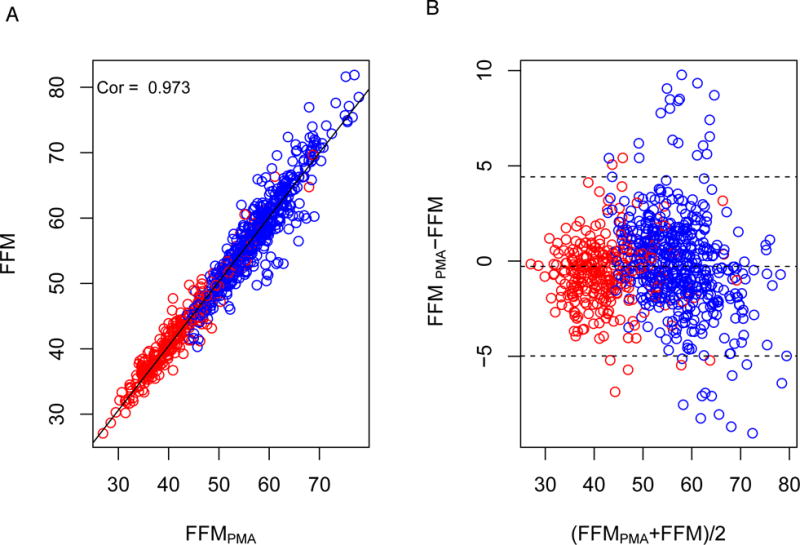
A. Relationship between FFMPMA and FFM in test group comprised of 783 ECLIPSE COPD cases. B. Bland-Altman plot comparing FFMPMA and FFM in the test group. Abbreviations: FFMI – fat-free mass index, FFMIPMA – fat-free mass index derived from pectoralis muscle area, COPD – chronic obstructive pulmonary disease
Low FFMI in overweight and obese COPD cases
We next used equation (1) to calculate FFMPMA in COPDGene. We divided FFMPMA by height squared to generate the index (FFMIPMA) in order to classify cases who have low FFMIPMA. In Table 3 we noted no COPD case with BMI greater than 25 was classified as having low FFMIPMA using the Schols classification criteria[1]. When we used the UK Biobank normative values based on the 5th percentile values from age, sex and BMI stratified groups reported by Franssen et al[13], 11% of COPD cases who were overweight and 4.2% of COPD cases who were obese had low FFMPMA. Further, using the UK Biobank normative values, significantly fewer COPD cases with low BMI had low FFMIPMA (Table 3: 92% versus 19.3%).
Table 3.
Criteria used to define low FFMI stratified by BMI category in COPD cases in COPDGene. Values denote N with percentage of BMI category in parentheses.
| BMI Category | Schols Classification | UK Biobank Normative Values | ||
|---|---|---|---|---|
| Normal | Low FFMIPMA | Normal | Low FFMIPMA | |
| <18.5 | 7 (8%) | 81(92%) | 71 (81%) | 17 (19%) |
| 18.5-25 | 668 (71%) | 277 (29%) | 720 (76%) | 225 (24%) |
| 25-30 | 1028 (100%) | 0 (0%) | 915 (89%) | 113(11%) |
| >30 | 1060 (100%) | 0 (0%) | 1015 (96%) | 45 (4%) |
Abbreviations: N – number of individuals, BMI – body mass index, FFMIPMA – fat-free mass index derived from pectoralis muscle area, UK – United Kingdom.
Relationship between FFMI derived from chest CT and survival in COPDGene
Next, we aimed to examine the survival experience stratified by low FFMIPMA. We began by exploring anthropometric and lung function characteristics between COPD cases in ECLIPSE and COPDGene. Overall, COPD cases from COPDGene had more females, African Americans and current smokers, weighed more, had higher FEV1 percent predicted, a lower FEV1/FVC and had less emphysema than COPD cases found in ECLIPSE (Table 4).
Table 4.
Characteristics of COPD cases from ECLIPSE and COPDGene used in the current analyses. Unless otherwise denoted values represent median with interquartile range in parentheses.
| Characteristic of COPD cases | ECLIPSE | COPDGene | P | |
|---|---|---|---|---|
| N | 1518 | 3121 | ||
| Sex (% Male) | 64.3 | 55.5 | <0.01 | |
| Age | 64 (10) | 63.7 (12.8) | 0.42 | |
| Weight-kg | 74.8 (23) | 80 (25.9) | <0.01 | |
| Height - m | 1.7 (0.13) | 1.7 (0.14) | 0.1 | |
| BMI (%) | <18.5 | 4.9 | 2.8 | <0.01 |
| 18.5-25 | 36.3 | 30.3 | ||
| 25-30 | 36.6 | 32.9 | ||
| >30 | 22.3 | 34 | ||
| Race (%) | NHW | 97.5 | 80.3 | <0.01 |
| AA | 1.8 | 19.7 | ||
| Other | <1 | 0 | ||
| Current Smoking (%) | 35.6 | 39.5 | 0.07 | |
| FEV1pp | 47.4 (24.3) | 51.3 (30) | <0.01 | |
| FEV1/FVC | 0.57 (0.22) | 0.5 (0.22) | <0.01 | |
| % Emphysema | 15.3 (17.4) | 8.7 (18.2) | <0.01 | |
| FFMIPMA | 18 (3.3) | 18.4 (3.6) | <0.01 | |
Abbreviations: BMI – body mass index, FEV1pp – Forced Expiratory Volume in 1 second percent predicted, FEV1/FVC – Forced Expiratory Volume in 1 second / Forced Vital Capacity.
The median FFMIPMA derived from CT in COPDGene was slightly higher (median: 18.4 kg/m2, range: 11.1 – 29.5) than COPD cases in ECLIPSE (median 18.0 kg/m2, range: 11.5 – 28.1). Initially, we used a FFMIPMA <16 kg/m2 in male COPD cases and FFMIPMA <15 kg/m2 in female COPD cases to define low FFMIPMA. In our analysis among 3121 subjects with PMA data and longitudinal follow-up in COPDGene, there were 729 deaths with a median follow-up of 6.25 years. COPD cases with low FFMIPMA were more likely to die than those whose FFMIPMA was above this threshold (Figure 2a). We further examined the relationship between all-cause mortality and low FFMIPMA in COPD cases using Cox regression modeling accounting for sex, age, race, smoking duration, GOLD category and number of comorbidities. When controlling for these additional covariates, a low FFMIPMA was still associated with a 60% increased risk of death (Table 5 Model I: HR: 1.6, 95% CI 1.2-1.9, p<0.001) in COPDGene. These results were also observed when we defined a low FFMPMA using the UK Biobank normative values (Table 5 Model II: HR: 1.5, 95% CI 1.2-1.8, p<0.001).
Figure 2. Kaplan Meier Survival Stratified by Low FFMI.
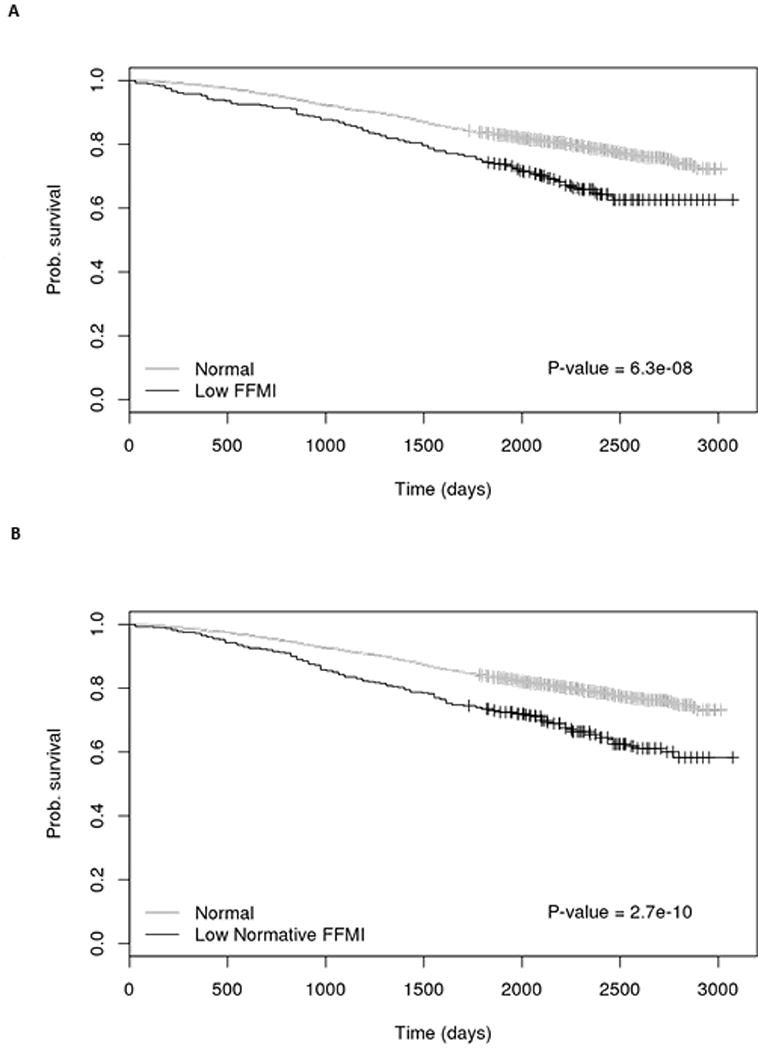
A. Low FFMIPMA defined using Schols cut-points of FFMIPMA <16 in men and FFMIPMA <15 in women[1] B. Low FFMIPMA defined by 5th percentile of normative FFMIPMA stratified by gender, age and BMI categories in the UK Biobank cohort[13]. Abbreviations: FFMI – fat-free mass index, FFMIPMA – fat-free mass index derived from pectoralis muscle area, COPD – chronic obstructive pulmonary disease
Table 5.
Relationship between risk of death from any cause with low FFMI in COPD cases from COPDGene.
| Covariate | HR (95% CI) | P-value | ||
|---|---|---|---|---|
| Model I | Low FFMIPMA | 1.6(1.2-1.9) | <0.001 | |
| Gender (ref - Male) | 0.82 (0.69-0.97) | 0.017 | ||
| Age (ref 45-55 years) | 55-65 | 1.1 (0.88-1.5) | 0.31 | |
| 65-55 | 1.6 (1.3-2.1) | <0.001 | ||
| >75 | 2.9 (2.2-3.9) | <0.001 | ||
| Race (ref NHW) | 1.2 (0.98-1.5) | 0.08 | ||
| log10(ATS Pack-Years Smoking) | 1.8 (1.3-2.6) | 0.001 | ||
| Final GOLD (ref GOLD=2) | 3 | 1.8 (1.5-2.2) | <0.001 | |
| 4 | 4.8 (4.0-5.8) | <0.001 | ||
| Self-Reported Co-Morbidities | 1-2 | 1.1(0.9-1.3) | 0.31 | |
| 3 or more | 1.5(1.2-1.8) | <0.001 | ||
| Model II | Low Normative FFMIPMA | 1.5 (1.2-1.8) | <0.001 | |
| Gender (ref - Male) | 0.94 (0.81-1.1) | 0.46 | ||
| Age (ref 45-55 years) | 55-65 | 1.1 (0.88-1.5) | 0.32 | |
| 65-55 | 1.7 (1.3-2.2) | <0.001 | ||
| >75 | 3.0 (2.2-4.0) | <0.001 | ||
| Race (ref NHW) | 1.2 (1.0-1.5) | 0.049 | ||
| log10(ATS Pack-Years Smoking) | 1.8 (1.3-2.6) | <0.001 | ||
| Final GOLD (ref GOLD=2) | 3 | 1.8 (1.5-2.1) | <0.001 | |
| 4 | 4.9 (4.1-5.9) | <0.001 | ||
| Self-Reported Co-Morbidities | 1-2 | 1.1(0.91-1.3) | 0.39 | |
| 3 or more | 1.4 (1.2-1.8) | 0.001 | ||
Abbreviations: FFMIPMA- Fat-free mass index derived from pectoralis muscle area, GOLD – Global Initiative for Chronic Lung Disease, ATS – American Thoracic Society.
Discussion
We have generated a formula to derive the FFMPMA and the FFMIPMA using the PMA obtained from chest CT scans of ever smokers with COPD. We have shown that a low FFMIPMA is a significant predictor of all-cause mortality in COPD cases independent of sex, age, race, smoking duration, GOLD grade and number of comorbidities. Our research has highlighted the need for further research defining thresholds for low FFMI in overweight and obese COPD cases but we believe that our methods will permit additional investigation of FFMI in COPD populations who may only have a thoracic CT scan available for monitoring body composition.
In the current report, we used a measure of muscle mass obtained from the trunk region to derive FFMIPMA in COPD cases and demonstrated a strong correlation between the PMA and BIA based assessment of FFM in unadjusted and models which include sex, height and weight. Improvement in model performance with the inclusion of such anthropomorphics is not surprising given the sexually dimorphic nature of body composition. Differential wasting in COPD has been observed at both whole-body and extremity levels [30, 31]. The high performance of our equation to derive total body FFM from PMA in the test group may indicate muscle wasting in COPD is more uniformly distributed than previously determined or that our benchmark BIA is insensitive to these regional differences.
In this study, we demonstrated decreased survival associated with low FFMIPMA derived from PMA measured on CT when adjusting for lung function categorized by GOLD stage. Although COPD is still primarily diagnosed based on airflow obstruction, the significant endpoints for patients such as those affecting quality of life and disease progression are often independent of degree of airflow obstruction[32]. The prognostic utility of FFMI is well-supported, as several studies have demonstrated increased morbidity and mortality among COPD cases with low FFMI[1, 2, 33]. Thus assessing muscle function (including mass and strength) is encouraged in clinical practice. In fact, the identification of patients with low muscle mass is useful to tailor treatments to improve muscle function including exercise training, nutrition, and hormone therapy[34]. Although our analysis does not allow us to have insight into the mechanisms linking low muscle mass and all-cause mortality, prior data in patients with COPD suggested that one of potential mechanisms is systemic inflammation. COPD subjects with elevated levels of plasma fibrinogen -a blood biomarker for systemic inflammation- had low FFM[2], and higher risk of death compared to those with low fibrinogen levels[35]. Another possibility is that low muscle mass might reflect decreased physical activity, which in turn is associated with increased all-cause mortality[36].
It is interesting that when the Schols criteria were used to classify low FFMIPMA in COPD cases, we observed no overweight COPD case in COPDGene with low FFMIPMA. Other studies in COPD cases have also observed few COPD cases with low FFMI in overweight and obese cases using this cut-point[37, 38]. The converse is observed in populations with lower BMI than in the United States, such as in Asia, where a higher number of cases would have low FFMI based on the Schols cut-point and lower cut-points are recommended to define low muscle mass to diagnose sarcopenia[39]. As populations become more admixed, information on race group will become harder to use to characterize individuals. Cut-points defined using information such as BMI, age and gender irrespective of race group are more relevant. Further, obesity is a risk factor for COPD and the prevalence of obesity continues to rise[40]. As current cut-points for low FFMI indicate decreased survival for those with low FFMI, under-diagnosing low FFMI in an ever-increasing population of overweight and obese COPD cases is problematic. Cut-points for low FFMI that give consideration to BMI, age and gender will have greater utility over the long-term.
There are several limitations to the current investigation, which must be addressed. We used total body FFM from BIA to generate the equation to derive total body FFM from PMA. The gold standard research method for measuring FFM in research is DXA, as BIA has been show to underestimate FFM and is sensitive to the patient’s hydration status[41]. In our analysis, we employed a formula to convert BIA-derived total body FFM to a value that accounts for underestimation of FFM from BIA in COPD cases[28]. We do not a present a method for deriving FFM from DXA, we present a method for deriving FFM from BIA using PMA from chest CT. BIA is a validated technique for monitoring for FFM. For example, the European Society of Clinical Nutrition and Metabolism (ESPEN)[42], the European Respiratory Society[43] and the international consensus paper on cancer cachexia[44] list BIA as a method for monitoring FFMI, an essential component for assessing malnutrition, cachexia, sarcopenia and frailty. However, additional research focused on generating a formula to derive FFM measured via DXA from PMA may refine the equation we present in the current manuscript. Further, we used PMA from chest CT and FFM from BIA at the baseline visit in ECLIPSE to generate the prediction formula and not every participant had chest CT and BIA measured on the same day. To address this limitation, we restricted our analyses to include COPD cases from ECLIPSE with chest CT and BIA within 60 days. Further for the cox regression analyses, self-reported comorbidities were used in our analyses and are subject to recall bias. Nonetheless, COPD cases with low FFMIPMA had similar increased risk of death survival (HRSchols cut-point: 1.6, 95%CI: 1.2-1.8) to other COPD cases in other studies examining the relationship of low FFMI and all-cause mortality. For example, in a cohort of 1898 COPD cases, Vestbo et al. reported an increased risk of death (HR: 1.5, 95%CI: 1.2-1.8) associated with low FFMI[2].
Thus, we report a formula which can be used to establish an estimate of total body FFM in COPD cases using information from chest CT and assist in characterizing COPD patient prognosis. This formula can be used in COPD cases with chest CT in order to expand research on muscle wasting, an understudied marker of increased risk of all-cause mortality among COPD cases. In summary, we have demonstrated that subjects with COPD and low FFMI derived from an easy-to-acquire PMA measurement on CT have an increased risk of death.
Supplementary Material
Supplementary Figure 1: Computed tomography (CT) axial slice showing the segmentation of the pectoralis muscles at the aortic arch level. The areas of the muscles were aggregated to calculate the total pectoralis muscle area (PMA)
Supplementary Figure 2: A. Relationship between FFMPMA and FFM in test group comprised of male ECLIPSE COPD cases. B. Bland-Altman plot comparing FFMPMA and FFM in the male test group. C. Relationship between FFMPMA and FFM in test group comprised of female ECLIPSE COPD cases. D. Bland-Altman plot comparing FFMPMA and FFM in the female test group. Female COPD cases are represented in red and male COPD cases are represented in blue. Note: FFM units are kg. Abbreviations: FFMI – fat-free mass index, FFMIPMA – fat-free mass index derived from pectoralis muscle area, COPD – chronic obstructive pulmonary disease
Acknowledgments
We would like to thank Mr. Andy Currie for his technical assistance in the preparation of this manuscript.
Funding: R00HL121087, R01HL122464, R01HL089856, R01HL089897 from the National Heart, Lung, and Blood Institute and the Parker B. Francis Foundation. The COPDGene® project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion. ECLIPSE was funded by GSK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agents.
Footnotes
Conception and design: MNM, ER, RH, AAD, RSJE, EFW, SIR, RC, CPH, MD, EKS, GRW
Data collection: RH, EFW, RIS, RC, MD, EKS, GRW
Data analysis: MNM, SML, AAD
Manuscript editing/ writing: MNM, ER, SML, RH, AAD, RSJE, GK, JH, BAG, EFW, SIR, RC, CPH, MD, EKS, GRW
References
- 1.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. 2005/07/09. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sorensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. 2005/12/22. [DOI] [PubMed] [Google Scholar]
- 3.Sanders KJC, Kneppers AEM, van de Bool C, Langen RCJ, Schols AMWJ. Cachexia in chronic obstructive pulmonary disease: New insights and therapeutic perspective. J Cachexia Sarcopenia Muscle. 2016;7:5–22. doi: 10.1002/jcsm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prado CMM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38:940–953. doi: 10.1177/0148607114550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner MC, Barton RL, Singh SJ, Morgan MD. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002;19:626–631. doi: 10.1183/09031936.02.00279602. 2002/05/10. [DOI] [PubMed] [Google Scholar]
- 6.Cooper C, Fielding R, Visser M, Van Loon LJ, Rolland Y, Orwoll E, Reid K, Boonen S, Dere W, Epstein S, Mitlak B, Tsouderos Y, Sayer AA, Rizzoli R, Reginster JY, Kanis JA. Tools in the assessment of sarcopenia. Calcif Tissue Int. 2013;93:201–210. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am J Clin Nutr. 2002:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 8.Ellegard LH, Ahlen M, Korner U, Lundholm KG, Plank LD, Bosaeus IG. Bioelectric impedance spectroscopy underestimates fat-free mass compared to dual energy X-ray absorptiometry in incurable cancer patients. Eur J Clin Nutr. 2009;63:794–801. doi: 10.1038/ejcn.2008.35. 2008/05/15. [DOI] [PubMed] [Google Scholar]
- 9.Thibault R, Genton L, Pichard C. Body composition: why, when and for who? Clin Nutr. 2012;31:435–447. doi: 10.1016/j.clnu.2011.12.011. 2012/02/03. [DOI] [PubMed] [Google Scholar]
- 10.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkov?? E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–672. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 13.Franssen FME, Rutten EPA, Groenen MTJ, Vanfleteren LE, Wouters EFM, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: Results from the UK biobank. J Am Med Dir Assoc. 2014;15 doi: 10.1016/j.jamda.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. 2002/09/17. [DOI] [PubMed] [Google Scholar]
- 15.Guerri R, Gayete A, Balcells E, Ramirez-Sarmiento A, Vollmer I, Garcia-Aymerich J, Gea J, Orozco-Levi M. Mass of intercostal muscles associates with risk of multiple exacerbations in COPD. Respir Med. 2010;104:378–388. doi: 10.1016/j.rmed.2009.10.015. 2009/11/26. [DOI] [PubMed] [Google Scholar]
- 16.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. 2008/10/17. [DOI] [PubMed] [Google Scholar]
- 17.Thibault R, Pichard C. The evaluation of body composition: a useful tool for clinical practice. Ann Nutr Metab. 2012;60:6–16. doi: 10.1159/000334879. [DOI] [PubMed] [Google Scholar]
- 18.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S–1137S. doi: 10.3945/ajcn.2010.28608C. 2010/02/19. [DOI] [PubMed] [Google Scholar]
- 19.McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, Eckbo E, Muralidhar N, Come CE, Cho MH, Hersh CP, Lange C, Wouters E, Casaburi RH, Coxson HO, Macnee W, Rennard SI, Lomas DA, Agusti A, Celli BR, Black-Shinn JL, Kinney GL, Lutz SM, Hokanson JE, Silverman EK, Washko GR. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. 2014/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz AA, Zhou L, Young TP, McDonald M-L, Harmouche R, Ross JC, San Jose Estepar R, Wouters EFM, Coxson HO, MacNee W, Rennard S, Maltais F, Kinney GL, Hokanson JE, Washko GR. Chest CT Measures of Muscle and Adipose Tissue in COPD. Acad Radiol. 2014;21:1255–1261. doi: 10.1016/j.acra.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsey CM, San Jose Estepar R, Van der Velden J, Cole BF, Christiani DC, Washko GR. Lower Pectoralis Muscle Area is Associated with a Worse Overall Survival in Non- Small Cell Lung Cancer. Cancer Epidemiol Biomarkers Prev. 2016 doi: 10.1158/1055-9965.EPI-15-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.S-I Go, Park MJ, Song H-N, Kim H-G, Kang MH, Lee HR, Kim Y, Kim RB, Lee Lee S, II, Lee G-W. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle. 2016 doi: 10.1002/jcsm.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiener RS, Gould MK, Arenberg DA, Au DH, Fennig K, Lamb CR, Mazzone PJ, Midthun DE, Napoli M, Ost DE, Powell CA, Rivera MP, Slatore CG, Tanner NT, Vachani A, Wisnivesky JP, Yoon SH, Black WC, Detterbeck F, Hirschowitz E, Jett J, Kinsinger L. An official American Thoracic Society/American College of Chest Physicians policy statement: Implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192:881–891. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyer VA, U.S. Preventive Services Task Force Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 25.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, Hagan G, Knobil K, Lomas DA, MacNee W, Silverman EK, Tal-Singer R. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 26.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. 2007/05/18. [DOI] [PubMed] [Google Scholar]
- 28.Rutten EPA, Spruit MA, Wouters EFM. Critical view on diagnosing muscle wasting by single-frequency bio-electrical impedance in COPD. Respir Med. 2010;104:91–98. doi: 10.1016/j.rmed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Diaz AA, Zhou L, Young TP, McDonald ML, Harmouche R, Ross JC, San Jose Estepar R, Wouters EFM, Coxson HO, MacNee W, Rennard S, Maltais F, Kinney GL, Hokanson JE, Washko GR, investigators E . Acad Radiol. Vol. 21. Elsevier USA; 2014. Chest CT Measures of Muscle and Adipose Tissue in COPD: Gender-based Differences in Content and in Relationships with Blood Biomarkers; pp. 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelen MPKJ, Schols AMWJ, Does JD, Wouters EFM. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–738. doi: 10.1093/ajcn/71.3.733. 2000/03/04. [DOI] [PubMed] [Google Scholar]
- 31.Engelen MP, Schols AM, Lamers RJ, Wouters EF. Different patterns of chronic tissue wasting among patients with chronic obstructive pulmonary disease. Clin Nutr. 1999;18:275–280. doi: 10.1016/s0261-5614(98)80024-1. 1999/12/22. [DOI] [PubMed] [Google Scholar]
- 32.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2012;187:347–365. doi: 10.1164/rccm.201204-0596PP. 2012/08/11. [DOI] [PubMed] [Google Scholar]
- 33.Mostert R, Goris A, Weling-Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:859–867. doi: 10.1053/rmed.2000.0829. 2000/09/23. [DOI] [PubMed] [Google Scholar]
- 34.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debiga??e R, Richard Dekhuijzen PN, Franssen F, Gayan-Ramirez G, Gea J, Gosker HR, Gosselink R, Hayot M, Hussain SNA, Janssens W, Polkey MI, Roca J, Saey D, Schols AMWJ, Spruit MA, Steiner M, Taivassalo T, Troosters T, Vogiatzis I, Wagner PD. An official American thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:15–62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller BE, Tal-Singer R, Rennard SI, Furtwaengler A, Leidy N, Lowings M, Martin UJ, Martin TR, Merrill DD, Snyder J, Walsh J, Mannino DM. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease: Perspective of the chronic obstructive pulmonary disease biomarker qualification consortium. Am J Respir Crit Care Med. 2016;193:607–613. doi: 10.1164/rccm.201509-1722PP. [DOI] [PubMed] [Google Scholar]
- 36.Vaes AW, Garcia-Aymerich J, Marott JL, Benet M, Groenen MTJ, Schnohr P, Franssen FME, Vestbo J, Wouters EFM, Lange P, Spruit MA. Changes in physical activity and all-cause mortality in COPD. Eur Respir J. 2014;44:1199–1209. doi: 10.1183/09031936.00023214. [DOI] [PubMed] [Google Scholar]
- 37.Vanfleteren LEGW, Spruit MA, Groenen M, Gaffron S, Van Empel VPM, Bruijnzeel PLB, Rutten EPA, Roodt JOt, Wouters EFM, Franssen FME. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 38.Breyer M-K, Spruit Ma, Celis APM, Rutten EPa, Janssen PP, Wouters EFM. Highly elevated C-reactive protein levels in obese patients with COPD: a fat chance? Clin Nutr. 2009;28:642–647. doi: 10.1016/j.clnu.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JSW, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. J Am Med Dir Assoc. Vol. 15. Elsevier Ltd; 2014. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia; pp. 95–101. [DOI] [PubMed] [Google Scholar]
- 40.Hanson C, Rutten EP, Wouters EFM, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J COPD. 2014;9:723–733. doi: 10.2147/COPD.S50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19:433–446. doi: 10.1177/0115426504019005433. 2005/10/11. [DOI] [PubMed] [Google Scholar]
- 42.Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MAE, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, Pison C, Rutten-Van Mölken M, Slinde F, Steiner MC, Tkacova R, Singh SJ. Nutritional assessment and therapy in COPD: A European respiratory society statement. Eur Respir J. 2014;44:1504–1520. doi: 10.1183/09031936.00070914. [DOI] [PubMed] [Google Scholar]
- 44.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Lancet Oncol. Vol. 12. Elsevier Ltd; 2011. Definition and classification of cancer cachexia: An international consensus; pp. 489–495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Computed tomography (CT) axial slice showing the segmentation of the pectoralis muscles at the aortic arch level. The areas of the muscles were aggregated to calculate the total pectoralis muscle area (PMA)
Supplementary Figure 2: A. Relationship between FFMPMA and FFM in test group comprised of male ECLIPSE COPD cases. B. Bland-Altman plot comparing FFMPMA and FFM in the male test group. C. Relationship between FFMPMA and FFM in test group comprised of female ECLIPSE COPD cases. D. Bland-Altman plot comparing FFMPMA and FFM in the female test group. Female COPD cases are represented in red and male COPD cases are represented in blue. Note: FFM units are kg. Abbreviations: FFMI – fat-free mass index, FFMIPMA – fat-free mass index derived from pectoralis muscle area, COPD – chronic obstructive pulmonary disease


